The Effect of Hydrodynamic Conditions on the Selective Flotation of Fully Liberated Low Grade Copper-Nickel Ore
Abstract
1. Introduction
2. Materials and Methods
Image Processing
3. Results and Discussion
3.1. Mineralogical Analysis of the Kevitsa Cu-Ni Sulfide Ore
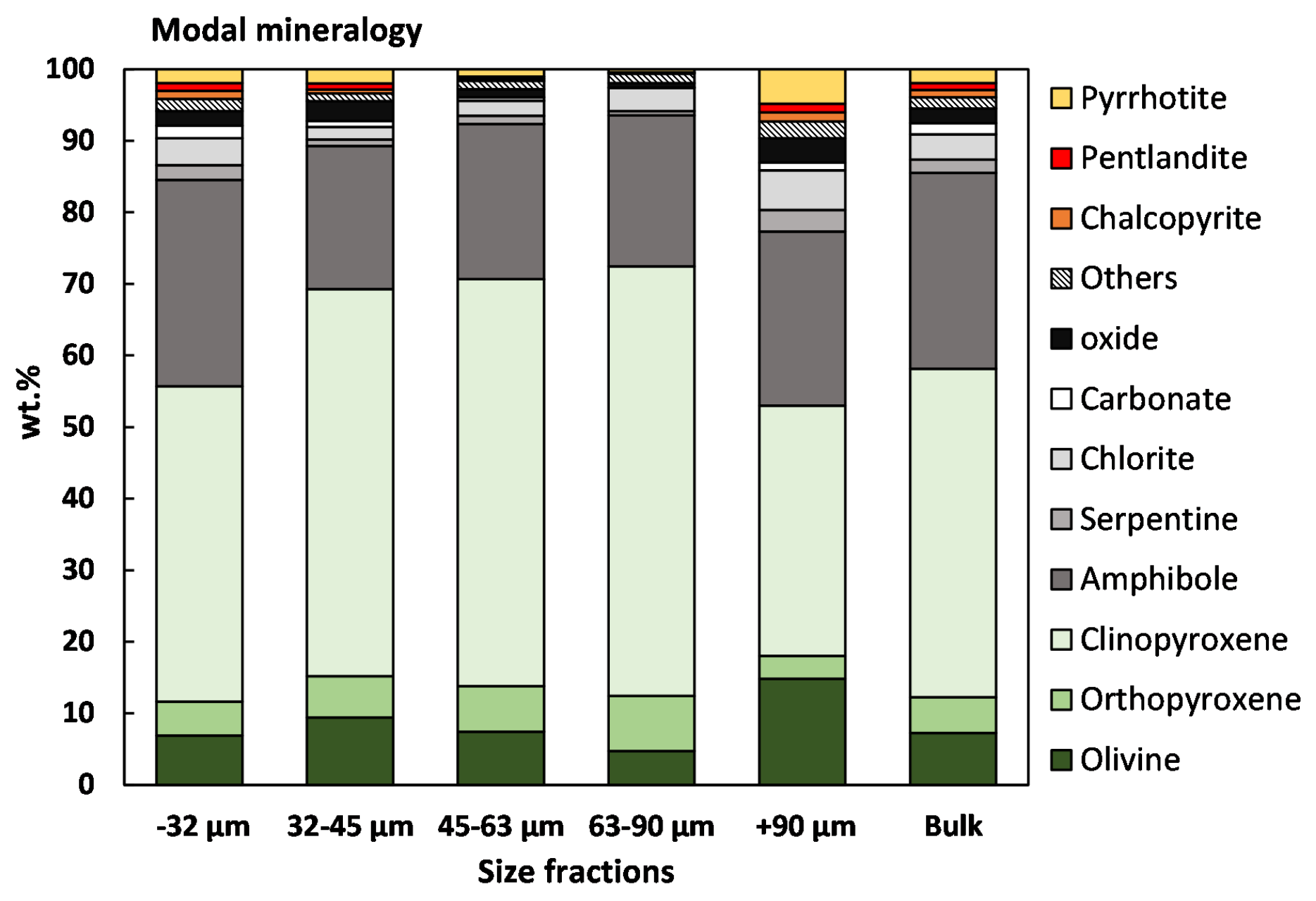
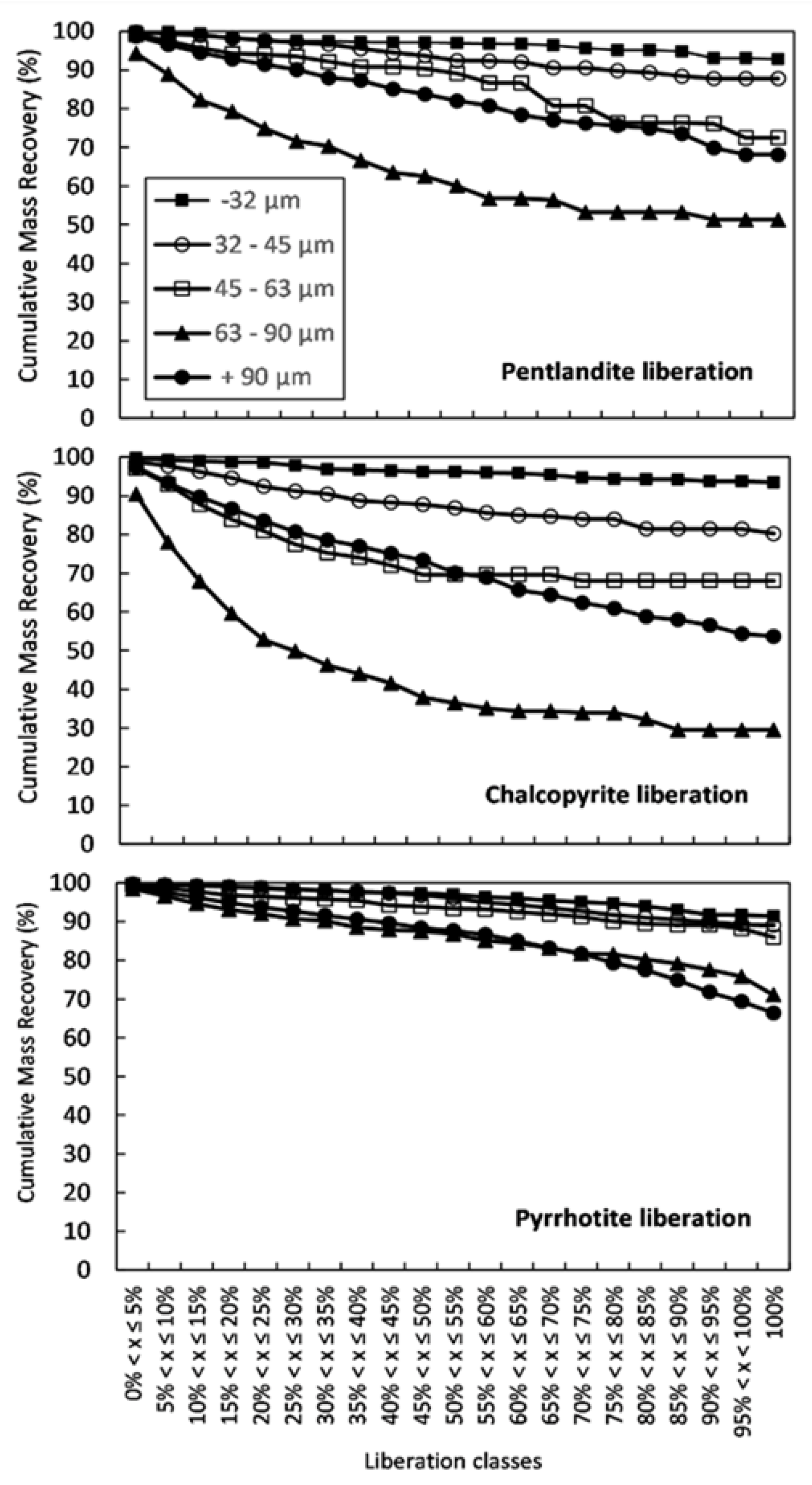
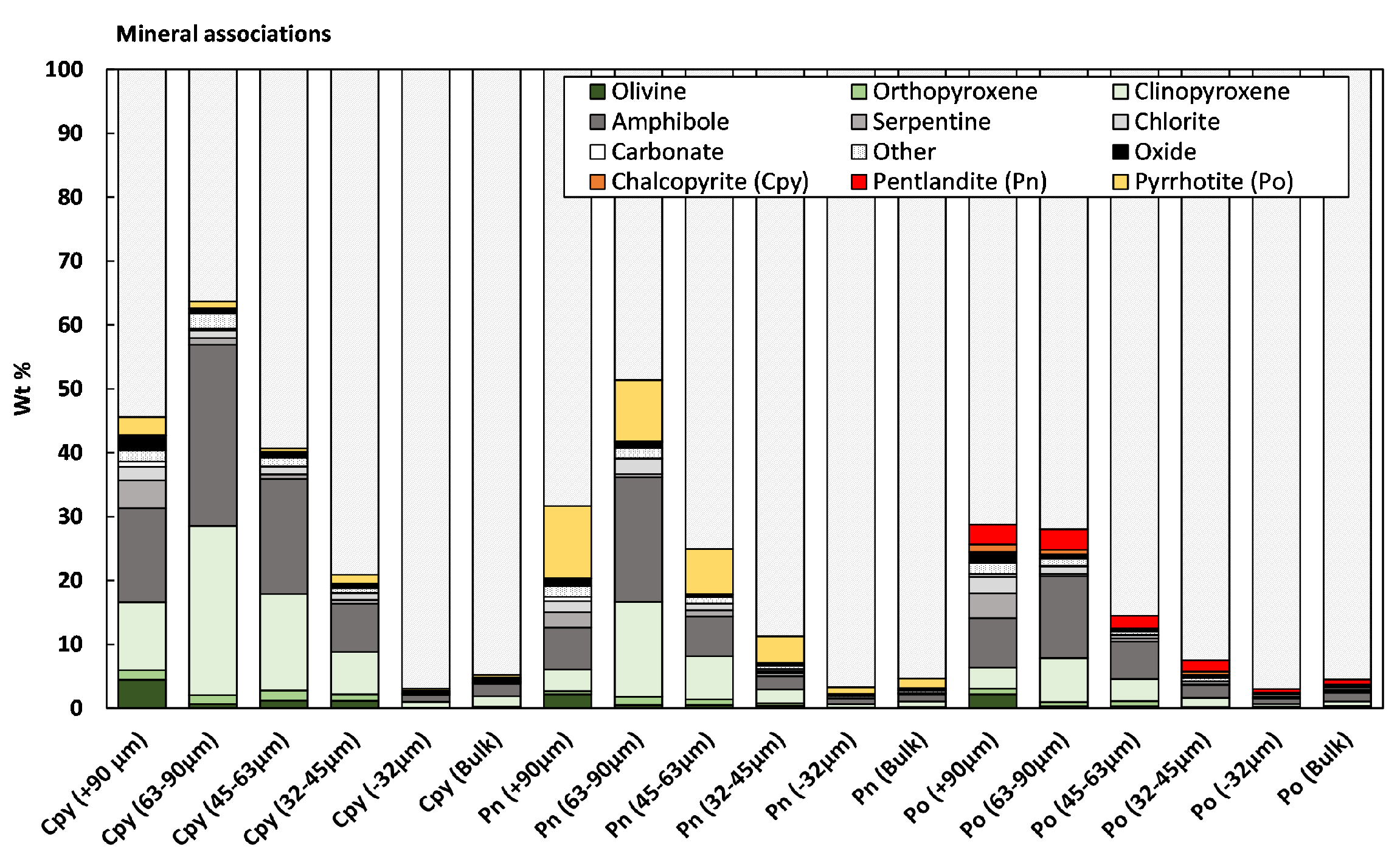
3.1.1. Feed Analysis
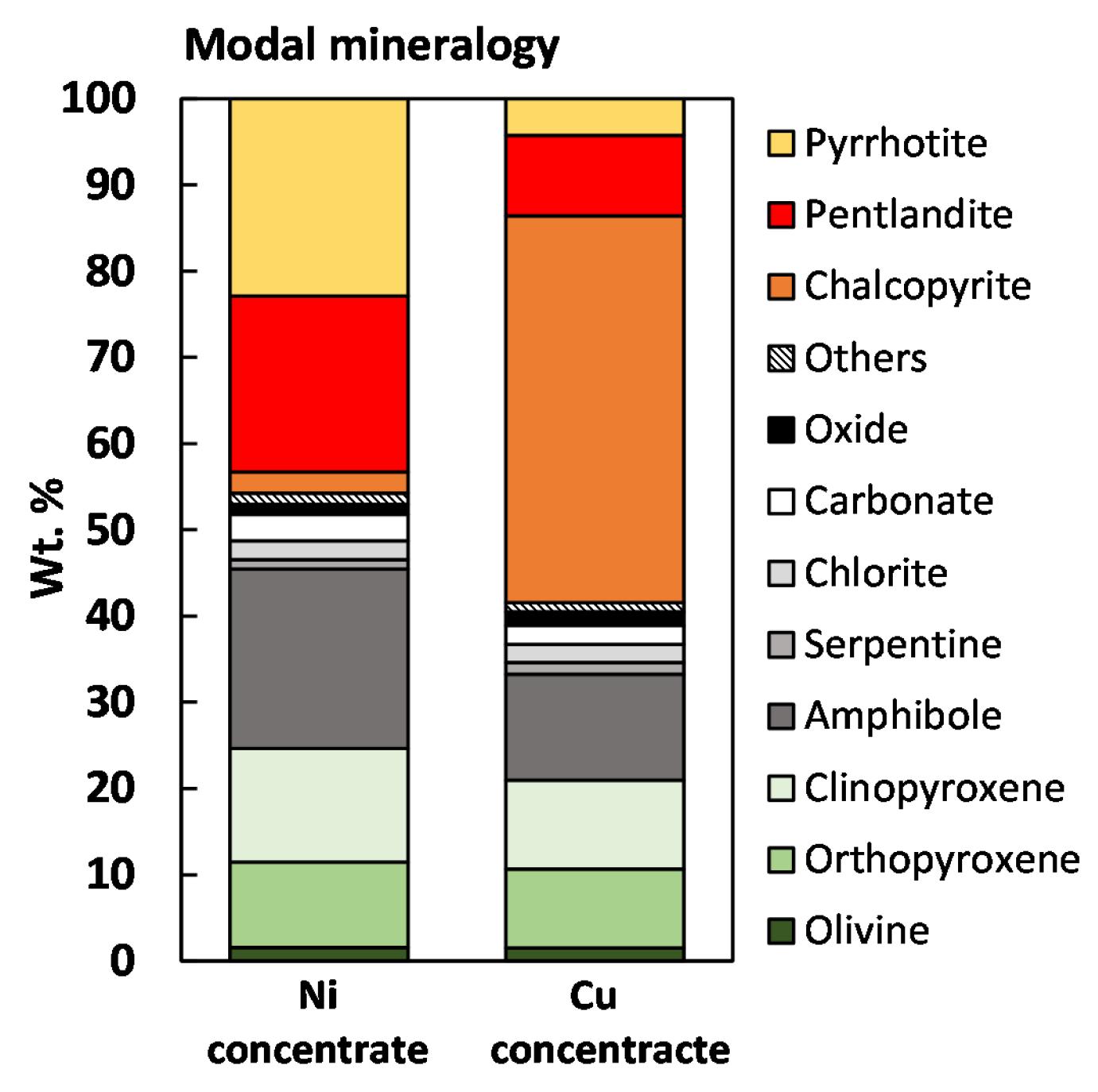
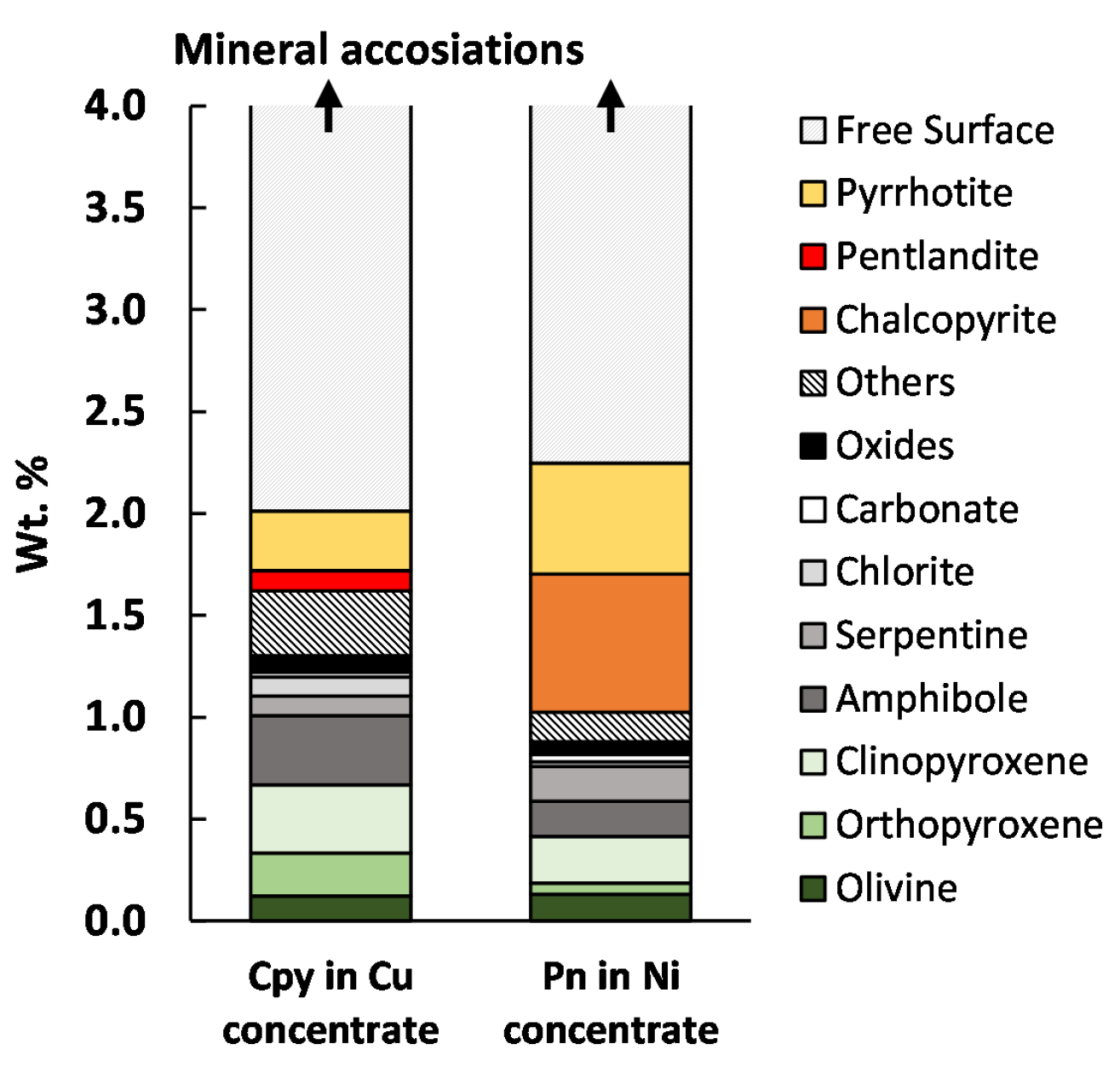
3.1.2. Concentrate Analysis
3.1.3. Tailings Analysis
3.2. Effect of the Bubble Surface Area Flux on the Bubble Size Distribution
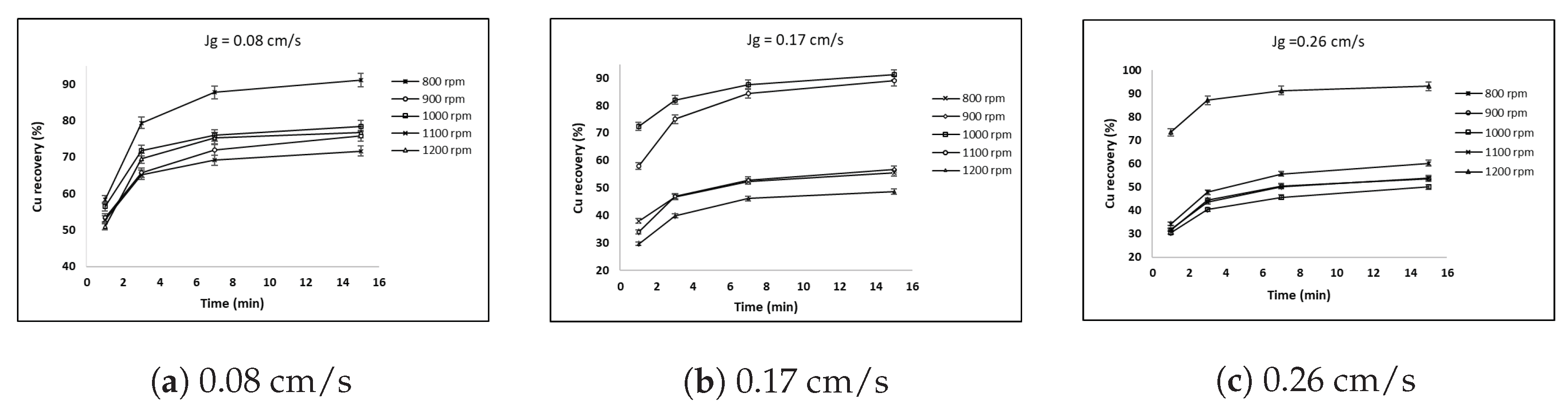
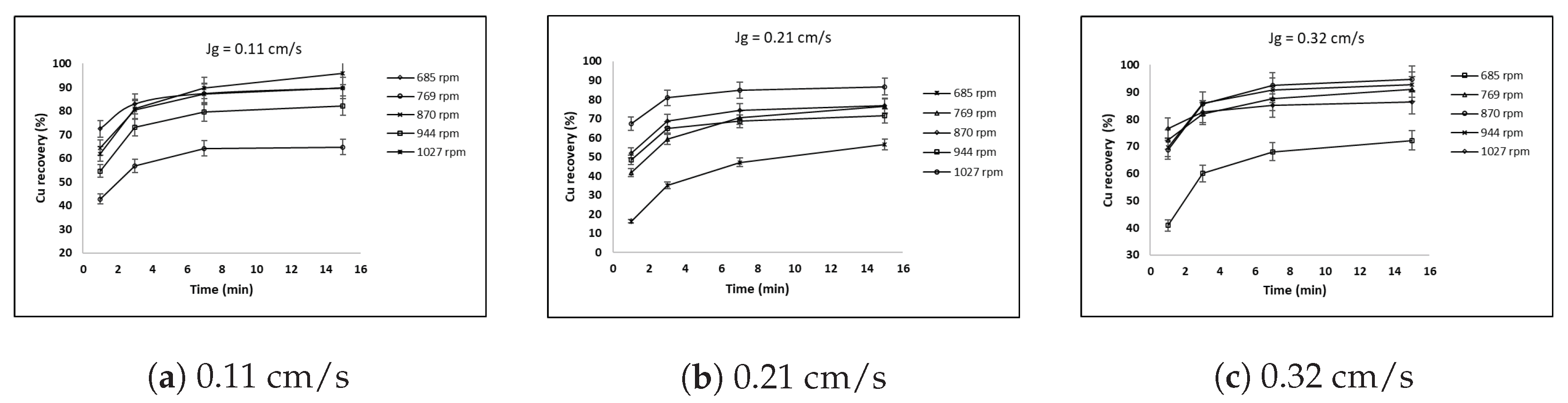
3.3. Effect of Superficial Gas Velocity and Impeller Speed on Copper Recovery with 6 and 7 cm Impellers
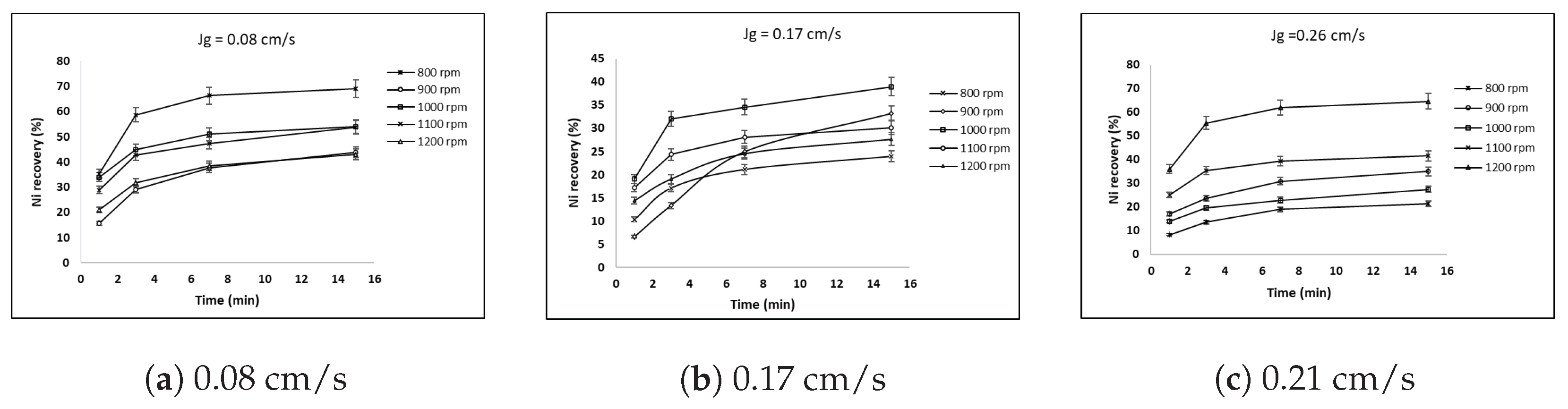
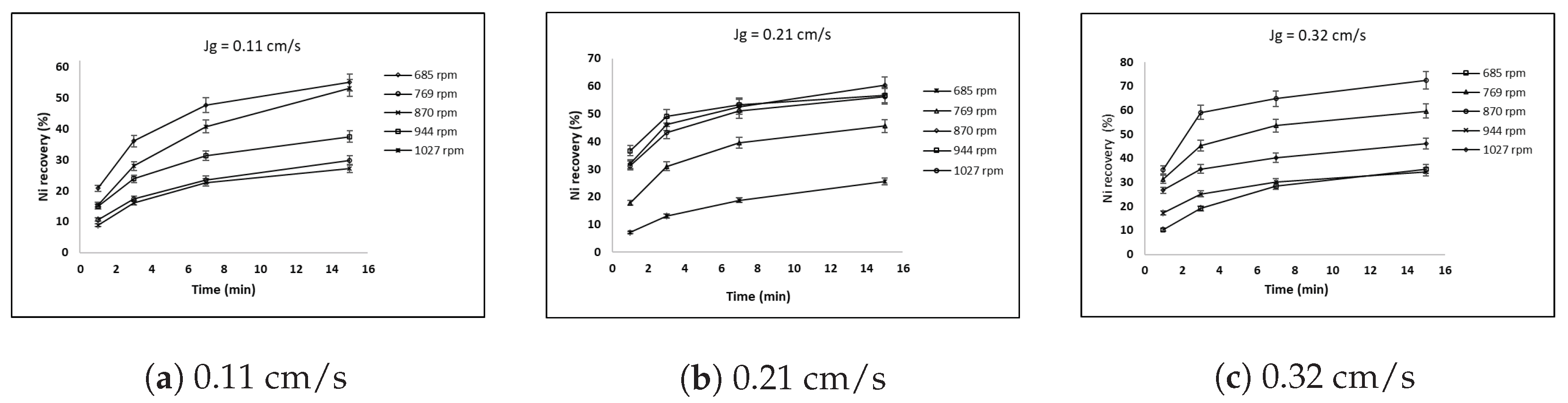
3.4. Effect of Superficial Gas Velocity and Impeller Speed on Nickel Recovery
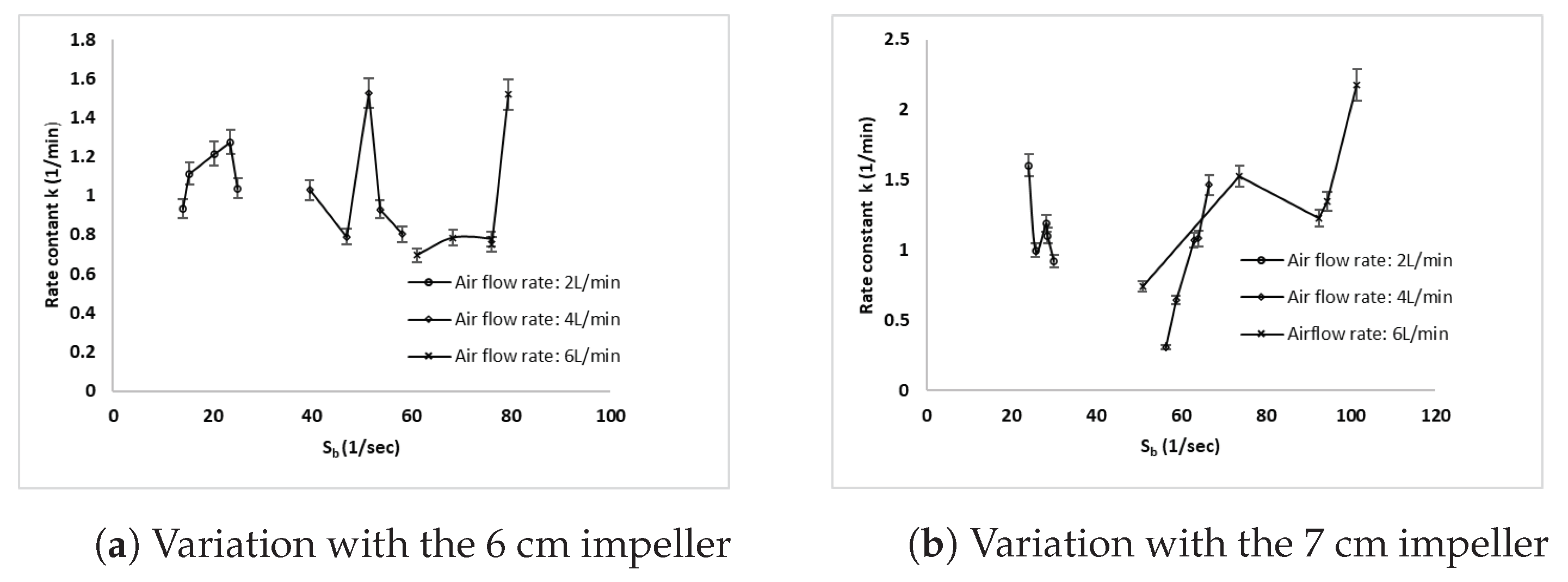
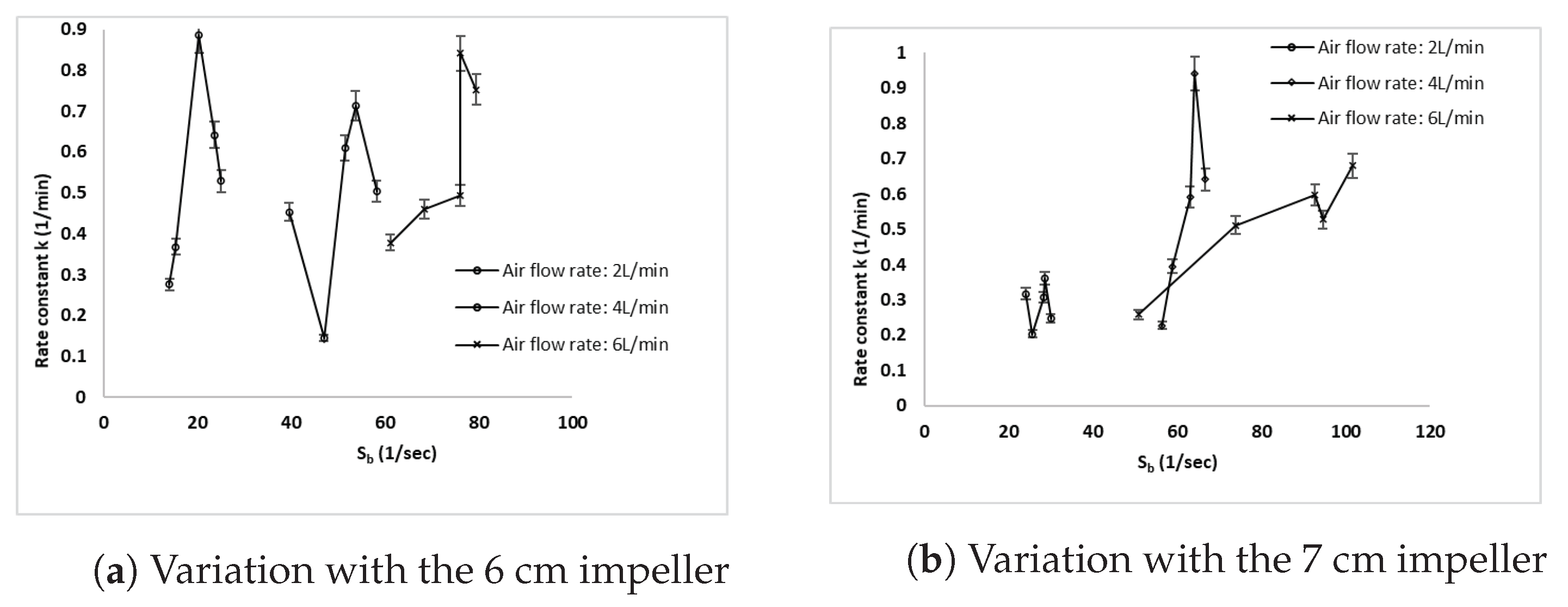
3.5. Effect of the Bubble Surface Area Flux on the Copper Rate Constant
3.6. Effect of the Bubble the Surface Area Flux on the Nickel Rate Constant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirjavainen, V.; Heiskanen, K. Some factors that affect beneficiation of sulfide nickel-copper ores. Miner. Eng. 2007, 20, 629–633. [Google Scholar] [CrossRef]
- Sibanda, V.; Khan, R.; Danha, G. The effect of chemical reagents on flotation performance of a pentlandite ore: An attainable region approach. Powder Technol. 2019, 352, 462–469. [Google Scholar] [CrossRef]
- Musuku, B.; Muzinda, I.; Lumsden, B. Cu-Ni processing improvements at First Quantum’s Kevitsa mine. Miner. Eng. 2016, 88, 9–17. [Google Scholar] [CrossRef]
- Edwards, C.; Kipkie, W.; Agar, G. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Lynch, A.J.; Johnson, N.; Manlapig, E.; Thorne, C. Mineral and Coal Flotation Circuits: Their Simulation and Control; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. The effect of high intensity conditioning on the flotation of a nickel ore. Part 1: Size-by-size analysis. Miner. Eng. 1999, 12, 1185–1200. [Google Scholar] [CrossRef]
- Cilek, E.C. The effect of hydrodynamic conditions on true flotation and entrainment in flotation of a complex sulfide ore. Int. J. Miner. Process. 2009, 90, 35–44. [Google Scholar] [CrossRef]
- Ahmed, N.; Jameson, G. The effect of bubble size on the rate of flotation of fine particles. Int. J. Miner. Process. 1985, 14, 195–215. [Google Scholar] [CrossRef]
- Yoon, R.H. Microbubble flotation. Miner. Eng. 1993, 6, 619–630. [Google Scholar] [CrossRef]
- Finch, J.; Xiao, J.; Hardie, C.; Gomez, C. Gas dispersion properties: Bubble surface area flux and gas holdup. Miner. Eng. 2000, 13, 365–372. [Google Scholar] [CrossRef]
- Gomez, C.; Mesías, J.; Alvarez, J. Bubble Surface Area Flux and Performance in Laboratory Flotation Testing. In Proceedings of the XXVIII International Mineral Processing Congress Proceedings, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Gorain, B.; Franzidis, J.P.; Manlapig, E. Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell—Part 1: Effect on bubble size distribution. Miner. Eng. 1995, 8, 615–635. [Google Scholar] [CrossRef]
- Gorain, B.; Franzidis, J.P.; Manlapig, E. Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell. Part 3: Effect on superficial gas velocity. Miner. Eng. 1996, 9, 639–654. [Google Scholar] [CrossRef]
- Gorain, B.; Franzidis, J.; Manlapig, E. Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell. Part 4: Effect of bubble surface area flux on flotation performance. Miner. Eng. 1997, 10, 367–379. [Google Scholar] [CrossRef]
- Heiskanen, K. On the relationship between flotation rate and bubble surface area flux. Miner. Eng. 2000, 13, 141–149. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Drzymala, J. Physical meaning of the Sauter mean diameter of spherical particulate matter. Part. Sci. Technol. 2016, 34, 645–647. [Google Scholar] [CrossRef]
- Welcome to Python.org. Available online: https://www.python.org/ (accessed on 19 March 2021).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Devernay, F. A non-maxima suppression method for edge detection with sub-pixel accuracy. Ph.D. Thesis, INRIA, Rocquencourt, France, 1995. [Google Scholar]
- Grau, R.A.; Laskowski, J.S.; Heiskanen, K. Effect of frothers on bubble size. Int. J. Miner. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Leiva, J.; Vinnett, L.; Contreras, F.; Yianatos, J. Estimation of the actual bubble surface area flux in flotation. Miner. Eng. 2010, 23, 888–894. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Maoming, F. Effects of nanobubble and hydrodynamic parameters on coarse quartz flotation. Int. J. Min. Sci. Technol. 2019, 29, 289–295. [Google Scholar] [CrossRef]
- Gibson, C.; Kelebek, S. Sensitivity of pentlandite flotation in complex sulfide ores towards pH control by lime versus soda ash: Effect on ore type. Int. J. Miner. Process. 2014, 127, 44–51. [Google Scholar] [CrossRef]
- Laplante, A.; Toguri, J.; Smith, H. The effect of air flow rate on the kinetics of flotation. Part 1: The transfer of material from the slurry to the froth. Int. J. Miner. Process. 1983, 11, 203–219. [Google Scholar] [CrossRef]
- Shahbazi, B.; Rezai, B.; Koleini, S.M.J.; Noparast, M. The effect of bubble surface area flux on flotation efficiency of pyrite particles. Iran. J. Chem. Chem. Eng. 2013, 32, 109–118. [Google Scholar]
- Hernandez-Aguilar, J.; Rao, S.; Finch, J. Testing the k-Sb relationship at the microscale. Miner. Eng. 2005, 18, 591–598. [Google Scholar] [CrossRef]



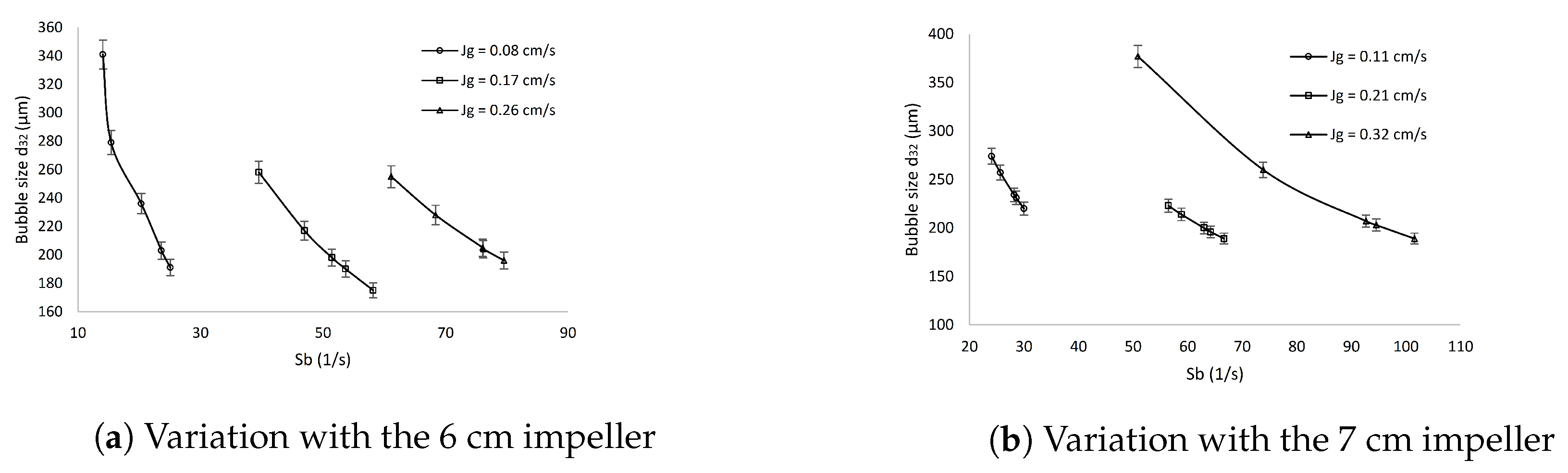
| Aeration Rate | Parameter with Units | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Air flow rate (2 L/min) | Impeller diameter (cm) | 6 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| Impeller speed (rpm) | 800 | 900 | 1000 | 1100 | 1200 | 685 | 769 | 870 | 944 | 1027 | |
| (µm) | 341 | 279 | 236 | 203 | 191 | 274 | 257 | 234 | 231 | 220 | |
| (cm/s) | 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | |
| (s) | 14.07 | 15.42 | 20.34 | 23.64 | 25.09 | 24.08 | 25.68 | 28.20 | 28.58 | 30 | |
| Air flow rate (2 L/min) | Impeller diameter (cm) | 6 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| Impeller speed (rpm) | 800 | 900 | 1000 | 1100 | 1200 | 685 | 769 | 870 | 944 | 1027 | |
| (µm) | 258 | 217 | 198 | 190 | 175 | 223 | 214 | 200 | 196 | 189 | |
| (cm/s) | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 | 0.21 | 0.21 | 0.21 | 0.21 | 0.21 | |
| (s) | 39.54 | 46.98 | 51.48 | 53.7 | 58.20 | 54.46 | 58.86 | 63.00 | 64.20 | 66.66 | |
| Air flow rate (2 L/min) | Impeller diameter (cm) | 6 | 6 | 6 | 6 | 6 | 7 | 7 | 7 | 7 | 7 |
| Impeller speed (rpm) | 800 | 900 | 1000 | 1100 | 1200 | 685 | 769 | 870 | 944 | 1027 | |
| (µm) | 255 | 228 | 205 | 204 | 196 | 377 | 260 | 207 | 203 | 189 | |
| (cm/s) | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | |
| (s) | 61.14 | 68.40 | 76.09 | 76.20 | 79.56 | 51.88 | 73.86 | 92.70 | 94.56 | 101.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Luolavirta, K.; Akram, S.U.; Mehmood, H.; Luukkanen, S. The Effect of Hydrodynamic Conditions on the Selective Flotation of Fully Liberated Low Grade Copper-Nickel Ore. Minerals 2021, 11, 328. https://doi.org/10.3390/min11030328
Kumar H, Luolavirta K, Akram SU, Mehmood H, Luukkanen S. The Effect of Hydrodynamic Conditions on the Selective Flotation of Fully Liberated Low Grade Copper-Nickel Ore. Minerals. 2021; 11(3):328. https://doi.org/10.3390/min11030328
Chicago/Turabian StyleKumar, Haresh, Kirsi Luolavirta, Saad Ullah Akram, Hassan Mehmood, and Saija Luukkanen. 2021. "The Effect of Hydrodynamic Conditions on the Selective Flotation of Fully Liberated Low Grade Copper-Nickel Ore" Minerals 11, no. 3: 328. https://doi.org/10.3390/min11030328
APA StyleKumar, H., Luolavirta, K., Akram, S. U., Mehmood, H., & Luukkanen, S. (2021). The Effect of Hydrodynamic Conditions on the Selective Flotation of Fully Liberated Low Grade Copper-Nickel Ore. Minerals, 11(3), 328. https://doi.org/10.3390/min11030328






