Origin and Evolution of the Late Cretaceous Reworked Phosphorite in the Sirhan-Turayf Basin, Northern Saudi Arabia
Abstract
1. Introduction
2. Geological Setting
2.1. Tectonics and Paleogeography
2.2. Stratigraphy
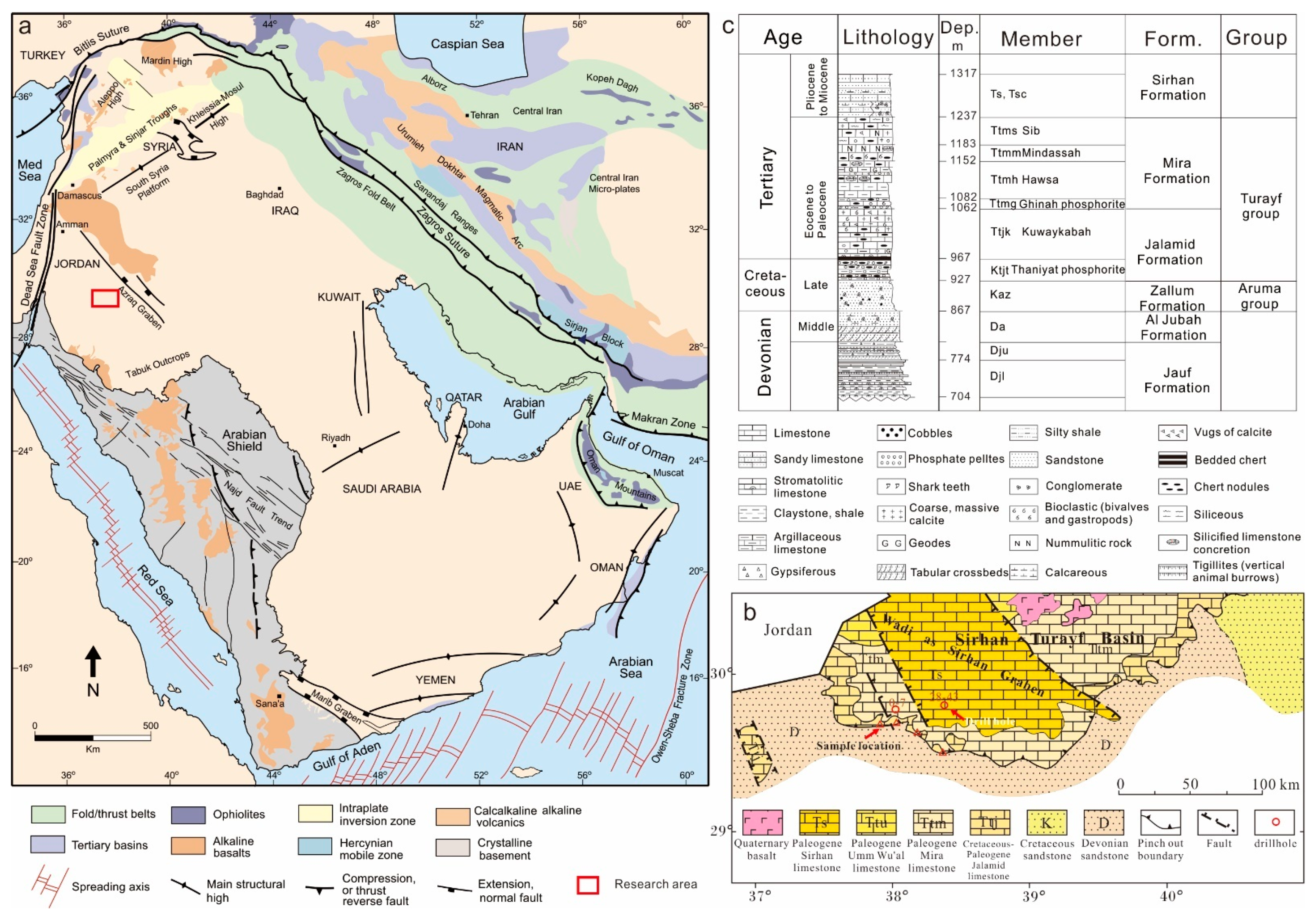
2.3. Phosphorite Occurrence
3. Materials and Methods
4. Results
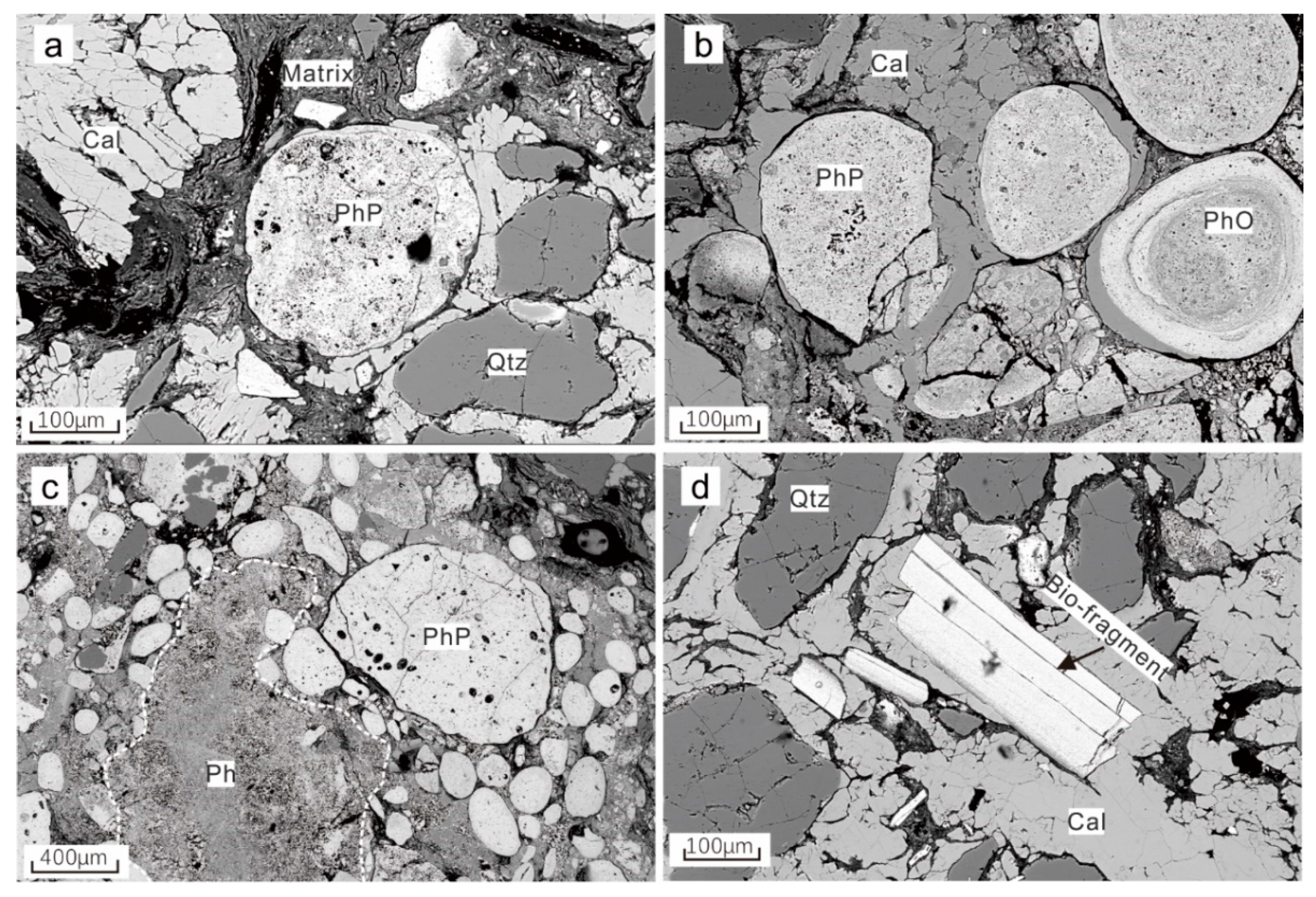
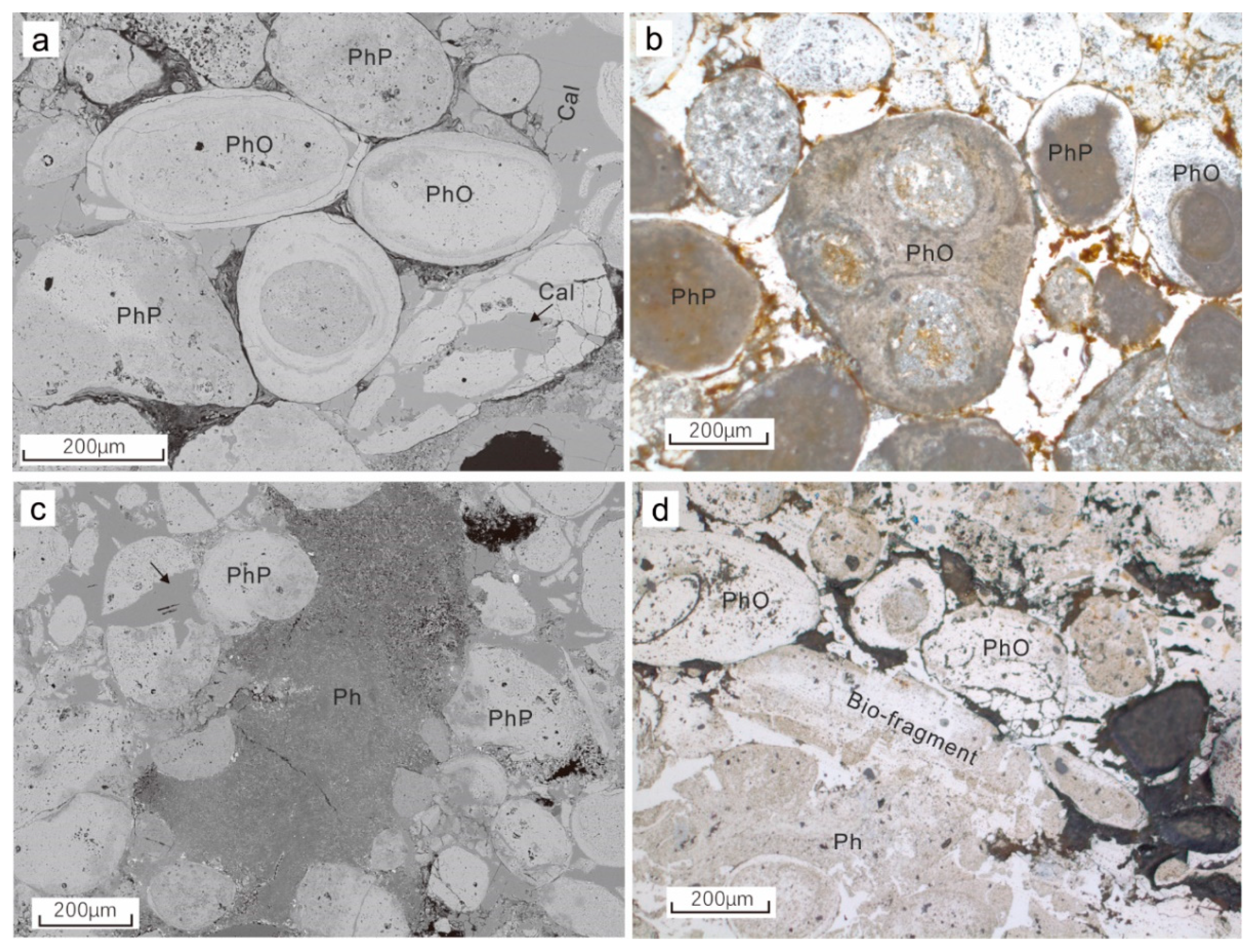
4.1. Petrography of the Phosphorite
4.1.1. Sandy Phosphorite
4.1.2. Calcareous Phosphorite
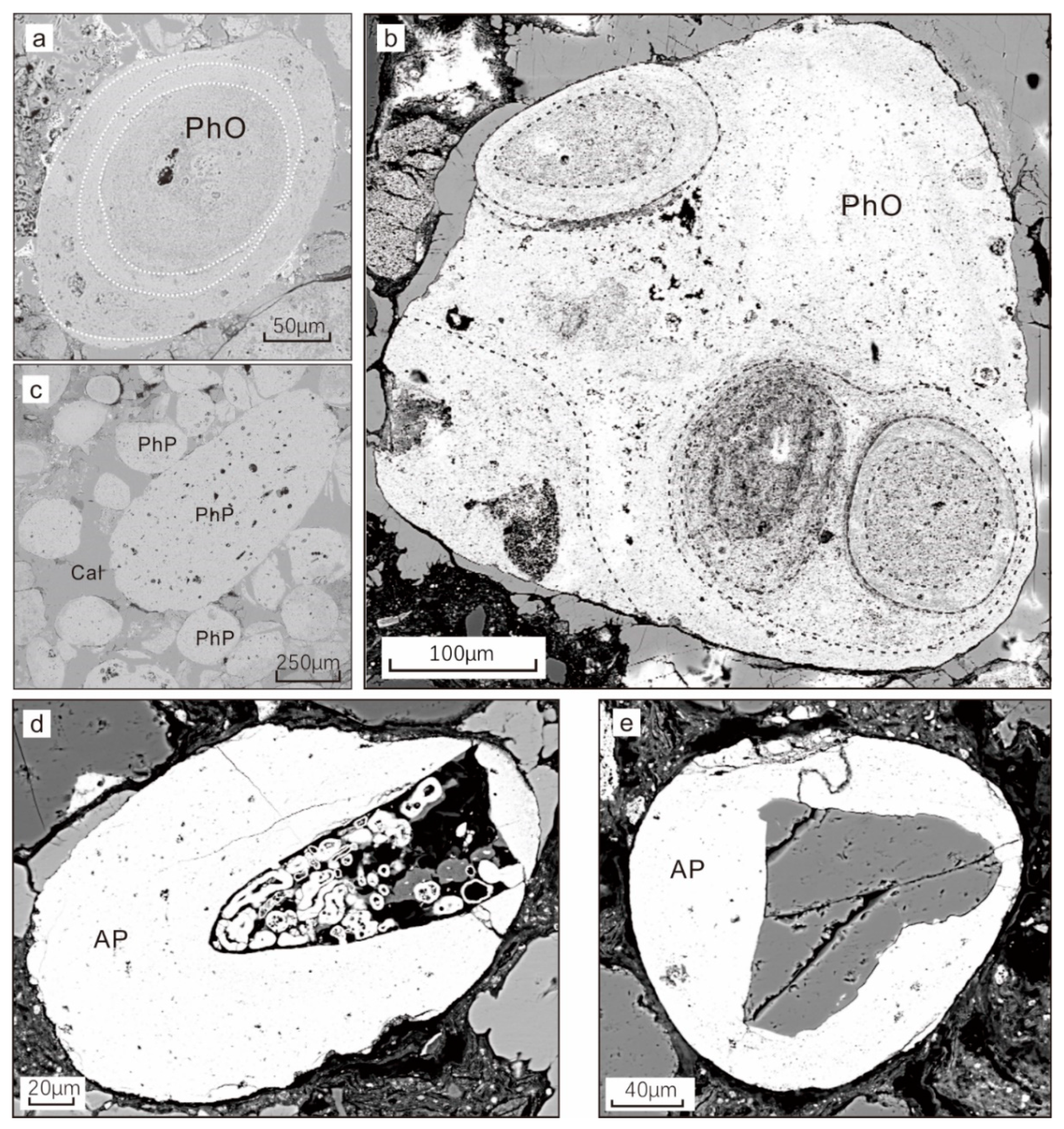
4.1.3. The Phosphatic Component
4.2. Element Distribution
4.2.1. Major, Minor, and Trace Elements of the Phosphorite
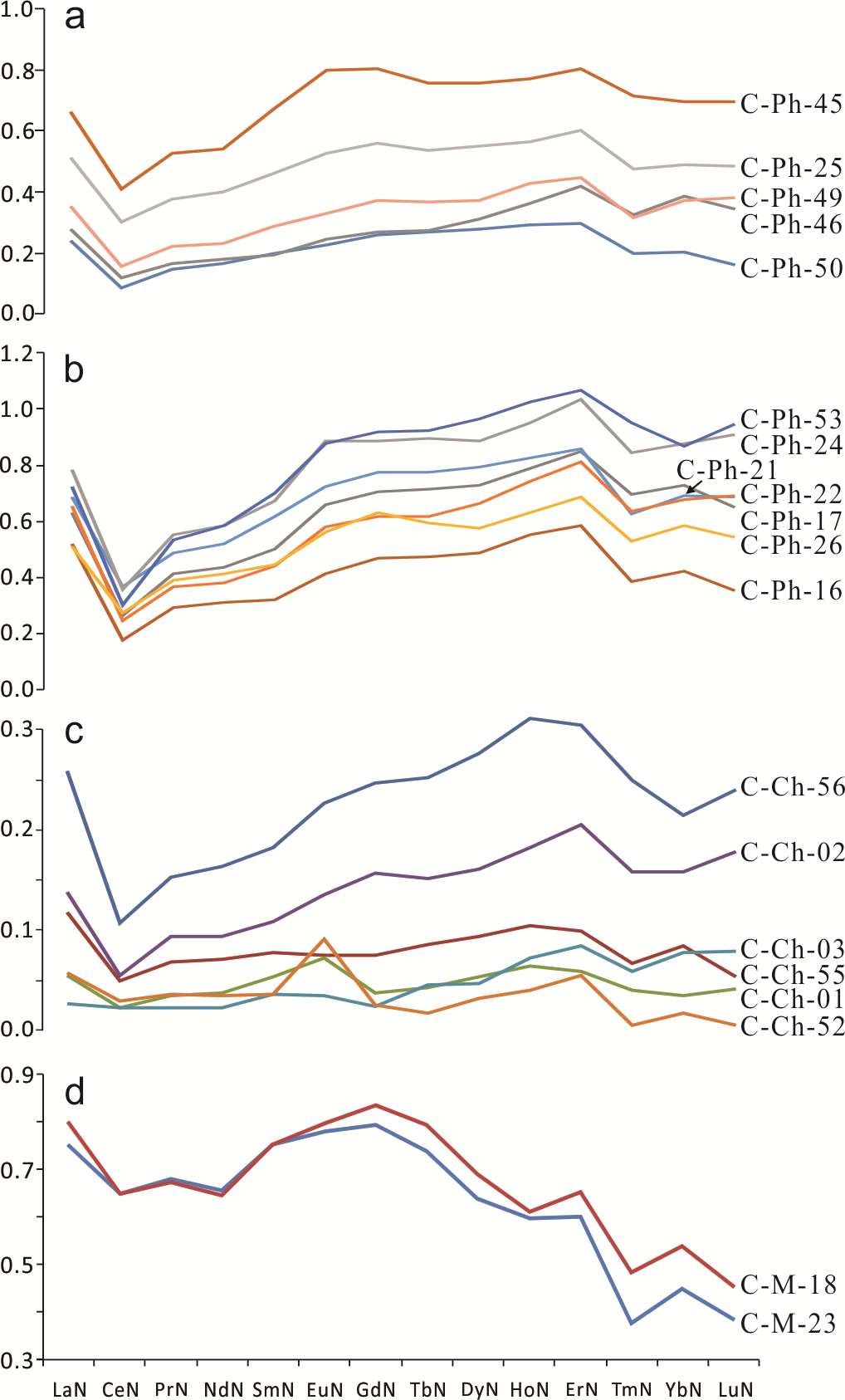
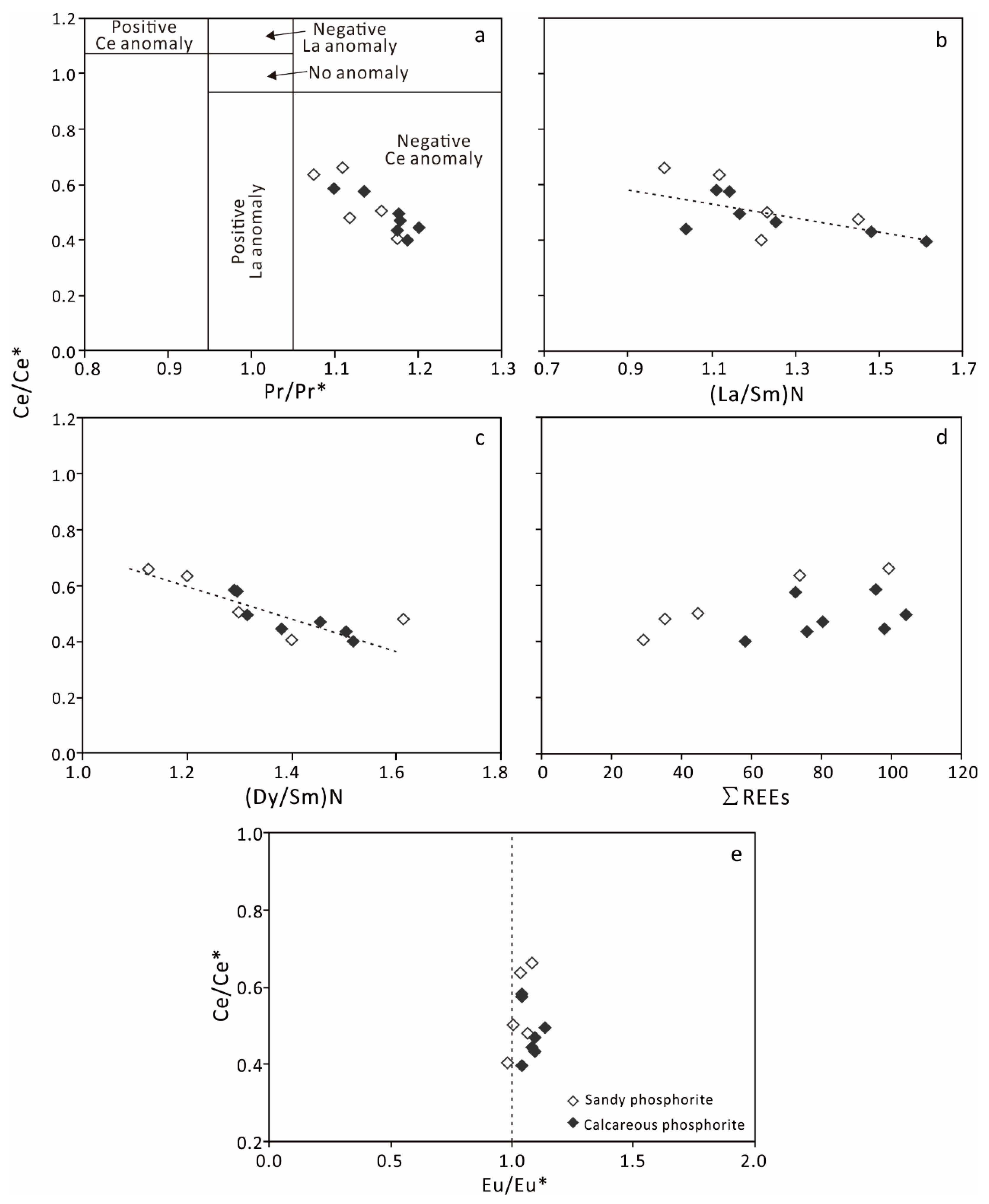
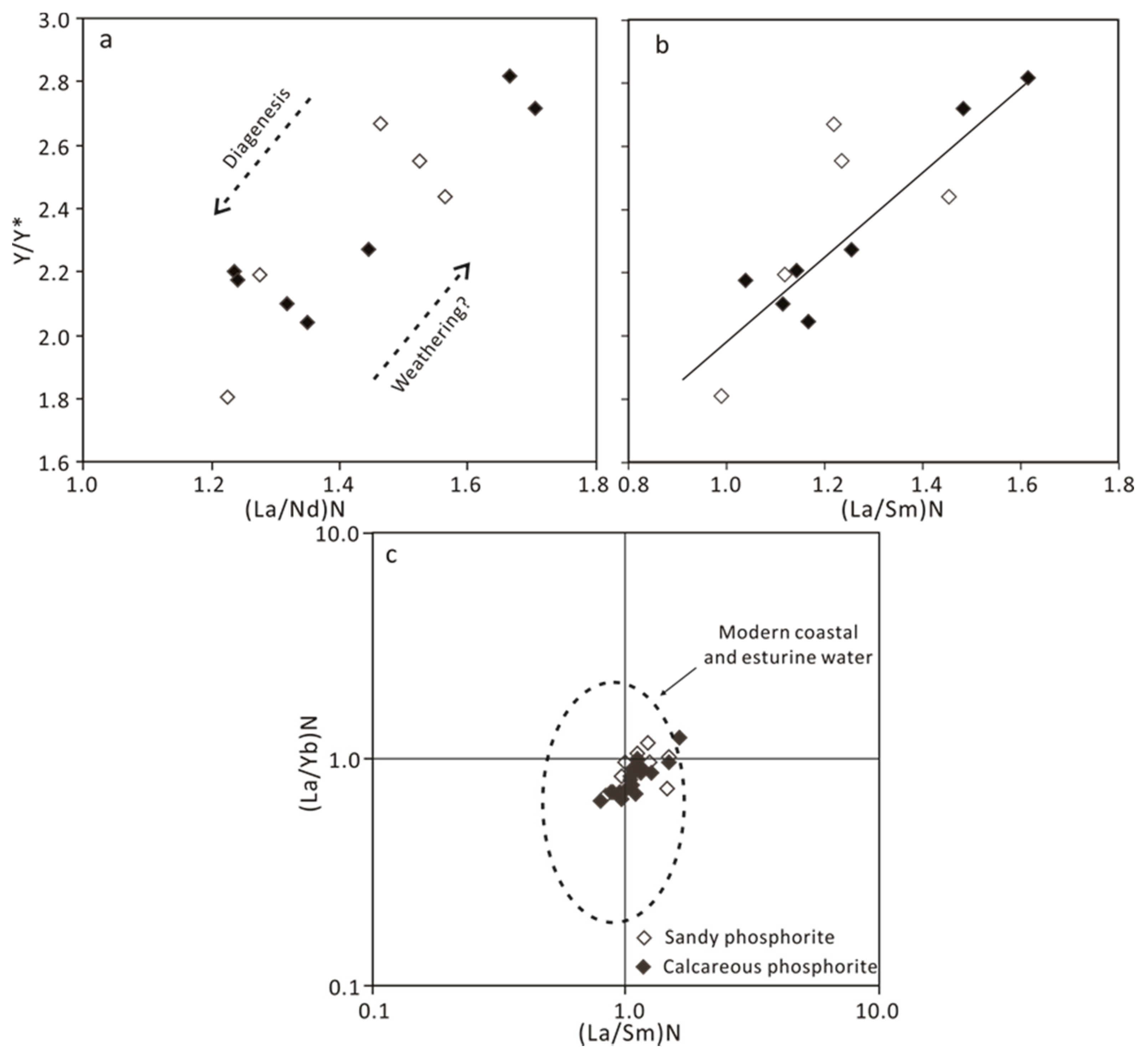
4.2.2. Rare Earth Elements
4.2.3. Element Distribution in a Phosphatic Grain
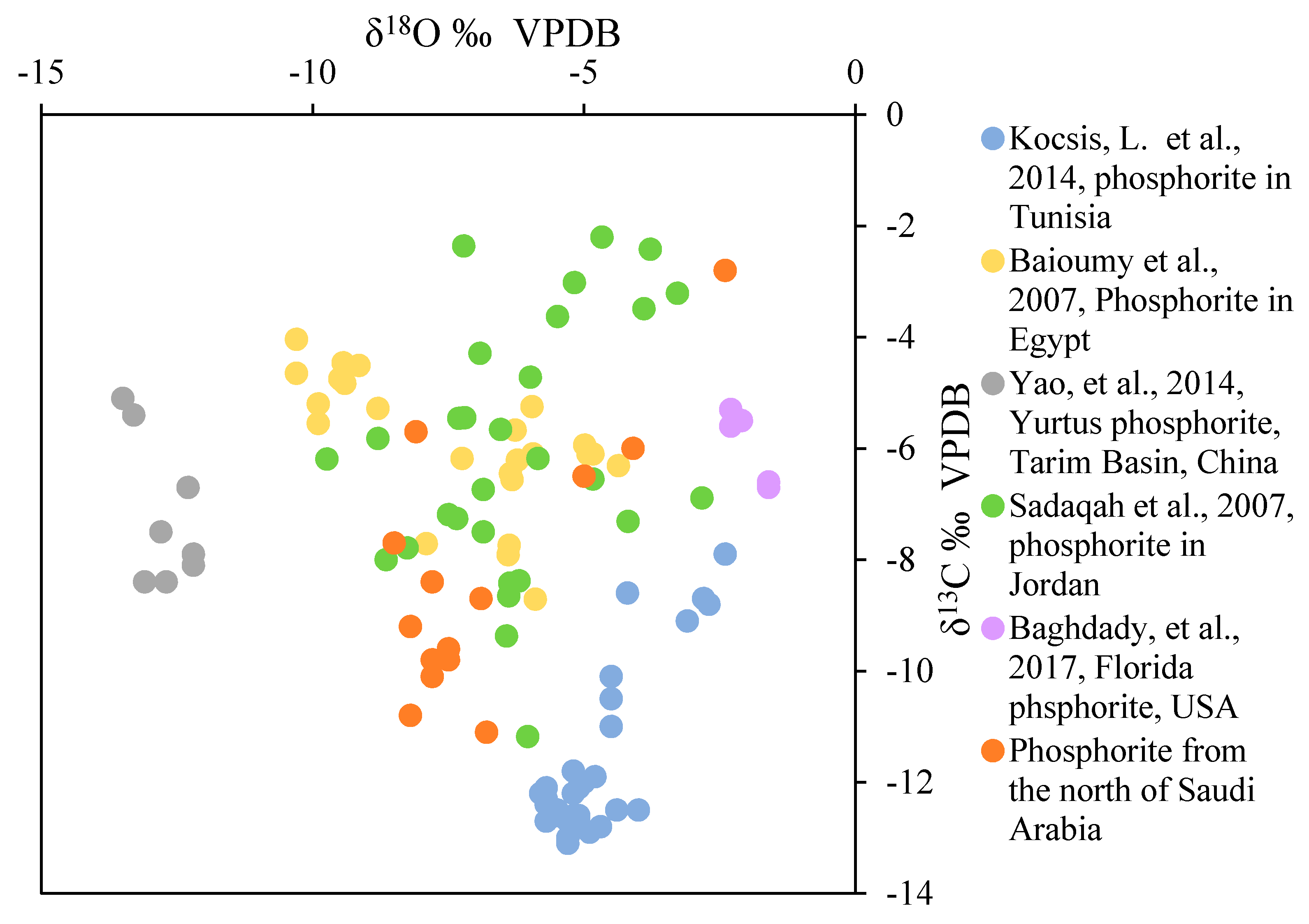
4.3. Isotope Composition
5. Discussion
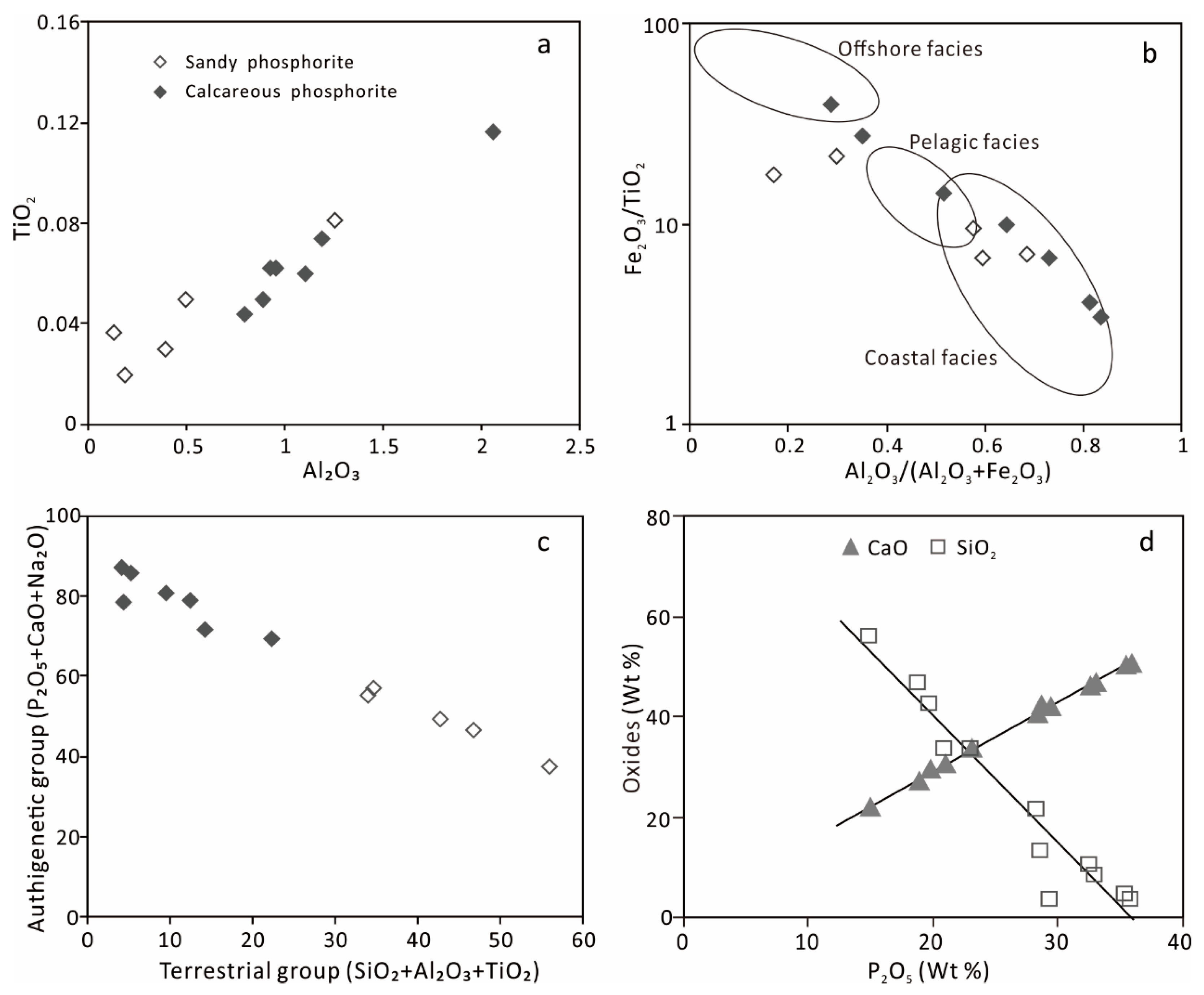
5.1. Major Elements Component
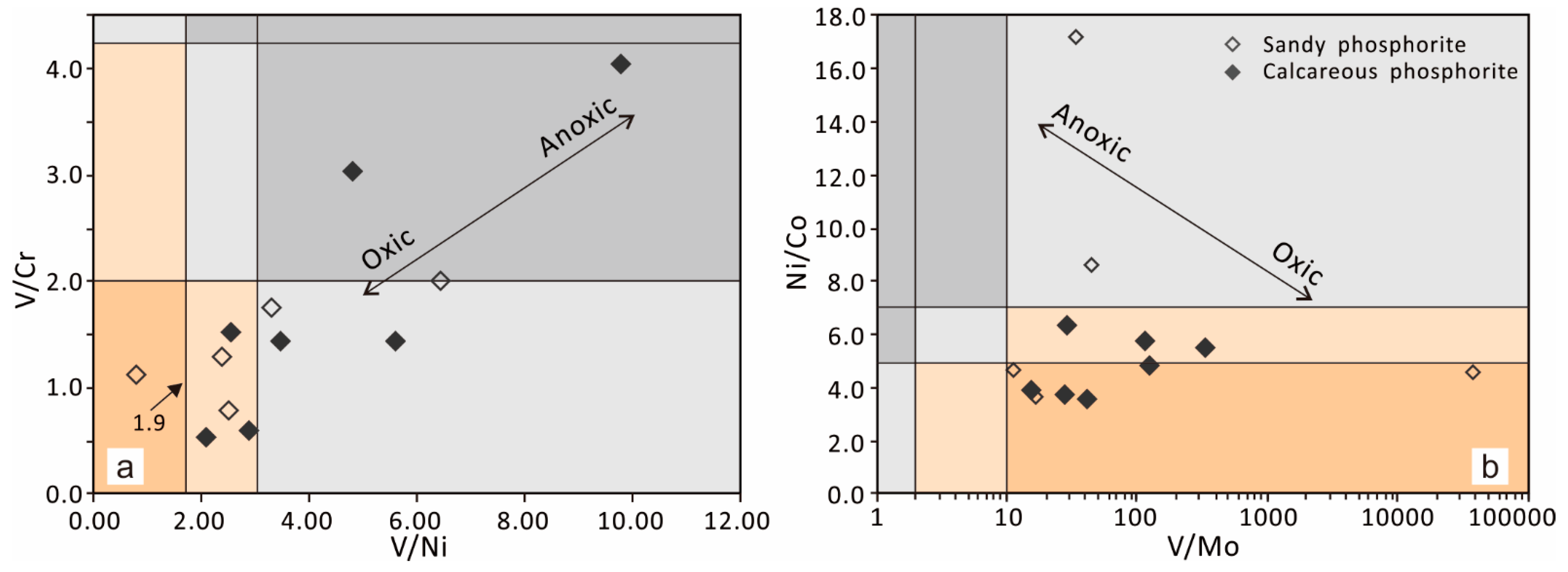
5.2. Trace Elements Implications
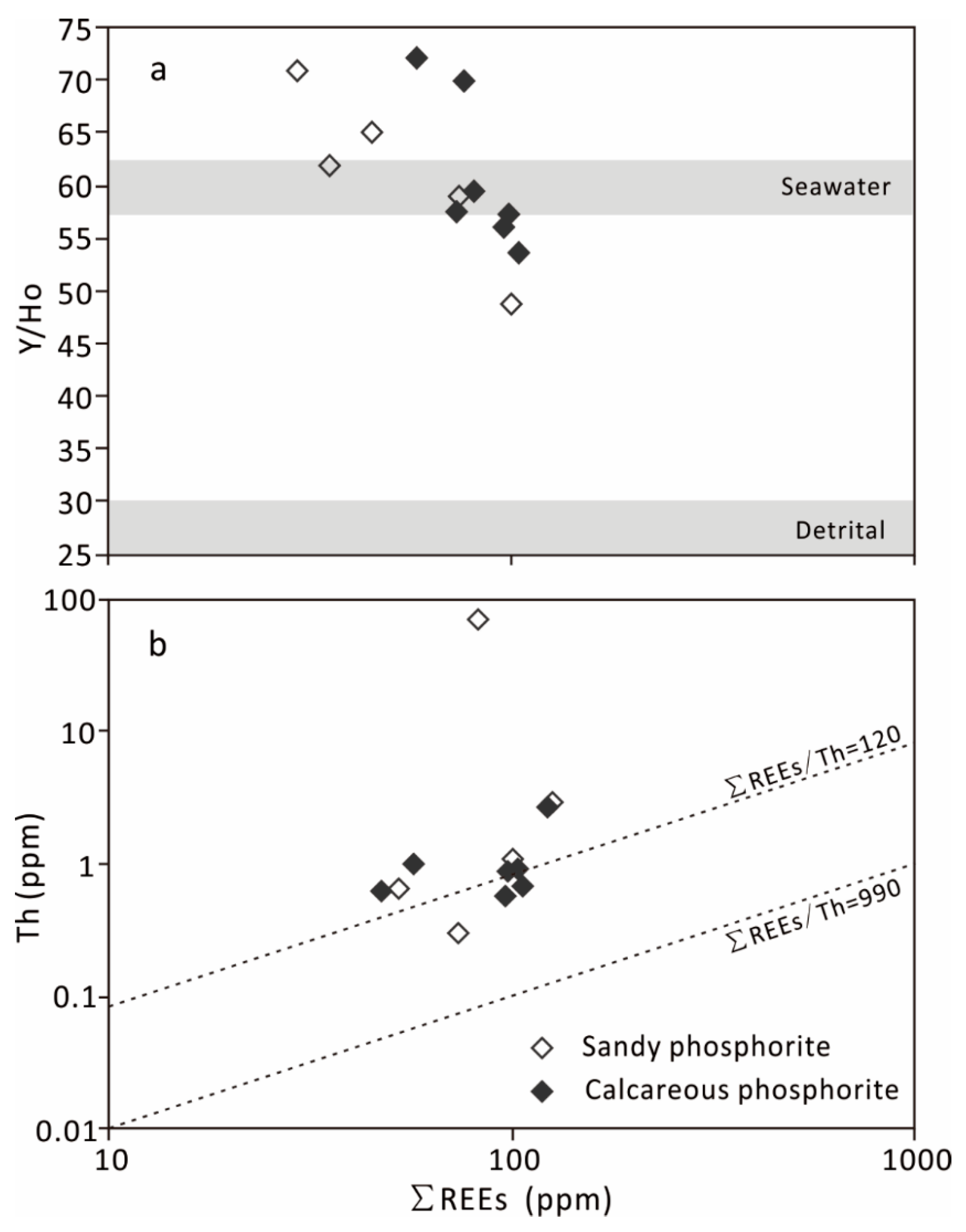
5.3. REE Patterns and Their Implications
5.4. Redox Conditions during the Reworked Phosphorite Deposition
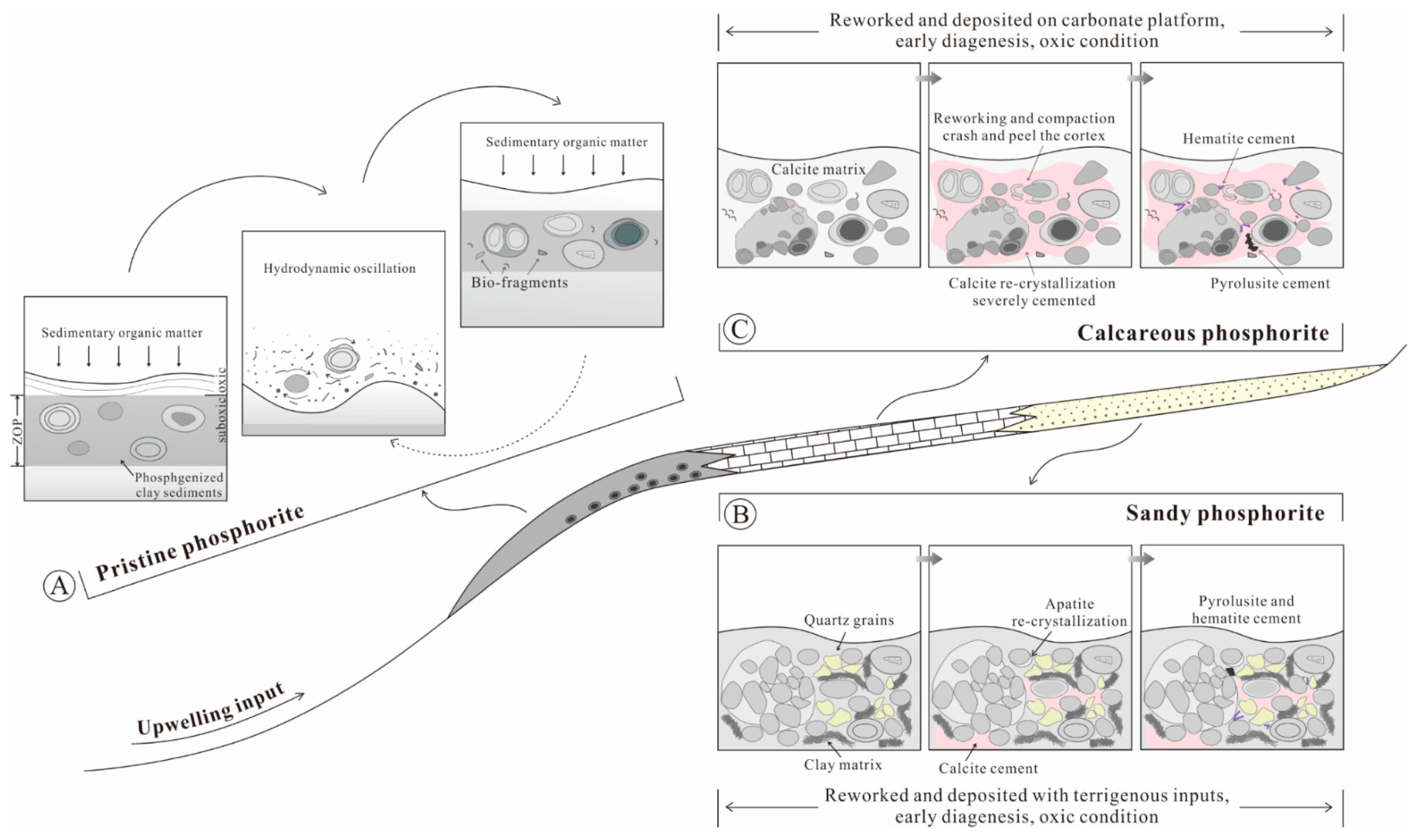
5.5. Formation of the Reworked Phosphorite
5.5.1. Depositional Processes
5.5.2. Diagenesis
5.5.3. Scenario of Phosphorite Occurrence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, P.J.; Shergold, J.H. Phosphorus, phosphorites and skeletal evolution at the Precambrian—Cambrian boundary. Nature 1984, 308, 231–236. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Ingall, E.D. Redox Stabilization of the Atmosphere and Oceans by Phosphorus-Limited Marine Productivity. Science 1996, 275, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, Y.; Knoll, A.H. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 1998, 391, 553–558. [Google Scholar] [CrossRef]
- Gál, P.; Polgári, M.; Józsa, S.; Gyollai, I.; Kovács, I.; Szabó, M.; Fintor, K. Contribution to the origin of Mn-U-Be-HREE-enrichment in phosphorite, near Bükkszentkereszt, NE Hungary. Ore Geol. Rev. 2020, 125, 103665. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, D.; Ni, J.; Zeng, X. Optimizing phosphate fertilizer application to reduce nutrient loss in a mustard (Brassica juncea var. tumida)-maize (Zea mays L.) rotation system in Three Gorges Reservoir area. Soil Tillage Res. 2019, 190, 78–85. [Google Scholar] [CrossRef]
- Barra, P.J.; Pontigo, S.; Delgado, M.; Parra–Almuna, L.; Duran, P.; Valentine, A.J.; Jorquera, M.A.; Mora, M.d.l.L. Phosphobacteria inoculation enhances the benefit of P–fertilization on Lolium perenne in soils contrasting in P–availability. Soil Biol. Biochem. 2019, 136, 107516. [Google Scholar] [CrossRef]
- Bi, Q.-F.; Zheng, B.-X.; Lin, X.-Y.; Li, K.-J.; Liu, X.-P.; Hao, X.-L.; Zhang, H.; Zhang, J.-B.; Jaisi, D.P.; Zhu, Y.-G. The microbial cycling of phosphorus on long-term fertilized soil: Insights from phosphate oxygen isotope ratios. Chem. Geol. 2018, 483, 56–64. [Google Scholar] [CrossRef]
- Naureen, Z.; Sham, A.; Al Ashram, H.; Gilani, S.A.; Al Gheilani, S.; Mabood, F.; Hussain, J.; Al Harrasi, A.; AbuQamar, S.F. Effect of phosphate nutrition on growth, physiology and phosphate transporter expression of cucumber seedlings. Plant Physiol. Biochem. 2018, 127, 211–222. [Google Scholar] [CrossRef]
- Li, B.; Bicknell, K.B.; Renwick, A. Peak phosphorus, demand trends and implications for the sustainable management of phosphorus in China. Resour. Conserv. Recycl. 2019, 146, 316–328. [Google Scholar] [CrossRef]
- Geissler, B.; Mew, M.C.; Steiner, G. Phosphate supply security for importing countries: Developments and the current situation. Sci. Total Environ. 2019, 677, 511–523. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Ruan, W.-Y.; Yi, K.-K. Internal phosphate starvation signaling and external phosphate availability have no obvious effect on the accumulation of cadmium in rice. J. Integr. Agric. 2019, 18, 2153–2161. [Google Scholar] [CrossRef]
- Edelman-Furstenberg, Y. Distribution and paleoecology of molluscan skeletal remains along an upwelling tract: Benguela system, Namibian shelf. Mar. Geol. 2014, 353, 153–162. [Google Scholar] [CrossRef]
- Arning, E.T.; Lueckge, A.; Breuer, C.; Gussone, N.; Birgel, D.; Peckmann, J. Genesis of phosphorite crusts off Peru. Mar. Geol. 2009, 262, 68–81. [Google Scholar] [CrossRef]
- Papineau, D. Global Biogeochemical Changes at Both Ends of the Proterozoic: Insights from Phosphorites. Astrobiology 2010, 10, 165–181. [Google Scholar] [CrossRef]
- Brandano, M.; Westphal, H.; Mateu-Vicens, G.; Preto, N.; Obrador, A. Ancient upwelling record in a phosphate hardground (Tortonian of Menorca, Balearic Islands, Spain). Mar. Pet. Geol. 2016, 78, 593–605. [Google Scholar] [CrossRef]
- Albuquerque, A.L.; Meyers, P.; Belem, A.L.; Turcq, B.; Siffedine, A.; Mendoza, U.; Capilla, R. Mineral and elemental indicators of post-glacial changes in sediment delivery and deposition under a western boundary upwelling system (Cabo Frio, southeastern Brazil). Palaeogeogr. Palaeoclim. Palaeoecol. 2016, 445, 72–82. [Google Scholar] [CrossRef]
- Defforey, D.; Paytan, A. Phosphorus cycling in marine sediments: Advances and challenges. Chem. Geol. 2018, 477, 1–11. [Google Scholar] [CrossRef]
- Drummond, J.B.R.; Pufahl, P.K.; Porto, C.G.; Carvalho, M. Neoproterozoic peritidal phosphorite from the Sete Lagoas Formation (Brazil) and the Precambrian phosphorus cycle. Sedimentology 2015, 62, 1978–2008. [Google Scholar] [CrossRef]
- Wigley, R.; Compton, J.S. Oligocene to Holocene glauconite-phosphorite grains from the Head of the Cape Canyon on the western margin of South Africa. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1375–1395. [Google Scholar] [CrossRef]
- Al-Hobaib, A.S.; Baioumy, H.M.; Al-Ateeq, M.A. Geochemistry and origin of the Paleocene phosphorites from the Hazm Al-Jalamid area, northern Saudi Arabia. J. Geochem. Explor. 2013, 132, 15–25. [Google Scholar] [CrossRef]
- Kechiched, R.; Laouar, R.; Bruguier, O.; Salmi-Laouar, S.; Kocsis, L.; Bosch, D.; Foufou, A.; Ameur-Zaimeche, O.; Larit, H. Glauconite-bearing sedimentary phosphorites from the Tebessa region (eastern Algeria): Evidence of REE enrichment and geochemical constraints on their origin. J. Afr. Earth Sci. 2018, 145, 190–200. [Google Scholar] [CrossRef]
- Kocsis, L.; Ounis, A.; Baumgartner, C.; Pirkenseer, C.; Harding, I.C.; Adatte, T.; Chaabani, F.; Neili, S.M. Paleocene-Eocene palaeoenvironmental conditions of the main phosphorite deposits (Chouabine Formation) in the Gafsa Basin, Tunisia. J. Afr. Earth Sci. 2014, 100, 586–597. [Google Scholar] [CrossRef]
- Stern, R.J.; Johnson, P. Continental lithosphere of the Arabian Plate: A geologic, petrologic, and geophysical synthesis. Earth Sci. Rev. 2010, 101, 29–67. [Google Scholar] [CrossRef]
- Beyazpirinç, M.; Akçay, A.E.; Yilmaz, A.; Sönmez, M.K. A Late Cretaceous ensimatic arc developed during closure of the northern branch of Neo-Tethys (central-northern Turkey). Geosci. Front. 2019, 10, 1015–1028. [Google Scholar] [CrossRef]
- Golonka, J. Plate tectonic evolution of the southern margin of Eurasia in the Mesozoic and Cenozoic. Tectonophysics 2004, 381, 235–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Qin, M.; Bao, Z.; Li, Z.; Yi, L.; Li, G. Sedimentology and stratigraphy sequence of the north of Saudi Arabia: Implications for the evolution of the Neo-Tethys in the Late Cretaceous. Geol. J. 2021, 56, 1510–1530. [Google Scholar] [CrossRef]
- Alqudah, M.; Ali Hussein, M.; van den Boorn, S.; Podlaha, O.G.; Mutterlose, J. Biostratigraphy and depositional setting of Maastrichtian—Eocene oil shales from Jordan. Mar. Pet. Geol. 2015, 60, 87–104. [Google Scholar] [CrossRef]
- Abed, A.M.; Arouri, X.R.; Boreham, C.J. Source rock potential of the phosphorite-bituminous chalk-marl sequence in Jordan. Mar. Pet. Geol. 2005, 22, 413–425. [Google Scholar] [CrossRef]
- Farouk, S.; Marzouk, A.M.; Ahmad, F. The Cretaceous/Paleogene boundary in Jordan. J. Asian Earth Sci. 2014, 94, 113–125. [Google Scholar] [CrossRef]
- Abed, A.M.; Amireh, B.S. Sedimentology, geochemistry, economic potential and palaeogeography of an Upper Cretaceous phosphorite belt in the southeastern desert of Jordan. Cretac. Res. 1999, 20, 119–133. [Google Scholar] [CrossRef]
- Abed, A.M.; Sadaqah, R.; Al-Jazi, M. Sequence stratigraphy and evolution of Eshidiyya phosphorite platform, southern Jordan. Sediment. Geol. 2007, 198, 209–219. [Google Scholar] [CrossRef]
- Meissner, C.R.; Dini, S.M.; Farasani, A.M.; Riddler, G.P.; Smith, G.H.; Griffin, M.B.; Eck, M.V. Preliminary Geologic Map of the Thaniyat Turayf Quadrangle, Sheet 29C, Kingdom of Saudi Arabia; U.S. Geological Survey: Reston, VA, USA; Saudi Geological Survey: Jeddah, Saudi Arabia, 1990.
- Meissner, R.C.; Ankary, A. Geology of Phosphate Deposits in the Sirhan-Turayf Basin, Kingdom of Saudi Arabia; U.S. Geological Survey: Reston, VA, USA; Saudi Geological Survey: Jeddah, Saudi Arabia, 1970.
- Haq, B.U.; Al-Qahtani, A.M. Phanerozoic cycles of sea-level change on the Arabian Platform. GeoArabia 2005, 10, 127–160. [Google Scholar]
- Ashckenazi-Polivoda, S.; Abramovich, S.; Almogi-Labin, A.; Schneider-Mor, A.; Feinstein, S.; Püttmann, W.; Berner, Z. Paleoenvironments of the latest Cretaceous oil shale sequence, Southern Tethys, Israel, as an integral part of the prevailing upwelling system. Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 305, 93–108. [Google Scholar] [CrossRef]
- Notholt, A.J.G.; Jarvis, I. A decade of phosphorite research and development. J. Geol. Soc. Lond. 1989, 146, 873–876. [Google Scholar] [CrossRef]
- Föllmi, K.B. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Abed, A.M.; Fakhouri, K. On the chemical variability of phosphatic particles from Jordanian phosphorite deposits. Geochem. Geol. 1996, 131, 1–13. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Chen, J.; Algeo, T.J.; Zhao, L.; Chen, Z.-Q.; Cao, L.; Zhang, L.; Li, Y. Diagenetic uptake of rare earth elements by bioapatite, with an example from Lower Triassic conodonts of South China. Earth Sci. Rev. 2015, 149, 181–202. [Google Scholar] [CrossRef]
- Fazio, A.M.; Scasso, R.A.; Castro, L.N.; Carey, S. Geochemistry of rare earth elements in early-diagenetic miocene phosphatic concretions of Patagonia, Argentina: Phosphogenetic implications. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1414–1432. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 1989, 21, 169–200. [Google Scholar]
- Scholz, F.; McManus, J.; Mix, A.C.; Hensen, C.; Schneider, R.R. The impact of ocean deoxygenation on iron release from continental margin sediments. Nat. Geosci. 2014, 7, 433–437. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Grimm, K.A.; Abed, A.M.; Sadaqah, R.M.Y. Upper Cretaceous (Campanian) phosphorites in Jordan implications for the formation of a south Tethyan phosphorite giant. Sediment. Geol. 2003, 161, 175–205. [Google Scholar] [CrossRef]
- Garnit, H.; Bouhlel, S.; Jarvis, I. Geochemistry and depositional environments of Paleocene Eocene phosphorites: Metlaoui Group, Tunisia. J. Afr. Earth Sci. 2017, 134, 704–736. [Google Scholar] [CrossRef]
- Abedini, A.; Calagari, A.A. REEs geochemical characteristics of lower Cambrian phosphatic rocks in the Gorgan-Rasht Zone, northern Iran: Implications for diagenetic effects and depositional conditions. J. Afr. Earth Sci. 2017, 135, 115–124. [Google Scholar] [CrossRef]
- Morad, S.; Felitsyn, S. Identification of primary Ce-anomaly signatures in fossil biogenic apatite: Implication for the Cambrian oceanic anoxia and phosphogenesis. Sediment. Geol. 2001, 143, 259–264. [Google Scholar] [CrossRef]
- Baioumy, H.M.; Tada, R.; Gharaie, M.H.M. Geochemistry of Late Cretaceous phosphorites in Egypt: Implication for their genesis and diagenesis. J. Afr. Earth Sci. 2007, 49, 12–28. [Google Scholar] [CrossRef]
- Murray, R.W. Chemical criteria to identify the depositional environment of chert: General principles and applications. Sedimen. Geol. 1994, 90, 213–232. [Google Scholar] [CrossRef]
- Baioumy, H.; Lehmann, B. Anomalous enrichment of redox-sensitive trace elements in the marine black shales from the Duwi Formation, Egypt: Evidence for the late Cretaceous Tethys anoxia. J. Afr. Earth Sci. 2017, 133, 7–14. [Google Scholar] [CrossRef]
- Garnit, H.; Bouhlel, S.; Barca, D.; Chtara, C. Application of LA-ICP-MS to sedimentary phosphatic particles from Tunisian phosphorite deposits: Insights from trace elements and REE into paleo-depositional environments. Chemie Erde Geochem. 2012, 72, 127–139. [Google Scholar] [CrossRef]
- Pi, D.-H.; Jiang, S.-Y.; Luo, L.; Yang, J.-H.; Ling, H.-F. Depositional environments for stratiform witherite deposits in the Lower Cambrian black shale sequence of the Yangtze Platform, southern Qinling region, SW China: Evidence from redox-sensitive trace element geochemistry. Palaeogeogr. Palaeoclim. Palaeoecol. 2014, 398, 125–131. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Algeo, T.J.; Maynard, J.B. Trace-element behavior and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems. Geochem. Geol. 2004, 206, 289–318. [Google Scholar] [CrossRef]
- Yao, C.; Ma, D.; Ding, H.; Zhang, X.; Huang, H. Trace elements and stable isotopic geochemistry of an Early Cambrian chert-phosphorite unit from the lower Yurtus Formation of the Sugetbrak section in the Tarim Basin. Sci. China Earth Sci. 2014, 57, 454–464. [Google Scholar] [CrossRef]
- Gallego-Torres, D.; Martinez-Ruiz, F.; De Lange, G.J.; Jimenez-Espejo, F.J.; Ortega-Huertas, M. Trace-elemental derived paleoceanographic and paleoclimatic conditions for Pleistocene Eastern Mediterranean sapropels. Palaeogeogr. Palaeoclim. Palaeoecol. 2010, 293, 76–89. [Google Scholar] [CrossRef]
- Piper, D.Z.; Calvert, S.E. A marine biogeochemical perspective on black shale deposition. Earth Sci. Rev. 2009, 95, 63–96. [Google Scholar] [CrossRef]
- Heggie, D.; Lewis, T. Cobalt in pore waters of marine sediments. Nature 1984, 311, 453–455. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A.C. Composition of Geochemical indices used for the interpretation of paleoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Galarraga, F.; Reategui, K.; Martïnez, A.; Martínez, M.; Llamas, J.F.; Marquez, G. V/Ni ratio as a parameter in palaeoenvironmental characterisation of non-mature medium-crude oils from several Latin American basins. J. Pet. Sci. Eng. 2008, 61, 9–14. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Jarvis, I.; Burnett, W.C.; Nathan, Y.; Almbaydin, F.S.M.; Attia, A.K.M.; Castro, L.N.; Flicoteaux, R.; Hilmy, M.E.; Husain, V.; Qutawnah, A.A.; et al. Phosphorite geochemistry-state-of-the-art and environmental concerns. Eclogae Geol. Helv. 1994, 87, 643–700. [Google Scholar]
- Fleet, A.J. Aqueous and Sedimentary Geochemistry of the Rare Earth Elements. In Rare Earth Element Geochemistry; Henderson, P., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 2, pp. 343–347. [Google Scholar]
- Elderfield, H.; Upstill-Goddard, R.; Sholkovitz, E.R. The rare earth elements in rivers, estuaries, and coastal seas and their significance to the composition of ocean waters. Geochim. Cosmochim. Acta 1990, 54, 971–991. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, X.; Wood, R.A.; Shi, Y.; Gao, Z.; Poulton, S.W. Oxygenation of the Mesoproterozoic ocean and the evolution of complex eukaryotes. Nat. Geosci. 2018, 11, 345–350. [Google Scholar] [CrossRef]
- Webb, G.E.; Kamber, B.S. Rare earth elements in Holocene reefal microbialites: A new shallow seawater proxy. Geochim. Cosmochim. Acta 2000, 64, 1557–1565. [Google Scholar] [CrossRef]
- Liao, J.; Sun, X.; Li, D.; Sa, R.; Lu, Y.; Lin, Z.; Xu, L.; Zhan, R.; Pan, Y.; Xu, H. New insights into nanostructure and geochemistry of bioapatite in REE-rich deep-sea sediments: LA-ICP-MS, TEM, and Z-contrast imaging studies. Chem. Geol. 2019, 512, 58–68. [Google Scholar] [CrossRef]
- Elderfield, H.; Pagett, R. REE in ichthyoliths: Variations with redox conditions and depositional environment. Sci. Total Environ. 1986, 49, 175–197. [Google Scholar] [CrossRef]
- Soudry, D.; Glenn, C.R.; Nathan, Y.; Segal, I.; VonderHaar, D. Evolution of Tethyan phosphogenesis along the northern edges of the Arabian–African shield during the Cretaceous–Eocene as deduced from temporal variations of Ca and Nd isotopes and rates of P accumulation. Earth Sci. Rev. 2006, 78, 27–57. [Google Scholar] [CrossRef]
- Caetano-Filho, S.; Paula-Santos, G.M.; Dias-Brito, D. Carbonate REE + Y signatures from the restricted early marine phase of South Atlantic Ocean (late Aptian—Albian): The influence of early anoxic diagenesis on shale-normalized REE + Y patterns of ancient carbonate rocks. Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 500, 69–83. [Google Scholar] [CrossRef]
- Shields, G.; Stille, P. Diagenetic constraints on the use of cerium anomalies as palaeoseawater redox proxies: An isotopic and REE study of Cambrian phosphorites. Chem. Geol. 2001, 175, 29–48. [Google Scholar] [CrossRef]
- Awadalla, G.S. Geochemistry and microprobe investigations of Abu Tartur REE-bearing phosphorite, Western Desert, Egypt. J. Afr. Earth Sci. 2010, 57, 431–443. [Google Scholar] [CrossRef]
- Khan, K.F.; Dar, S.A.; Khan, S.A. Rare earth element (REE) geochemistry of phosphorites of the Sonrai area of Paleoproterozoic Bijawar basin, Uttar Pradesh, India. J. Rare Earths 2012, 30, 507–514. [Google Scholar] [CrossRef]
- Soudry, D. Microbial phosphate sediment. In Microbial Sediments; Riding, R.E., Awramik, S.M., Eds.; Spinger: Berlin/Heidelberg, Germany, 2000; pp. 127–136. [Google Scholar]
- Schulz, H.N.; Schulz, H.D. Large sulfur bacteria and the formation of phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef]
- O’Brien, G.W.; Harris, J.R.; Milnes, A.R.; Veeh, H.H. Bacterial origin of East Australian continental margin phosphorites. Nature 1981, 294, 442–444. [Google Scholar] [CrossRef]
- Compton, J.S.; Bergh, E.W. Phosphorite deposits on the Namibian shelf. Mar. Geol. 2016, 380, 290–314. [Google Scholar] [CrossRef]
- Godfrey, L.V.; Falkowski, P.G. The cycling and redox state of nitrogen in the Archaean ocean. Nat. Geosci. 2009, 2, 725. [Google Scholar] [CrossRef]
- Goldhammer, T.; Brüchert, V.; Ferdelman, T.G.; Zabel, M. Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 2010, 3, 557–560. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Grimm, K.A. Coated phosphate grains: Proxy for physical, chemical, and ecological changes in seawater. Geology 2003, 31, 801–804. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Weir-Murphy, S. Early diagenesis of inner-shelf phosphorite and iron-silicate minerals, Lower Cretaceous of the Orpheus graben, southeastern Canada: Implications for the origin of chlorite rims. AAPG Bull. 2008, 92, 1153–1168. [Google Scholar] [CrossRef]
- Javier Alvaro, J.; Shields-Zhou, G.A.; Ahlberg, P.; Jensen, S.; Palacios, T. Ediacaran-Cambrian phosphorites from the western margins of Gondwana and Baltica. Sedimentology 2016, 63, 350–377. [Google Scholar] [CrossRef]
- Birch, G.F.; Thomson, J.; McArthur, J.M.; Burnett, W.C. Pleistocene phosphorites off the west coast of South Africa. Nature 1983, 302, 601–603. [Google Scholar] [CrossRef]
- Ahmad, F.; Farouk, S.; Abd El-Moghny, M.W. A regional stratigraphic correlation for the upper Campanian phosphorites and associated rocks in Egypt and Jordan. Proc. Geol. Assoc. 2014, 125, 419–431. [Google Scholar] [CrossRef]
- Simmons, M.D.; Sharland, P.R.; Casey, D.M.; Davies, R.B.; Sutcliffe, O.E. Arabian Plate sequence stratigraphy: Potential implications for global chronostratigraphy. GeoArabia 2007, 12, 101–130. [Google Scholar]
- Hannigan, R.E.; Sholkovitz, E.R. The development of middle rare earth element enrichments in freshwaters: Weathering of phosphatic minerals. Chem. Geol. 2001, 175, 495–508. [Google Scholar] [CrossRef]
- Reynard, B.; Lécuyer, C.; Grandjean, P. Crystal-chemical controls on rare-earth element concentrations in fossil biogenic apatites and implications for paleoenvironmental reconstructions. Chem. Geol. 1999, 155, 233–241. [Google Scholar] [CrossRef]
- Lumiste, K.; Mänd, K.; Bailey, J.; Paiste, P.; Lang, L.; Lepland, A.; Kirsimäe, K. REE+Y uptake and diagenesis in Recent sedimentary apatites. Chem. Geol. 2019, 525, 268–281. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Landing, W.M.; Lewis, B.L. Ocean particle chemistry: The fractionation of rare earth elements between suspended particles and seawater. Geochim. Cosmochim. Acta 1994, 58, 1567–1579. [Google Scholar] [CrossRef]
- Edelman-Furstenberg, Y. Cyclic upwelling facies along the Late Cretaceous southern Tethys (Israel): Taphonomic and ichnofacies evidence of a high-productivity mosaic. Cretac. Res. 2009, 30, 847–863. [Google Scholar] [CrossRef]
- Soudry, D.; Nathan, Y.; Ehrlich, S. Geochemical diagenetic trends during phosphorite formation—Economic implications: The case of the Negev Campanian phosphorites, Southern Israel. Sedimentology 2013, 60, 800–819. [Google Scholar] [CrossRef]
- Bar, O.; Zilberman, E.; Feinstein, S.; Calvo, R.; Gvirtzman, Z. The uplift history of the Arabian Plateau as inferred from geomorphologic analysis of its northwestern edge. Tectonophysics 2016, 671, 9–23. [Google Scholar] [CrossRef]




| Sediment Features | Calcareous Phosphorite | Sandy Phosphorite |
|---|---|---|
| Bedding | Relative thin, interbedded with thin chert, carbonate, and mudstone layers | Thick, varies in the top part of the Devonian sandstones (unconsolidated). |
| Phosphate fraction | Pellet grains, ooids, amalgamated greater grains and boundary-unclear concretions, and bio-derived particles | Same with calcareous phosphorite, fewer ooids but more pellets. |
| Vertebrate fragments | Sporadic | Sporadic |
| Allochthonous non-phosphate clasts | Rare or absent | Often present, quartz grains and terrestrial matrix (clay minerals etc.) |
| Carbonate fraction | High (calcareous matrix) | Low (carbonate cement) |
| Diagenesis affection | Apatite and calcite cemented densely, distinguished compaction, hematite, and pyrolusite cement. | Calcite and clay minerals cemented partially, apatite re-crystallization, hematite, and pyrolusite cement |
| Spatial distribution | Spreading under the carbonate and over the sandy phosphorite | In the uppermost part of the sandstone layer, beneath the calcareous phosphorite |
| Sample ID | Lithology | SiO₂ | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | MnO | TiO2 | P2O5 | LOI a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C–Ph–16 | Calcareous phosphorite | 12.83 | 1.19 | 2.92 | 1.35 | 42.4 | 0.82 | 0.104 | 0.029 | 0.074 | 28.57 | 5.64 |
| C–Ph–17 | 3.38 | 0.893 | 0.204 | 0.657 | 42.06 | 7.03 | 0.124 | 0.022 | 0.05 | 29.35 | 13.70 | |
| C–Ph–21 | 21.32 | 0.958 | 0.891 | 0.278 | 40.6 | 0.497 | 0.067 | 0.017 | 0.062 | 28.31 | 3.00 | |
| C–Ph–22 | 3.21 | 0.797 | 0.437 | 0.323 | 50.67 | 0.791 | 0.052 | 0.015 | 0.044 | 35.91 | 3.22 | |
| C–Ph–24 | 10.22 | 2.06 | 0.399 | 0.644 | 46.04 | 0.511 | 0.119 | 0.039 | 0.116 | 32.5 | 3.31 | |
| C–Ph–26 | 8.3 | 1.11 | 0.404 | 0.63 | 46.71 | 1.19 | 0.067 | 0.009 | 0.06 | 33.08 | 4.41 | |
| C–Ph–53 | 4.29 | 0.927 | 1.71 | 0.296 | 50.15 | 0.37 | 0.058 | 0.021 | 0.062 | 35.42 | 2.66 | |
| C–Ph–25 | Sandy phosphorite | 33.33 | 0.502 | 0.34 | 0.28 | 30.68 | 3.53 | 0.059 | 0.007 | 0.05 | 20.93 | 7.28 |
| C–Ph–45 | 33.24 | 1.26 | 0.573 | 0.493 | 33.62 | 0.473 | 0.263 | 0.023 | 0.081 | 23.13 | 3.13 | |
| C–Ph–46 | 42.37 | 0.393 | 0.285 | 0.353 | 29.48 | 0.361 | 0.08 | 0.01 | 0.03 | 19.8 | 3.11 | |
| C–Ph–49 | 46.62 | 0.134 | 0.648 | 0.19 | 27.2 | 0.706 | 0.028 | 0.02 | 0.037 | 18.81 | 2.60 | |
| C–Ph–50 | 55.74 | 0.187 | 0.433 | 0.26 | 21.91 | 0.751 | 0.036 | 0.047 | 0.02 | 14.92 | 2.67 |
| Sample ID | Lithology | V | Cr | Co | Ni | Cu | Zn | Sr | Mo | V/Ni | V/Cr | Ni/Co | V/Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-Ph-16 | Calcareous phosphorite | 428 | 106 | 7.97 | 43.8 | 76.6 | 291 | 914 | 1.28 | 9.77 | 4.04 | 5.50 | 334.38 |
| C-Ph-17 | 240 | 78.9 | 8.74 | 50 | 87.3 | 241 | 552 | 2.06 | 4.80 | 3.04 | 5.72 | 116.50 | |
| C-Ph-21 | 88.3 | 58 | 8.98 | 35 | 62.4 | 206 | 215 | 5.8 | 2.52 | 1.52 | 3.90 | 15.22 | |
| C-Ph-22 | 178 | 123 | 8.53 | 31.8 | 56.7 | 147 | 214 | 6.52 | 5.60 | 1.45 | 3.73 | 27.30 | |
| C-Ph-24 | 81.1 | 134 | 8.07 | 28.2 | 51.5 | 171 | 169 | 1.96 | 2.88 | 0.61 | 3.49 | 41.38 | |
| C-Ph-26 | 82.7 | 156 | 8.35 | 40 | 46.8 | 127 | 155 | 0.67 | 2.07 | 0.53 | 4.79 | 123.43 | |
| C-Ph-53 | 186 | 130 | 8.56 | 54.1 | 40.7 | 253 | 155 | 6.56 | 3.44 | 1.43 | 6.32 | 28.35 | |
| C-Ph-25 | Sandy phosphorite | 58.7 | 45.3 | 6.96 | 24.8 | 73.1 | 148 | 198 | 3.52 | 2.37 | 1.30 | 3.56 | 16.68 |
| C-Ph-45 | 56 | 71.2 | 4.82 | 22.4 | 60.3 | 195 | 296 | 4.96 | 2.50 | 0.79 | 4.65 | 11.29 | |
| C-Ph-46 | 60.1 | 53.2 | 4.53 | 77.6 | 48.9 | 829 | 211 | 1.76 | 0.77 | 1.13 | 17.13 | 34.15 | |
| C-Ph-49 | 127 | 72 | 4.5 | 38.5 | 50.6 | 205 | 155 | 2.88 | 3.30 | 1.76 | 8.56 | 44.10 | |
| C-Ph-50 | 74.7 | 37.3 | 2.58 | 11.6 | 27.5 | 87.8 | 122 | <0.002 | 6.44 | 2.00 | 4.50 | 37350.00 |
| Lithology | Calcareous Phosphorite | Sandy Phosphorite | Chert | Mudstone | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | C–Ph–16 | C–Ph–17 | C–Ph–21 | C–Ph–22 | C–Ph–24 | C–Ph–26 | C–Ph–53 | C–Ph–25 | C–Ph–45 | C–Ph–46 | C–Ph–49 | C–Ph–50 | C–Ch–01 | C–Ch–02 | C–Ch–03 | C–Ch–52 | C–Ch–55 | C–Ch–56 | C–M–18 | C–M–23 |
| LaN | 0.521 | 0.631 | 0.688 | 0.654 | 0.788 | 0.51 | 0.728 | 0.516 | 0.665 | 0.283 | 0.356 | 0.244 | 0.055 | 0.137 | 0.027 | 0.058 | 0.118 | 0.259 | 0.798 | 0.751 |
| CeN | 0.18 | 0.265 | 0.369 | 0.245 | 0.357 | 0.275 | 0.302 | 0.304 | 0.412 | 0.119 | 0.158 | 0.088 | 0.022 | 0.055 | 0.022 | 0.03 | 0.05 | 0.107 | 0.649 | 0.648 |
| PrN | 0.292 | 0.413 | 0.49 | 0.369 | 0.554 | 0.391 | 0.533 | 0.381 | 0.53 | 0.168 | 0.227 | 0.149 | 0.034 | 0.094 | 0.022 | 0.036 | 0.069 | 0.153 | 0.674 | 0.681 |
| NdN | 0.313 | 0.437 | 0.522 | 0.383 | 0.584 | 0.413 | 0.587 | 0.404 | 0.543 | 0.181 | 0.233 | 0.166 | 0.037 | 0.094 | 0.023 | 0.035 | 0.071 | 0.163 | 0.646 | 0.655 |
| SmN | 0.323 | 0.503 | 0.618 | 0.441 | 0.676 | 0.447 | 0.701 | 0.461 | 0.672 | 0.195 | 0.288 | 0.2 | 0.054 | 0.109 | 0.036 | 0.036 | 0.078 | 0.182 | 0.751 | 0.751 |
| EuN | 0.413 | 0.663 | 0.727 | 0.582 | 0.891 | 0.564 | 0.88 | 0.53 | 0.8 | 0.248 | 0.333 | 0.228 | 0.072 | 0.135 | 0.034 | 0.091 | 0.075 | 0.226 | 0.797 | 0.78 |
| GdN | 0.472 | 0.708 | 0.777 | 0.62 | 0.891 | 0.633 | 0.921 | 0.562 | 0.803 | 0.273 | 0.373 | 0.264 | 0.037 | 0.156 | 0.024 | 0.026 | 0.076 | 0.247 | 0.835 | 0.794 |
| TbN | 0.477 | 0.718 | 0.776 | 0.62 | 0.897 | 0.597 | 0.925 | 0.537 | 0.76 | 0.278 | 0.37 | 0.271 | 0.043 | 0.151 | 0.045 | 0.017 | 0.085 | 0.252 | 0.792 | 0.736 |
| DyN | 0.489 | 0.731 | 0.797 | 0.665 | 0.889 | 0.579 | 0.968 | 0.553 | 0.756 | 0.314 | 0.374 | 0.28 | 0.053 | 0.161 | 0.047 | 0.032 | 0.093 | 0.276 | 0.69 | 0.637 |
| HoN | 0.555 | 0.792 | 0.829 | 0.745 | 0.956 | 0.631 | 1.029 | 0.565 | 0.772 | 0.363 | 0.43 | 0.295 | 0.064 | 0.182 | 0.072 | 0.039 | 0.105 | 0.311 | 0.612 | 0.595 |
| ErN | 0.586 | 0.849 | 0.86 | 0.814 | 1.039 | 0.688 | 1.07 | 0.604 | 0.807 | 0.421 | 0.449 | 0.297 | 0.059 | 0.205 | 0.084 | 0.055 | 0.099 | 0.304 | 0.653 | 0.6 |
| TmN | 0.385 | 0.696 | 0.63 | 0.64 | 0.847 | 0.528 | 0.953 | 0.479 | 0.714 | 0.326 | 0.319 | 0.202 | 0.04 | 0.158 | 0.059 | 0.005 | 0.067 | 0.249 | 0.484 | 0.375 |
| YbN | 0.422 | 0.73 | 0.695 | 0.677 | 0.879 | 0.585 | 0.869 | 0.489 | 0.695 | 0.387 | 0.372 | 0.208 | 0.035 | 0.159 | 0.078 | 0.017 | 0.084 | 0.214 | 0.539 | 0.447 |
| LuN | 0.353 | 0.654 | 0.691 | 0.693 | 0.91 | 0.545 | 0.949 | 0.485 | 0.697 | 0.346 | 0.381 | 0.162 | 0.042 | 0.178 | 0.079 | 0.005 | 0.053 | 0.24 | 0.453 | 0.381 |
| YN | 1.47 | 1.73 | 1.707 | 1.915 | 1.885 | 1.333 | 2.17 | 1.226 | 1.381 | 0.826 | 1.026 | 0.767 | 0.127 | 0.378 | 0.159 | 0.129 | 0.271 | 0.73 | 1.041 | 0.881 |
| ∑REEs | 58.194 | 80.262 | 95.682 | 75.846 | 104.03 | 72.746 | 98.103 | 73.782 | 99.358 | 35.125 | 44.896 | 29.252 | 6.62 | 17.328 | 4.967 | 6.909 | 13.495 | 31.162 | 127.192 | 124.649 |
| (MREEs/LREEs)N | 2.09 | 2.36 | 2.19 | 2.22 | 2.28 | 2.17 | 2.52 | 2.00 | 2.12 | 2.23 | 2.23 | 2.37 | 2.17 | 2.35 | 2.72 | 1.52 | 1.67 | 2.19 | 1.62 | 1.57 |
| (MREEs/HREEs)N | 1.56 | 1.40 | 1.57 | 1.30 | 1.41 | 1.47 | 1.41 | 1.56 | 1.57 | 1.13 | 1.43 | 1.77 | 1.84 | 1.28 | 0.86 | 2.95 | 1.69 | 1.48 | 2.10 | 2.38 |
| Eu/Eu* | 1.04 | 1.10 | 1.04 | 1.10 | 1.14 | 1.04 | 1.08 | 1.03 | 1.09 | 1.06 | 1.01 | 0.98 | 1.58 | 1.02 | 1.15 | 2.95 | 0.98 | 1.05 | 1.01 | 1.01 |
| Ce/Ce* | 0.40 | 0.47 | 0.58 | 0.43 | 0.50 | 0.58 | 0.44 | 0.64 | 0.66 | 0.48 | 0.50 | 0.40 | 0.45 | 0.45 | 0.86 | 0.59 | 0.49 | 0.47 | 0.87 | 0.90 |
| Y/Y* | 2.82 | 2.27 | 2.10 | 2.72 | 2.04 | 2.20 | 2.17 | 2.19 | 1.81 | 2.44 | 2.55 | 2.67 | 2.18 | 2.20 | 2.69 | 3.59 | 2.73 | 2.49 | 1.60 | 1.43 |
| Pr/Pr* | 1.19 | 1.18 | 1.10 | 1.17 | 1.18 | 1.14 | 1.20 | 1.07 | 1.11 | 1.12 | 1.16 | 1.18 | 1.16 | 1.26 | 0.98 | 1.12 | 1.14 | 1.13 | 1.04 | 1.04 |
| (La/Sm)N | 1.62 | 1.25 | 1.11 | 1.48 | 1.17 | 1.14 | 1.04 | 1.12 | 0.99 | 1.45 | 1.23 | 1.22 | 1.02 | 0.76 | 1.61 | 1.51 | 1.42 | 1.06 | 1.00 | |
| (La/Yb)N | 1.23 | 0.86 | 0.99 | 0.97 | 0.90 | 0.87 | 0.84 | 1.05 | 0.96 | 0.73 | 0.96 | 1.17 | 1.57 | 1.26 | 0.35 | 3.33 | 1.41 | 1.21 | 1.48 | 1.68 |
| (Dy/Sm)N | 1.52 | 1.45 | 1.29 | 1.51 | 1.32 | 1.30 | 1.38 | 1.20 | 1.13 | 1.61 | 1.30 | 1.40 | 0.98 | 1.48 | 1.30 | 0.90 | 1.19 | 1.51 | 0.92 | 0.85 |
| (La/Nd)N | 1.66 | 1.44 | 1.32 | 1.71 | 1.35 | 1.23 | 1.24 | 1.28 | 1.22 | 1.56 | 1.53 | 1.47 | 1.49 | 1.46 | 1.17 | 1.66 | 1.66 | 1.59 | 1.24 | 1.15 |
| Y/Ho | 72.18 | 59.49 | 56.08 | 70.05 | 53.75 | 57.60 | 57.45 | 59.11 | 48.76 | 61.94 | 65.02 | 70.89 | 54.44 | 56.67 | 60.56 | 88.97 | 70.29 | 63.96 | 46.37 | 40.34 |
| Sample ID | Lithology | δ13C | δ18O |
|---|---|---|---|
| C–Ph–24–04 | Phosphatic chert | −9.6 | −7.5 |
| C–Ph–24–05 | Phosphorite | −9.2 | −8.2 |
| C–Ph–24–06 | Phosphorite | −9.8 | −7.8 |
| C–Ph–24–07 | Phosphatic sandstone | −6.5 | −5 |
| C-Ph–24–50 | Phosphorite | −5.7 | −8.1 |
| C–Ph–24–52 | Phosphorite | −6 | −4.1 |
| C–Ph–24–54 | Phosphorite | −2.8 | −2.4 |
| C–Ph–24–76 | Phosphorite | −7.7 | −8.5 |
| C–Ph–24–77 | Phosphatic mudstone | −8.4 | −7.8 |
| C–Ph–24–80 | Phosphorite | −9.8 | −7.5 |
| C–Ph–24–81 | Phosphorite | −8.7 | −6.9 |
| C–Ph–18 | Sandy Phosphorite | −10.1 | −7.8 |
| C–Ph–20 | Sandy Phosphorite | −11.1 | −6.8 |
| C–Ph–22 | Sandy Phosphorite | −10.8 | −8.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Z.; Dini, S.M.; Qin, M.; Banakhar, A.S.; Li, Z.; Yi, L.; Memesh, A.M.; Shammari, A.M.; Li, G. Origin and Evolution of the Late Cretaceous Reworked Phosphorite in the Sirhan-Turayf Basin, Northern Saudi Arabia. Minerals 2021, 11, 350. https://doi.org/10.3390/min11040350
Zhang Y, Li Z, Dini SM, Qin M, Banakhar AS, Li Z, Yi L, Memesh AM, Shammari AM, Li G. Origin and Evolution of the Late Cretaceous Reworked Phosphorite in the Sirhan-Turayf Basin, Northern Saudi Arabia. Minerals. 2021; 11(4):350. https://doi.org/10.3390/min11040350
Chicago/Turabian StyleZhang, Yunlong, Ziying Li, Saleh M. Dini, Mingkuan Qin, Ahmed S. Banakhar, Zhixing Li, Longsheng Yi, Abdullah M. Memesh, Abdullah M. Shammari, and Guochen Li. 2021. "Origin and Evolution of the Late Cretaceous Reworked Phosphorite in the Sirhan-Turayf Basin, Northern Saudi Arabia" Minerals 11, no. 4: 350. https://doi.org/10.3390/min11040350
APA StyleZhang, Y., Li, Z., Dini, S. M., Qin, M., Banakhar, A. S., Li, Z., Yi, L., Memesh, A. M., Shammari, A. M., & Li, G. (2021). Origin and Evolution of the Late Cretaceous Reworked Phosphorite in the Sirhan-Turayf Basin, Northern Saudi Arabia. Minerals, 11(4), 350. https://doi.org/10.3390/min11040350








