Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China
Abstract
:1. Introduction
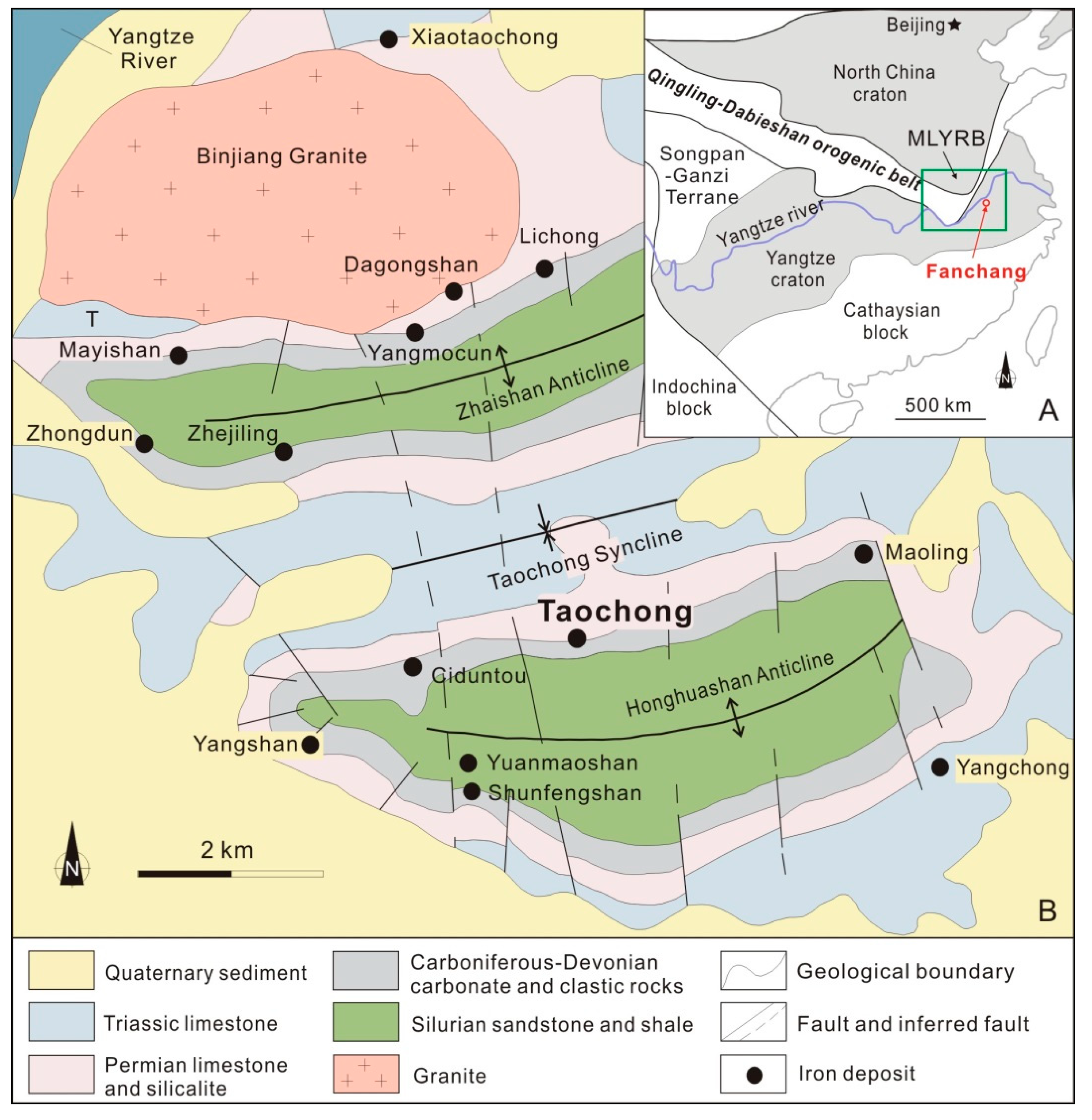
2. Geological Setting
3. Mineralization Styles and Paragenetic Sequence
4. Samples and Analytical Techniques
5. Results
6. Discussion
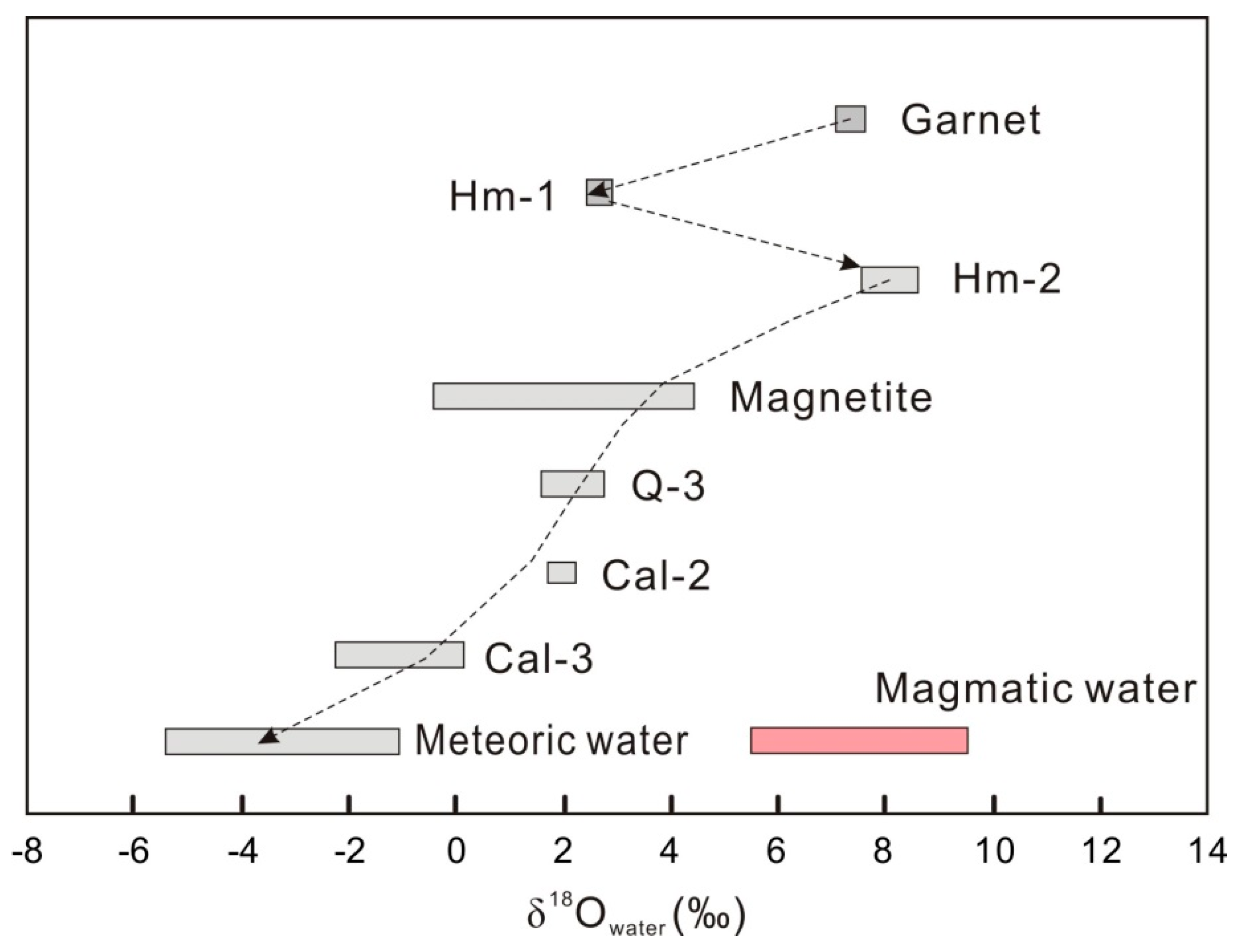
6.1. Sources of Hydrothermal Fluids
6.2. Modeling of Hydrothermal Fluid Processes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnes, H.L. Geochemistry of Hydrothemal Ore Deposits, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1979. [Google Scholar]
- Barnes, H.L. Geochemistry of Hydrothemal ORE deposits, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Kwak, T.A.P. Fluid inclusions in skarns (carbonate replacement deposits). J. Metamorph. Geol. 1986, 4, 362–384. [Google Scholar] [CrossRef]
- Cao, Y.; Du, Y.S.; Pang, Z.S.; Du, Y.L.; Kou, S.L.; Chen, L.J.; Gao, F.P.; Zhou, G.B. Geologic, fluid inclusion and stable isotope constraints on mechanisms of ore deposition at the Datuanshan copper deposit, Middle–Lower Yangtze Valley, Eastern China. Acta Geol. Sin Engl. 2015, 89, 801–840. [Google Scholar]
- Ding, K.; Seyfried, W.E. Determination of Fe–Cl complexing in the low pressure supercritical region (NaCl fluid). iron solubility constraints on pH of subseafloor hydrothermal fluids. Geochim. Cosmochim. Acta 1992, 56, 3681–3692. [Google Scholar] [CrossRef]
- Hu, W.; Li, J.W.; Lentz, D.; Ren, Z.; Zhao, X.F.; Deng, X.D.; Hall, D. Dissolution-reprecipitation process of magnetite from the Chengchao iron deposit: Insights into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- Cao, Y.; Du, Y.S.; Gao, F.P.; Hu, L.F.; Xin, F.P.; Pang, Z.S. Origin and evolution of hydrothermal fluids in the Taochong iron deposit, Middle–Lower Yangtze Valley, Eastern China: Evidence from microthermometric and stable isotope analyses of fluid inclusions. Ore Geol. Rev. 2012, 48, 225–235. [Google Scholar] [CrossRef]
- Xie, G.Q.; Mao, J.W.; Zhu, Q.Q.; Zheng, J.H. Mineralogy, fluid inclusion, and stable isotope studies of the Chengchao deposit, Hubei Province, Eastern China: Implications for the formation of high-grade Fe skarn deposits. Econ. Geol. 2019, 114, 325–352. [Google Scholar]
- Loughrey, L.; Marshall, D.; Ihlen, P.; Jones, P. Boiling as a mechanism for colour zonations observed at the Byrud emerald deposit, Eidsvoll, Norway: Fluid inclusion, stable isotope and Ar–Ar studies. Geofluids 2013, 13, 542–558. [Google Scholar] [CrossRef]
- Moura, M.A.; Botelho, N.F.; Olivo, G.R.; Kyser, K.; Pontes, R.M. Genesis of the Proterozoic Mangabeira tin–indium mineralization, Central Brazil: Evidence from geology, petrology, fluid inclusion and stable isotope data. Ore Geol. Rev. 2014, 60, 36–49. [Google Scholar] [CrossRef]
- Gao, X.; Yang, L.Q.; Yan, H.; Meng, J.Y. Ore-forming processes and mechanisms of the Hongshan skarn Cu–Mo deposit, Southwest China: Insights from mineral chemistry, fluid inclusions, and stable isotopes. Ore Energy Resour. Geol. 2020, 4, 100007. [Google Scholar] [CrossRef]
- Mei, W.; Lu, X.B.; Cao, X.F.; Liu, Z.; Zhao, Y.; Ai, Z.L.; Tang, R.K.; Abfaua, M.M. Ore genesis and hydrothermal evolution of the Huanggang skarn iron-tin polymetallic deposit, southern Great Xing’an Range: Evidence from fluid inclusions and isotope analyses. Ore Geol. Rev. 2015, 64, 239–252. [Google Scholar] [CrossRef]
- Wen, G.; Bi, S.J.; Li, J.W. Role of evaporitic sulfates in iron skarn mineralization: A fluid inclusion and sulfur isotope study from the Xishimen deposit, Handan-Xingtai district, North China Craton. Miner. Deposita 2017, 52, 495–514. [Google Scholar] [CrossRef]
- Meinert, L.D.; Hefton, K.K.; Mayes, D.; Tasiran, I. Geology, zonation, and fluid evolution of the Big Gossan Cu-Au skarn deposit, Ertsberg District, Irian Jaya. Econ. Geol. 1997, 92, 509–534. [Google Scholar] [CrossRef]
- Yang, Y.L.; Ni, P.; Pan, J.Y.; Wang, G.G.; Xu, Y.F. Constraints on the mineralization processes of the Makeng iron deposit, eastern China: Fluid inclusion, H-O isotope and magnetite trace element analysis. Ore Geol. Rev. 2017, 88, 791–808. [Google Scholar] [CrossRef]
- Lu, H.Z.; Liu, Y.M.; Wang, C.L.; Xu, Y.Z.; Li, H.Q. Mineralization and fluid inclusion study of the Shizhuyuan W-Sn-Bi-Mo-F skarn deposit, Hunan Province, China. Econ. Geol. 2003, 98, 955–974. [Google Scholar] [CrossRef]
- Shmulovich, K.I.; Landwehr, D.; Simon, K.; Heinrich, W. Stable isotope fractionation between liquid and vapour in water–salt systems up to 600 °C. Chem. Geol. 1999, 157, 343–354. [Google Scholar] [CrossRef]
- Pan, Y.M.; Dong, P. The Lower Changjiang (Yangzi/Yangtze River) metallogenic belt, east central China: Intrusion– and wall rock–hosted Cu–Fe–Au, Mo, Zn, Pb, Ag deposits. Ore Geol. Rev. 1999, 15, 177–242. [Google Scholar] [CrossRef]
- Chang, Y.F.; Liu, X.P.; Wu, Y.C. The Copper–Iron Belt of the Lower and Middle Reaches of the Changjiang River; Geological Publishing House: Beijing, China, 1991; p. 379, (In Chinese with English abstract). [Google Scholar]
- Mao, J.W.; Wang, Y.T.; Lehmann, B.; Yu, J.J.; Du, A.D.; Mei, Y.X.; Li, Y.F.; Zang, W.S.; Stein, H.; Zhou, T.F. Molybdenite Re–Os and albite 40Ar/39Ar dating of Cu–Au–Mo and magnetite porphyry systems in the Yangtze River valley and metallogenic implications. Ore Geol. Rev. 2006, 29, 307–324. [Google Scholar] [CrossRef]
- Mao, J.W.; Xie, G.Q.; Duan, C.; Pirajno, F.; Ishiyama, D.; Chen, Y.C. A tectono–genetic model for porphyry–skarn–stratabound Cu–Au–Mo–Fe and magnetite–apatite deposits along the Middle–Lower Yangtze River Valley, Eastern China. Ore Geol. Rev. 2011, 43, 294–314. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Yang, Z.S.; Lü, Q.T.; Zeng, P.S.; Xie, Y.L.; Meng, Y.F.; Tian, S.H.; Xu, W.Y.; Li, H.Y.; Jiang, Z.P.; et al. The large scale Dongguashan deposit, Shizishan district in East China: Carboniferous sedex-type massive sulfides overprinted by Late Jurassic skarn Cu mineralization. Acta Geol. Sin. 2011, 85, 659–686, (In Chinese with English abstract). [Google Scholar]
- Tang, Y.C.; Wu, Y.C.; Chu, G.Z.; Xing, F.M.; Wang, Y.M.; Cao, F.Y.; Chang, Y.F. Geology of Copper–Gold Polymetallic Deposits Along the Yangtz River, Anhui Province; Geological Publishing House: Beijing, China, 1998; p. 351, (In Chinese with English abstract). [Google Scholar]
- Xu, G.; Lin, X. Geology and geochemistry of the Changlongshan skarn iron deposit, Anhui Province, China. Ore Geol. Rev. 2000, 16, 91–106. [Google Scholar] [CrossRef]
- Xu, G.; Lin, X. On the origin of banded iron ore of the Changlongshan deposit, southern Anhui Province. Contrib. Geol. Mineral Resour. Res. 1991, 6, 69–75, (In Chinese with English abstract). [Google Scholar]
- Ren, G.L.; He, W.; Liu, J.P.; Wang, B.; Wu, Y.F.; Huang, C.Y. Geochemical characteristics and genesis of Taochong iron deposit in Fanchang district, Anhui Province, China. Earth Sci. Front. 2012, 19, 82–95, (In Chinese with English abstract). [Google Scholar]
- Wagner, T.; Mlynarczyk, M.S.J.; Williams–Jones, A.E.; Boyce, A.J. Stable isotope constraints on ore formation at the San Rafael tin–copper deposit, southeast Peru. Econ. Geol. 2009, 104, 223–248. [Google Scholar] [CrossRef]
- Wagner, T.; Boyce, A.J. Pyrite metamorpohism in the Devonian Hunsruck slate of Germany insights from Laser microprobe sulfur isotope analysis and thermodynamic modeling. J. Sci. 2006, 306, 525–552. [Google Scholar]
- Wagner, T.; Williams–Jones, A.E.; Boyce, A.J. Stable isotope–based modeling of the origin and genesis of an unusual Au–Ag–Sn–W epithermal system at Cirotan, Indonesia. Chem. Geol. 2005, 219, 237–260. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Z.Y.; Wang, Y. Geological Characteristics of iron deposits in Fanchang area, Anhui Province, East China. Express Inf. Min. Ind. 2007, 462, 75–77. (In Chinese) [Google Scholar]
- Metcalfe, I. Tectonic framework and Phanerozoic evolution of Sundaland. Gondwana Res. 2011, 19, 3–21. [Google Scholar] [CrossRef]
- 321 Geological Team of Bureau of Geology and Mineral Exploration of Anhui Province. Report of 1/50000 Regional Geological Survey for the Fanchang Area. 1989. Available online: http://www.ngac.cn/125cms/c/qggnew/index.htm (accessed on 1 April 2021). (In Chinese).
- 803 Geological Team of East China Metallurgical and Geological Exploration. Internal Exploration Report for the Changlonshan Iron Deposit. 1963. Available online: http://www.ngac.cn/125cms/c/qggnew/index.htm (accessed on 1 April 2021). (In Chinese).
- Ni, R.S.; Wang, D.H. An investigation of the genesis of the Taochong iron deposit in Fanchang County, Anhui Province. Miner. Depos. 1983, 2, 16–23, (In Chinese with English abstract). [Google Scholar]
- Cao, Y.; Du, Y.S.; Pang, Z.S.; Gao, F.P.; Du, Y.L.; Liu, X.M. Iron transport and deposition mechanisms in the Taochong iron-rich skarn deposit, Middle–Lower Yangtze Valley, Eastern China. Ore Geol. Rev. 2016, 72, 1037–1052. [Google Scholar] [CrossRef]
- Clayton, R.N.; Mayeda, T.K. The use of bromine pentafiuoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen isotope exchange between quartz and water. J. Geophys. Res. 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Chen, J.F. Stable Isotope Geochemistry; Science Press: Beijing, China, 2000; pp. 1–316. (In Chinese) [Google Scholar]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Zhang, G.L. The Application of the Stable Isotope to Geology; Shaanxi Science and Technology Publishing House: Xi’an, China, 1985; pp. 1–267, (In Chinese with English abstract). [Google Scholar]
- Zhou, T.F.; Yue, S.C. Isotope geochemistry of copper mineralization in Yueshan, Anhui province. Miner. Depos. 1996, 15, 341–350, (In Chinese with English abstract). [Google Scholar]
- Horita, J.; Cole, D.R.; Wesolowski, D.J. The activity–composition relationship of oxygen and hydrogen isotopes in aqueous salt solutions: III. Vapor–liquid water equilibration of NaCl solutions to 350 °C. Geochim. Cosmochim. Acta 1995, 59, 1139–1151. [Google Scholar] [CrossRef]
- Horita, J.; Wesolowski, D.J.; Cole, D.R. The activity–composition relationship of oxygen and hydrogen isotopes in aqueous salt solutions: I. Vapor–liquid water equilibration of single salt solutions from 50 to 100 °C. Geochim. Cosmochim. Acta 1993, 57, 2797–2817. [Google Scholar] [CrossRef]
- Truesdell, A.H.; Nathenson, M. The effects of boiling and dilution on the isotopic compositions of Yellowstone thermal waters. J. Geophys. Res. 1977, 82, 3694–3704. [Google Scholar] [CrossRef]
- Horita, J.; Wesolowski, D.J. Liquid–vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochim. Cosmochim. Acta 1994, 58, 3425–3437. [Google Scholar] [CrossRef]
- Taylor, H.P. Water/rock interactions and the origin of H2O in granitic batholiths. J. Geol. Soc. 1977, 133, 509–558. [Google Scholar] [CrossRef]
- Taylor, H.P. Oxygen and hydrogen isotope relationships in hydrothermal mineral deposits. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1997; pp. 229–302. [Google Scholar]
- Reed, M.H.; Spycher, N.F. Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim. Cosmochim. Acta. 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Spycher, N.; Reed, M.H. Evolution of a Broadlands–type epithermal ore fluid along alternative P–T paths: Implications for the transport and deposition of base, precious, and volatile metals. Econ. Geol. 1989, 84, 328–359. [Google Scholar] [CrossRef]
- Schwinn, G.; Wagner, T.; Markl, G.; Baatartsogt, B. Quantification of mixing processes in ore–forming hydrothermal systems by combination of stable isotope and fluid inclusion analyses. Geochim. Cosmochim. Acta. 2006, 70, 965–982. [Google Scholar] [CrossRef]



| Sample | Mineral | Temperature (°C) a | δ18O‰ | δ18Owater (‰) b |
|---|---|---|---|---|
| TCYQ101 | Hematite (Hm-1) | 380 | −6.86 | 2.67 |
| TCYQ102 | Hematite (Hm-2) | 320 | −1.60 | 8.54 |
| TCYQ103 | Hematite (Hm-2) | 320 | −2.50 | 7.64 |
| TCYQ104 | Hematite (Hm-2) | 320 | −2.10 | 8.04 |
| TCYQ129 | Magnetite | 320 | −5.00 | 3.08 |
| TCYQ139 | Magnetite | 320 | −3.91 | 4.17 |
| TCYQ183 | Magnetite | 320 | −8.20 | −0.12 |
| TCYQ100 c | Magnetite | 320 | −4.70 | 3.38 |
| TCYQ106-1 d | Quartz (Q-3) | 300 | 8.74 | 1.85 |
| TCYQ128 d | Quartz (Q-3) | 320 | 7.89 | 1.68 |
| TCYQ132 d | Quartz (Q-3) | 320 | 8.80 | 2.59 |
| TCYQ143 d | Quartz (Q-3) | 320 | 7.94 | 1.73 |
| TCYQ101 d | Calcite (Cal-2) | 260 | 8.96 | 1.88 |
| TCYQ132 d | Calcite (Cal-2) | 260 | 9.15 | 2.07 |
| TCYQ139 d | Calcite (Cal-3) | 250 | 7.81 | 0.36 |
| TCYQ003 c | Calcite (Cal-3) | 250 | 5.80 | −1.65 |
| TCYQ004 c | Calcite (Cal-3) | 250 | 5.50 | −1.95 |
| TCYQ005 c | Calcite (Cal-3) | 250 | 5.30 | −2.15 |
| TCYQ006 c | Calcite (Cal-3) | 250 | 5.28 | −2.17 |
| F = Mass | Tmix (°C) | δ18Owater | δ18Omineral | |||
|---|---|---|---|---|---|---|
| Fraction Brine | Quartz | Hematite | Magnetite | Calcite | ||
| The Minerals in Equilibrium with A Magmatic Brine (T = 500 °C, δ18O = 8.6‰) That Is Mixing with Meteoric Water (T = 200 °C, δ18O = −3.2‰) | ||||||
| 1.00 | 500 | 8.60 | 10.9 | 0.24 | 1.61 | 10.99 |
| 0.95 | 485 | 8.01 | 10.5 | −0.49 | 0.93 | 10.55 |
| 0.90 | 470 | 7.42 | 10.1 | −1.22 | 0.24 | 10.12 |
| 0.85 | 455 | 6.83 | 9.8 | −1.96 | −0.45 | 9.70 |
| 0.80 | 440 | 6.24 | 9.5 | −2.69 | −1.14 | 9.30 |
| 0.75 | 425 | 5.65 | 9.2 | −3.43 | −1.82 | 8.91 |
| 0.70 | 410 | 5.06 | 8.9 | −4.17 | −2.51 | 8.54 |
| 0.65 | 395 | 4.47 | 8.6 | −4.91 | −3.50 | 8.19 |
| 0.60 | 380 | 3.88 | 8.4 | −5.65 | −3.87 | 7.86 |
| 0.55 | 365 | 3.29 | 8.2 | −6.39 | −4.55 | 7.54 |
| 0.50 | 350 | 2.70 | 8.0 | −7.14 | −5.22 | 7.26 |
| 0.45 | 335 | 2.11 | 7.8 | −7.88 | −5.89 | 7.00 |
| 0.40 | 320 | 1.52 | 7.7 | −8.62 | −6.56 | 6.77 |
| 0.35 | 305 | 0.93 | 7.6 | −9.36 | −7.21 | 6.58 |
| 0.30 | 290 | 0.34 | 7.6 | −10.10 | −7.86 | 6.42 |
| 0.25 | 275 | −0.25 | 7.6 | −10.83 | −8.50 | 6.30 |
| 0.20 | 260 | −0.84 | 7.7 | −11.56 | −9.13 | 6.24 |
| 0.15 | 245 | −1.43 | 7.8 | −12.28 | −9.74 | 6.22 |
| 0.10 | 230 | −2.02 | 7.9 | −13.00 | −10.33 | 6.27 |
| 0.05 | 215 | −2.61 | 8.2 | −13.70 | −10.91 | 6.38 |
| 0.00 | 200 | −3.20 | 8.5 | −14.39 | −11.45 | 6.57 |
| The Minerals in Equilibrium with A Magmatic Brine (T = 500 °C, δ18O = 8.6‰) That Is Mixing with Meteoric Water (T = 200 °C, δ18O = −5.0‰) | ||||||
| 1.00 | 500 | 8.60 | 10.85 | 0.24 | 1.61 | 10.99 |
| 0.95 | 485 | 7.92 | 10.40 | −0.58 | 0.84 | 10.46 |
| 0.90 | 470 | 7.24 | 9.96 | −1.40 | 0.06 | 9.94 |
| 0.85 | 455 | 6.56 | 9.53 | −2.23 | −0.72 | 9.43 |
| 0.80 | 440 | 5.88 | 9.13 | −3.05 | −1.50 | 8.94 |
| 0.75 | 425 | 5.20 | 8.73 | −3.88 | −2.27 | 8.46 |
| 0.70 | 410 | 4.52 | 8.36 | −4.71 | −3.05 | 8.00 |
| 0.65 | 395 | 3.84 | 8.01 | −5.54 | −3.82 | 7.56 |
| 0.60 | 380 | 3.16 | 7.68 | −6.37 | −5.05 | 7.14 |
| 0.55 | 365 | 2.48 | 7.38 | −7.20 | −5.36 | 6.73 |
| 0.50 | 350 | 1.80 | 7.10 | −8.04 | −6.12 | 6.36 |
| 0.45 | 335 | 1.12 | 6.86 | −8.87 | −6.88 | 6.01 |
| 0.40 | 320 | 0.44 | 6.65 | −9.70 | −7.64 | 5.69 |
| 0.35 | 305 | −0.24 | 6.47 | −10.53 | −8.38 | 5.41 |
| 0.30 | 290 | −0.92 | 6.34 | −11.36 | −9.12 | 5.16 |
| 0.25 | 275 | −1.60 | 6.25 | −12.18 | −9.85 | 4.95 |
| 0.20 | 260 | −2.28 | 6.21 | −13.00 | −10.57 | 4.80 |
| 0.15 | 245 | −2.96 | 6.23 | −13.81 | −11.27 | 4.69 |
| 0.10 | 230 | −3.64 | 6.31 | −14.62 | −11.95 | 4.65 |
| 0.05 | 215 | −4.32 | 6.46 | −15.41 | −12.62 | 4.67 |
| 0.00 | 200 | −5.00 | 6.70 | −16.19 | −13.25 | 4.77 |
| T (°C) | δ18Owater | δ18Omineral | |||
|---|---|---|---|---|---|
| Quartz | Hematite | Magnetite | Calcite | ||
| 500 | 8.60 | 10.9 | 0.24 | 1.61 | 10.99 |
| 480 | 8.60 | 11.2 | 0.05 | 1.48 | 11.19 |
| 460 | 8.60 | 11.5 | −0.14 | 1.35 | 11.41 |
| 440 | 8.60 | 11.8 | −0.33 | 1.22 | 11.66 |
| 420 | 8.60 | 12.2 | −0.53 | 1.10 | 11.93 |
| 400 | 8.60 | 12.7 | −0.73 | 0.97 | 12.24 |
| 380 | 8.60 | 13.1 | −0.93 | 0.85 | 12.58 |
| 360 | 8.60 | 13.6 | −1.13 | 0.73 | 12.95 |
| 340 | 8.60 | 14.2 | −1.34 | 0.62 | 13.38 |
| 320 | 8.60 | 14.8 | −1.54 | 0.52 | 13.85 |
| 300 | 8.60 | 15.5 | −1.74 | 0.44 | 14.39 |
| 280 | 8.60 | 16.2 | −1.93 | 0.37 | 14.99 |
| 260 | 8.60 | 17.1 | −2.12 | 0.31 | 15.68 |
| 240 | 8.60 | 18.0 | −2.29 | 0.29 | 16.46 |
| 220 | 8.60 | 19.1 | −2.45 | 0.30 | 17.35 |
| 200 | 8.60 | 20.3 | −2.59 | 0.35 | 18.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Cao, Y.; Zhang, Z.; Du, Y.; Guo, C. Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China. Minerals 2021, 11, 375. https://doi.org/10.3390/min11040375
Li N, Cao Y, Zhang Z, Du Y, Guo C. Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China. Minerals. 2021; 11(4):375. https://doi.org/10.3390/min11040375
Chicago/Turabian StyleLi, Niannian, Yi Cao, Zhaonian Zhang, Yilun Du, and Chenfang Guo. 2021. "Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China" Minerals 11, no. 4: 375. https://doi.org/10.3390/min11040375
APA StyleLi, N., Cao, Y., Zhang, Z., Du, Y., & Guo, C. (2021). Oxygen-Isotope-Based Modeling of the Hydrothermal Fluid Processes of the Taochong Skarn Iron Deposit, Anhui Province, China. Minerals, 11(4), 375. https://doi.org/10.3390/min11040375






