Study on Chromaticity Removal from Mineral Processing Wastewater with Salicylic Hydroxamic Acid by Granular Activated Carbon Catalyzed Ozonation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Experimental Wastewater
2.1.2. Chemicals
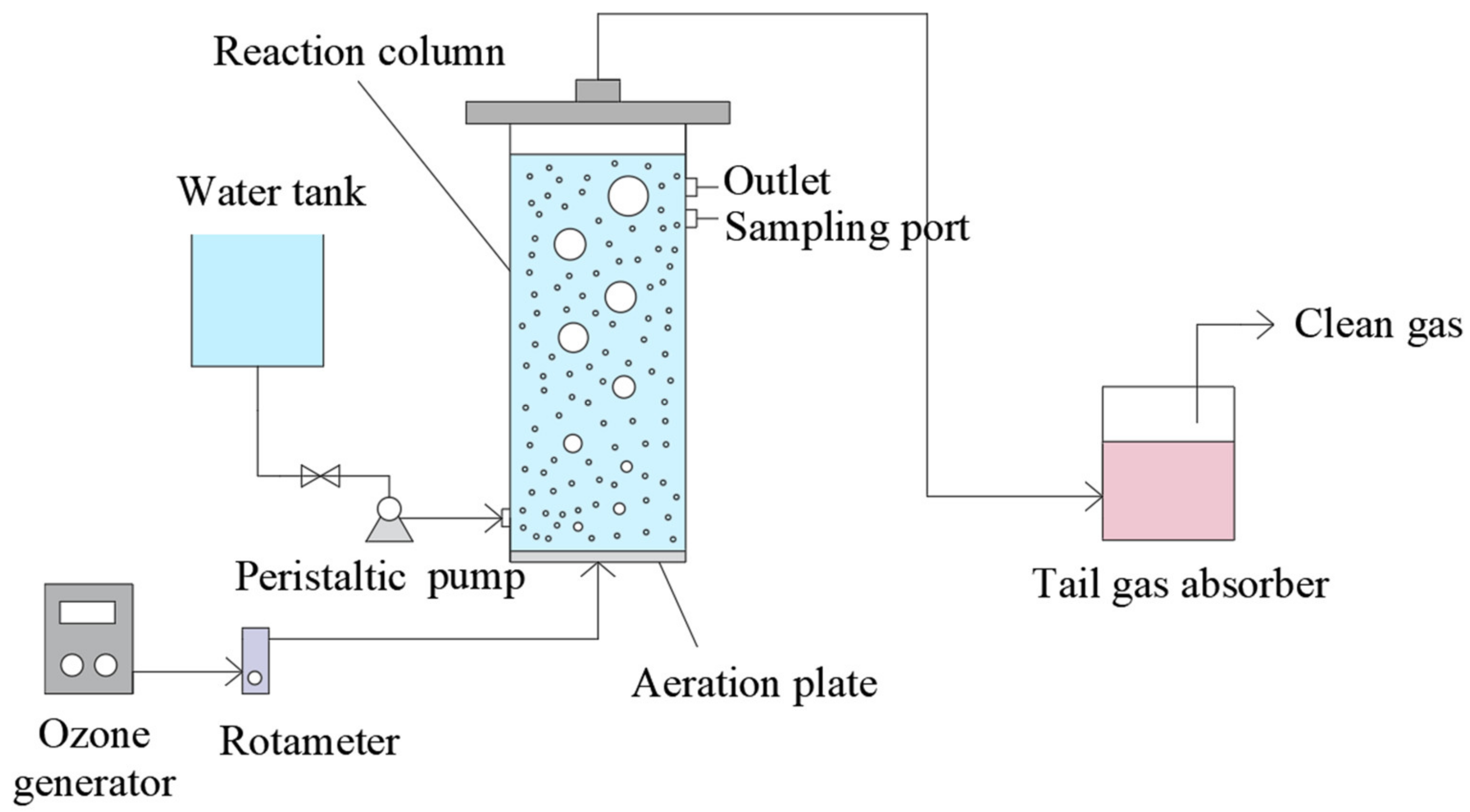
2.2. Experimental Setup
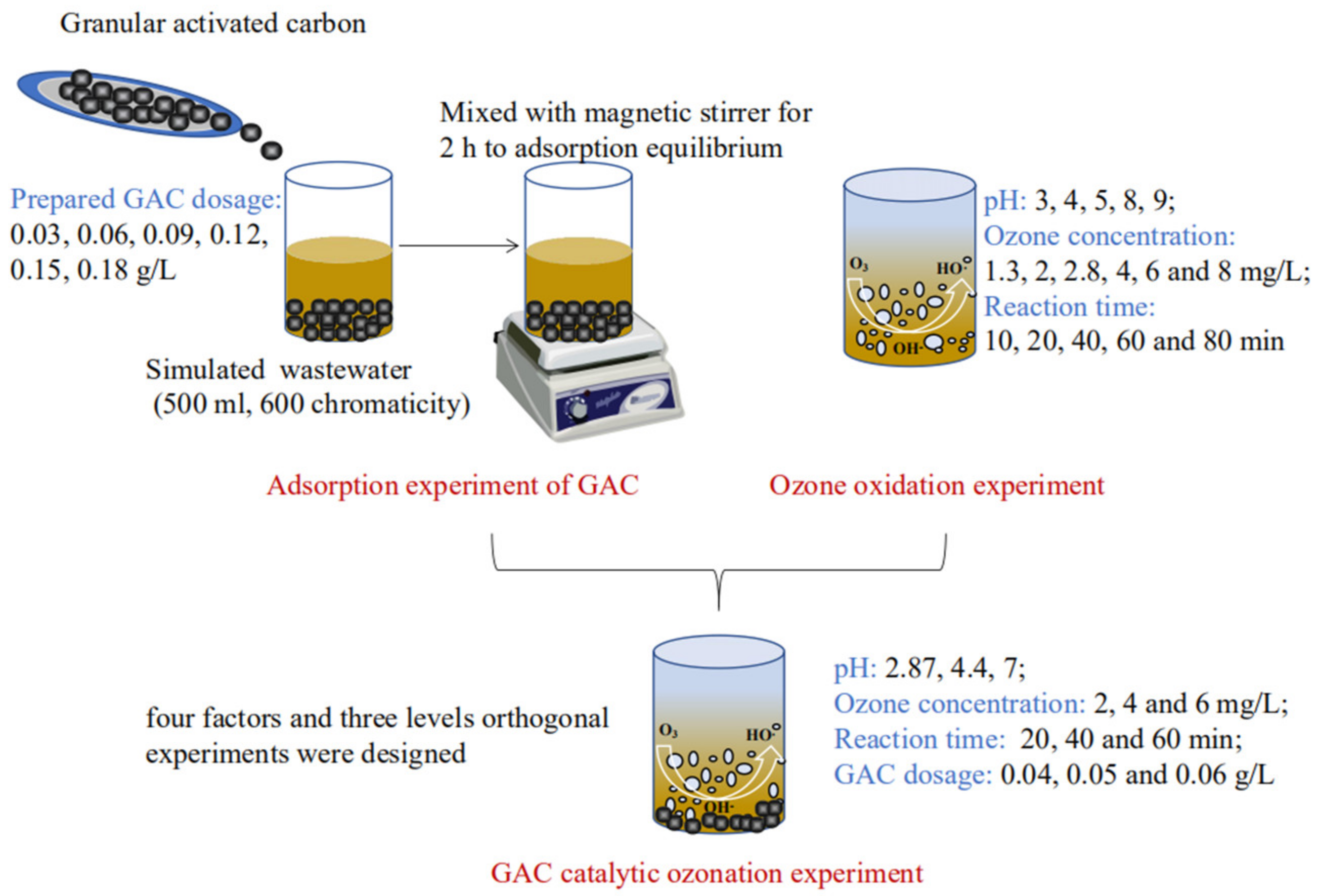
2.3. Experimental Procedure
2.3.1. Adsorption Experiment of GAC
2.3.2. Ozone Oxidation Experiment
2.3.3. GAC Catalytic Ozonation Experiment
2.4. Analysis
3. Results and Discussion
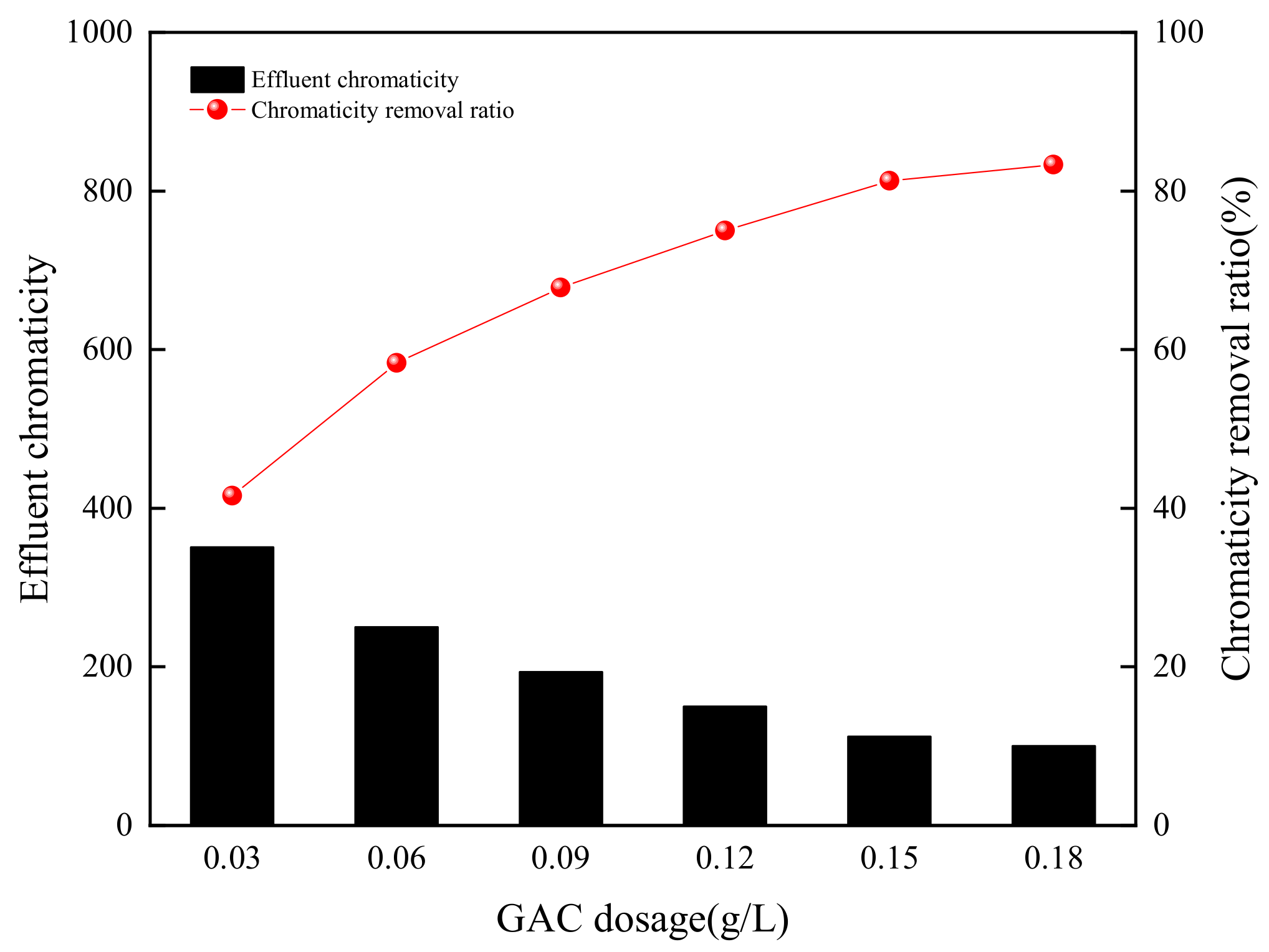
3.1. Effect of GAC Dosage on Chromaticity Removal
3.2. Ozonation Effects of Operation Parameters on Chromaticity Removal
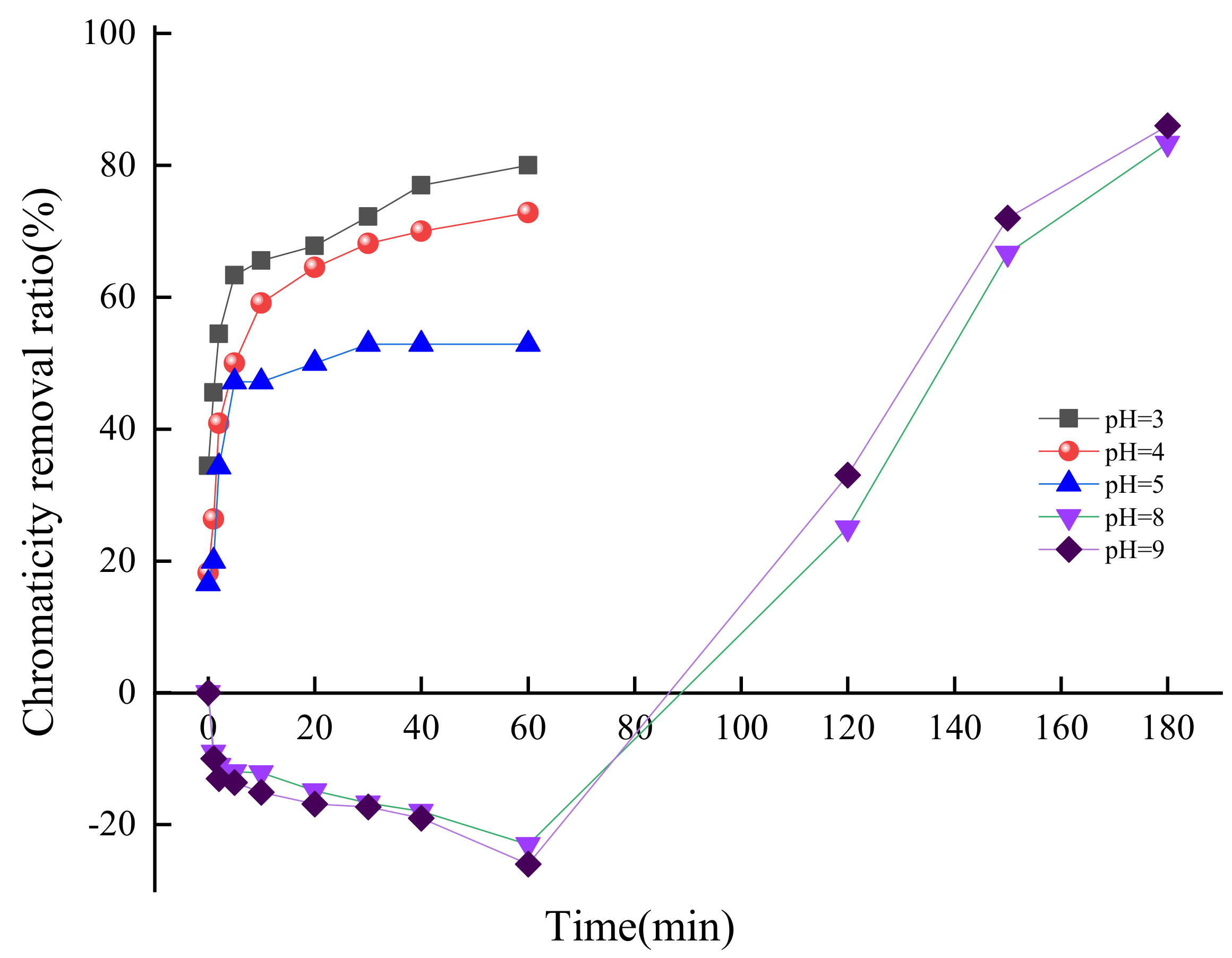
3.2.1. pH Value
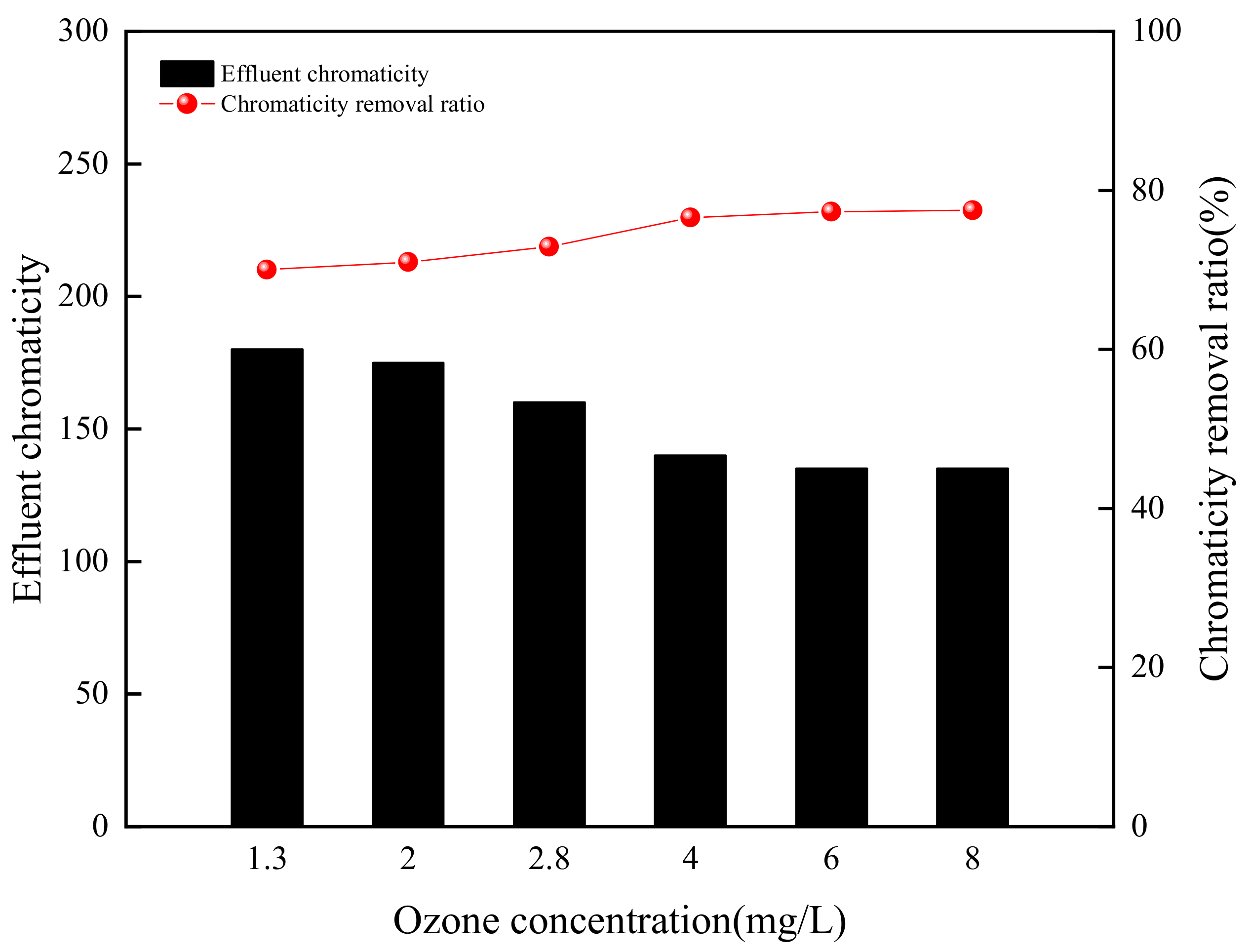
3.2.2. Ozone Concentration
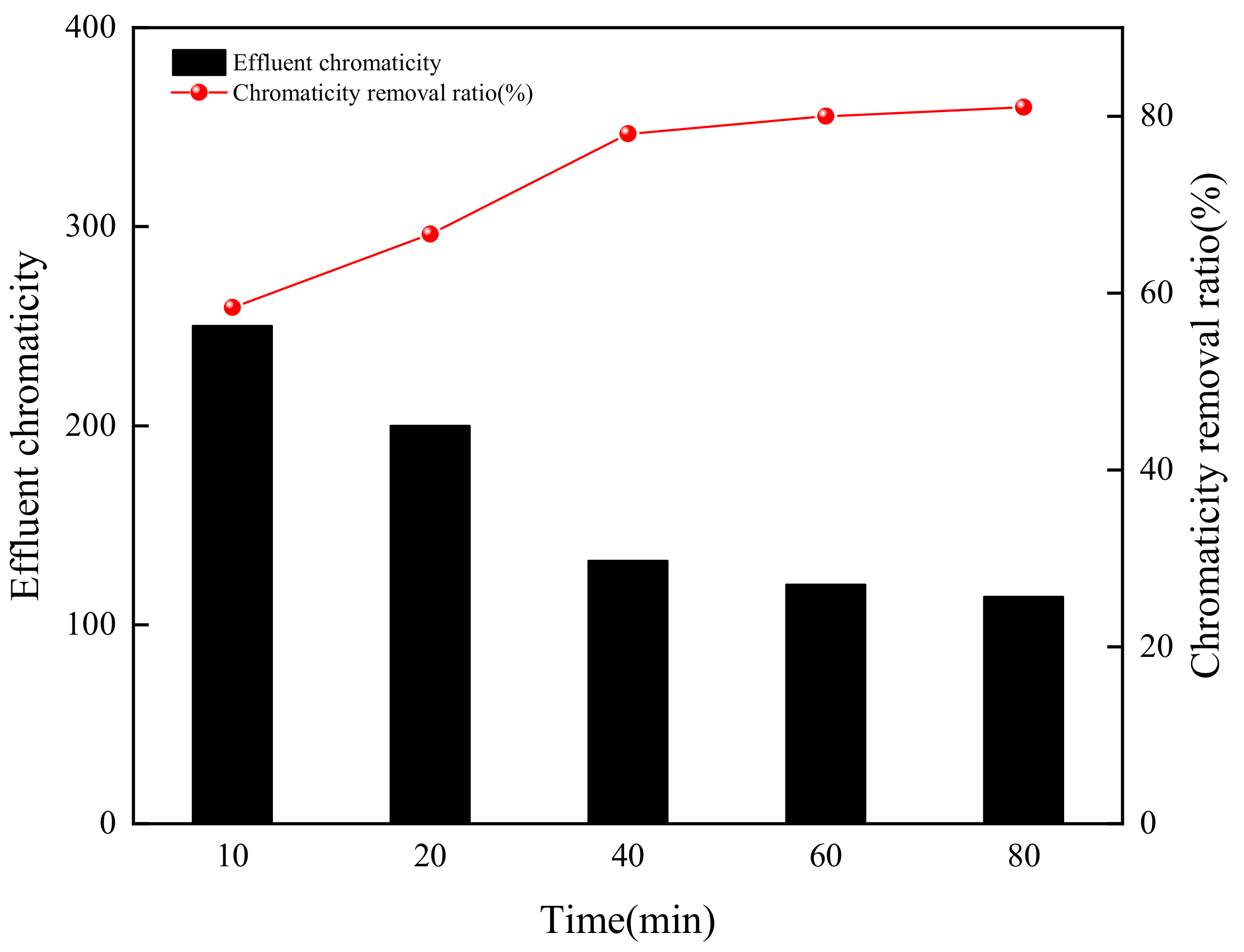
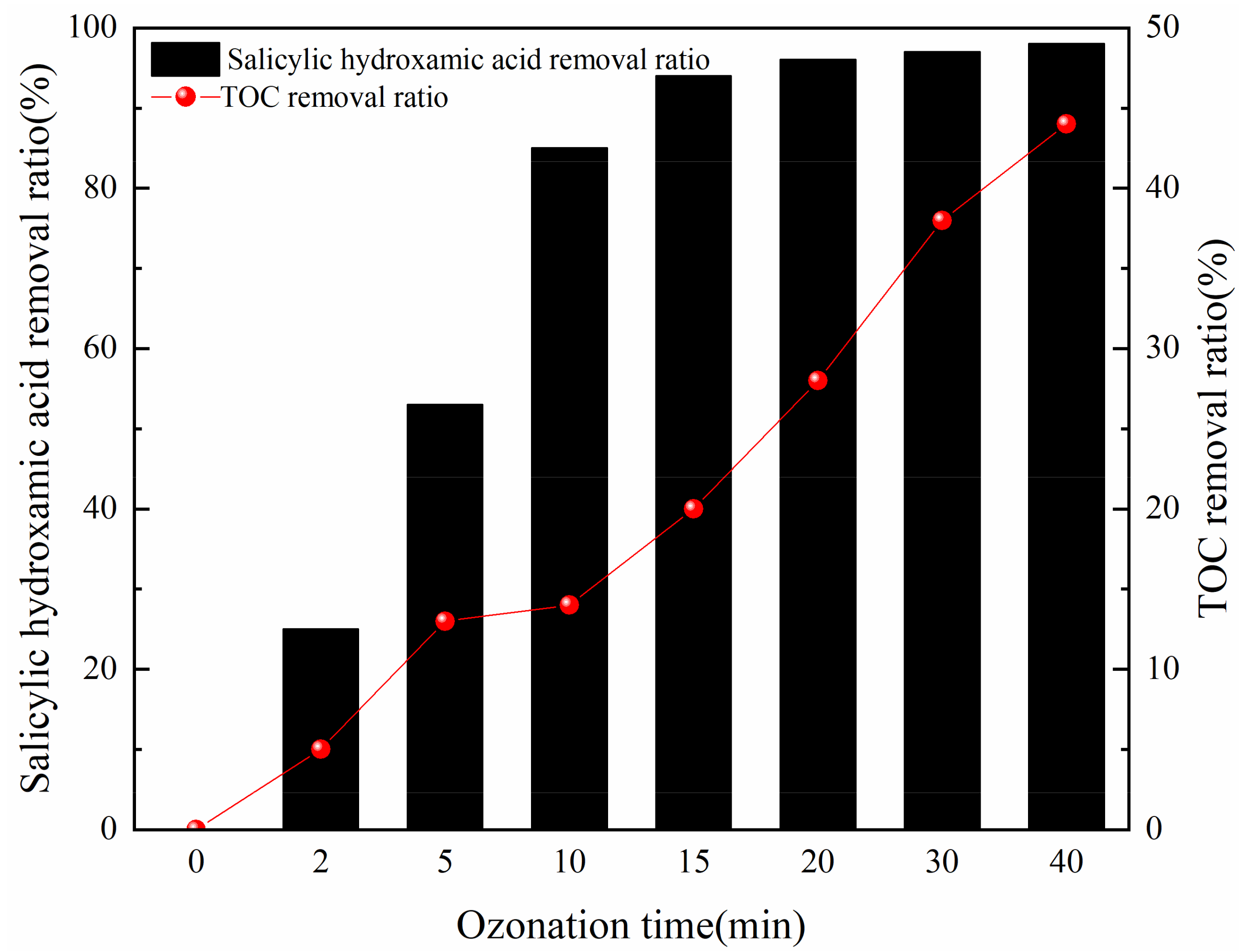
3.2.3. Ozonation Time
3.3. Catalytic Ozonation Effects on Chromaticity Removal
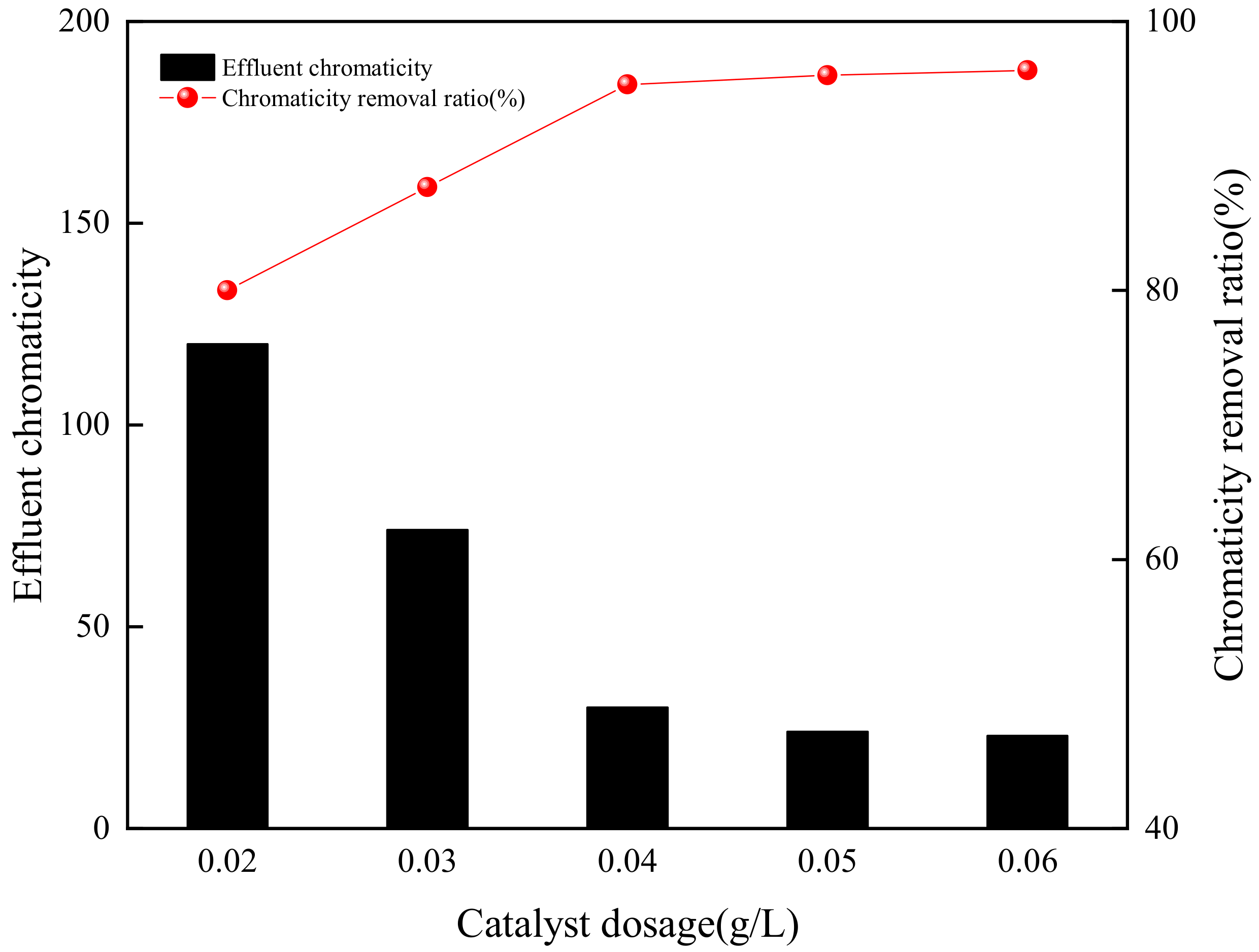
3.3.1. GAC Dosage
3.3.2. Orthogonal Experiment of Catalytic Ozonation
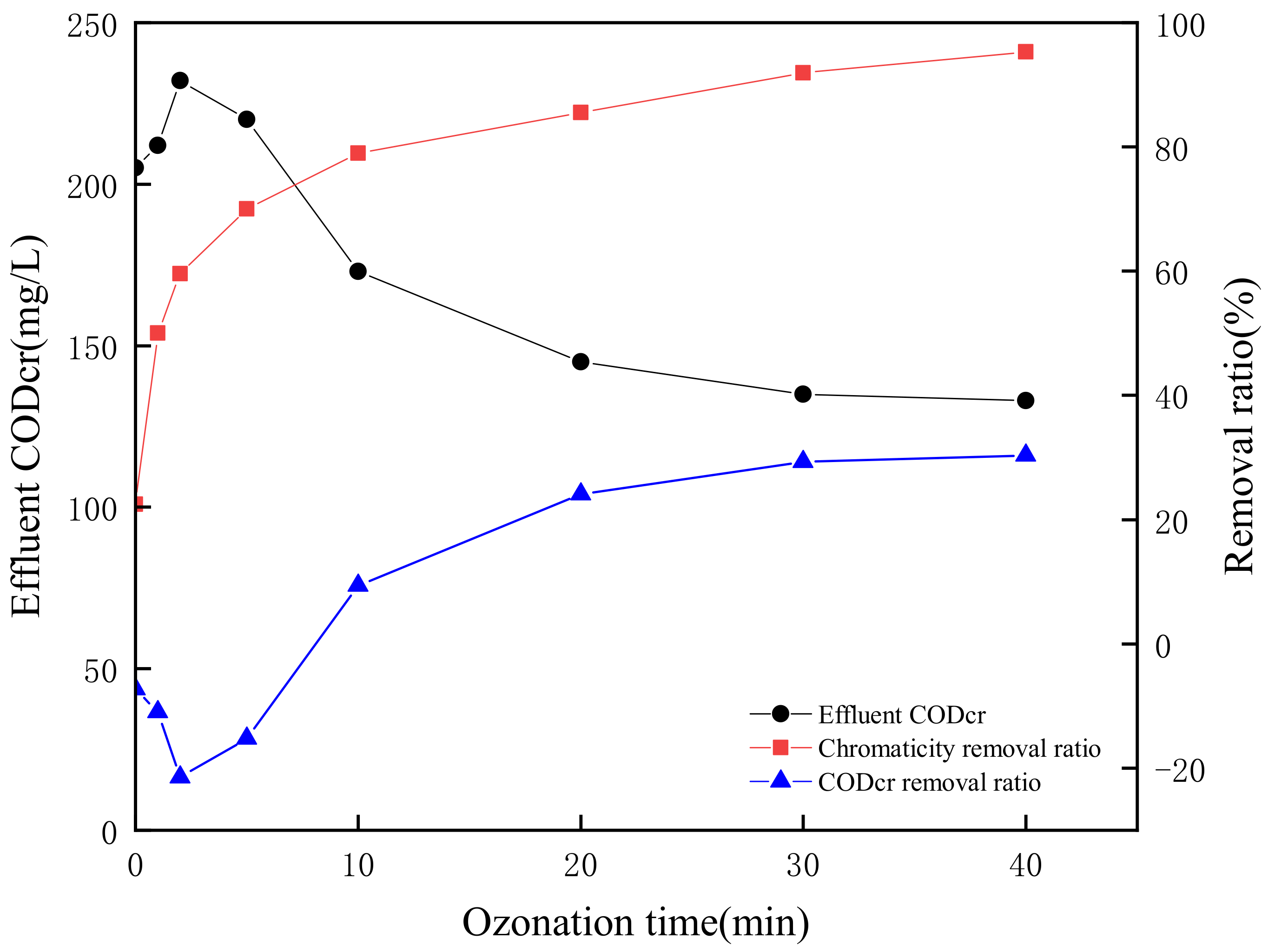
3.4. Catalytic Ozonation Effects on CODCr, TOC and Chromaticity
3.5. Degradation Mechanism of Chromaticity
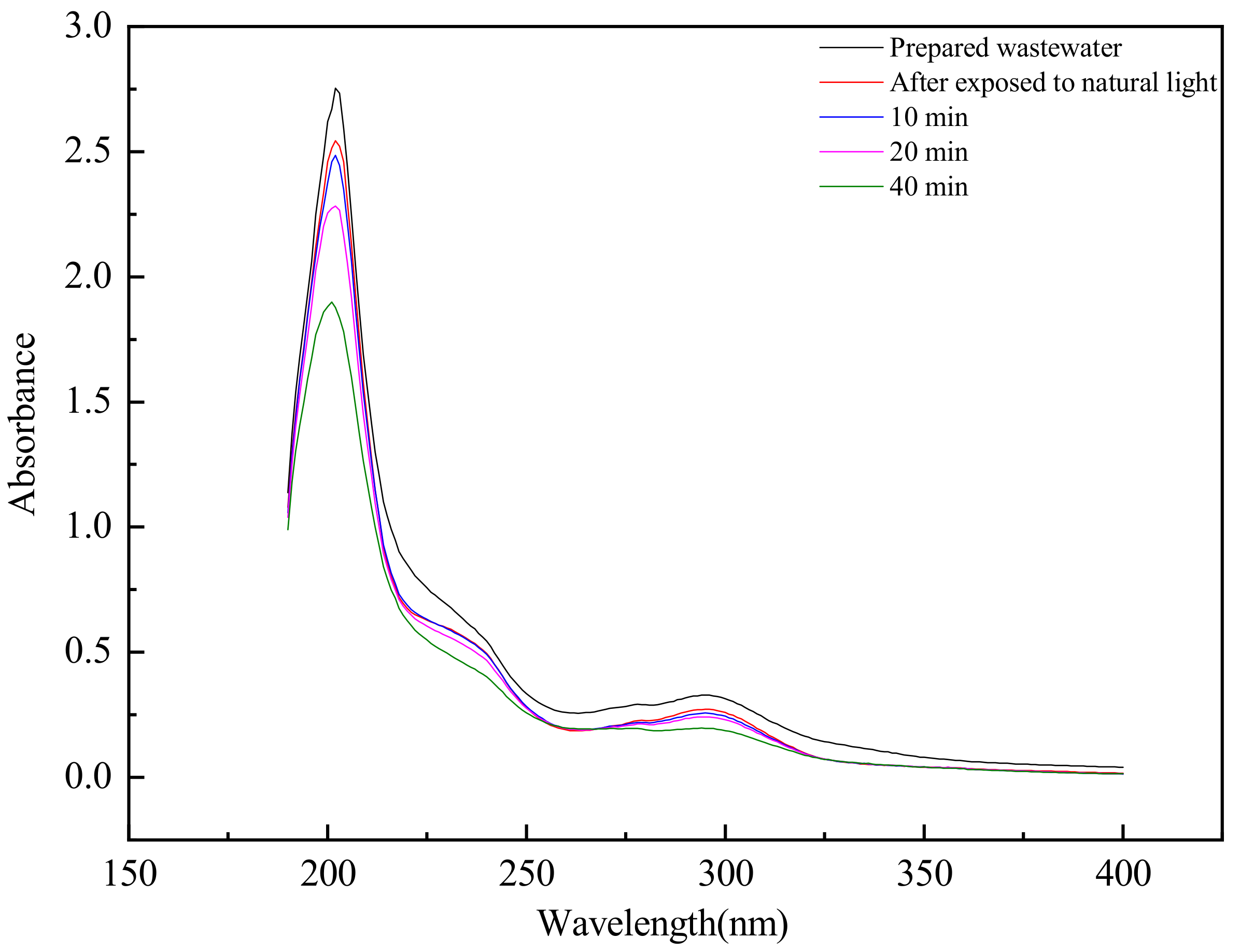
3.5.1. Ultraviolet Absorption Spectrum
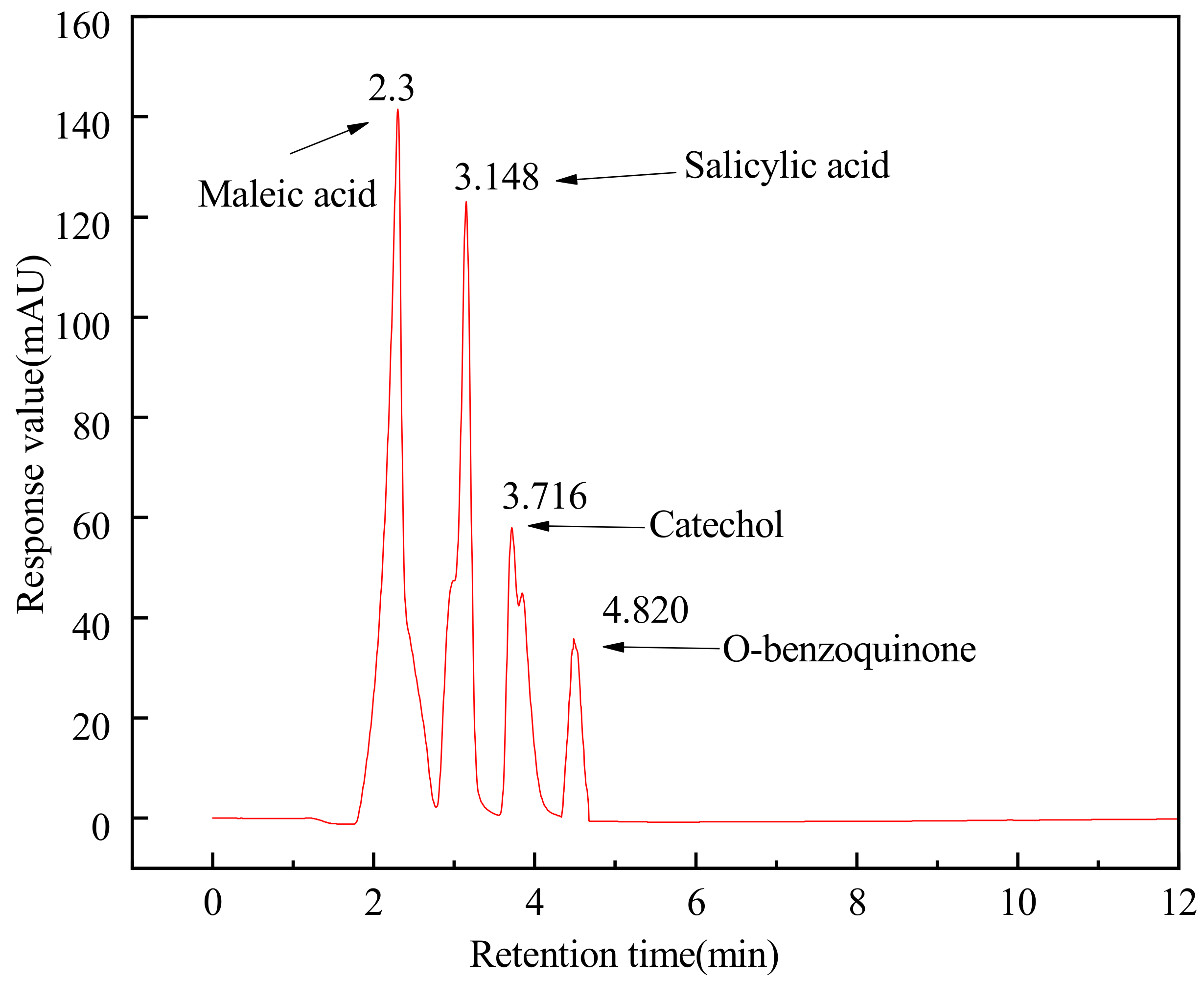
3.5.2. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kholmogorov, A.G.; Kononova, O.N. Processing mineral raw materials in Siberia: Ores of molybdenum, tungsten, lead and gold. Hydrometallurgy 2005, 76, 37–54. [Google Scholar] [CrossRef]
- Zhang, L.P.; Zhang, X.; Xiang, J.; Xue, J.W.; Song, X.J. Study on Treatment of Salicylhydroxamic Acid Wastewater from Tungsten Molybdenum Mineral Processing. J. Chem. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.X.; Zhou, X.T.; Qiu, X.Y.; He, X.J. The Application of Floatation Reagents of Scheelite. China Tungsten Ind. 2010, 25, 19–22. [Google Scholar]
- Huang, J.P.; Zhong, H.; Qiu, X.Y.; Wang, S. Flotation behavior and adsorption mechanism of cyclohexyl hydroxamic acid to wolframite. Chin. J. Nonferrous Met. 2013, 23, 2033–2039. [Google Scholar]
- Wang, X.; Song, H.; Jiao, F.; Qin, W.Q.; Yang, C.R.; Cui, Y.F.; Zhang, Z.Q.; Zhang, J.; Li, H.B. Utilization of wastewater from zeolite production in synthesis of flotation reagents. Trans. Nonferrous Met. Soc. China 2020, 30, 3093–3102. [Google Scholar] [CrossRef]
- Al-Saati, N.; Hussein, T.; Abbas, M.; Hashim, K.S.; Al-Saati, Z.; Kot, P.; Sadique, M.M.; Aljefery, M.; Carnacina, I. Statistical modelling of turbidity removal applied to non-toxic natural coagulants in water treatment: A case study. Desalin. Water Treat. 2019, 150, 406–412. [Google Scholar] [CrossRef]
- Tian, Y.P.; Chen, S.P. Research on the Effect of Coagulation on Decolorization and Degradation of Red Dye Wastewater. Adv. Mater. Res. 2013, 610–613, 2401–2404. [Google Scholar] [CrossRef]
- Jovan, K.; Kenneth, S.S.; Hans, K.; Susiana, P.; Asaf, K.S. Optimization study of leucaena leucocephala seed extract as natural, coagulant on decolorization of aqueous congo red solutions. Arab. J. Sci. Eng. 2020, 2020, 1–12. [Google Scholar]
- Li, Z.Y.; Han, H.J. Study on Fenton oxidation-coagulation method of biologically treated coal-chemical engineering wastewater. Water Wastewater Eng. 2013, 39, 316–319. [Google Scholar]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Integrated Wastewater Discharge Standard, GB 8978–1996; The State Bureau of Quality and Technical Supervision: Beijing, China, 1996.
- Venkatesha, B.M.; Radhika, R.T.; Ananda, S.; Byrappa, K. Oxidative decolorization of indigo caramine dye with chloramine-T catalyzed by cobalt(II). Res. Chem. Intermed. 2011, 37, 195–199. [Google Scholar] [CrossRef]
- Huang, L.J.; Xu, T.B.; Wang, S.F. Decolorization of methyl orange simulated wastewater by chlorine dioxide with ultrasound. Adv. Mater. Res. 2011, 255–260, 2904–2908. [Google Scholar] [CrossRef]
- Yan, W.Y.; Chai, Y.H. The treatment of H-GL dye wastewater by Fenton oxidation. Adv. Mater. Res. 2012, 518–523, 2274–2277. [Google Scholar] [CrossRef]
- Torrades, F.; García-Montaño, J. Using central composite experimental design to optimize the degradation of real dye wastewater by Fenton and photo-Fenton reactions. Dye. Pigment. 2014, 100, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Değermenci, N.; Değermenci, G.D.; Ulu, H.B. Decolorization of reactive azo dye from aqueous solutions with Fenton oxidation process: Effect of system parameters and kinetic study. Desalination Water Treat. 2019, 169, 363–371. [Google Scholar] [CrossRef]
- Nurbasa, M.; Kutukcuoglu, S.B. Investigation of water decolorization by Fenton oxidation process in batch and continuous systems. Desalination Water Treat. 2015, 55, 3731–3736. [Google Scholar] [CrossRef]
- Konsowa, A.H. Decolorization of wastewater containing direct dye by ozonation in a batch bubble column reactor. Desalination 2003, 158, 233–240. [Google Scholar] [CrossRef]
- Konsowa, A.H.; Ossman, M.E.; Chen, Y.S.; Crittendend, J.C. Decolorization of industrial wastewater by ozonation followed by adsorption on activated carbon. J. Hazard. Mater. 2010, 176, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Wang, J.; Zhang, Y.J.; Han, L.M.; Song, Y.H.; Liu, G.Q.; Su, B.S. Treatment of biologically-treated effluent of dye wastewater by heterogeneous catalytic ozonation technology. Chin. J. Environ. Eng. 2017, 11, 2820–2827. [Google Scholar]
- Zheng, X.Y.; Wang, J.L.; Li, X.W.; Tian, W.J.; Li, K.X. Advanced treatment of secondary effluent by ozonation. China Environ. Sci. 2014, 34, 1159–1165. [Google Scholar]
- Jiang, G.A.; Zhao, Y.; Li, B.Z.; Guo, H.S. Treatment of acid brilliant scarlet dye wastewater by ozone catalytic oxidation over activated carbon-based catalyst. Mod. Chem. Ind. 2018, 5, 124–127. [Google Scholar]
- Liu, L.J.; Dong, J.; Zhang, L.X.; Wang, W.; Xiong, W.; Cao, B.Q.; Zhang, S.F. Pilot experiment on advanced treatment of printing and dyeing wastewater by powder activated carbon membrane bioreactor. Chem. Bioeng. 2021, 38, 43–46. [Google Scholar]
- Rajaha, Z.; Guiza, M.; Solísb, R.R.; Rivas, F.J.; Ouederni, A. Catalytic and photocatalytic ozonation with activated carbon as technologies in the removal of aqueous micropollutants. J. Photochem. Photobiol. A: Chem. 2019, 382, 1–9. [Google Scholar]
- Gümüs, D.; Akbal, F. A comparative study of ozonation, iron coated zeolite catalyzed ozonation and granular activated carbon catalyzed ozonation of humic acid. Chemosphere 2017, 174, 218–231. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, 3381–3393. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Kolar, P.; Shah, S.B.; Cheng, J.J.; Lim, P.K. Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind. Crop. Prod. 2016, 92, 34–41. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, T.; Liu, W. The Reuse of Urban Recycling Water-Water Quality Standard for Industrial Uses, GB/T 19923-2005; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2005. [Google Scholar]
- Zi, L.; Li, X.Z.; Liu, Z.Q.; Liu, L.Z.; Chen, J.X. The enhanced mechanism of benzoquinone(BQ) on poly-silicate-ferric(PSF) in heterogeneous UV-Fenton system. China Environ. Sci. 2020, 40, 2943–2951. [Google Scholar]
- Ruan, C.; Huang, Q. Experimental study on decolorization of dyeing and printing wastewater by activated carbon adsorption. Sichuan Environ. 2006, 25, 29–58. [Google Scholar]
- Jiang, W.; Peng, X.; Zhang, L.; Li, Z.; Wei, G. Research progress in treatment of xanthate in mineral concentration wastewater by advanced oxidation technology. Met. Mine 2017, 498, 123–129. [Google Scholar]
- Scheck, C.K.; Frimmel, F.H. Degradation of phenol and salicylic acid by ultraviolet radiation/hydrogen peroxide/oxygen. Water Res. 1995, 29, 2346–2352. [Google Scholar] [CrossRef]
- Deng, S.; Bai, M.; Bai, X.; Liu, X.W. Characteristics and chemical reaction of hyaroxyl radical. J. Dalian Marit. Univ. 2004, 30, 62–64. [Google Scholar]
- Chu, W.; Ma, C.W. Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res. 2000, 34, 3153–3160. [Google Scholar] [CrossRef]
- Shu, H.Y. Degradation of dyehouse effluent containing C.I. direct blue 199 by process of ozonation with UV/H2O2. J. Hazard. Mater 2006, 133, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. Comparison of the new Cl2/O3/UV process with different ozone- and UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Sci. Total Environ. 2021, 765, 142720. [Google Scholar] [CrossRef]
- Chang, J.; Wang, S.P.; Zhang, Y.X.; Wang, Y.B.; Zhang, W.J.; Lu, J.F. Oxidation of salicylic acid in water by the O3 and UV/O3 processes: Removal and reaction byproducts. Water Sci. Technol. 2020, 81, 753–762. [Google Scholar]
- Cai, H.; Li, K.L.; Chen, Y.Z.; Wang, L.P. Advanced treatment of printing and dyeing wastewater by activated carbon catalytic ozonation method. J. Chang. Univ. 2010, 22, 38–41. [Google Scholar]
- Wei, K.J.; Wang, Z.; Ouyang, C.P.; Cao, X.X.; Liang, P.; Huang, X.; Zhang, X.Y. A hybrid fluidized-bed reactor (HFBR) based on arrayed ceramic membranes (ACMs) coupled with powdered activated carbon (PAC) for efficient catalytic ozonation: A comprehensive study on a pilot scale. Water Res. 2020, 173, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.A.M.; Hishammudin, M.N.A.M.; Hamdan, R. Factor Affecting Textile Dye Removal Using Adsorbent From Activated Carbon: A Review. In Proceedings of the International Symposium on Civil and Environmental Engineering, Melaka, Malaysia, 5–6 December 2016; Volume 103, p. 06015. [Google Scholar]
- Dong, J.W.; Xing, B.; Yang, G.; Jia, M.; Liu, X.Y. Treatment of methylene blue wastewater by catalytic ozonation using Mn-Ce bimetal/activated carbon as catalyst. Ind. Water Treat. 2019, 39, 60–65. [Google Scholar]
- Zhang, L.P.; Wu, S.N.; Xiang, J.; Jiao, X.F.; Wang, J. Research on treatment and mechanism of salicylhydroxamic acid flotation wastewater by O3-BAF process. Water Sci. Technol. 2020, 82, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Han, W. Study on the Charateristics and Mechanism of Biodegradation of Typical Hydroxamic Acid Floatation Collectors; Wuhan University of Technology: Wuhan, China, 2012. [Google Scholar]

| No. | pH Value | Ozone Concentration (mg/L) | GAC Dosage (g/L) | Reaction Time (min) | Chromaticity Removal Ratio (%) |
|---|---|---|---|---|---|
| 1 | 2.87 | 2 | 0.04 | 20 | 68.57 |
| 2 | 2.87 | 4 | 0.05 | 40 | 95.16 |
| 3 | 2.87 | 6 | 0.06 | 60 | 96.36 |
| 4 | 4.4 | 2 | 0.05 | 60 | 72.5 |
| 5 | 4.4 | 4 | 0.06 | 20 | 85 |
| 6 | 4.4 | 6 | 0.04 | 40 | 78.33 |
| 7 | 7 | 2 | 0.06 | 40 | 20 |
| 8 | 7 | 4 | 0.04 | 60 | 20 |
| 9 | 7 | 6 | 0.05 | 20 | 0 |
| K1 | 260.09 | 161.07 | 166.9 | 153.57 | |
| K2 | 235.83 | 200.16 | 167.66 | 193.49 | |
| K3 | 40 | 174.69 | 201.36 | 188.86 | |
| Mean value 1 | 86.70 | 53.69 | 55.63 | 51.19 | |
| Mean value 2 | 78.61 | 66.72 | 55.89 | 64.50 | |
| Mean value 3 | 13.33 | 58.23 | 67.12 | 62.95 | |
| Range | 73.36 | 13.03 | 11.49 | 13.31 | |
| Mean Square | 4854.308 | 131.236 | 129.098 | 158.913 | |
| F value | 37.618 | 1.016 | 1.000 | 1.231 | |
| Order of precedence | pH value > reaction time > ozone concentration > GAC dosage | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wu, S.; Zhang, N.; Yao, R.; Wu, E. Study on Chromaticity Removal from Mineral Processing Wastewater with Salicylic Hydroxamic Acid by Granular Activated Carbon Catalyzed Ozonation. Minerals 2021, 11, 359. https://doi.org/10.3390/min11040359
Zhang L, Wu S, Zhang N, Yao R, Wu E. Study on Chromaticity Removal from Mineral Processing Wastewater with Salicylic Hydroxamic Acid by Granular Activated Carbon Catalyzed Ozonation. Minerals. 2021; 11(4):359. https://doi.org/10.3390/min11040359
Chicago/Turabian StyleZhang, Liping, Shengnian Wu, Nan Zhang, Ruihan Yao, and Eryong Wu. 2021. "Study on Chromaticity Removal from Mineral Processing Wastewater with Salicylic Hydroxamic Acid by Granular Activated Carbon Catalyzed Ozonation" Minerals 11, no. 4: 359. https://doi.org/10.3390/min11040359
APA StyleZhang, L., Wu, S., Zhang, N., Yao, R., & Wu, E. (2021). Study on Chromaticity Removal from Mineral Processing Wastewater with Salicylic Hydroxamic Acid by Granular Activated Carbon Catalyzed Ozonation. Minerals, 11(4), 359. https://doi.org/10.3390/min11040359






