Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition
Abstract
1. Introduction
2. RCMD Mass Measurement Methods
3. RCMD Particle Size Characterization
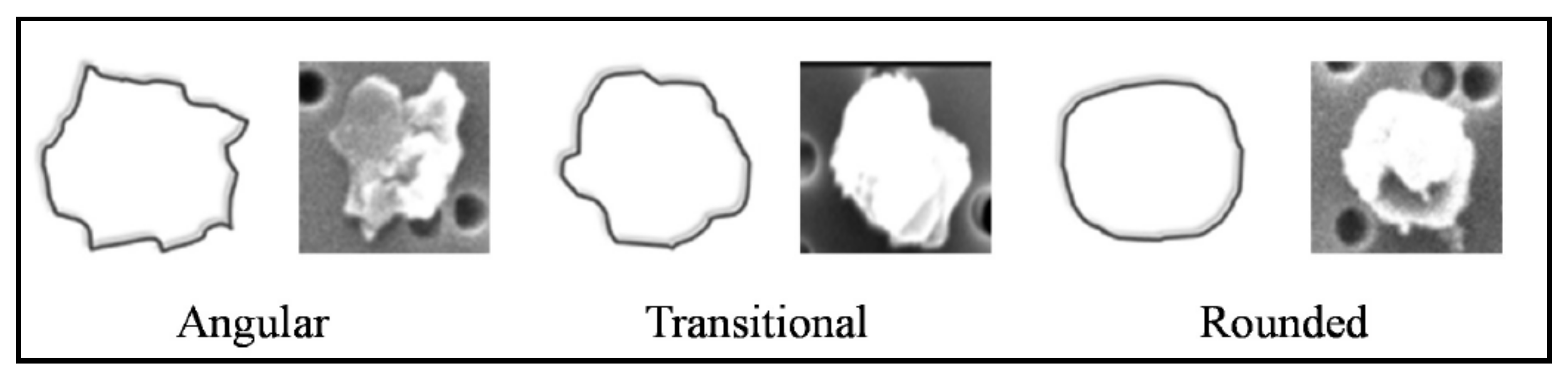
3.1. Microscopic Imaging
3.2. Aerodynamic Particle Sizing
3.2.1. Cascade Impactors
3.2.2. Electrical Low Pressure Impactor (ELPI)
3.2.3. Aerodynamic Particle Sizer (APS)
3.2.4. Aerodynamic Aerosol Classifier (AAC)
3.3. Optical Particle Sizing
3.4. Electrical Mobility Particle Sizing
3.5. Evaluation for Size Distribution Measurements in Mines
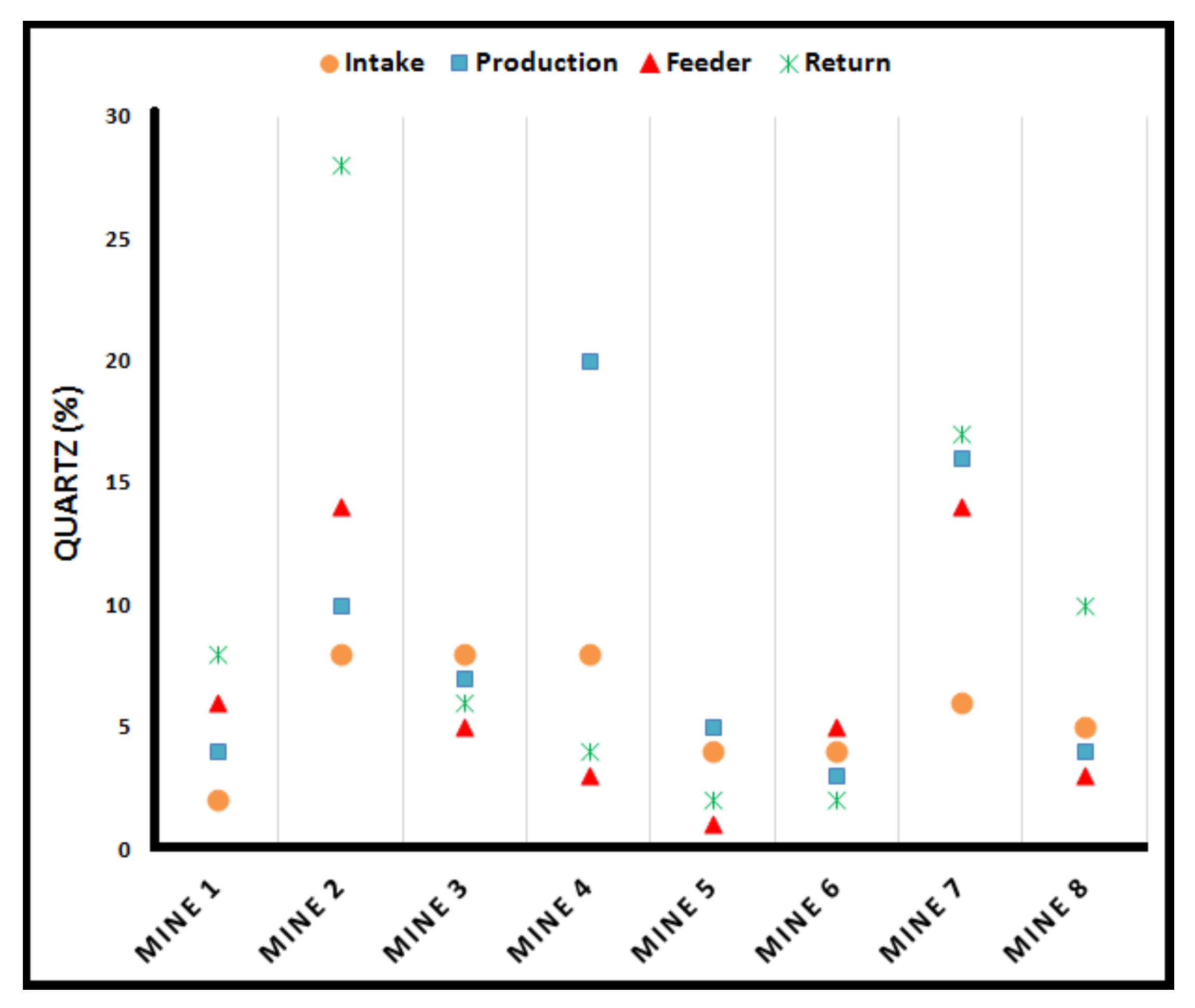
4. Chemical Composition of RCMD
4.1. Thermal Analysis Methods
4.1.1. Differential Thermal Analysis (DTA)
4.1.2. Differential Scanning Calorimetry (DSC)
4.1.3. Thermogravimetric Analysis (TGA)
4.1.4. Thermal/Optical Analysis by Reflectance and Transmittance (TOR/TOT)
4.1.5. Thermal Desorption (TD)
4.2. Spectroscopic Analysis for Chemical Compounds
4.2.1. Energy Dispersive X-ray (EDX)
4.2.2. X-Ray Fluorescence (XRF)
4.2.3. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
4.2.4. X-ray Diffraction (XRD)
4.2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
4.2.6. Raman Spectroscopy
4.2.7. 13C and 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
4.2.8. Example of Comprehensive RCMD Chemical Characterization
5. Summary and Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castranova, V.; Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 2000, 108, 675–684. [Google Scholar] [CrossRef]
- Halldin, C.N.; Wolfe, A.L.; Laney, A.S. Comparative respiratory morbidity of former and current US coal miners. Am. J. Public Health 2015, 105, 2576–2577. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Coal Workers’ Health Surveillance Program—Data Query System; NIOSH: Morgantown, WV, USA, 2016. [Google Scholar]
- Antao, V.C.; Petsonk, E.L.; Attfield, M.D. Advanced Cases of Coal Workers’ Pneumoconiosis—Two Counties, Virginia, 2006. JAMA 2006, 296, 2085. [Google Scholar] [CrossRef]
- Fitzhugh, P.; Margach, E. Department of Health and Human Services Coal mine dust exposures and associated health outcomes- A review. In Coal Mine Dust: Health Reviews and Key Studies (with accompanying CD); Nova Science Publishers Inc: Hauppauge, NY, USA, 2013; pp. 25–67. ISBN 9781624170973. [Google Scholar]
- Laney, A.S.; Petsonk, E.L.; Attfield, M.D. Pneumoconiosis among underground bituminous coal miners in the United States: Is silicosis becoming more frequent? Occup. Environ. Med. 2010, 67, 652–656. [Google Scholar] [CrossRef]
- Blackley, D.J.; Reynolds, L.E.; Short, C.; Carson, R.; Storey, E.; Halldin, C.N.; Laney, A.S. Progressive massive fibrosis in coal miners from 3 clinics in Virginia. JAMA 2018, 319, 500–501. [Google Scholar] [CrossRef]
- Blackley, D.J.; Crum, J.B.; Halldin, C.N.; Storey, E.; Laney, A.S. Resurgence of progressive massive fibrosis in coal miners — Eastern Kentucky, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Laney, A.S.; Petsonk, E.L.; Hale, J.M.; Wolfe, A.L.; Attfield, M.D. Potential determinants of coal workers’ pneumoconiosis, advanced pneumoconiosis, and progressive massive fibrosis among underground coal miners in the United States, 2005-2009. Am. J. Public Health 2012, 102, 2005–2009. [Google Scholar] [CrossRef]
- Suarthana, E.; Laney, A.S.; Storey, E.; Hale, J.M.; Attfield, M.D. Coal workers’ pneumoconiosis in the United States: Regional differences 40 years after implementation of the 1969 Federal Coal Mine Health and Safety Act. Occup. Environ. Med. 2011, 68, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services Coal Mine Dust Exposures and Associated Health Outcomes: A Review of Information Published Since 1995; National Institute for Occupational Safety and Health (NIOSH): Morgantown, WV, USA, 2011; Volume 64.
- MSHA Lowering Miners’ Exposure to Respirable Coal Mine Dust, Including Continuous Personal Dust Monitors. Fed. Regist. 2014, 79, 24814–24994. [CrossRef][Green Version]
- U.S. EPA National ambient air quality standards for particulate matter: Proposed rule. Fed. Regist. 2012, 77, 38890–39055.
- Madl, A.K.; Donovan, E.P.; Gaffney, S.H.; McKinley, M.A.; Moody, E.C.; Henshaw, J.L.; Paustenbach, D.J. State-of-the-science review of the occupational health hazards of crystalline silica in abrasive blasting operations and related requirements for respiratory protection. J. Toxicol. Environ. Health. B. Crit. Rev. 2008, 11, 548–608. [Google Scholar] [CrossRef]
- Vincent, J. Particle size-selective sampling for particulate air contaminants; American Conference of Governmental Industrial Hygienists (ACGIH): Cincinnati, OH, USA, 1999; ISBN 978-1882417308. [Google Scholar]
- Johann-Essex, V.; Keles, C.; Sarver, E. A Computer-Controlled SEM-EDX Routine for Characterizing Respirable Coal Mine Dust. Minerals 2017, 7, 15. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health - where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Sreenath, A. Performance of a respirable multi-inlet cyclone sampler. J. Aerosol Sci. 1997, 28, 1265–1281. [Google Scholar] [CrossRef]
- Vincent, J.H. Health-related aerosol measurement: A review of existing sampling criteria and proposals for new ones. J. Environ. Monit. 2005, 7, 1037–1053. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Monitoring and Sampling Approaches to Assess Underground Coal Mine Dust Exposures; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Li, Q.; Wang, K.; Zheng, Y.; Ruan, M.; Mei, X.; Lin, B. Experimental research of particle size and size dispersity on the explosibility characteristics of coal dust. Powder Technol. 2016, 292, 290–297. [Google Scholar] [CrossRef]
- Birch, M.E.; Noll, J.D. Submicrometer elemental carbon as a selective measure of diesel particulate matter in coal mines. J. Environ. Monit. 2004, 6, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, J.E.; McCawley, M.A.; Wheeler, R.W. Particle Size Distributions in Underground Coal Mines. Am. Ind. Hyg. Assoc. J. 1987, 48, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization–A review. Fuel 2010, 89, 904–911. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Beer, C.; Kolstad, H.A.; Søndergaard, K.; Bendstrup, E.; Heederik, D.; Olsen, K.E.; Omland, Ø.; Petsonk, E.; Sigsgaard, T.; Sherson, D.L.; et al. A systematic review of occupational exposure to coal dust and the risk of interstitial lung diseases. Eur. Clin. Respir. J. 2017, 4, 1264711. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.A.; Petsonk, E.L.; Rose, C.; Young, B.; Regier, M.; Najmuddin, A.; Abraham, J.L.; Churg, A.; Green, F.H.Y. Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am. J. Respir. Crit. Care Med. 2016, 193, 673–680. [Google Scholar] [CrossRef]
- Mine Safety and Health Administration (MSHA). 30 CFR Part 74: Coal Mine Dust Sampling Devices, Federal Register; U.S. Department of Labor: Washington, DC, USA, 2016.
- Bartley, D.L.; Chen, C.C.; Song, R.; Fischbach, T.J. Respirable Aerosol Sampler Performance Testing. Am. Ind. Hyg. Assoc. J. 1994, 55, 1036–1046. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Shah, J.J.; Pace, T.G. The effect of sampling inlets on the PM10 and PM15 to TSP concentration ratios. J. Air Pollut. Control Assoc. 1983, 33, 114–119. [Google Scholar] [CrossRef]
- Page, S.J.; Volkwein, J.C. A revised conversion factor relating respirable dust concentrations measured by 10 mm Dorr-Oliver nylon cyclones operated at 1.7 and 2.0 L min−1. J. Environ. Monit. 2009, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.G.; Tropp, R.J.; Kohl, S.D.; Wang, X.L.; Chow, J.C. Filter processing and gravimetric analysis for suspended particulate matter samples. Aerosol Sci. Eng. 2017, 1, 193–205. [Google Scholar] [CrossRef]
- Blachman, M.W.; Lippmann, M. Performance Characteristics of the Multicyclone Aerosol Sampler. Am. Ind. Hyg. Assoc. J. 1974, 35, 311–326. [Google Scholar] [CrossRef]
- Kar, K.; Gautam, M. Orientation Bias of the Isolated 10-mm Nylon Cyclone at Low Stream Velocity. Am. Ind. Hyg. Assoc. J. 1995, 56, 1090–1098. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Shiau, H.-G.; Lin, K.-C.; Shih, T.-S. Effect of deposited particles and particle charge on the penetration of small sampling cyclones. J. Aerosol Sci. 1999, 30, 313–323. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Personal Dust Monitor (Model PDM3700) Instruction Manual; Thermo Fisher Scientific: Franklin, MA, USA, 2016. [Google Scholar]
- Patashnick, H.; Rupprecht, E.G. Continuous PM-10 Measurements Using the Tapered Element Oscillating Microbalance. J. Air Waste Manag.. Assoc. 1991, 41, 1079–1083. [Google Scholar] [CrossRef]
- Maynard, A.D.; Kenny, L.C. Performance assessment of three personal cyclone models, using an Aerodynamic Particle Sizer. J. Aerosol Sci. 1995, 26, 671–684. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Vinson, R.P.; McWilliams, L.J.; Tuchman, D.P.; Mischler, S.E. Mining Publication: Performance of a New Personal Respirable Dust Monitor for Mine Use, Report of Investigations 9663; National Institute for Occupational Safety and Health (NIOSH): Pittsburgh, PA, USA, 2004. [Google Scholar]
- Chow, J.C.; Fairley, D.; Watson, J.G.; de Mandel, R.; Fujita, E.M.; Lowenthal, D.H.; Lu, Z.; Frazier, C.A.; Long, G.; Cordova, J. Source apportionment of wintertime PM10 at San Jose, CA. J. Environ. Eng 1995, 21, 378–387. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Vinson, R.P.; Page, S.J.; McWilliams, L.J.; Joy, G.J.; Mischler, S.E.; Tuchman, D.P. Report of investigations (National Institute for Occupational Safety and Health. In Laboratory and Field Performance of a Continuously Measuring Personal Respirable Dust Monitor; Department of Healthy and Human Services: Cincinnati, OH, USA, 2006. [Google Scholar]
- Page, S.J.; Volkwein, J.C.; Vinson, R.P.; Joy, G.J.; Mischler, S.E.; Tuchman, D.P.; McWilliams, L.J. Equivalency of a personal dust monitor to the current United States coal mine respirable dust sampler. J. Environ. Monit. 2008, 10, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Halterman, A.; Sousan, S.; Peters, T.M. Comparison of Respirable Mass Concentrations Measured by a Personal Dust Monitor and a Personal DataRAM to Gravimetric Measurements. Ann. Work Expo. Heal. 2017, 62, 62–71. [Google Scholar] [CrossRef]
- Thorpe, A.; Walsh, P.T. Direct-Reading Inhalable Dust Monitoring—An Assessment of Current Measurement Methods. Ann. Occup. Hyg. 2013, 57, 824–841. [Google Scholar] [CrossRef] [PubMed]
- Noll, J.; Volkwein, J.; Janisko, S.; Patts, L. Portable instruments for measuring tailpipe diesel particulate in underground mines. Min. Eng. 2013, 65, 42–49. [Google Scholar] [PubMed]
- Bulpitt, S.; Price, M. The composition of PM10 as collected by a conventional TEOM, a modified TEOM and a Partisol gravimetric monitor at a kerbside site in the North East of England. Environ. Monit. Assess. 2006, 121, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Nosratabadi, A.R.; Graff, P.; Karlsson, H.; Ljungman, A.; Leanderson, P. Use of TEOM monitors for continuous long-term sampling of ambient particles for analysis of constituents and biological effects. Air Qual. Atmos. Heal. 2019, 12, 161–171. [Google Scholar] [CrossRef]
- Patts, J.R.; Tuchman, D.P.; Rubinstein, E.N.; Cauda, E.G.; Cecala, A.B. Performance Comparison of Real-Time Light Scattering Dust Monitors Across Dust Types and Humidity Levels. Min. Met. Explor. 2019, 36, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Pui, D.Y.H. Direct-reading instrumentation for workplace aerosol measurements. A review. Analyst 1996, 121, 1215. [Google Scholar] [CrossRef]
- Thorpe, A. Assessment of Personal Direct-Reading Dust Monitors for the Measurement of Airborne Inhalable Dust. Ann. Occup. Hyg. 2007, 51, 97–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.L.; Chancellor, G.; Evenstad, J.; Farnsworth, J.E.; Hase, A.; Olson, G.M.; Sreenath, A.; Agarwal, J.K. A Novel Optical Instrument for Estimating Size Segregated Aerosol Mass Concentration in Real Time. Aerosol Sci. Technol. 2009, 43, 939–950. [Google Scholar] [CrossRef]
- Hagler, G.S.W.; Solomon, P.A.; Hunt, S.W. New Technology for Low-Cost, Real-Time Air Monitoring; Air and Waste Management Association’s Magazine for Environmental Managers; Air & Waste Management Association: Pittsburgh, PA, USA, 2014. [Google Scholar]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-Sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M.; et al. Applications of low-cost sensing technologies for air quality monitoring and exposure assessment: How far have they gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef]
- McMurry, P.H. A Review of Atmospheric Aerosol Measurement. Atmos. Environ. 2000, 34, 1959–1999. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Maricq, M.; Ntziachristos, L.; Dardiotis, C.; Wang, X.; Axmann, H.; Bergmann, A.; Schindler, W. Review of motor vehicle particulate emissions sampling and measurement: From smoke and filter mass to particle number. J. Aerosol Sci. 2014, 67, 48–86. [Google Scholar] [CrossRef]
- Shamir, L.; Delaney, J.D.; Orlov, N.; Eckley, D.M.; Goldberg, I.G. Pattern recognition software and techniques for biological image analysis. PLoS Comput. Biol. 2010, 6, e1000974. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J. Relationships between Mass and Number Concentrations of Respirable Airborne Dust in British Coal Mines: Part 1—Mass-Number Relationships for Coal-Dust Clouds. Ann. Occup. Hyg. 1965, 8, 193–206. [Google Scholar] [CrossRef]
- Stein, F.; Corn, M. Shape factors of narrow size range samples of respirable coal mine dust. Powder Technol. 1975, 13, 133–141. [Google Scholar] [CrossRef]
- Johann-essex, V.; Keles, C.; Rezaee, M.; Scaggs-witte, M.; Sarver, E. International Journal of Coal Geology Respirable coal mine dust characteristics in samples collected in central and northern Appalachia. Int. J. Coal Geol. 2017, 182, 85–93. [Google Scholar] [CrossRef]
- Sellaro, R.; Sarver, E.; Baxter, D. A Standard Characterization Methodology for Respirable Coal Mine Dust Using SEM-EDX. Resources 2015, 939–957. [Google Scholar] [CrossRef]
- Sarver, E.; Keles, C.; Rezaee, M. International Journal of Coal Geology Beyond conventional metrics: Comprehensive characterization of respirable coal mine dust. Int. J. Coal Geol. 2019, 207, 84–95. [Google Scholar] [CrossRef]
- Powers, M.C. A New Roundness Scale for Sedimentary Particles. Sepm J. Sediment. Res. 1953, 23, 117–119. [Google Scholar] [CrossRef]
- Arffman, A.; Kuuluvainen, H.; Harra, J.; Vuorinen, O.; Juuti, P.; Yli-Ojanperä, J.; Mäkelä, J.M.; Keskinen, J. The critical velocity of rebound determined for sub-micron silver particles with a variable nozzle area impactor. J. Aerosol Sci. 2015, 86, 32–43. [Google Scholar] [CrossRef]
- Marple, V.A. History of Impactors—The First 110 Years. Aerosol Sci. Technol. 2004, 38, 247–292. [Google Scholar] [CrossRef]
- Ramachandran, G.; Johnson, E.W.; Vincent, J.H. Inversion techniques for personal cascade impactor data. J. Aerosol Sci. 1996, 27, 1083–1097. [Google Scholar] [CrossRef]
- Winklmayr, W.; Wang, H.-C.; John, W. Adaptation of the Twomey Algorithm to the Inversion of Cascade Impactor Data. Aerosol Sci. Technol. 1990, 13, 322–331. [Google Scholar] [CrossRef]
- Potts, J.D.; McCawley, M.A.; Jankowski, R.A. Thoracic Dust Exposures on Longwall and Continuous Mining Sections. Appl. Occup. Environ. Hyg. 1990, 5, 440–447. [Google Scholar] [CrossRef]
- Rubow, K.L.; Cantrell, B.K.; Marple, V.A. Measurement of coal dust and diesel exhaust aerosols in underground mines. In Proceedings of the 7th International Pneumoconioses Conference, Pittsburgh, PA, USA, 23–26 August 1988; pp. 23–26. [Google Scholar]
- Seixas, N.S.; Hewett, P.; Robins, T.G.; Haney, R. Variability of Particle Size-Specific Fractions of Personal Coal Mine Dust Exposures. Am. Ind. Hyg. Assoc. J. 1995, 56, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rubow, K.L.; Marple, V.A.; Olin, J.; McCawley, M.A. A Personal Cascade Impactor Design, Evaluation and Calibration. AIHAJ 1987, 48, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Misra, C.; Singh, M.; Shen, S.; Sioutas, C.; Hall, P.M. Development and evaluation of a personal cascade impactor sampler (PCIS). J. Aerosol Sci. 2002, 33, 1027–1047. [Google Scholar] [CrossRef]
- Chen, M.; Romay, F.J.; Marple, V.A. Design and evaluation of a low flow personal cascade impactor. Aerosol Sci. Technol. 2018, 52, 192–197. [Google Scholar] [CrossRef]
- Marple, V.A.; Rubow, K.L.; Behm, S.M. A Microorifice Uniform Deposit Impactor (MOUDI): Description, Calibration, and Use. Aerosol Sci. Technol. 1991, 14, 434–446. [Google Scholar] [CrossRef]
- Cantrell, B.K. Source apportionment analysis applied to mine dust aerosols: Coal dust and diesel emissions aerosol measurement. In Proceedings of the 3rd Mine Ventilation Symposium, University Park, PA, USA, 12–14 October 1987; Society of Mining Engineering: Littleton, CO, USA, 1987; pp. 495–501. [Google Scholar]
- Cantrell, B.K.; Volkwein, J.C. Mine Aerosol Measurement. In Aerosol Measurement: Principles, Techniques, and Applications; Baron, P.A., Willeke, K., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 801–820. [Google Scholar]
- Chen, M.; Romay, F.J.; Li, L.; Naqwi, A.; Marple, V.A. A novel quartz crystal cascade impactor for real- time aerosol mass distribution measurement measurement. Aerosol Sci. Technol. 2016, 50, 971–983. [Google Scholar] [CrossRef]
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360. [Google Scholar] [CrossRef]
- Lemmetty, M.; Keskinen, J.; Marjamäki, M. The ELPI Response and Data Reduction II: Properties of Kernels and Data Inversion. Aerosol Sci. Technol. 2005, 39, 583–595. [Google Scholar] [CrossRef]
- Marjamäki, M.; Lemmetty, M.; Keskinen, J. ELPI Response and Data Reduction I: Response Functions. Aerosol Sci. Technol. 2005, 39, 575–582. [Google Scholar] [CrossRef]
- Saari, S.; Arffman, A.; Harra, J.; Rönkkö, T.; Keskinen, J. Performance evaluation of the HR-ELPI + inversion. Aerosol Sci. Technol. 2018, 52, 1037–1047. [Google Scholar] [CrossRef]
- Moisio, M. Real time size distribution measurement of combustion aerosols. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 1999. [Google Scholar]
- Lehmann, U.; Niemelä, V.; Mohr, M. New Method for Time-Resolved Diesel Engine Exhaust Particle Mass Measurement. Environ. Sci. Technol. 2004, 38, 5704–5711. [Google Scholar] [CrossRef] [PubMed]
- Maricq, M.M.; Szente, J.; Loos, M.; Vogt, R. Motor Vehicle PM Emissions Measurement at LEV III Levels. SAE Int. J. Engines 2011, 4, 597–609. [Google Scholar] [CrossRef]
- Maricq, M.M. Monitoring Motor Vehicle PM Emissions: An Evaluation of Three Portable Low-Cost Aerosol Instruments. Aerosol Sci. Technol. 2013, 47, 564–573. [Google Scholar] [CrossRef]
- Maricq, M.M.; Szente, J.J.; Harwell, A.L.; Loos, M.J. How well can aerosol instruments measure particulate mass and solid particle number in engine exhaust? Aerosol Sci. Technol. 2016, 50, 605–614. [Google Scholar] [CrossRef]
- Jiang, J.; Kim, C.; Wang, X.; Stolzenburg, M.R.; Kaufman, S.L.; Qi, C.; Sem, G.J.; Sakurai, H.; Hama, N.; McMurry, P.H. Aerosol Charge Fractions Downstream of Six Bipolar Chargers: Effects of Ion Source, Source Activity, and Flowrate. Aerosol Sci. Technol. 2014, 48, 1207–1216. [Google Scholar] [CrossRef]
- Wang, X.L.; Grose, M.A.; Caldow, R.; Osmondson, B.L.; Swanson, J.J.; Chow, J.C.; Watson, J.G.; Kittelson, D.B.; Li, Y.; Xue, J.; et al. Improvement of Engine Exhaust Particle Sizer (EEPS) size distribution measurement: II. Engine exhaust aerosols. J. Aerosol Sci. 2016, 92, 83–94. [Google Scholar] [CrossRef]
- Järvinen, A.; Aitomaa, M.; Rostedt, A.; Keskinen, J.; Yli-Ojanperä, J. Calibration of the new electrical low pressure impactor (ELPI+). J. Aerosol Sci. 2014, 69, 150–159. [Google Scholar] [CrossRef]
- Marjamäki, M.; Keskinen, J.; Chen, D.-R.; Pui, D.Y.H. Performance evaluation of the electrical low-pressure impactor (ELPI). J. Aerosol Sci. 2000, 31, 249–261. [Google Scholar] [CrossRef]
- Bugarski, A.D.; Hummer, J.A.; Vanderslice, S.; Shahan, M.R. Characterization of Aerosols in an Underground Mine during a Longwall Move. Min. Met. Explor. 2020, 37, 1065–1078. [Google Scholar] [CrossRef]
- Wilson, J.C.; Liu, B.Y.H. Aerodynamic particle size measurement by laser-doppler velocimetry. J. Aerosol Sci. 1980, 11, 139–150. [Google Scholar] [CrossRef]
- Agarwal, J.K.; Remiarz, R.J.; Quant, F.R.; Sem, G.J. Real-time aerodynamic particle size analyzer. J. Aerosol Sci 1982, 13, 222–223. [Google Scholar] [CrossRef]
- Baron, P.A. Calibration and Use of the Aerodynamic Particle Sizer (APS 3300). Aerosol Sci. Technol. 1986, 5, 55–67. [Google Scholar] [CrossRef]
- Baron, P.A.; Mazumder, M.K.; Cheng, Y.-S.; Peters, T.M. Real-Time Techniques for Aerodynamic Size Measurement. Aerosol Meas. 2011, 36, 313–338. [Google Scholar] [CrossRef]
- Ananth, G.; Wilson, J.C. Theoretical Analysis of the Performance of the TSI Aerodynamic Particle Sizer The Effect of Density on Response. Aerosol Sci. Technol. 1988, 9, 189–199. [Google Scholar] [CrossRef]
- Rader, D.J.; Brockmann, J.E.; Ceman, D.L.; Lucero, D.A. A Method To Employ the Aerodynamic Particle Sizer Factory Calibration Under Different Operating Conditions. Aerosol Sci. Technol. 1990, 13, 514–521. [Google Scholar] [CrossRef]
- Wang, H.C.; John, W. Particle Density Correction for the Aerodynamic Particle Sizer. Aerosol Sci. Technol. 1987, 6, 191–198. [Google Scholar] [CrossRef]
- Baron, P.; Deye, G.J.; Martinez, A.B.; Jones, E.N.; Bennett, J.S. Size Shifts in Measurements of Droplets with the Aerodynamic Particle Sizer and the Aerosizer. Aerosol Sci. Technol. 2008, 42, 201–209. [Google Scholar] [CrossRef]
- Bartley, D.L.; Martinez, A.B.; Baron, P.A.; Secker, D.R.; Hirst, E. Droplet Distortion in Accelerating Flow. J. Aerosol Sci. 2000, 31, 1447–1460. [Google Scholar] [CrossRef]
- Volckens, J.; Peters, T.M. Counting and particle transmission efficiency of the aerodynamic particle sizer. J. Aerosol Sci. 2005, 36, 1400–1408. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, S.H. Shift of Aerosol Penetration in Respirable Cyclone Samplers. Am. Ind. Hyg. Assoc. J. 1999, 60, 720–729. [Google Scholar] [CrossRef]
- Kurth, L.M.; McCawley, M.; Hendryx, M.; Lusk, S. Atmospheric particulate matter size distribution and concentration in West Virginia coal mining and non-mining areas. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.M. Use of the Aerodynamic Particle Sizer to Measure Ambient PM10–2.5: The Coarse Fraction of PM10. J. Air Waste Manag. Assoc. 2006, 56, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.S.; Jonsson, H.H.; Maring, H.B.; Smirnov, A.; Savoie, D.L.; Cliff, S.S.; Reid, E.A.; Livingston, J.M.; Meier, M.M.; Dubovik, O.; et al. Comparison of size and morphological measurements of coarse mode dust particles from Africa. J. Geophys. Res. 2003, 108, 8593. [Google Scholar] [CrossRef]
- Sioutas, C. Evaluation of the Measurement Performance of the Scanning Mobility Particle Sizer and Aerodynamic Particle Sizer. Aerosol Sci. Technol. 1999, 30, 84–92. [Google Scholar] [CrossRef]
- DeCarlo, P.; Slowik, J.; Worsnop, D.; Davidovits, P.; Jimenez, J. Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory. Aerosol Sci. Technol. 2004, 38, 1185–1205. [Google Scholar] [CrossRef]
- Park, K.; Dutcher, D.; Emery, M.; Pagels, J.; Sakurai, H.; Scheckman, J.; Qian, S.; Stolzenburg, M.R.; Wang, X.; Yang, J.; et al. Tandem Measurements of Aerosol Properties—A Review of Mobility Techniques with Extensions. Aerosol Sci. Technol. 2008, 42, 801–816. [Google Scholar] [CrossRef]
- Stanier, C.O.; Khlystov, A.Y.; Pandis, S.N. Ambient aerosol size distributions and number concentrations measured during the Pittsburgh Air Quality Study (PAQS). Atmos. Environ. 2004, 38, 3275–3284. [Google Scholar] [CrossRef]
- Saarikoski, S.; Teinilä, K.; Timonen, H.; Aurela, M.; Laaksovirta, T.; Reyes, F.; Vásques, Y.; Oyola, P.; Artaxo, P.; Pennanen, A.S.; et al. Particulate matter characteristics, dynamics, and sources in an underground mine. Aerosol Sci. Technol. 2018, 52, 114–122. [Google Scholar] [CrossRef]
- Johnson, T.J.; Irwin, M.; Symonds, J.P.R.; Olfert, J.S.; Boies, A.M. Measuring aerosol size distributions with the aerodynamic aerosol classifier. Aerosol Sci. Technol. 2018, 52, 655–665. [Google Scholar] [CrossRef]
- Tavakoli, F.; Olfert, J.S. An Instrument for the Classification of Aerosols by Particle Relaxation Time: Theoretical Models of the Aerodynamic Aerosol Classifier. Aerosol Sci. Technol. 2013, 47, 916–926. [Google Scholar] [CrossRef]
- Tavakoli, F.; Olfert, J.S. Determination of particle mass, effective density, mass–mobility exponent, and dynamic shape factor using an aerodynamic aerosol classifier and a differential mobility analyzer in tandem. J. Aerosol Sci. 2014, 75, 35–42. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 21501-1:2009 Determination of Particle Size Distribution—Single Particle Light Interaction Methods—Part 1: Light Scattering Aerosol Spectrometer; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Sorensen, C.M.; Gebhart, J.; O’Hern, T.J.; Rader, D.J. Optical Measurement Techniques: Fundamentals and Applications. In Aerosol Measurement; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 269–312. ISBN 9781118001684. [Google Scholar]
- Pinnick, R.G.; Pendleton, J.D.; Videen, G. Response Characteristics of the Particle Measuring Systems Active Scattering Aerosol Spectrometer Probes. Aerosol Sci. Technol. 2000, 33, 334–352. [Google Scholar] [CrossRef]
- Whitby, K.T.; Liu, B.Y.H. Generation of countable pulses by high concentrations of subcountable sized particles in the sensing volume of optical counters. J. Colloid Interface Sci. 1967, 25, 537–546. [Google Scholar] [CrossRef]
- Whitby, K.T.; Willeke, K. Single particle optical counters: Principles and field use. In Aerosol Measurement; Lundgren, D.A., Lippmann, M., Harris, F.S., Clark, W.E., Marlow, W.H., Durham, M.D., Eds.; University Presses of Florida: Gainesville, FL, USA, 1979; pp. 145–182. [Google Scholar]
- Wang, X.; Zhou, H.; Arnott, W.P.; Meyer, M.E.; Taylor, S.; Firouzkouhi, H.; Moosmüller, H.; Chow, J.C.; Watson, J.G. Evaluation of gas and particle sensors for detecting spacecraft-relevant fire emissions. Fire Saf. J. 2020, 113, 102977. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Marpple, V.A.; Whitby, K.T.; Barsic, N.J. Size Distribution Measurement of Airborne Coal Dust by Optical Particle Counters. Am. Ind. Hyg. Assoc. J. 1974, 35, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Whitby, K.T.; Vomela, R.A. Response of single particle optical counters to nonideal particles. Environ. Sci. Technol. 1967, 1, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Barone, T.L.; Hesse, E.; Seaman, C.E.; Baran, A.J.; Beck, T.W.; Harris, M.L.; Jaques, P.A.; Lee, T.; Mischler, S.E. Calibration of the cloud and aerosol spectrometer for coal dust composition and morphology. Adv. Powder Technol. 2019, 30, 1805–1814. [Google Scholar] [CrossRef]
- Marple, V.A.; Rubow, K.L. Aerodynamic particle size calibration of optical particle counters. J. Aerosol Sci. 1976, 7, 425–433. [Google Scholar] [CrossRef]
- Marple, V.A.; Rubow, K.L. A portable optical particle counter system for measuring dust aerosols. Am. Ind. Hyg. Assoc. J. 1978, 39, 210–218. [Google Scholar] [CrossRef]
- Flagan, R.C. History of Electrical Aerosol Measurements. Aerosol Sci. Technol. 1998, 28, 301–380. [Google Scholar] [CrossRef]
- Wang, S.C.; Flagan, R.C. Scanning Electrical Mobility Spectrometer. Aerosol Sci. Technol. 1990, 13, 230–240. [Google Scholar] [CrossRef]
- Fuchs, N.A. On the stationary charge distribution on aerosol particles in a bipolar ionic atmosphere. Geofis. Pura E Appl. 1963, 56, 185–193. [Google Scholar] [CrossRef]
- Wiedensohler, A. An approximation of the bipolar charge distribution for particles in the submicron size range. J. Aerosol Sci. 1988, 19, 387–389. [Google Scholar] [CrossRef]
- Kallinger, P.; Steiner, G.; Szymanski, W.W. Characterization of four different bipolar charging devices for nanoparticle charge conditioning. J. Nanoparticle Res. 2012, 14, 944. [Google Scholar] [CrossRef]
- Lee, H.M.; Soo Kim, C.; Shimada, M.; Okuyama, K. Bipolar diffusion charging for aerosol nanoparticle measurement using a soft X-ray charger. J. Aerosol Sci. 2005, 36, 813–829. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Pui, D.Y.H. Equilibrium bipolar charge distribution of aerosols. J. Colloid Interface Sci. 1974, 49, 305–312. [Google Scholar] [CrossRef]
- Liu, B.Y.H.; Pui, D.Y.H. Electrical neutralization of aerosols. J. Aerosol Sci. 1974, 5, 465–472. [Google Scholar] [CrossRef]
- Knutson, E.O.; Whitby, K.T. Aerosol classification by electric mobility: Apparatus, theory, and applications. J. Aerosol Sci. 1975, 6, 443–451. [Google Scholar] [CrossRef]
- McMurry, P.H. The History of Condensation Nucleus Counters. Aerosol Sci. Technol. 2000, 33, 297–322. [Google Scholar] [CrossRef]
- Mai, H.; Flagan, R.C. Scanning DMA Data Analysis I. Classification Transfer Function. Aerosol Sci. Technol. 2018, 52, 1382–1399. [Google Scholar] [CrossRef]
- Mai, H.; Kong, W.; Seinfeld, J.H.; Flagan, R.C. Scanning DMA data analysis II. Integrated DMA-CPC instrument response and data inversion. Aerosol Sci. Technol. 2018, 52, 1400–1414. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Sodeman, D.A.; Lowenthal, D.H.; Chang, M.-C.O.; Park, K.; Wang, X.L. Comparison of four scanning mobility particle sizers at the Fresno Supersite. Particuology 2011, 9, 204–209. [Google Scholar] [CrossRef]
- Brunelli, N.A.; Flagan, R.C.; Giapis, K.P. Radial Differential Mobility Analyzer for One Nanometer Particle Classification. Aerosol Sci. Technol. 2009, 43, 53–59. [Google Scholar] [CrossRef]
- Cai, R.; Chen, D.-R.; Hao, J.; Jiang, J. A miniature cylindrical differential mobility analyzer for sub-3 nm particle sizing. J. Aerosol Sci. 2017, 106, 111–119. [Google Scholar] [CrossRef]
- Franchin, A.; Downard, A.; Kangasluoma, J.; Nieminen, T.; Lehtipalo, K.; Steiner, G.; Manninen, H.E.; Petäjä, T.; Flagan, R.C.; Kulmala, M. A new high-transmission inlet for the Caltech nano-RDMA for size distribution measurements of sub-3nm ions at ambient concentrations. Atmos. Meas. Tech. 2016, 9, 2709–2720. [Google Scholar] [CrossRef]
- Iida, K.; Stolzenburg, M.R.; McMurry, P.H. Effect of Working Fluid on Sub-2 nm Particle Detection with a Laminar Flow Ultrafine Condensation Particle Counter. Aerosol Sci. Technol. 2008, 43, 81–96. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, M.; Kuang, C.; Attoui, M.; McMurry, P.H. Electrical Mobility Spectrometer Using a Diethylene Glycol Condensation Particle Counter for Measurement of Aerosol Size Distributions Down to 1 nm. Aerosol Sci. Technol. 2011, 45, 510–521. [Google Scholar] [CrossRef]
- Kangasluoma, J.; Cai, R.; Jiang, J.; Deng, C.; Stolzenburg, D.; Ahonen, L.R.; Chan, T.; Fu, Y.; Kim, C.; Laurila, T.M.; et al. Overview of measurements and current instrumentation for 1–10 nm aerosol particle number size distributions. J. Aerosol Sci. 2020, 148, 105584. [Google Scholar] [CrossRef]
- Kuang, C.; Chen, M.; McMurry, P.H.; Wang, J. Modification of Laminar Flow Ultrafine Condensation Particle Counters for the Enhanced Detection of 1 nm Condensation Nuclei. Aerosol Sci. Technol. 2012, 46, 309–315. [Google Scholar] [CrossRef]
- Stolzenburg, M.R.; Scheckman, J.H.T.; Attoui, M.; Han, H.-S.; McMurry, P.H. Characterization of the TSI model 3086 differential mobility analyzer for classifying aerosols down to 1 nm. Aerosol Sci. Technol. 2018, 52, 748–756. [Google Scholar] [CrossRef]
- Enroth, J.; Kangasluoma, J.; Korhonen, F.; Hering, S.; Picard, D.; Lewis, G.; Attoui, M.; Petäjä, T. On the time response determination of condensation particle counters. Aerosol Sci. Technol. 2018, 52, 778–787. [Google Scholar] [CrossRef]
- Fernandez de la Mora, J.; Perez-Lorenzo, L.J.; Arranz, G.; Amo-Gonzalez, M.; Burtscher, H. Fast high-resolution nanoDMA measurements with a 25 ms response time electrometer. Aerosol Sci. Technol. 2017, 51, 724–734. [Google Scholar] [CrossRef]
- Shah, S.D.; Cocker, D.R. A Fast Scanning Mobility Particle Spectrometer for Monitoring Transient Particle Size Distributions. Aerosol Sci. Technol. 2005, 39, 519–526. [Google Scholar] [CrossRef]
- Tröstl, J.; Tritscher, T.; Bischof, O.F.; Horn, H.-G.; Krinke, T.; Baltensperger, U.; Gysel, M. Fast and precise measurement in the sub-20nm size range using a Scanning Mobility Particle Sizer. J. Aerosol Sci. 2015, 87, 75–87. [Google Scholar] [CrossRef]
- Amanatidis, S.; Kim, C.; Spielman, S.R.; Lewis, G.S.; Hering, S.V.; Flagan, R.C. The Spider DMA: A miniature radial differential mobility analyzer. Aerosol Sci. Technol. 2020, 54, 175–189. [Google Scholar] [CrossRef]
- Hering, S.V.; Lewis, G.S.; Spielman, S.R.; Eiguren-Fernandez, A. A MAGIC concept for self-sustained, water-based, ultrafine particle counting. Aerosol Sci. Technol. 2019, 53, 63–72. [Google Scholar] [CrossRef]
- Khlystov, A.; Stanier, C.; Pandis, S.N. An Algorithm for Combining Electrical Mobility and Aerodynamic Size Distributions Data when Measuring Ambient Aerosol Special Issue ofAerosol Science and Technologyon Findings from the Fine Particulate Matter Supersites Program. Aerosol Sci. Technol. 2004, 38, 229–238. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Zhang, Q.; Deng, J.; Hao, J. A spectrometer for measuring particle size distributions in the range of 3 nm to 10 μm. Front. Environ. Sci. Eng. 2016, 10, 63–72. [Google Scholar] [CrossRef]
- Hand, J.L.; Kreidenweis, S.M. A New Method for Retrieving Particle Refractive Index and Effective Density from Aerosol Size Distribution Data. Aerosol Sci. Technol. 2002, 36, 1012–1026. [Google Scholar] [CrossRef]
- Wang, X.L.; Watson, J.G.; Chow, J.C.; Gronstal, S.; Kohl, S.D. An efficient multipollutant system for measuring real-world emissions from stationary and mobile sources. Aerosol Air Qual. Res. 2012, 12, 145–160. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Lowenthal, D.H.; Magliano, K.L. Estimating aerosol light scattering at the Fresno Supersite. Atmos. Environ. 2008, 42, 1186–1196. [Google Scholar] [CrossRef]
- Woo, K.-S. Measurement of Atmospheric Aerosols: Size Distribution of Nanoparticles, Estimation of Distribution Moments and Control of Relative Humidity; University of Minnesota: Minneapolis, MN, USA, 2002. [Google Scholar]
- Skubacz, K.; Wojtecki, Ł.; Urban, P. Aerosol concentration and particle size distributions in underground excavations of a hard coal mine. Int. J. Occup. Saf. Erg. 2017, 23, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, S.; Salo, L.; Bloss, M.; Alanen, J.; Teinilä, K.; Reyes, F.; Vázquezuez, Y.; Keskinen, J.; Oyola, P.; Rönkkö, T.; et al. Sources and Characteristics of Particulate Matter at Five Locations in an Underground Mine. Aerosol Air Qual. Res. 2019, 9, 2613–2624. [Google Scholar] [CrossRef]
- Biskos, G.; Reavell, K.; Collings, N. Description and Theoretical Analysis of a Differential Mobility Spectrometer. Aerosol Sci. Technol. 2005, 39, 527–541. [Google Scholar] [CrossRef]
- Johnson, T.; Caldow, R.; Pöcher, A.; Mirme, A.; Kittelson, D. A New Electrical Mobility Particle Sizer Spectrometer for Engine Exhaust Particle Measurements; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Symonds, J.P.R.; Reavell, K.S.J.; Olfert, J.S.; Campbell, B.W.; Swift, S.J. Diesel soot mass calculation in real-time with a differential mobility spectrometer. J. Aerosol Sci. 2007, 38, 52–68. [Google Scholar] [CrossRef]
- Wang, X.L.; Grose, M.A.; Avenido, A.; Stolzenburg, M.R.; Caldow, R.; Osmondson, B.L.; Chow, J.C.; Watson, J.G. Improvement of Engine Exhaust Particle Sizer (EEPS) size distribution measurement: I. Algorithm and Applications to Compact Aerosols. J. Aerosol Sci. 2016, 92, 95–108. [Google Scholar] [CrossRef]
- Nakamura, K.; Oshikiri, T.; Ueno, K.; Wang, Y.; Kamata, Y.; Kotake, Y.; Misawa, H. Properties of Plasmon-Induced Photoelectric Conversion on a TiO2 /NiO p–n Junction with Au Nanoparticles. J. Phys. Chem. Lett. 2016, 7, 1004–1009. [Google Scholar] [CrossRef]
- Reavell, K.; Hands, T.; Collings, N. A Fast Response Particulate Spectrometer for Combustion Aerosols. SAE Technical Paper2002-01-2714. Sae Trans. 2002, 111, 1338–1344. [Google Scholar] [CrossRef]
- Bugarski, A.D.; Hummer, J.A. Contribution of various types and categories of diesel-powered vehicles to aerosols in an underground mine. J. Occup. Environ. Hyg. 2020, 17, 121–134. [Google Scholar] [CrossRef]
- Bugarski, A.D.; Barone, T.L.; Hummer, J.A. Diesel and welding aerosols in an underground mine. Int. J. Min. Sci. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Marcel Dekker Šrám, R.J.; Holá, N.; Kotešovec, F.; Vávra, R. Chromosomal abnormalities in soft coal open-cast mining workers. Mutat. Res. Lett. 1985, 144, 271–275. [Google Scholar] [CrossRef]
- Braunstein, R.; Copenhaver, E.D.; Pfuderer, R. Environmental, Health, and Control Aspects of Coal Conversion: An Information Overview: Prepared for the Energy Research and Development Administration, Assistant Administrator for Environment and Safety; Information Center Complex: Oak Ridge, TN, USA, 1977; Volume 1. [Google Scholar]
- Walton, W.H.; Dodgson, J.; Hadden, G.G.; Jacobsen, M. The effect of quartz and other non-coal dusts in coal workers’ pneumoconiosis. Part I: Epidemiological studies. Inhaled Part. 1975, 669–690. [Google Scholar]
- Warae, S.S.J. Coal Properties, Analysis and Effective Use; Institute of Coal Research, University of Newcastle: Newcastle, NSW, Australia, 1982; pp. 113–136. [Google Scholar]
- Finkelman, R.B.; Dai, S.; French, D. The importance of minerals in coal as the hosts of chemical elements: A review. Int. J. Coal Geol. 2019, 212, 103251. [Google Scholar] [CrossRef]
- Huang, X.; Gordon, T.; Rom, W.N.; Finkelman, R.B. Interaction of iron and calcium minerals in coals and their roles in coal dust-induced health and environmental problems. Rev. Miner. Geochem. 2006, 64, 153–178. [Google Scholar] [CrossRef]
- Harrington, A.D.; Hylton, S.; Schoonen, M.A.A. Pyrite-driven reactive oxygen species formation in simulated lung fluid: Implications for coal workers’ pneumoconiosis. Environ. Geochem. Health 2012, 34, 527–538. [Google Scholar] [CrossRef] [PubMed]
- MSHA Mine Safety and Health Administration. 30 CFR Part 72 Diesel Particulate Matter Exposure of Coal Miners. Proposed Rule. Fed. Regist 2001, 68, 5526. [Google Scholar]
- MSHA Mine Safety and Health Administration. 30 CFR Part 57 Diesel Particulate Matter Exposure of Underground Metal and Nonmetal Miners; Final Rule. Fed. Regist 2005, 70, 32868. [Google Scholar]
- Cram, K.; Glover, D. Gravimetric Respirable Dust Sampling Experience in NSWand Dust Improvements Relating to Mining Methods and Equipment. In Proceedings of the Underground Mining Seminar, Brisbane, QLD, Australia, 4–7 September 1995. [Google Scholar]
- Leiteritz, H.; Bauer, D.; Bruckmann, E. Mineralogical characteristics of airborne dust in coal mines of Western Germany and their relations to pulmonary changes of coal hewers. Inhaled Part. 1970, 2, 729–743. [Google Scholar]
- Tomb, T.F.; Gero, A.J.; Kogut, J. Analysis of Quartz Exposure Data Obtained from Underground and Surface Coal Mining Operations. Appl. Occup. Environ. Hyg. 1995, 10, 1019–1026. [Google Scholar] [CrossRef]
- Cauda, E.; Joy, G.; Miller, A.; Mischler, S. Analysis of the Silica Percent in Airborne Respirable Mine Dust Samples From U.S. Operations. In Silica and Associated Respirable Mineral Particles; Harper, M., Lee, T., Eds.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 12–27. [Google Scholar]
- Page, S.J.; Organiscak, J.A. Suggestion of a cause-and-effect relationship among coal rank, airborne dust, and incidence of workers’ pneumoconiosis. AIHAJ 2000, 61, 785–787. [Google Scholar] [PubMed]
- Pollock, D.E.; Potts, J.D.; Joy, G.J. Investigation into dust exposures and mining practices in mines in the southern Appalachian Region. Min. Eng. 2010, 62, 44–49. [Google Scholar]
- Landen, D.D.; Wassell, J.T.; McWilliams, L.; Patel, A. Coal dust exposure and mortality from ischemic heart disease among a cohort of U.S. coal miners. Am. J. Ind. Med. 2011, 54, 727–733. [Google Scholar] [CrossRef]
- Joy, G.J. Evaluation of the approach to respirable quartz exposure control in U.S. coal mines. J. Occup. Environ. Hyg. 2012, 9, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Mischler, S.E.; Cauda, E.G.; Di Giuseppe, M.; Ortiz, L.A. A multi-cyclone sampling array for the collection of size-segregated occupational aerosols. J. Occup. Environ. Hyg. 2013, 10, 685–693. [Google Scholar] [CrossRef]
- Chen, Y.; Mori, S.; Pan, W.P. Estimating the Combustibility of Various Coals by TG-DTA. Energy Fuels 1995, 9, 71–74. [Google Scholar] [CrossRef]
- Klaja, J.; Przelaskowska, A.; Kulinowski, P.; Bujok, P.; Klempa, M. Journal of Petroleum Science and Engineering Investigation of fine-grained siliciclastic rocks of different clay content using thermal methods. J. Pet. Sci. Eng. 2020, 184, 106531. [Google Scholar] [CrossRef]
- Earnest, C.M. Thermal Analysis of Selected Illite and Smectite Clay Minerals. Part I. Illite Clay Specimens BT—Thermal Analysis in the Geosciences; Smykatz-Kloss, W., Warne, S.S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 270–286. [Google Scholar]
- Langier-Kuzniarowa, A. Remarks on the applicability of thermal analysis for the investigations of clays and related materials BT - Thermal Analysis in the Geosciences; Smykatz-Kloss, W., Warne, S.S.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 314–326. [Google Scholar]
- Thornley, D.M.; Primmer, T.J. Thermogravimetry/evolved water analysis (TG/EWA) combined with XRD for improved quantitative whole-rock analysis of clay minerals in sandstones. Clay Min. 1995, 30, 27–38. [Google Scholar] [CrossRef]
- Kaljuvee, T.; Keelmann, M.; Trikkel, A.; Kuusik, R. Thermooxidative decomposition of oil shales. J. Therm. Anal. Calorim. 2010, 105, 395–403. [Google Scholar] [CrossRef]
- Kok, M.V. Thermal investigation of Seyitomer oil shale. Thermochim. Acta 2001, 369, 149–155. [Google Scholar] [CrossRef]
- Labus, M.; Labus, K.; Bujok, P. Determination of the pore space parameters in microporous rocks by means of thermal methods. J. Pet. Sci. Eng. 2015, 127, 482–489. [Google Scholar] [CrossRef]
- Labus, M.; Lempart, M. Studies of Polish Paleozoic shale rocks using FTIR and TG/DSC methods. J. Pet. Sci. Eng. 2018, 161, 311–318. [Google Scholar] [CrossRef]
- Wieckowska, J. The study of coal by thermal analysis, advantages and disadvantages. Thermochim. Acta 1988, 134, 359–364. [Google Scholar] [CrossRef]
- Sharkey Jr, A.G.; McCartney, J.T. Physical properties of coal and its products. In Chemistry of Coal Utilization; Wiley: New York, NY, USA, 1981; Volume 2, pp. 173–186. [Google Scholar]
- Warne, S.S.J. Differential thermal analysis of coal minerals. In Analytical methods for coal and coal products; Elsevier: Amsterdam, The Netherlands, 1979; pp. 447–477. [Google Scholar]
- Warne, S.S.J.; Dubrawski, J. V Applications of DTA and DSC to coal and oil shale evaluation. J. Therm. Anal. 1989, 35, 219–242. [Google Scholar] [CrossRef]
- Warne, S.S.J. Detection and Identification of Silica Minerals Quartz, Chalcedony, Agete and Opal, by Diffrential Thermal Analysis. J. Inst. Fuel 1970, 43, 240. [Google Scholar]
- Sułkowski, J.; Sankiewicz, J. Magnezowy kalcyt jedwabisty w kopalni węgla kamiennego “Ziemowit”. Przegląd Geol. 1974, 22, 496–498. [Google Scholar]
- Warne, S.S.J. Identification and evaluation of minerals in coal by differential thermal analysis. J. Inst. Fuel 1965, 38, 207–217. [Google Scholar]
- Gupta, R. Advanced Coal Characterization: A Review. Energy Fuels 2007, 451–460. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, G.; Ding, J.; Li, S.; Wang, G. Preparation and characterization of an agglomeration-cementing agent for dust suppression in open pit coal mining. Cellulose 2018, 25, 4011–4029. [Google Scholar] [CrossRef]
- Fan, T.; Zhou, G.; Wang, J. Preparation and characterization of a wetting-agglomeration-based hybrid coal dust suppressant. Process Saf. Environ. Prot. 2018, 113, 282–291. [Google Scholar] [CrossRef]
- Zhou, G.; Fan, T.; Ma, Y. Preparation and chemical characterization of an environmentally-friendly coal dust cementing agent. J. Chem. Technol. Biotechnol. 2017, 92, 2699–2708. [Google Scholar] [CrossRef]
- Breuer, K.H.; Eysel, W. The calorimetric calibration of differential scanning calorimetry cells. Thermochim. Acta 1982, 57, 317–329. [Google Scholar] [CrossRef]
- Dubrawski, J.V.; Warne, S.S.J. The application of differential scanning calorimetry to mineralogical analysis. Thermochim. Acta 1986, 107, 51–59. [Google Scholar] [CrossRef]
- Dubrawski, J.V.; Warne, S.S.J. Use of differential scanning calorimetry in measuring the thermal decomposition of mineral carbonates occurring in coal. Fuel 1987, 66, 1733–1736. [Google Scholar] [CrossRef]
- Roberts, D. Intrinsic Reaction Kinetics of Coal Chars with O2, CO2 and H2O at Elevated Pressures. Ph.D. Thesis, University of Newcastle, Newcastle, UK, 2000. [Google Scholar]
- Tang, L.G.; Gupta, R.P.; Sheng, C.D.; Wall, T.F. The estimation of char reactivity from coal reflectogram. Fuel 2005, 84, 127–134. [Google Scholar] [CrossRef]
- Li, H.; Shen, X.-Z.; Sisk, B.; Orndorff, W.; Li, D.; Pan, W.-P.; Riley, J.T. Studies of fly ash using thermal analysis techniques. J. Therm. Anal. 1997, 49, 943–951. [Google Scholar] [CrossRef]
- Agioutanti, E.; Keles, C.; Sarver, E. A thermogravimetric analysis application to determine coal, carbonate, and non-carbonate minerals mass fractions in respirable mine dust. J. Occup. Environ. Hyg. 2020, 17, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Keles, C.; Sarver, E.; Scaggs-Witte, M. Coal and Mineral Mass Fractions in Personal Respirable Dust Samples Collected by Central Appalachian Miners. Min. Eng. 2018, 70, 16–30. [Google Scholar]
- Coats, A.W.; Redfern, J.P. Thermogravimetric analysis. A review. Analyst 1963, 88, 906–924. [Google Scholar] [CrossRef]
- Colinet, J.F.; Listak, J.M. Silica and Respirable Content in Rock Dust Samples. Coal Age. 2012, 117, 48–52. [Google Scholar]
- Watson, J.G.; Chow, J.C.; Chen, L.-W.A. Summary of Organic and Elemental Carbon/Black Carbon Analysis Methods and Intercomparisons. Aerosol Air Qual. Res. 2005, 5, 65–102. [Google Scholar] [CrossRef]
- Birch, M.E.; Cary, R.A. Elemental carbon-based method for occupational monitoring of particulate diesel exhaust: Methodology and exposure issues. Analyst 1996, 121, 1183–1190. [Google Scholar] [CrossRef]
- Birch, M.E.; Cary, R.A. Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust. Aerosol Sci. Technol. 1996, 25, 221–241. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health (NIOSH). Diesel Particulate Matter (as Elemental Carbon) Method 5040, Issue 4. In NIOSH Manual of Analytical Methods (NMAM), 5th ed.; NIOSH: Washington, DC, USA, 2016. [Google Scholar]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Chang, M.C.O.; Robinson, N.F.; Trimble, D.; Kohl, S. The IMPROVE_A temperature protocol for thermal/optical carbon analysis: Maintaining consistency with a long-term database. J. Air Waste Manag. Assoc. 2007, 57, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The DRI thermal/optical reflectance carbon analysis system: Description, evaluation and applications in US air quality studies. Atmos. Environ. Part A. Gen. Top. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.A.; Arnott, W.P.; Moosmüller, H.; Fung, K. Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ. Sci. Technol. 2004, 38, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Watson, J.G.; Chen, L.-W.; Paredes-Miranda, G.; Chang, M.-C.; Trimble, D.; Fung, K.K.; Zhang, H.; Zhen Yu, J. Refining temperature measures in thermal/optical carbon analysis. Atmos. Chem. Phys. 2005, 5, 2961–2972. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Robles, J.; Wang, X.L.; Chen, L.-W.A.; Trimble, D.L.; Kohl, S.D.; Tropp, R.J.; Fung, K.K. Quality assurance and quality control for thermal/optical analysis of aerosol samples for organic and elemental carbon. Anal. Bioanal. Chem. 2011, 401, 3141–3152. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.A.; Chow, J.C.; Wang, X.L.; Robles, J.A.; Sumlin, B.J.; Lowenthal, D.H.; Zimmermann, R.; Watson, J.G. Multi-wavelength optical measurement to enhance thermal/optical analysis for carbonaceous aerosol. Atmos. Meas. Tech. 2015, 8, 451–461. [Google Scholar] [CrossRef]
- Chow, J.C.; Wang, X.L.; Sumlin, B.J.; Gronstal, S.B.; Chen, L.-W.A.; Trimble, D.L.; Kohl, S.D.; Mayorga, S.R.; Riggio, G.M.; Hurbain, P.R.; et al. Optical calibration and equivalence of a multiwavelength thermal/optical carbon analyzer. Aerosol Air Qual. Res. 2015, 15, 1145–1159. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Green, M.C.; Wang, X.L.; Chen, L.-W.A.; Trimble, D.L.; Cropper, P.M.; Kohl, S.D.; Gronstal, S.B. Separation of brown carbon from black carbon for IMPROVE and CSN PM2.5 samples. J. Air Waste Manag. Assoc. 2018, 68, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Chen, L.-W.A.; Wang, X.; Green, M.C.; Watson, J.G. Improved estimation of PM2. 5 brown carbon contributions to filter light attenuation. Particuology 2021, 56, 1–9. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Wang, X.; Zielinska, B.; Kohl, S.D.; Gronstal, S. Characterization of Real-World Emissions from Nonroad Mining Trucks in the Athabasca Oil Sands Region during September, 2009; Desert Research Institute: Reno, NV, USA, 2013. [Google Scholar]
- Waterman, D.; Horsfield, B.; Hall, K.; Smith, S. Application of micro-scale sealed vessel thermal desorption-gas chromatography-mass spectrometry for the organic analysis of airborne particulate matter: Linearity, reproducibility and quantification. J. Chromatogr. A 2001, 912, 143–150. [Google Scholar] [CrossRef]
- Greaves, R.C.; Barkley, R.M.; Sievers, R.E. Rapid sampling and analysis of volatile constituents of airborne particulate matter. Anal. Chem. 1985, 57, 2807–2815. [Google Scholar] [CrossRef]
- Jeon, S.J.; Meuzelaar, H.L.; Sheya, S.A.; Lighty, J.S.; Jarman, W.M.; Kasteler, C.; Sarofim, A.F.; Simoneit, B.R. Exploratory studies of PM10 receptor and source profiling by GC/MS and principal component analysis of temporally and spatially resolved ambient samples. J. Air Waste Manag. Assoc. 2001, 51, 766–784. [Google Scholar] [CrossRef] [PubMed]
- Hays, M.D.; Smith, N.D.; Kinsey, J.; Dong, Y.; Kariher, P. Polycyclic aromatic hydrocarbon size distributions in aerosols from appliances of residential wood combustion as determined by direct thermal desorption—GC/MS. J. Aerosol Sci. 2003, 34, 1061–1084. [Google Scholar] [CrossRef]
- Hallama, R.A.; Rosenberg, E.; Grasserbauer, M. Development and application of a thermal desorption method for the analysis of polar volatile organic compounds in workplace air. J. Chromatogr. A 1998, 809, 47–63. [Google Scholar] [CrossRef]
- Waterman; Horsfield; Leistner; Hall; Smith Quantification of polycyclic aromatic hydrocarbons in the NIST standard reference material (SRM1649A) urban dust using thermal desorption GC/MS. Anal. Chem. 2000, 72, 3563–3567. [CrossRef]
- Hamilton, J.F.; Webb, P.J.; Lewis, A.C.; Reviejo, M.M. Quantifying small molecules in secondary organic aerosol formed during the photo-oxidation of toluene with hydroxyl radicals. Atmos. Environ. 2005, 39, 7263–7275. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Chen, L.-W.A.; Engling, G.; Wang, X.L. Source apportionment: Principles and methods. In Airborne Particulate Matter: Sources, Atmospheric Processes and Health; Harrison, R.M., Ed.; Royal Society of Chemistry: London, UK, 2016; pp. 72–125. [Google Scholar]
- Chow, J.C.; Yu, J.Z.; Watson, J.G.; Hang Ho, S.S.; Bohannan, T.L.; Hays, M.D.; Fung, K.K. The application of thermal methods for determining chemical composition of carbonaceous aerosols: A review. J. Environ. Sci. Heal. Part A 2007, 42, 1521–1541. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G. Chemical Analyses of Particle Filter Deposits: Aerosolss Handbook: Measuerment, Dosimetry, and Health Efffects. In Aerosols Handbook; CRC Press/Tylor & Francis: New York, NY, USA, 2012; pp. 179–204. [Google Scholar]
- Willis, R.D.; Blanchard, F.T.; Conner, T.L. Guidelines for the Application of SEM/EDX Analytical Techniques to Particulate Matter Samples; #600/R-02/070: 2002; U.S. Environmental Protection Agency: Triangle Park, NC, USA, 2002. [Google Scholar]
- Jacobson, M.Z. Control of fossil-fuel particulate black carbon and organic matter, possibly the most effective method of slowing global warming. J. Geophys. Res. Atmos. 2002, 107, ACH-16. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.C.; Frazier, C.A. X-ray fluorescence analysis of ambient air samples. In Elemental Analysis of Airborne Particles, Vol. 1; Advances in Environmental, Industrial and Process Control Technologies; Landsberger, S., Creatchman, M., Eds.; Gordon and Breach Science: Amsterdam, The Netherlands, 1999; pp. 67–96. [Google Scholar]
- Heagney, J.M.; Heagney, J.S. Thin film X-ray fluorescence calibration standards. Nucl. Instrum. Methods 1979, 167, 137–138. [Google Scholar] [CrossRef]
- Shi, G.; Chen, Z.; Xu, S.; Zhang, J.; Wang, L.; Bi, C.; Teng, J. Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ. Pollut. 2008, 156, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Doraiswamy, P.; Watson, J.G.; Chen, L.W.A.; Ho, S.S.H.; Sodeman, D.A. Advances in integrated and continuous measurements for particle mass and chemical composition. J. Air Waste Manag. Assoc. 2008, 58, 141–163. [Google Scholar] [CrossRef]
- Herner, J.D.; Green, P.G.; Kleeman, M.J. Measuring the Trace Elemental Composition of Size-Resolved Airborne Particles. Environ. Sci. Technol. 2006, 40, 1925–1933. [Google Scholar] [CrossRef]
- Kulkarni, P.; Chellam, S.; Ghurye, G.; Fraser, M.P. In Situ Generation of Hydrofluoric Acid during Microwave Digestion of Atmospheric Particulate Matter Prior to Trace Element Analysis Using Inductively Coupled Plasma Mass Spectrometry. Environ. Eng. Sci. 2003, 20, 517–531. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; DeMinter, J.T.; Weinstein, J.P. Emissions of Metals Associated with Motor Vehicle Roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef]
- Pekney, N.J.; Davidson, C.I. Determination of trace elements in ambient aerosol samples. Anal. Chim. Acta 2005, 540, 269–277. [Google Scholar] [CrossRef]
- Wang, X.; Sato, T.; Xing, B.; Tamamura, S.; Tao, S. Source identification, size distribution and indicator screening of airborne trace metals in Kanazawa, Japan. J. Aerosol Sci. 2005, 36, 197–210. [Google Scholar] [CrossRef]
- Whittaker, A.; BeruBe, K.; Jones, T.; Maynard, R.; Richards, R. Killer smog of London, 50 years on: Particle properties and oxidative capacity. Sci. Total Environ. 2004, 334, 435–445. [Google Scholar] [CrossRef]
- Coedo, A.G.; Padilla, I.; Dorado, M.T. Determination of minor elements in steelmaking flue dusts using laser ablation inductively coupled plasma mass spectrometry. Talanta 2005, 67, 136–143. [Google Scholar] [CrossRef]
- Gligorovski, S.; Van Elteren, J.T.; Grgić, I. A multi-element mapping approach for size-segregated atmospheric particles using laser ablation ICP-MS combined with image analysis. Sci. Total Environ. 2008, 407, 594–602. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, J.; Zhang, W.; Tian, H.; Hu, Z.; Ruan, M.; Xu, H.; Liu, L.; Yan, X.; Chen, D. FT-IR and micro-Raman spectroscopic characterization of minerals in high-calcium coal ashes. J. Energy Inst. 2018, 91, 389–396. [Google Scholar] [CrossRef]
- Nowak, S.; Lafon, S.; Caquineau, S.; Journet, E.; Laurent, B. Quantitative study of the mineralogical composition of mineral dust aerosols by X-ray diffraction. Talanta 2018, 186, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Liu, Z.; Lin, S.; Li, X.; Hong, S.; Li, D. Characteristics Analysis of Post-Explosion Coal Dust Samples by X-ray Diffraction. Combust. Sci. Technol. 2018, 190, 740–754. [Google Scholar] [CrossRef]
- Engelbrecht, J.P.; Stenchikov, G.; Prakash, P.J.; Lersch, T.; Anisimov, A.; Shevchenko, I. Physical and chemical properties of deposited airborne particulates over the Arabian Red Sea coastal plain. Atmos. Chem. Phys. 2017, 17, 11467–11490. [Google Scholar] [CrossRef]
- Schoening, F.R.L. X-ray structural parameter for coal. Fuel 1982, 61, 695–699. [Google Scholar] [CrossRef]
- Dun, W.; Guijian, L.; Ruoyu, S.; Xiang, F. Investigation of Structural Characteristics of Thermally Metamorphosed Coal by FTIR Spectroscopy and X - ray Di ff raction. Energy Fuels 2013. [Google Scholar] [CrossRef]
- Hirsch, P.B. Recent results on the structure of dislocations in tetrahedrally coordinated semiconductors. J. Phys. Colloq. 1979, 40, 27–32. [Google Scholar] [CrossRef][Green Version]
- Diamond, R. A least-squares analysis of the diffuse X-ray scattering from carbons. Acta Cryst. 1958, 11, 129–138. [Google Scholar] [CrossRef]
- Warren, B.E. X-Ray Diffraction in Random Layer Lattices. Phys. Rev. 1941, 59, 693–698. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-Ray Study of Carbon Black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer programs for standardless quantitative analysis of minerals using the full powder diffraction profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef]
- Ward, C.R.; Spears, D.A.; Booth, C.A.; Staton, I.; Gurba, L.W. Mineral matter and trace elements in coals of the Gunnedah Basin, New South Wales, Australia. Int. J. Coal Geol. 1999, 40, 281–308. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Liu, Z.T.; Zhang, S.S.; Li, Z.H.; Zhao, E.L.; Lin, S.; Guo, R.L. Investigation on coal dust explosion residues using 20 L explosion sphere vessels. J. China Univ. Min. Technol. 2015, 44, 823–828. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). Crystalline by XRD (filter redeposition) Method 7500 Silica. In NIOSH Manual of Analytical Methods (NMAM), 4th ed.; NIOSH: Washington, DC, USA, 2003. [Google Scholar]
- Miola, W.; Ramani, R. V Quartz content in bulk-coal, host-rock and airborne dust samples: A comparative study of IR and XRD procedures. Trans. Soc. Min. Met. Explor. 1996, 298, 1845–1850. [Google Scholar]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Comparative performance of X-ray diffraction and Raman microprobe techniques for the study of carbon materials. J. Mater. Chem. 1998, 8, 2875–2879. [Google Scholar] [CrossRef]
- Painter, P.C.; Snyder, R.W.; Starsinic, M.; Coleman, M.M.; Kuehn, D.W.; Davis, A. Concerning the Application of FT-IR to the Study of Coal: A Critical Assessment of Band Assignments and the Application of Spectral Analysis Programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar] [CrossRef]
- Cancado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A.; Pimenta, M.A. Measuring the degree of stacking order in graphite by Raman spectroscopy. Carbon 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Cloke, M.; Gilfillan, A.; Lester, E. The characterization of coals and density separated coal fractions using FTIR and manual and automated petrographic analysis. Fuel 1997, 76, 1289–1296. [Google Scholar] [CrossRef]
- Gilfillan, A.; Lester, E.; Cloke, M.; Snape, C. The structure and reactivity of density separated coal fractions. Fuel 1999, 78, 1639–1644. [Google Scholar] [CrossRef]
- Department of Health and Human Services; Centers for Disease Control and Prevention; National Institute for Occupational Safety and Health (NIOSH). Mining Project: Feasibility Study For a Novel Field-Portable DPM Monitor; NIOSH: Washington, DC, USA, 2017. [Google Scholar]
- Tahmasebi, A.; Jiang, Y.; Yu, J.; Li, X.; Lucas, J. Solvent extraction of Chinese lignite and chemical structure changes of the residue during H2O2 oxidation. Fuel Process. Technol. 2015, 129, 213–221. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Deńca, A.; Strojek, J.W. Application of Attenuated Total Reflection Infrared Spectroscopy to the Characterization of Coal. Appl. Spectrosc. 1986, 40, 998–1004. [Google Scholar] [CrossRef]
- Gao, Z.; Zheng, M.; Zhang, D.; Zhang, W. Low temperature pyrolysis properties and kinetics of non-coking coal in Chinese western coals. J. Energy Inst. 2016, 89, 544–559. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, M.; Dou, G.; Wang, D.; Chen, Y.; Mo, Y.; Zhang, Y. Structural characterization and oxidation study of a Chinese lignite with the aid of ultrasonic extraction. J. Energy Inst. 2015, 88, 398–405. [Google Scholar] [CrossRef]
- El Hafid, K.; Hajjaji, M. Effects of the experimental factors on the microstructure and the properties of cured alkali-activated heated clay. Appl. Clay Sci. 2015, 116–117, 202–210. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Dimitrovska, S. Minerals from Macedonia: XIV. Identification of some sulfate minerals by vibrational (infrared and Raman) spectroscopy. Vib. Spectrosc. 2005, 39, 229–239. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Painter, P.C.; Coleman, M.M.; Jenkins, R.G.; Walker Jr, P.L. Fourier transform infrared study of acid-demineralized coal. Fuel 1978, 57, 125–126. [Google Scholar] [CrossRef]
- Mukherjee, S.; Srivastava, S.K. Minerals Transformations in Northeastern Region Coals of India on Heat Treatment. Energy Fuels 2006, 20, 1089–1096. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Li, B. Characterization of low-temperature coal ash behaviors at high temperatures under reducing atmosphere. Fuel 2008, 87, 583–591. [Google Scholar] [CrossRef]
- Mozgawa, W.; Krol, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2014, 132, 889–894. [Google Scholar] [CrossRef]
- Han, F.; Meng, A.; Li, Q.; Zhang, Y. Thermal decomposition and evolved gas analysis (TG-MS) of lignite coals from Southwest China. J. Energy Inst. 2016, 89, 94–100. [Google Scholar] [CrossRef]
- Painter, P.C.; Rimmer, S.M.; Snyder, R.W.; Davis, A. A Fourier Transform Infrared Study of Mineral Matter in Coal: The Application of a Least Squares Curve-Fitting Program. Appl. Spectrosc. 1981, 35, 102–106. [Google Scholar] [CrossRef]
- Bandopadhyay, A.K. Determination of quartz content for Indian coals using an FTIR technique. Int. J. Coal Geol. 2010, 81, 73–78. [Google Scholar] [CrossRef]
- Mine Safety and Health Administration (MSHA). Pittsburgh Safety and Health Technology Center: Infrared determination of quartz in respirable coal mine dust: Method P-7; U.S. Department of Labor: Pittsburgh, PA, USA, 2013. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). QUARTZ in coal mine dust, by IR ( redeposition)- NIOSH Method 7063. In NIOSH Manual of Analytical Methods (NMAM), 4th ed.; NIOSH: Washington, DC, USA, 2003. [Google Scholar]
- Mine Safety and Health Administration (MSHA). Infrared Determination of Quartz in Respirable Coal Mine Dust-Method No. MSHA P7; Mine Safety and Health Administration: Arlington, VA, USA; U.S. Department of Labor: Pittsburgh, PA, USA, 2008. [Google Scholar]
- Miller, A.L.; Weakley, A.T.; Griffiths, P.R.; Cauda, E.G.; Bayman, S. Direct-on-Filter α-Quartz Estimation in Respirable Coal Mine Dust Using Transmission Fourier Transform Infrared Spectrometry and Partial Least Squares Regression. Appl. Spectrosc. 2017, 71, 1014–1024. [Google Scholar] [CrossRef]
- Hart, J.F.; Autenrieth, D.A.; Cauda, E.; Chubb, L.; Spear, T.M.; Wock, S.; Rosenthal, S. A comparison of respirable crystalline silica concentration measurements using a direct-on-filter Fourier transform infrared (FT-IR) transmission method vs. a traditional laboratory X-ray diffraction method. J. Occup. Environ. Hyg. 2018, 15, 743–754. [Google Scholar] [CrossRef]
- Ojima, J. Determining of crystalline silica in respirable dust samples by infrared spectrophotometry in the presence of interferences. J. Occup. Health 2003, 45, 94–103. [Google Scholar] [CrossRef]
- Ashley, E.L.; Cauda, E.; Chubb, L.G.; Tuchman, D.P.; Rubinstein, E.N. Performance Comparison of Four Portable FTIR Instruments for Direct-on-Filter Measurement of Respirable Crystalline Silica. Ann. Work Expo. Heal. 2020, 64, 536–546. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in mineralogical crystallography; Walter de Gruyter GmbH: Berlin, Germany, 2016; pp. 1–29. [Google Scholar]
- Das, S.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.; Potgieter, J.H.; Van Grieken, R. The application of Raman spectrometry to investigate and characterize cement, Part I: A review. Cem. Concr. Res. 2006, 36, 656–662. [Google Scholar] [CrossRef]
- Akyuz, S.; Akyuz, T.; Basaran, S.; Bolcal, C.; Gulec, A. Analysis of ancient potteries using FT-IR, micro-Raman and EDXRF spectrometry. Vib. Spectrosc. 2008, 48, 276–280. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Sampaio, C.H.; Guedes, A.; Fdez-Ortiz de Vallejuelo, S.; Madariaga, J.M. Multianalytical approaches to the characterisation of minerals associated with coals and the diagnosis of their potential risk by using combined instrumental microspectroscopic techniques and thermodynamic speciation. Fuel 2012, 94, 52–63. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Sanz, A.; Flores, D.; Noronha, F. Characterization of fly ash from a power plant and surroundings by micro-Raman spectroscopy. Int. J. Coal Geol. 2008, 73, 359–370. [Google Scholar] [CrossRef]
- Health and Safety Laboratory Crystalline Silica in Respirable Airborne Dust. Methods for the Determination of Hazardous Substances 101/2; Health and Safety Executive (HSE): Bootle, UK, 2014; pp. 1–21. ISBN 0-8186-8084-9. [Google Scholar]
- Stacey, P.; Mader, K.T.; Sammon, C. Feasibility of the quantification of respirable crystalline silica by mass on aerosol sampling filters using Raman microscopy. J. Raman Spectrosc. 2017, 48, 720–725. [Google Scholar] [CrossRef]
- Zheng, L.; Kulkarni, P.; Birch, M.E.; Ashley, K.; Wei, S. Analysis of Crystalline Silica Aerosol Using Portable Raman Spectrometry: Feasibility of Near Real-Time Measurement. Anal. Chem. 2018, 90, 6229–6239. [Google Scholar] [CrossRef] [PubMed]
- Kingma, K.J.; Hemley, R.J. Raman spectroscopic study of microcrystalline silica. Am. Miner. 1994, 79, 269–273. [Google Scholar]
- Potgieter-Vermaak, S.; Maledi, N.; Wagner, N.; Van Heerden, J.H.P.; Van Grieken, R.; Potgieter, J.H. Raman spectroscopy for the analysis of coal: A review. J. Raman Spectrosc. 2011, 42, 123–129. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Friedel, R.A.; Carlson, G.L. Difficult carbonaceous materials and their infra-red and Raman spectra. Reassignments for coal spectra. Fuel 1972, 51, 194–198. [Google Scholar] [CrossRef]
- Deng, C.; Brooks, S.D.; Vidaurre, G.; Thornton, D.C.O. Using Raman Microspectroscopy to Determine Chemical Composition and Mixing State of Airborne Marine Aerosols over the Pacific Ocean. Aerosol Sci. Technol. 2014, 48, 193–206. [Google Scholar] [CrossRef]
- Stacey, P.; Kauffer, E.; Moulut, J.-C.; Dion, C.; Beauparlant, M.; Fernandez, P.; Key-Schwartz, R.; Friede, B.; Wake, D. An International Comparison of the Crystallinity of Calibration Materials for the Analysis of Respirable α-Quartz Using X-Ray Diffraction and a Comparison with Results from the Infrared KBr Disc Method. Ann. Occup. Hyg. 2009, 53, 639–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genetti, D.; Fletcher, T.H.; Pugmire, R.J. Development and Application of a Correlation of 13C NMR Chemical Structural Analyses of Coal Based on Elemental Composition and Volatile Matter Content. Energy Fuels 1999, 13, 60–68. [Google Scholar] [CrossRef]
- Smith, K.L.; Smoot, L.D.; Fletcher, T.H.; Pugmire, R.J. The Structure and Reaction Processes of Coal; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 148991322X. [Google Scholar]
- Retcofsky, H.L.; Schweighardt, F.K.; Hough, M. Determination of aromaticities of coal derivatives by nuclear magnetic resonance spectrometry and the Brown-Ladner equation. Anal. Chem. 1977, 49, 585–588. [Google Scholar] [CrossRef]

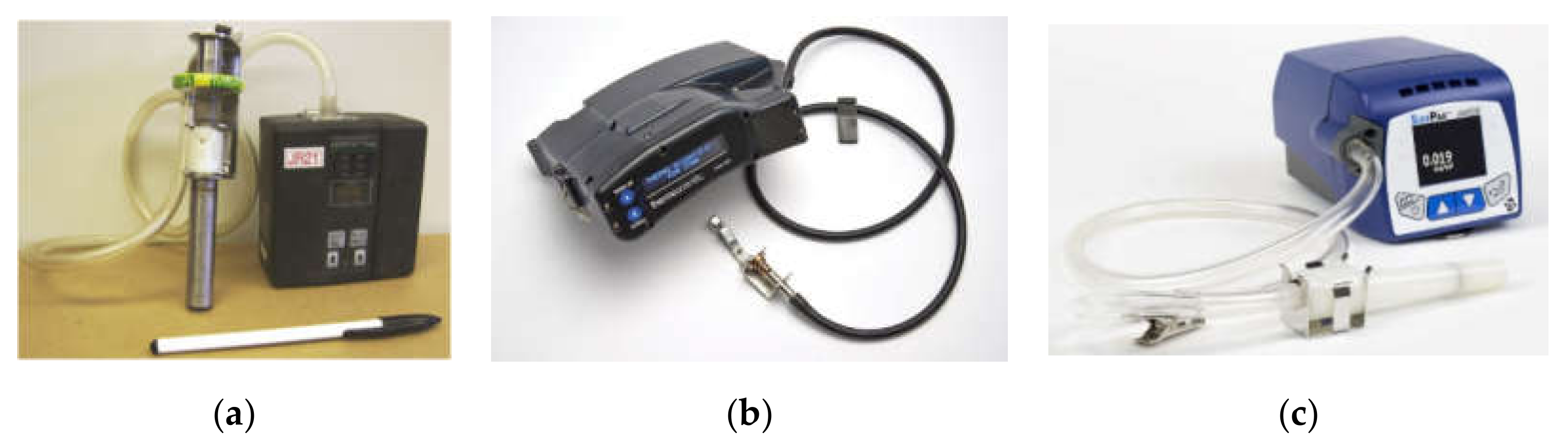

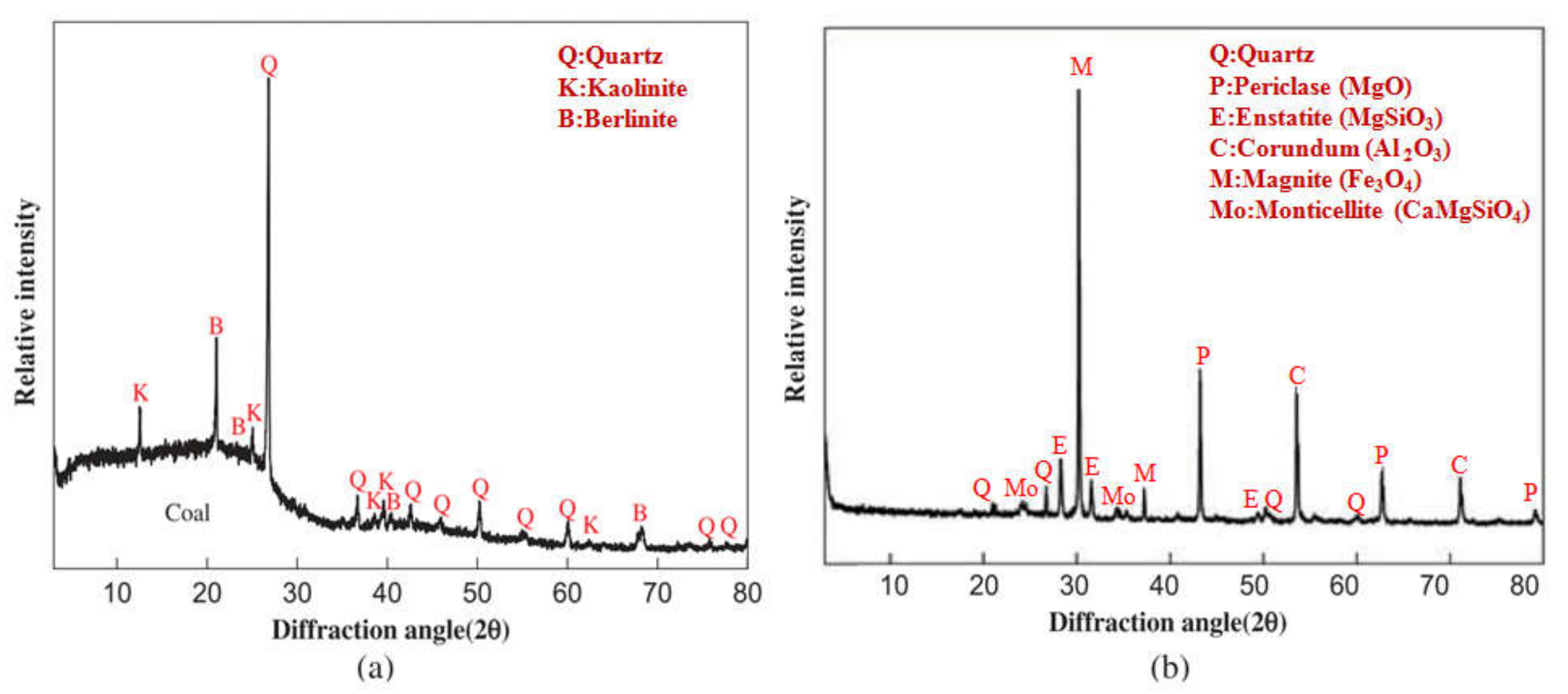

| Method | Description | Limitations and Challenges |
|---|---|---|
| Gravimetric sampler | Constant-flow sampling through a particle size-selective cyclone (e.g., Dorr–Oliver) onto a filter cartridge by a personal sampling pump The filter is submitted to gravimetric analysis and optionally for chemical analysis in the laboratory Reference method Relatively low cost | Ensuring that the cyclone assembly stays upright Labor intensive Low time resolution Data are not immediately available |
| Continuous personal dust monitor (CPDM) | A TEOM (tapered-element oscillating microbalance) obtains near real-time, gravimetric-equivalent measurement of RCMD mass concentrations Filter can be used for limited laboratory analysis Near real-time measurement (30-min average) Regulatory requirement Relatively independent of aerosol optical, physical, and chemical properties | High cost Size and weight are burdensome Regulatory requirement to report data to MSHA Potential evaporation losses |
| Photometer | Inferred mass concentration based on aerosol light scattering intensity Low cost Lightweight Fast response (~1 s) | Scattering-mass relationship varies with particle refractive index, shape, size distribution, density, and relative humidity Field calibration is needed |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Optical Microscopy Size range > 1µm | Visual size and morphology evaluation | Time consuming; not suitable for submicron particles; potential observational bias and errors |
| SEM Size range: ~0.01–10 µm | Morphology and size analysis; elemental characteristics; wide particle size range | Laboratory measurement; needs sample pre-preparation; slow and costly; may not be representative as a small fraction of particles are analyzed |
| Cascade Impactor Size range: ~0.01–10 µm | Wide aerodynamic diameter range; size segregated mass concentration and chemical composition; can be used for personal sampling; mechanically rugged | Ex situ analysis; long sampling duration to collect sufficient mass; particle bounce may cause bias; non-uniform deposition |
| ELPI Size range: 0.006–10 µm | In situ real-time aerodynamic size distribution; wide size and concentration ranges | Particle bouncing; blow-off from substrates; overloading of substrates; low size resolution; charging efficiency uncertainty |
| APS Size range: 0.5–20 µm | In situ real-time aerodynamic size distributions; high size resolution; easy operation | Not suitable for particles <0.5 µm; density-dependent non-Stokesian correction; liquid particle deformation and losses; low concentration limit |
| AAC Size range: 0.025–>5 µm | In situ aerodynamic size distributions; high size resolution; high transmission efficiency | Relatively slow scans (~2 min); fast rotating components; still under development/perfection |
| OPC Size range: ~0.3–10 µm | In situ real-time optical size distribution; compact and portable size; relatively low cost | Low concentration limit; dependence on particle shape and composition; non-monotonic dependence of light scattering on particle size |
| SMPS Size range: ~0.003–1 µm | In situ near real-time mobility size distribution; high size resolution and accuracy for submicron particles | Relatively slow scans; not suitable for >1 µm; limitation of using radioactive neutralizers |
| EEPS/FMPS/DMS Size range: 0.006–0.6 µm for EEPS and FMPS; 0.005–2.5 µm for DMS | In situ real-time mobility size distribution; high time resolution; suitable for rapidly changing aerosols | Lower size resolution than SMPS; dependence of charging efficiency on particle morphology |
| Classification | Mineral | Formula |
|---|---|---|
| Carbonates | Siderite | FeCO3 |
| Dolomite | CaMg(CO3)2 | |
| Ankerite | Ca(Fe, Mg, Mn)(CO3)2 | |
| Calcite | CaCO3 | |
| Magnesite | MgCO3 | |
| Silicates | Illite | K0.65(Al,Fe,Mg)2.0[Al0.65,Si3.5]O10(OH)2 |
| Kaolinite | Al2[Si2O5](OH)4 | |
| Sericite | KAl2(AlSi3O10)(OH)2 | |
| Smectite | Mx(Si4)(Al2-x,((Mg,Fe3+)x)O10(OH)2.nH2O | |
| Quartz | SiO2 | |
| Montmorillinite | Mx(Si4)(Al2-x,((Mg,Fe3+)x)O10(OH)2.nH2O | |
| Sulfides/Sulfates | Marcasite | FeS2 |
| Pyrite | FeS2 | |
| Melnikovite | FeS2 | |
| Sphalerite | ZnS | |
| Galena | PbS | |
| Chalcopyrite | CuFeS2 | |
| Gypsum | CaSO4.2H2O | |
| Jarosite | KFe3(SO4)2(OH)6 | |
| Hydrated iron sulfates | FeSO4·xH2O |
| Mineral Matter | Detection Limit (wt %) |
|---|---|
| Pyrite and marcasite | 0.5% |
| Calcite, magnesite, dolomite, and ankerite | 1% |
| Siderite and kaolinite | 2% |
| Quartz | 2 to 5% |
| Montmorillonite | 15% |
| Illite | Up to 30% |
| Band Wave Number (cm−1) | Functional Groups | Peak Intensity | ||
|---|---|---|---|---|
| L | H | A | ||
| 3419–3359 | –OH stretching vibration | W | S | S |
| 3080–3035 | Aromatic CH stretching vibration | S | M | W |
| 2975–2848 | Aliphatic CH stretching vibration | M | S | W |
| 1745–1695 | C=O | S | M | W |
| 1615–1585 | C=C | S | M | W |
| 1500–1450 | C–C stretching | W | M | S |
| 1300–1000 | C–O–C stretching | S | M | W |
| 900–700 | C–H out–plane bending | S | M | W |
| Mineral | FTIR Absorption Bands (cm−1) |
|---|---|
| Anhydrite | 1154, 1120, 679, 613, 595 |
| Quartz | 1164 a, 1082 a, 797, 778, 696 a, 513 |
| Calcite | 1797, 1447 a, 875, 713 |
| Aragonite | 1476, 857 |
| Microcline | 646, 534 a |
| Albite | 425 a |
| Amorphous silica | 1099 a, 1013 a |
| Metakaolinite | 1030 a, 562 a |
| Portlandite | 3641 |
| Nitrate | 1385 |
| Unknown aluminasilicate | 479 a, 445a |
| Channel 1 Teflon Membrane | Channel 2 Quartz Fiber | Channel 3 Polycarbonate |
|---|---|---|
| XRF (elemental analysis) Function: identify wide variety of elements (51 elements Na to U) Limitation: low concentrations of several rare-earth elements (lanthanide series) or light elements (Li, Be, and B) cannot be identified. | TOR/TOT (carbon analysis) Function: identify OC, EC, brown carbon (BrC), and carbonates Limitation: destructive process and uncertainty in char correction that separates EC from OC. | SEM-EDX: (morphological and elemental analysis) Function: size and shape analysis; Identify elements with atomic number larger than ~12 Limitation: captures only a small fraction of particles; labor intensive |
| ICP-MS (elemental analysis) Function: complement XRF with additional rare-earth elements and with lower minimum detection limits Limitation: destructive method; preparation and sample extraction may contaminate the sample or lead to incomplete analysis. | TD-GC-MS (organic molecules analysis) Function: quantify ~110 non-polar organic compounds, including alkanes, alkene, hopanes, steranes, and PAHs Limitation: destructive process and only a fraction of organic compounds are analyzed. | FTIR: (chemical composition analysis) Function: identify organic functional groups and mineral composition (including quartz) for both crystalline and amorphous states Limitation: overlapping bands often occur in infrared spectroscopy; minimum detection limit is high to detect quartz (between 3 and 10 μg) |
| XRD (mineralogy) Function: composition and structure of crystal components (e.g., gypsum, and metal oxides) Limitation: high minimum detection limit (between 3 and 10 μg); cannot characterize disordered materials quartz | Raman Spectroscopy: (chemical composition analysis) Function: complement FTIR results with lower minimum detection limit (crystalline silica and spectral fingerprints) Limitation: not suitable for particles with high absorption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, B.; Wang, X.; Chow, J.C.; Watson, J.G.; Peik, B.; Nasiri, V.; Riemenschnitter, K.B.; Elahifard, M. Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition. Minerals 2021, 11, 426. https://doi.org/10.3390/min11040426
Abbasi B, Wang X, Chow JC, Watson JG, Peik B, Nasiri V, Riemenschnitter KB, Elahifard M. Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition. Minerals. 2021; 11(4):426. https://doi.org/10.3390/min11040426
Chicago/Turabian StyleAbbasi, Behrooz, Xiaoliang Wang, Judith C. Chow, John G. Watson, Bijan Peik, Vahid Nasiri, Kyle B Riemenschnitter, and Mohammadreza Elahifard. 2021. "Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition" Minerals 11, no. 4: 426. https://doi.org/10.3390/min11040426
APA StyleAbbasi, B., Wang, X., Chow, J. C., Watson, J. G., Peik, B., Nasiri, V., Riemenschnitter, K. B., & Elahifard, M. (2021). Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition. Minerals, 11(4), 426. https://doi.org/10.3390/min11040426







