Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
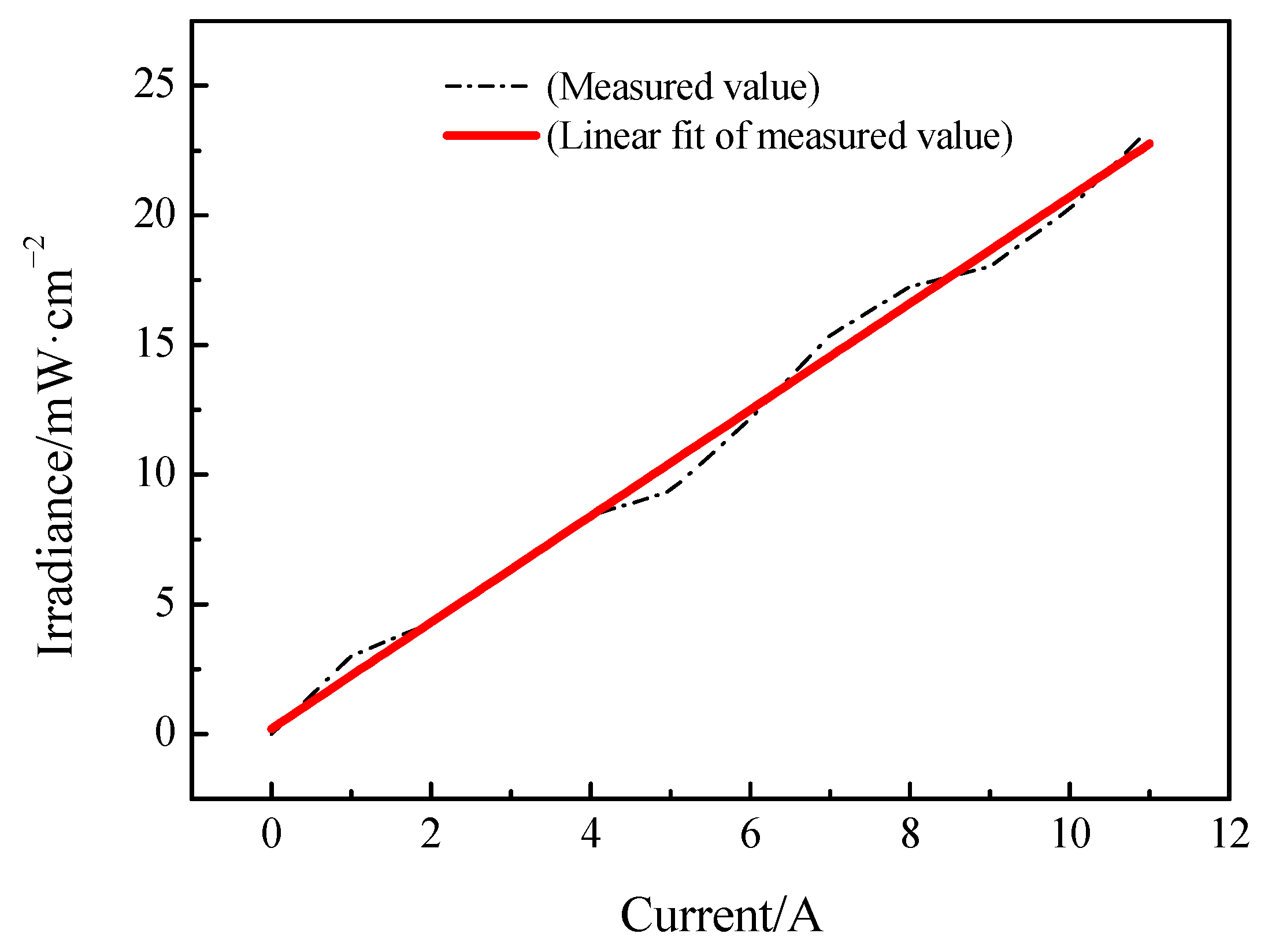
2.2. Light Source
2.3. Gold Ore Leaching Experimental Design
2.4. Quartz Crystal Microbalance with Dissipation (QCM-D)
3. Results and Discussion
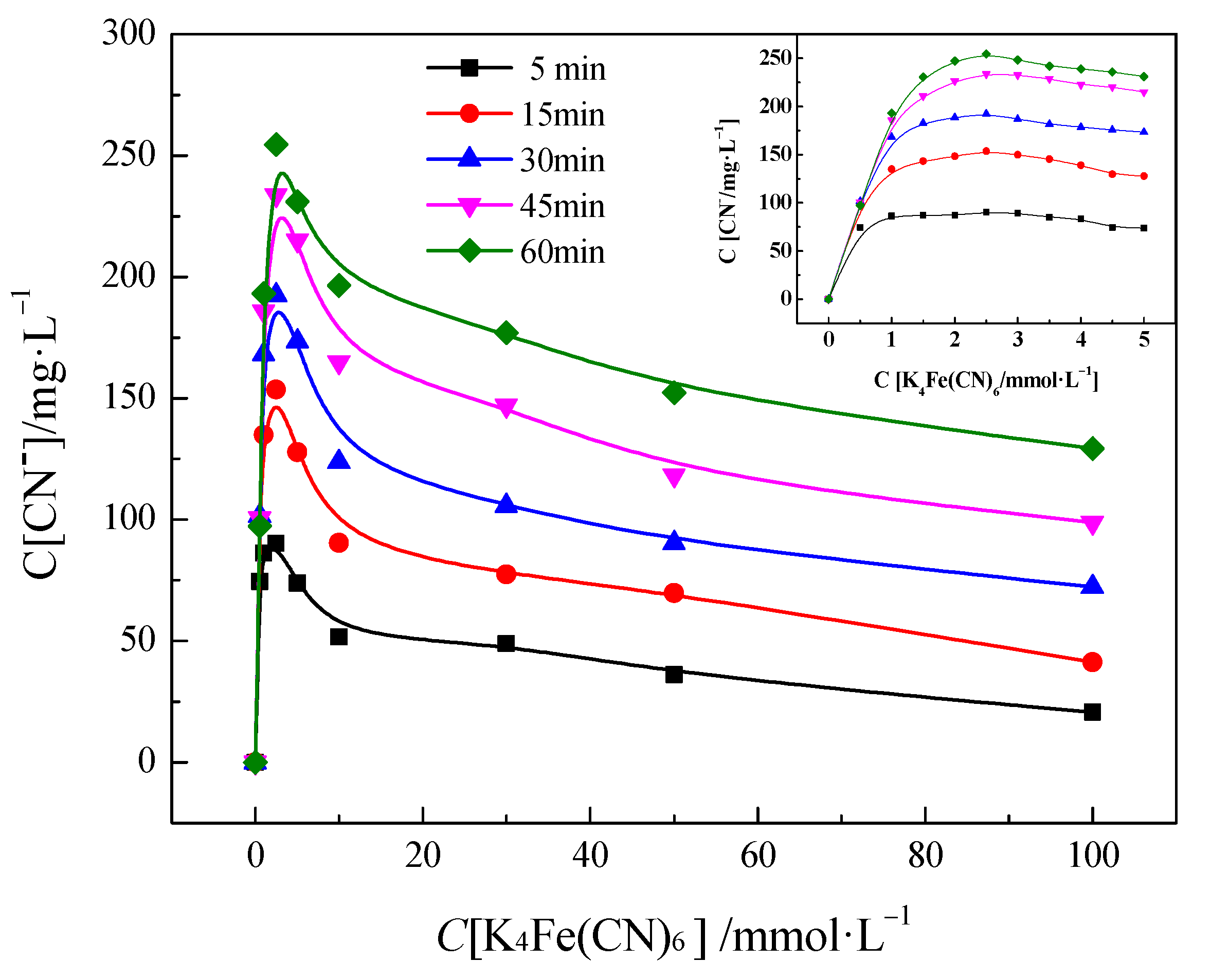
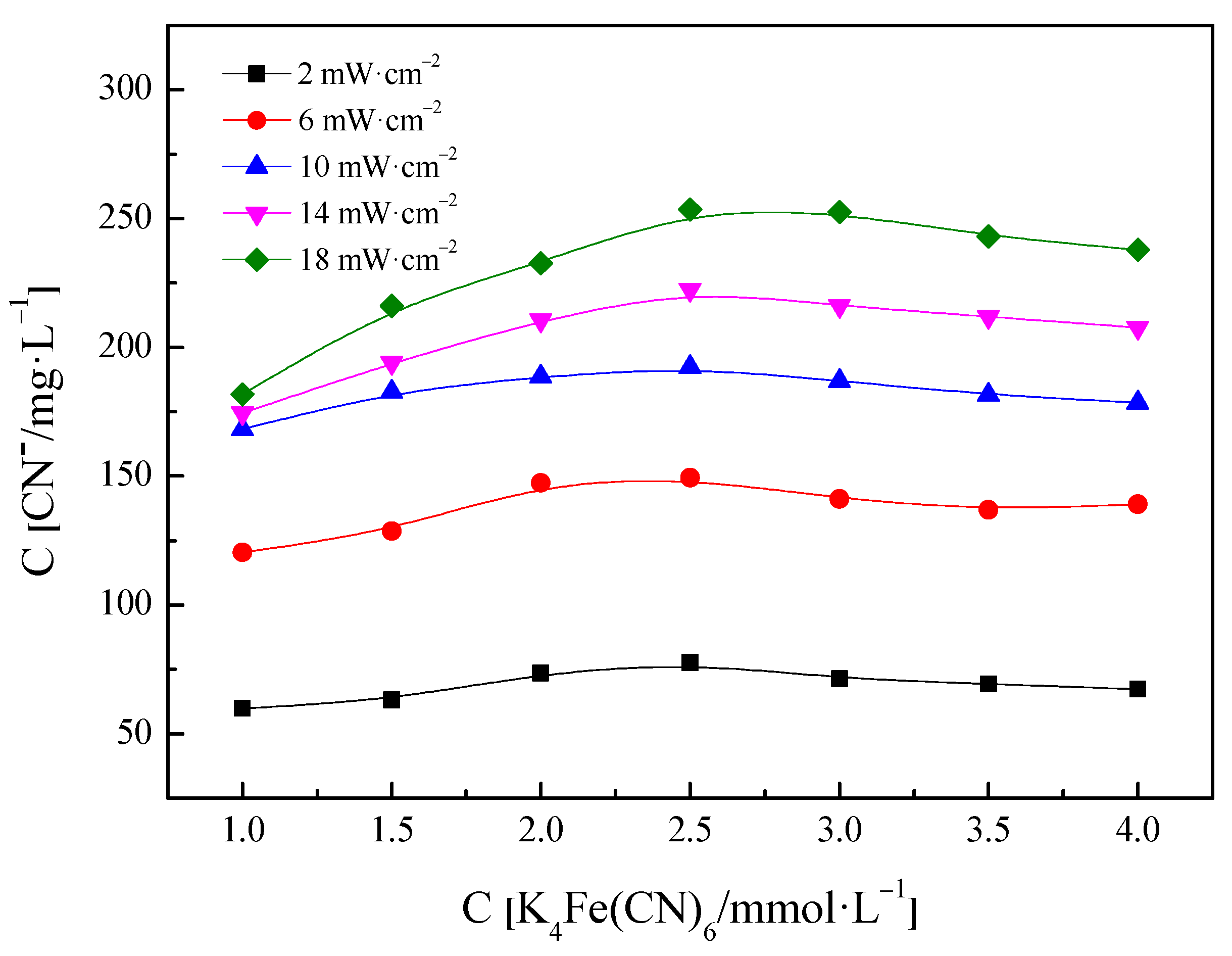
3.1. Decomposition of PF Solution under UV Light
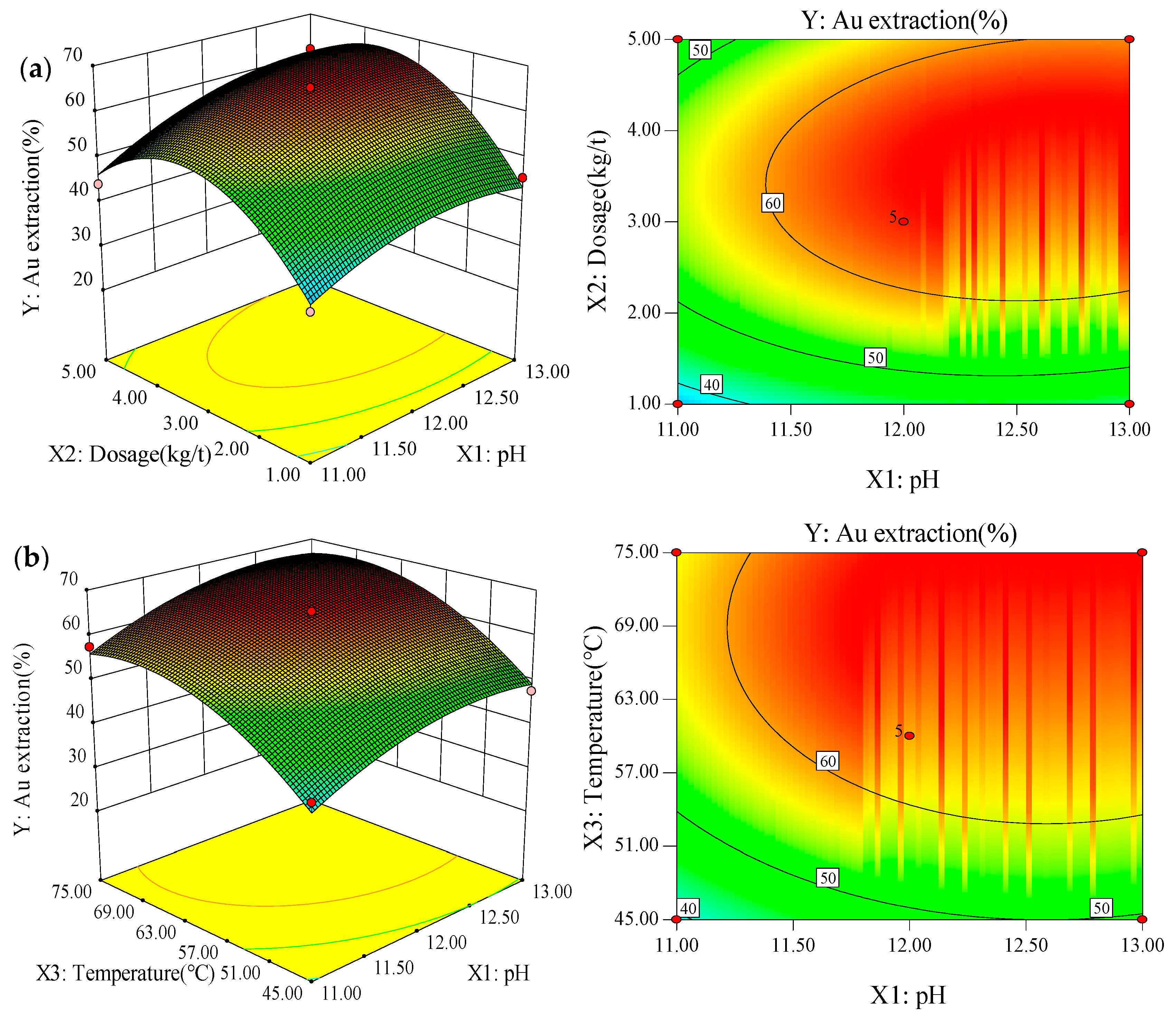
3.2. Box-Behnken Design and Response Surface Methodology
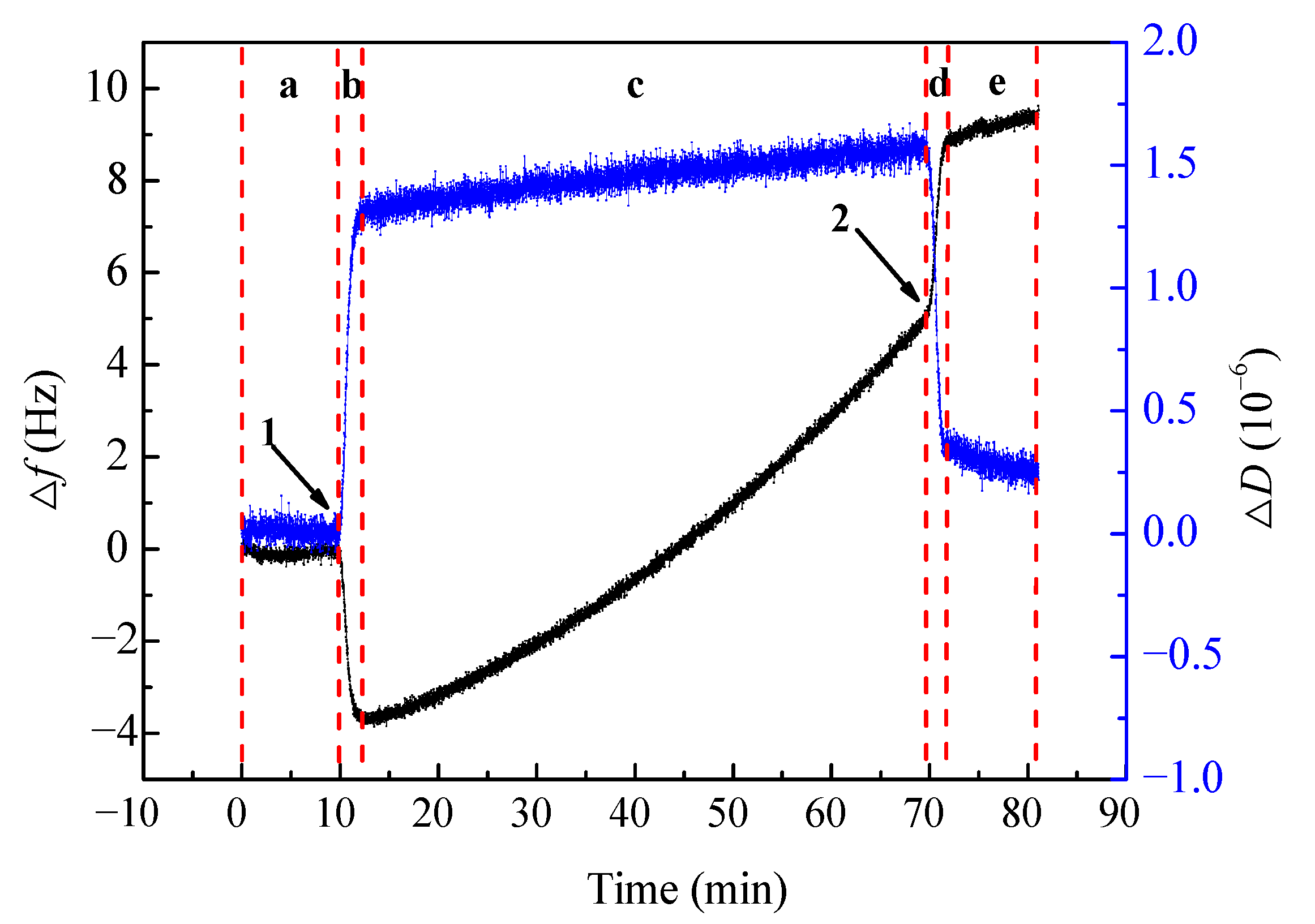
3.3. Kinetic Process of Gold Leaching on Au Sensor with PF by QCM-D
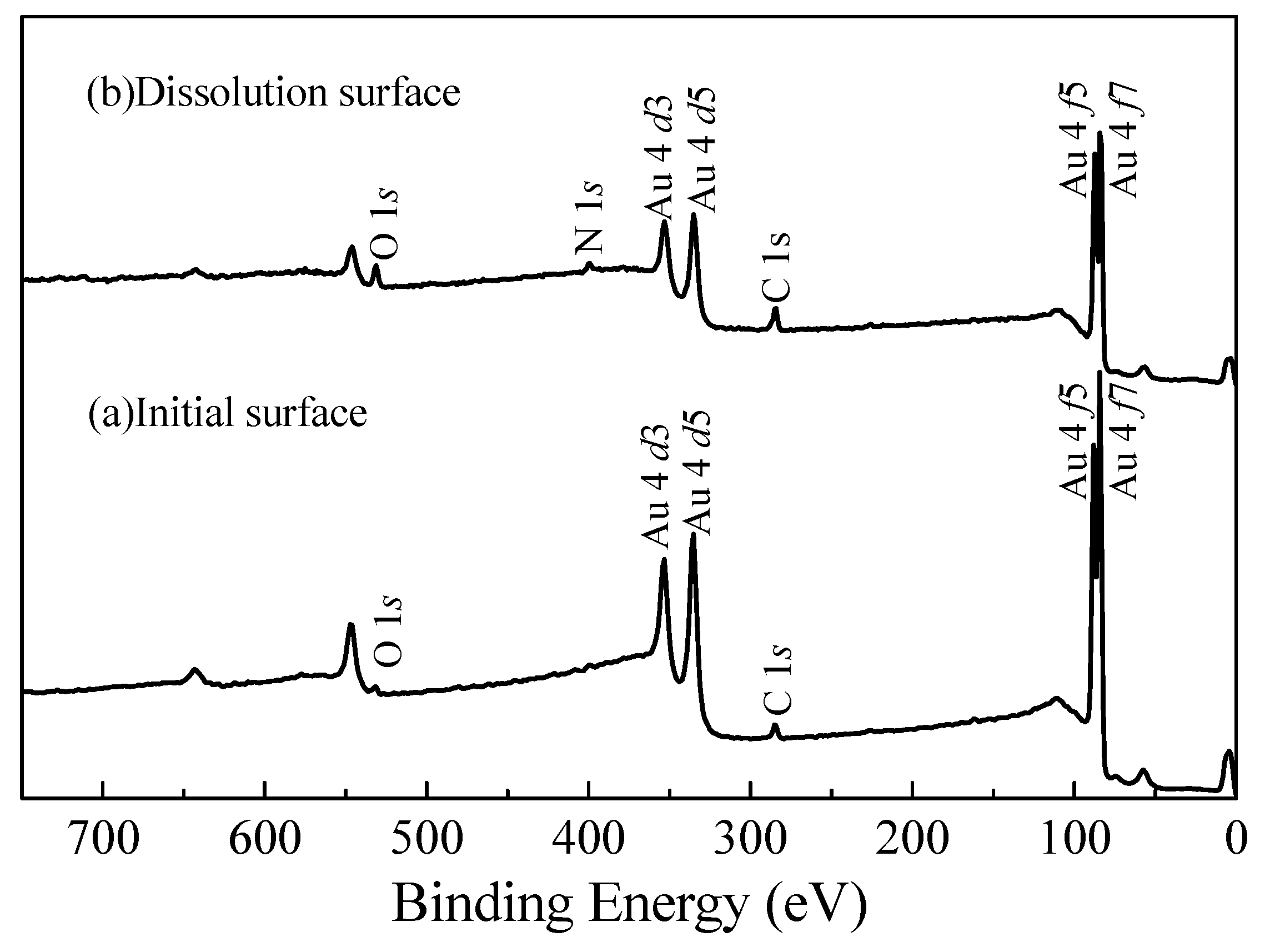
3.4. Surface Product Composition Analysis by XPS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas, C.; Navarro, P.; Espinoza, D.; Manríquez, J.; Mejía, E. Dissolution Behavior of Gold in Alkaline Media Using Thiourea. Int. J. Nonferrous Metall. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Y.; Huang, W.; Li, H.; Liao, S. The role of glycine in the ammonium thiocyanate leaching of gold. Hydrometallurgy 2019, 185, 111–116. [Google Scholar] [CrossRef]
- Liang, C.; Li, J. Recovery of gold in iodine-iodide system—A review. Sep. Sci. Technol. 2019, 54, 1055–1066. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, Y.; Li, Q. An eco-friendly and efficient process of low potential thiosulfate leaching-resin adsorption recovery for extracting gold from a roasted gold concentrate. J. Clean. Prod. 2019, 229, 387–398. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Breuer, P.L. The cyanide leaching of gold in solutions containing sulfide. Miner. Eng. 2000, 13, 1097–1106. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; Karrech, A.; Attar, M. Gold extraction from paleochannel ores using an aerated alkaline glycine lixiviant for consideration in heap and in-situ leaching applications. Miner. Eng. 2019, 138, 112–118. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Azizitorghabeh, A.; Wang, J.; Ramsay, J.A.; Ghahreman, A. A review of thiocyanate gold leaching—Chemistry, thermodynamics, kinetics and processing. Miner. Eng. 2021, 160, 106689. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J.; O’Connor, G. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Tran, Q.B.; Lohitnavy, M.; Phenrat, T. Assessing potential hydrogen cyanide exposure from cyanide-contaminated mine tailing management practices in Thailand’s gold mining. J. Environ. Manag. 2019, 249, 109357. [Google Scholar] [CrossRef]
- Meeussen, J.C.; Keizer, M.G.; De Haan, F.A. Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ. Sci. Technol. 1992, 26, 511–516. [Google Scholar] [CrossRef]
- Lim, H.S.; Hwang, J.Y.; Choi, E.; Lee, G.; Yun, S.S.; Kim, M. Assessment of ferrocyanide intake from food-grade salt in the Korean population. LWT Food Sci. Technol. 2018, 93, 620–627. [Google Scholar] [CrossRef]
- Van Nguyen, M.; Thorarinsdottir, K.A.; Thorkelsson, G.; Gudmundsdottir, A.; Arason, S. Influences of potassium ferrocyanide on lipid oxidation of salted cod (Gadus morhua) during processing, storage and rehydration. Food Chem. 2012, 131, 1322–1331. [Google Scholar] [CrossRef]
- Ašpergěr, S. Kinetics of the decomposition of potassium ferrocyanide in ultra-violet light. Trans. Faraday Soc. 1952, 48, 617–624. [Google Scholar] [CrossRef]
- Pommeret, S.; Naskrecki, R.; van der Meulen, P.; Ménard, M.; Vigneron, G.; Gustavsson, T. Ultrafast events in the electron photodetachment from the hexacyanoferrate (II) complex in solution. Chem. Phys. Lett. 1998, 288, 833–840. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.; Vásquez, C.; Veitia, L.; Ossa-Echeverry, O.; Rodriguez-Vallejo, J.; Barraza-Burgos, J.; Marriaga-Cabrales, N.; Machuca-Martínez, F. An approach to utilize the artificial high power LED UV-A radiation in photoreactors for the degradation of methylene blue. Photochem. Photobiol. Sci. 2017, 16, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hanela, S.; Durán, J.; Jacobo, S. Removal of iron–cyanide complexes from wastewaters by combined UV–ozone and modified zeolite treatment. J. Environ. Chem. Eng. 2015, 3, 1794–1801. [Google Scholar] [CrossRef]
- Chen, W.D.; Kang, S.-K.; Stark, W.J.; Rogers, J.A.; Grass, R.N. The light triggered dissolution of gold wires using potassium ferrocyanide solutions enables cumulative illumination sensing. Sens. Actuators B 2019, 282, 52–59. [Google Scholar] [CrossRef]
- Chemical Book Database. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB3329047.htm (accessed on 26 March 2021).
- Jeffrey, M.I.; Woods, R. Frequency changes observed with the EQCM resulting from hydrophilic/hydrophobic transitions. Colloids Surf. 2007, 302, 488–493. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S. In situ kinetics and conformation studies of dodecylamine adsorption onto zinc sulfide using a quartz crystal microbalance with dissipation (QCM-D). Colloids Surf. 2016, 490, 110–120. [Google Scholar] [CrossRef]
- Hieda, M.; Garcia, R.; Dixon, M.; Daniel, T.; Allara, D.; Chan, M. Ultrasensitive quartz crystal microbalance with porous gold electrodes. Appl. Phys. Lett. 2004, 84, 628–630. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Swiatek, S.; Komorek, P.; Jachimska, B. Adsorption of β-lactoglobulin A on gold surface determined in situ by QCM-D measurements. Food Hydrocoll. 2019, 91, 48–56. [Google Scholar] [CrossRef]
- Jeffrey, M.I.; Linda, L.; Breuer, P.L.; Chu, C.K. A kinetic and electrochemical study of the ammonia cyanide process for leaching gold in solutions containing copper. Miner. Eng. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Josefsson, P.; Henriksson, G.; Wågberg, L. The physical action of cellulases revealed by a quartz crystal microbalance study using ultrathin cellulose films and pure cellulases. Biomacromolecules 2008, 9, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gutig, C.; Grady, B.P.; Striolo, A. Experimental studies on the adsorption of two surfactants on solid—Aqueous interfaces: Adsorption isotherms and kinetics. Langmuir 2008, 24, 4806–4816. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Weng, L.-P.; Zhang, Q.-L.; Xu, H.; Ji, L.-N. Optimization of glucose oxidase production by Aspergillus niger in a benchtop bioreactor using response surface methodology. World J. Microbiol. Biotechnol. 2003, 19, 317–323. [Google Scholar] [CrossRef]
- Johnson, C.A. The fate of cyanide in leach wastes at gold mines: An environmental perspective. Appl. Geochem. 2015, 57, 194–205. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin Elmer Corp. 1992, 40, 221. [Google Scholar]
- Xu, S.; Kou, J.; Sun, T.; Jong, K. A study of adsorption mechanism of dodecylamine on sphalerite. Colloids Surf. 2015, 486, 145–152. [Google Scholar] [CrossRef]
- Ping, Z.; Xiaolin, W.; Jiangrong, Y.; Xiaoguo, F.; Lizhu, L. Effect of Vacuum Heat Treatment on oxidation of Uranium Surfaces. Rare Met. Mater. Eng. 2008, 1, 94–97. [Google Scholar]
- Xu, Y.; Sun, B.-L.; Ma, C.; Zhang, P.; Cai, M.-L.; Wu, X.-M. XPS and SEM spectroscopy study of hyperdispersant on atrazine surface. Spectrosc. Spectral Anal. 2011, 31, 2569–2573. [Google Scholar]
- Abbott, A.P.; Frisch, G.; Gurman, S.J.; Hillman, A.R.; Hartley, J.; Holyoak, F.; Ryder, K.S. Ionometallurgy: Designer redox properties for metal processing. Chem. Commun. (Camb.) 2011, 47, 10031–10033. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, Y. Investigation on the electrocatalytic characteristics of SnO2 electrodes with nanocoating prepared by electrodeposition method. Sci. China Ser. E Technol. Sci. 2009, 52, 1799–1803. [Google Scholar] [CrossRef]
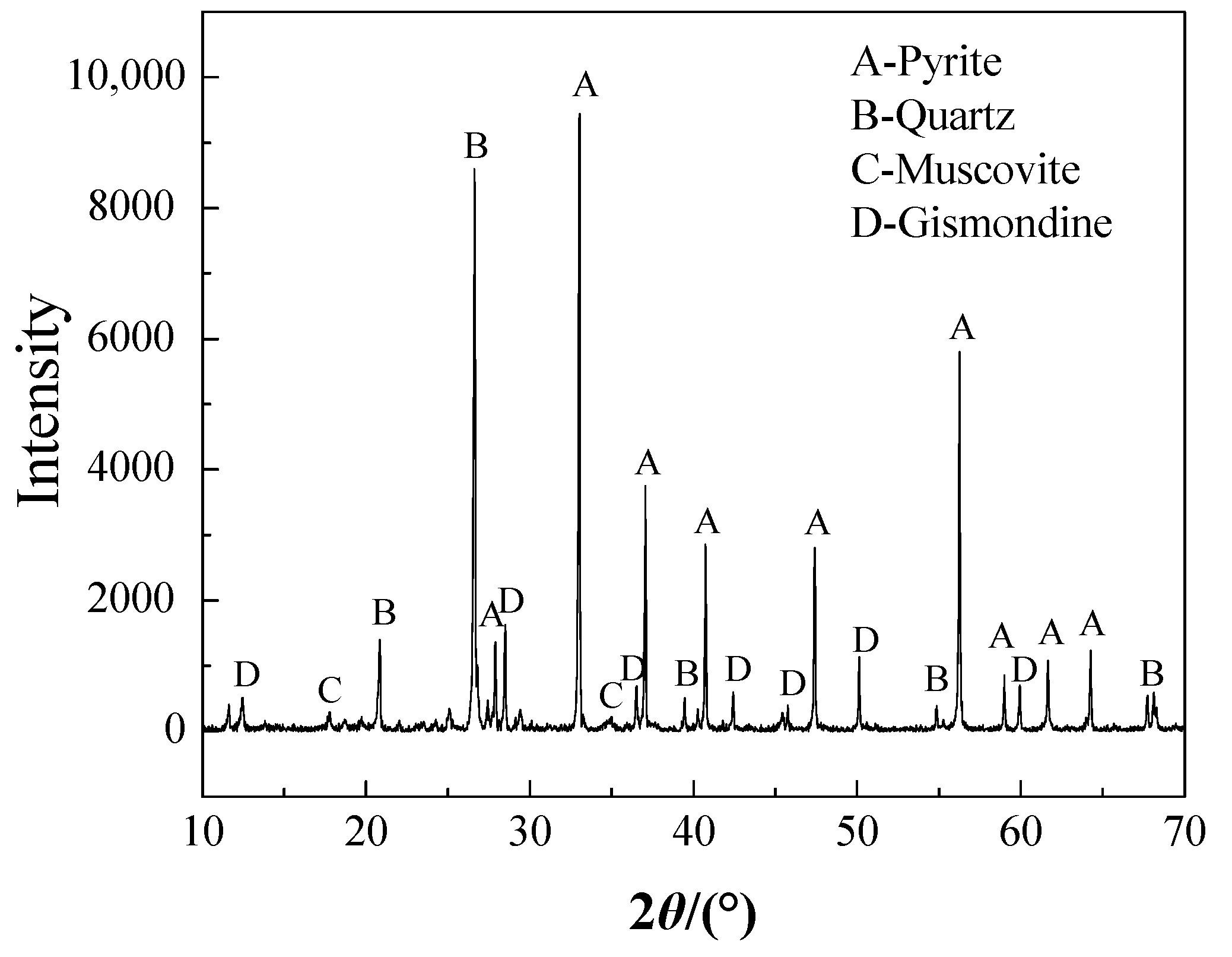
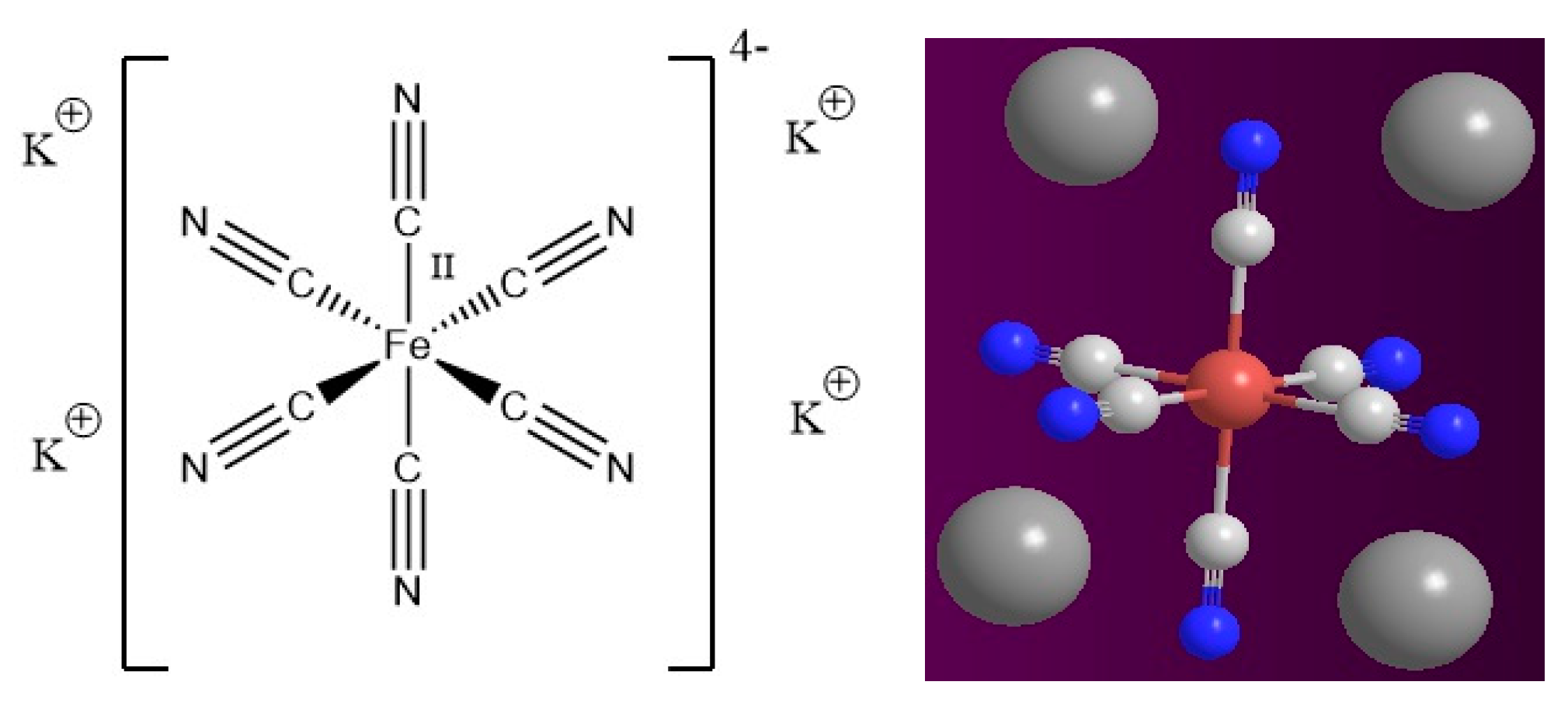
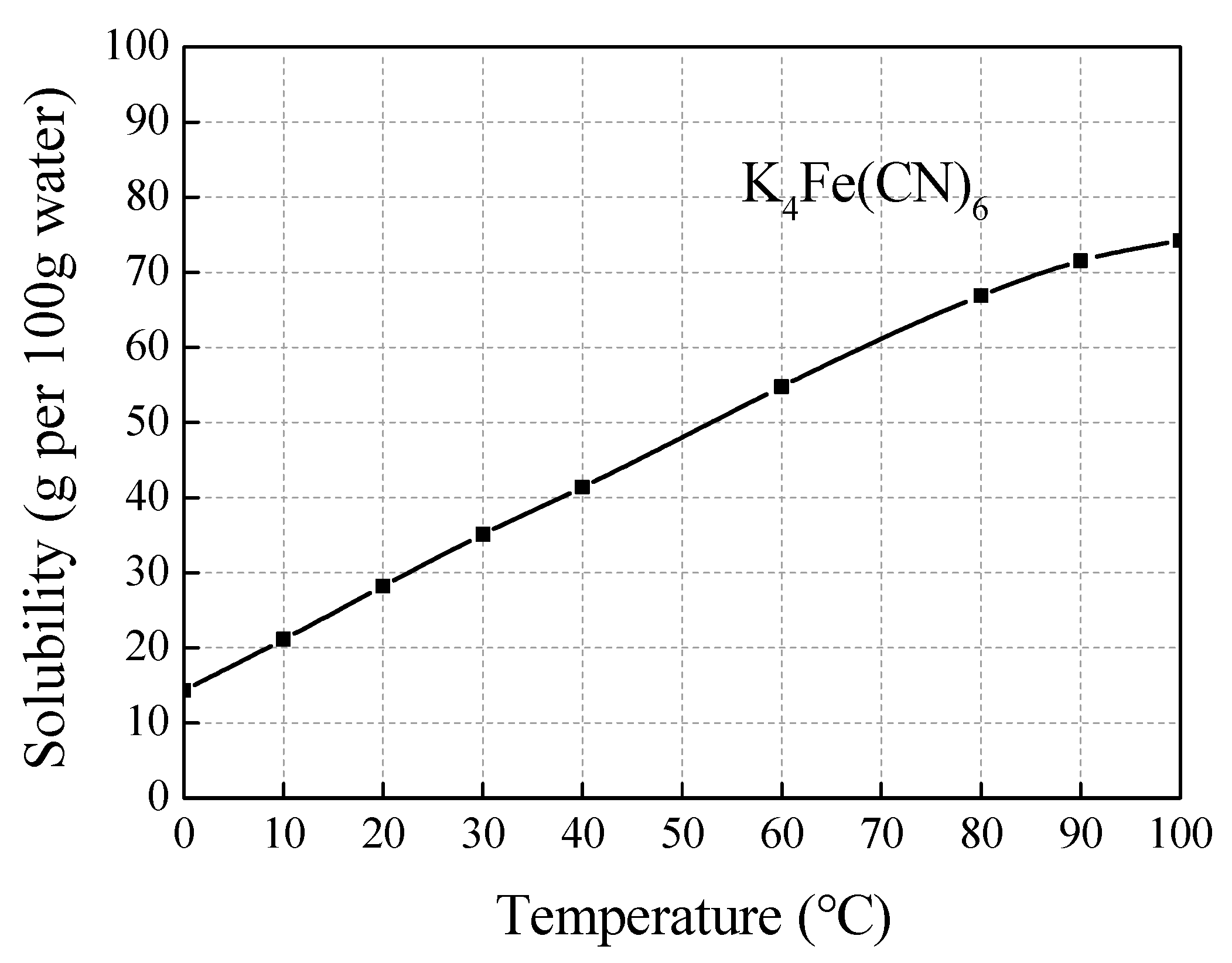
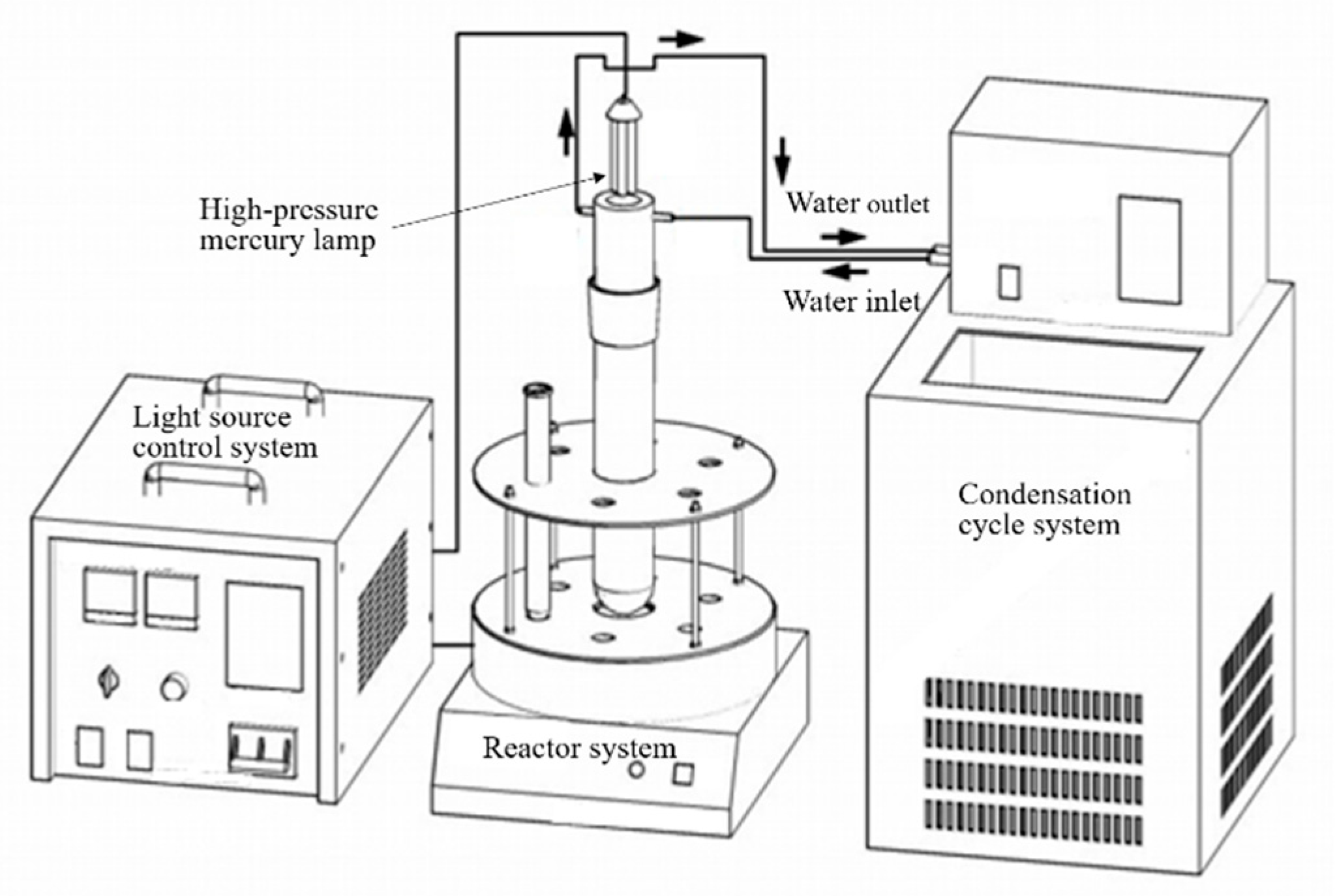



| Au * | Ag * | Fe | Cu | As | Zn |
| 56.78 | 38.8 | 26.03 | 0.11 | 0.082 | 0.044 |
| Pb | S | CaO | MgO | Al2O3 | SiO2 |
| 0.10 | 26.64 | 1.27 | 0.95 | 6.69 | 33.51 |
| Wavelength/nm | 250 | 313 | 365 | 400 | 510 | 620 | 720 |
| Relative intensity/% | 20 | 85 | 100 | 30 | 20 | 40 | 80 |
| Levels | X1: pH | X2: Dosage (kg/t) | X3: Temperature (°C) |
|---|---|---|---|
| −1 | 11 | 1 | 45 |
| 0 | 12 | 3 | 60 |
| 1 | 13 | 5 | 75 |
| Run | Factors | Response | ||
|---|---|---|---|---|
| X1: pH | X2: Dosage (kg/t) | X3: Temperature (°C) | Y: Au Extraction (%) | |
| 1 | 11 | 5 | 60 | 44.17 |
| 2 | 12 | 1 | 45 | 29.38 |
| 3 | 12 | 3 | 60 | 63.19 |
| 4 | 13 | 3 | 45 | 47.69 |
| 5 | 12 | 3 | 60 | 62.49 |
| 6 | 13 | 3 | 75 | 64.42 |
| 7 | 12 | 5 | 75 | 60.73 |
| 8 | 11 | 3 | 75 | 57.56 |
| 9 | 12 | 5 | 45 | 40.65 |
| 10 | 12 | 3 | 60 | 65.13 |
| 11 | 12 | 3 | 60 | 64.95 |
| 12 | 13 | 1 | 60 | 45.58 |
| 13 | 11 | 3 | 45 | 41.18 |
| 14 | 12 | 1 | 75 | 44.52 |
| 15 | 11 | 1 | 60 | 35.01 |
| 16 | 13 | 5 | 60 | 61.61 |
| 17 | 12 | 3 | 60 | 65.30 |
| Source | Sum of Squares | Degree of Freedom | Adjusted Mean Square | F Value | P Value Probability > F |
|---|---|---|---|---|---|
| Model | 2289.29 | 9 | 254.37 | 51.48 | <0.0001 |
| X1 | 214.04 | 1 | 214.04 | 43.32 | 0.0003 |
| X2 | 346.77 | 1 | 346.77 | 70.19 | <0.0001 |
| X3 | 583.62 | 1 | 583.62 | 118.13 | <0.0001 |
| X1X2 | 11.8 | 1 | 11.80 | 2.39 | 0.1662 |
| X1X3 | 0.031 | 1 | 0.031 | 0.0062 | 0.9394 |
| X2X3 | 6.1 | 1 | 6.10 | 1.23 | 0.3032 |
| X12 | 80.17 | 1 | 80.17 | 16.23 | 0.005 |
| X22 | 739.88 | 1 | 739.88 | 149.76 | <0.0001 |
| X32 | 214.41 | 1 | 214.41 | 43.40 | 0.0003 |
| Lack of Fit | 28 | 3 | 9.33 | 5.67 | 0.0634 |
| Reagent | pH | Dosage (kg/t) | Temperature (°C) | Cyanide Ion (mg/L) | Gold Extraction (Test Value %) |
|---|---|---|---|---|---|
| PF | 12.6 | 3.8 | 62 | 19.1 | 67.01 |
| PF | 12.6 | 3.8 | 62 | 17.9 | 67.47 |
| PF | 12.6 | 3.8 | 62 | 18.4 | 67.33 |
| Cyanide | 12.6 | 2 | 62 | 84.9 | 68.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Kou, J.; Xing, Y.; Sun, C. Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals 2021, 11, 387. https://doi.org/10.3390/min11040387
Liu Z, Kou J, Xing Y, Sun C. Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals. 2021; 11(4):387. https://doi.org/10.3390/min11040387
Chicago/Turabian StyleLiu, Ziyuan, Jue Kou, Yi Xing, and Chunbao Sun. 2021. "Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light" Minerals 11, no. 4: 387. https://doi.org/10.3390/min11040387
APA StyleLiu, Z., Kou, J., Xing, Y., & Sun, C. (2021). Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light. Minerals, 11(4), 387. https://doi.org/10.3390/min11040387







