Dissimilatory Iron-Reducing Microorganisms Are Present and Active in the Sediments of the Doce River and Tributaries Impacted by Iron Mine Tailings from the Collapsed Fundão Dam (Mariana, MG, Brazil)
Abstract
1. Introduction
2. Materials and Methods
3. Results
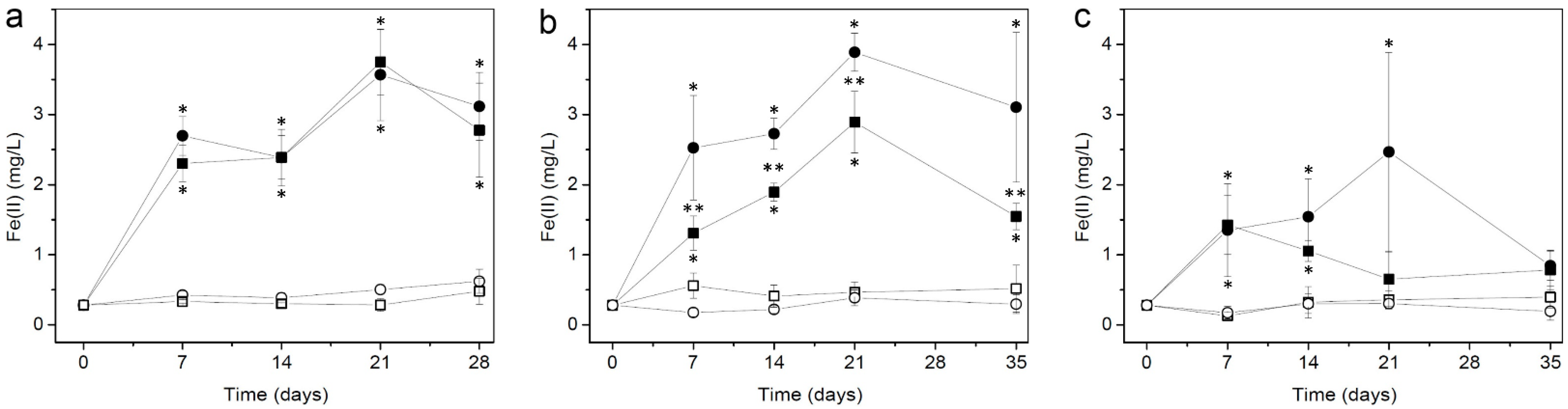
3.1. Fe(II) Concentrations over Time in the Liquid Phase of Short-Term Cultures
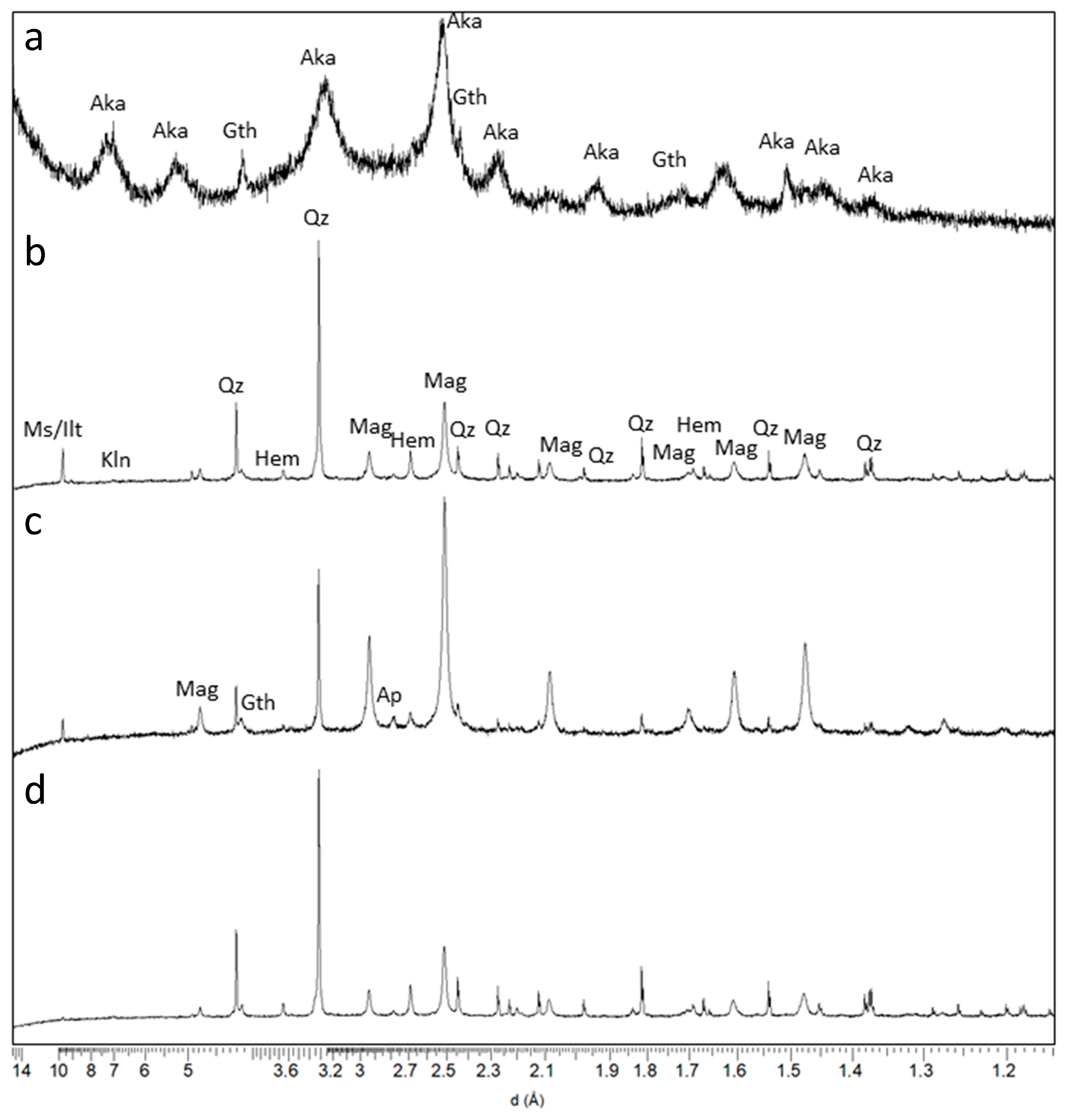
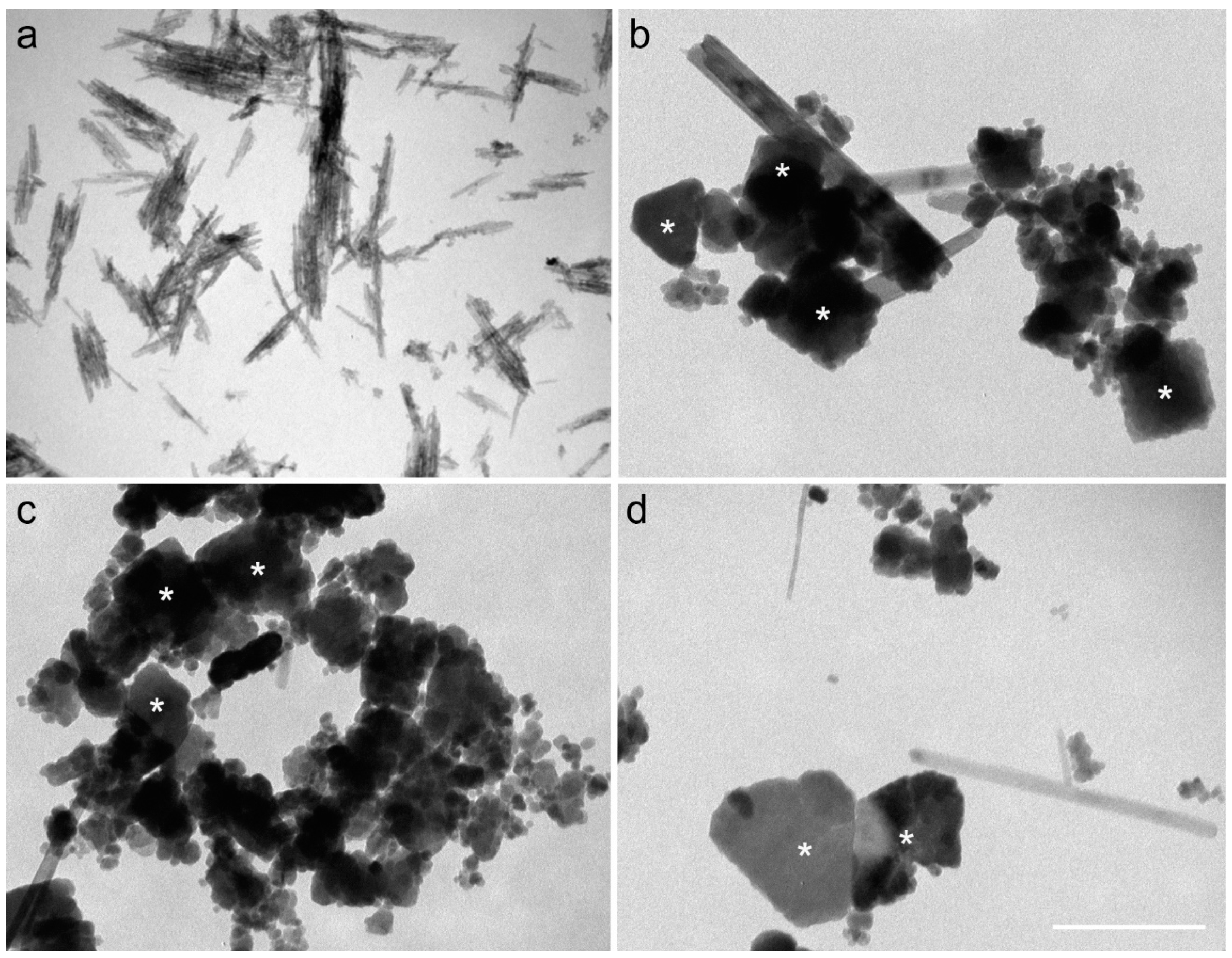
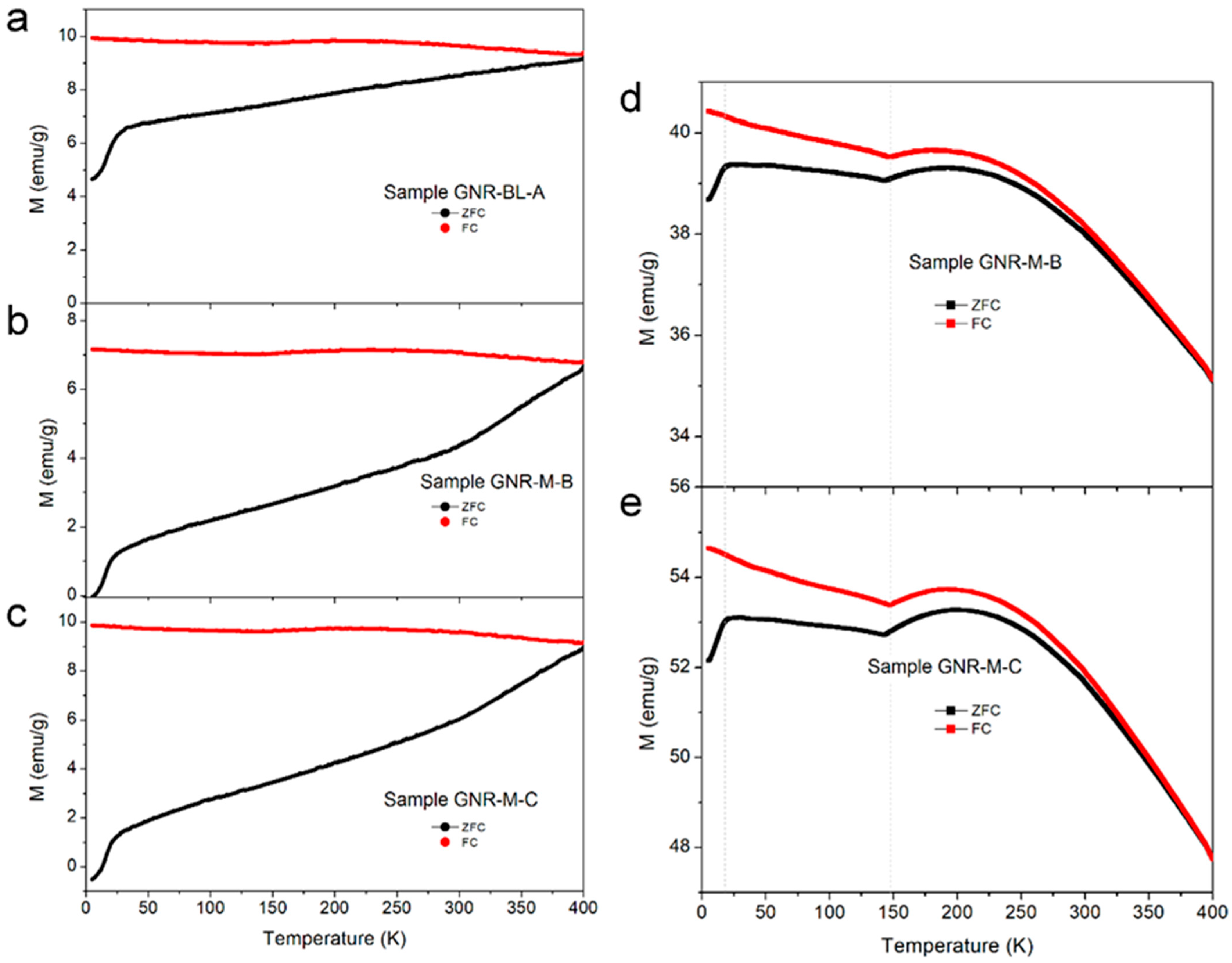
3.2. Long-Term Cultures and the Presence of Magnetite (FeO∙Fe2O3) in the Solid Phase
4. Discussion
4.1. Soluble Fe(II) and Magnetite as Proxies for DIRM Enrichment
4.2. Significance of Soluble Fe(II) and the Crystalline Phases Magnetite, Goethite, and Hematite in Crude Enrichment Cultures
4.3. Significance of DIRMs in the Context of Riverine Sediments Enriched in Tailings
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovley, D.R. Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol. J. 1987, 5, 375–399. [Google Scholar] [CrossRef]
- Lovley, D.R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 1991, 55, 259–287. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Dissimilatory metal reduction. Annu. Rev. Microbiol. 1993, 47, 263–290. [Google Scholar] [CrossRef]
- Myers, C.R.; Nealson, K.H. Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J. Bacteriol. 1990, 172, 6232–6238. [Google Scholar] [CrossRef]
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl. Environ. Microbiol. 1986, 52, 751–757. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Dollhopf, M.E.; Aller, R.; Stackebrandt, E.; Nealson, K.H. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int. J. Syst. Bacteriol. 1998, 48, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Caccavo Jr, F.; Spring, S.; Rosenzweig, R.F. Ferribacterium limneticum, gen. nov., sp. nov., an Fe(III)-reducing microorganism isolated from mining-impacted freshwater lake sediments. Arch. Microbiol. 1999, 171, 183–188. [Google Scholar] [CrossRef]
- Francis, C.A.; Obraztsova, A.Y.; Tebo, B.M. Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl. Environ. Microbiol. 2000, 66, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kostka, J.E.; Dalton, D.D.; Skelton, H.; Dollhopf, S.; Stucki, J.W. Growth of iron(III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. Appl. Environ. Microbiol. 2002, 68, 6256–6262. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Watanabe, J.-I.; Tani, Y.; Seyama, H.; Miyata, N. Removal of heavy metal cations by biogenic magnetite nanoparticles produced in Fe(III)-reducing microbial enrichment cultures. J. Biosci. Bioeng. 2014, 117, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, X.; Wang, C.; Wang, L.; Guo, R. Magnetite nanoparticles facilitate methane production from ethanol via acting as electron acceptors. Sci. Rep. 2015, 5, 16118. [Google Scholar] [CrossRef]
- Nixon, S.L.; Telling, J.P.; Wadham, J.L.; Cockell, C.S. Viable cold-tolerant iron-reducing microorganisms in geographically diverse subglacial environments. Biogeosciences 2017, 14, 1445–1455. [Google Scholar] [CrossRef]
- Fortney, N.W.; He, S.; Converse, B.J.; Beard, B.L.; Johnson, C.M.; Boyd, E.S.; Roden, E.E. Microbial Fe(III) oxide reduction potential in Chocolate Pots hot spring, Yellowstone National Park. Geobiology 2016, 14, 255–275. [Google Scholar] [CrossRef]
- Kostka, J.E.; Nealson, K.H. Dissolution and reduction of magnetite by bacteria. Environ. Sci. Technol. 1995, 29, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Roden, E.E.; Zachara, J.M. Microbial reduction of crystalline iron(III) oxides: Influence of oxides surface area and potential for cell growth. Environ. Sci. Technol. 1996, 30, 1618–1628. [Google Scholar] [CrossRef]
- Zachara, J.M.; Fredrickson, J.K.; Li, S.-M.; Kennedy, D.W.; Smith, S.C.; Gassman, P.L. Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am. Mineral. 1998, 83, 1426–1443. [Google Scholar] [CrossRef]
- Cummings, D.E.; Caccavo, F., Jr.; Fendorf, S.; Rosenzweig, R.F. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999, 33, 723–729. [Google Scholar] [CrossRef]
- Bonneville, S.; Van Cappellen, P.; Behrends, T. Microbial reduction of iron(III) oxyhydroxides: Effects of mineral solubility and availability. Chem. Geol. 2004, 212, 255–268. [Google Scholar] [CrossRef]
- Cutting, R.S.; Coker, V.S.; Telling, N.D.; Kimber, R.L.; van der Laan, G.; Pattrick, R.A.D.; Vaughan, D.J.; Arenholz, E.; Lloyd, J.R. Microbial reduction of arsenic-doped schwertmannite by Geobacter sulfurreducens. Environ. Sci. Technol. 2012, 46, 12591–12599. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Lu, X.; Liu, H.; Li, J.; Zhu, T.; Zhu, X.; Lu, J.; Wang, R. Reduction of jarosite by Shewanella oneidensis MR-1 and secondary mineralization. Geochim. Cosmochim. Acta 2014, 124, 54–71. [Google Scholar]
- Till, J.L.; Guyodo, Y.; Lagroix, F.; Ona-Nguema, G.; Brest, J. Magnetic comparison of abiogenic and biogenic alteration products of lepidocrocite. Earth Planet. Sci. Let. 2014, 395, 149–158. [Google Scholar] [CrossRef]
- Castro, L.; Blásquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Reductive leaching of jarosites by Aeromonas hydrophila. Min. Eng. 2016, 95, 21–28. [Google Scholar] [CrossRef]
- Han, R.; Liu, T.; Li, F.; Li, X.; Chen, D.; Wu, Y. Dependence of secondary mineral formation on Fe(II) production from ferrihydrite reduction by Shewanella oneidensis MR-1. ACS Earth Space Chem. 2018, 2, 399–409. [Google Scholar] [CrossRef]
- Poggenburg, C.; Mikutta, R.; Schippers, A.; Dohrmann, R.; Guggenberger, G. Impact of natural organic matter coatings on the microbial reduction of iron oxides. Geochim. Cosmochim. Acta 2018, 224, 223–248. [Google Scholar] [CrossRef]
- Maitte, B.; Jorand, F.P.A.; Grgic, D.; Abdelmoula, M.; Carteret, C. Remineralization of ferrous carbonate from bioreduction of natural goethite in the Lorraine iron ore (Minette) by Shewanella putrefaciens. Chem. Geol. 2015, 412, 48–58. [Google Scholar] [CrossRef]
- Jorand, F.; Zegeye, A.; Ghanbaja, J.; Abdelmoula, M. The formation of green rust induced by tropical river biofilm components. Sci. Total Environ. 2011, 409, 2586–2596. [Google Scholar] [CrossRef][Green Version]
- Crowe, S.A.; O’Neill, A.H.; Weisener, C.G.; Kulczycki, E.; Fowle, D.A.; Roberts, J.A. Reductive dissolution of trace metals from sediments. Geomicrobiol. J. 2007, 24, 157–165. [Google Scholar] [CrossRef]
- Landa, E.R.; Phillips, E.J.P.; Lovley, D.R. Release of 226Ra from uranium mill tailings by microbial Fe(III) reduction. Appl. Geochem. 1991, 6, 647–652. [Google Scholar] [CrossRef]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L.; Phillips, E.J.P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S.M. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Vali, H.; Weiss, B.; Li, Y.-L.; Sears, S.K.; Kim, S.S.; Kirschvink, J.L.; Zhang, C.L. Formation of tabular single-domain magnetite induced by Geobacter metallireducens GS-15. Proc. Nat. Acad. Sci. USA 2004, 101, 16121–16126. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, R.D., Jr.; Sabree, Z.L.; Palumbo, A.V.; Moyer, C.L.; Devol, A.H.; Roh, Y.; Zhou, J. Metal reduction at cold temperatures by Shewanella isolates from various marine environments. Aquat. Microb. Ecol. 2005, 38, 81–91. [Google Scholar] [CrossRef]
- Byrne, J.M.; Telling, N.D.; Coker, V.S.; Pattrick, R.A.D.; van der Laan, G.; Arenholz, E.; Tuna, F.; Lloyd, J.R. Control of nanoparticle size, reactivity and magnetic properties during the bioproduction of magnetite by Geobacter sulfurreducens. Nanotechnology 2011, 22, 455709. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Jimenez-Lopez, C.; Neal, A.L.; Rull-Perez, F.; Rodriguez-Navarro, A.; Fernandez-Vivas, A.; Iañez-Pareja, E. Magnetite biomineralization induced by Shewanella oneidensis. Geochim. Cosmochim. Acta 2010, 74, 967–979. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Valverde-Tercedor, C.; Yebra-Rodriguez, A.; Prozorov, T.; Gonzalez-Muñoz, M.T.; Arias-Peñalver, J.M.; Jimenez-Lopez, C. Chemical purity of Shewanella oneidensis-induced magnetites. Geomicrobiol. J. 2013, 30, 731–748. [Google Scholar] [CrossRef]
- Klueglein, N.; Lösekann-Behrens, T.; Obst, M.; Behrens, S.; Appel, E.; Kappler, A. Magnetite formation by the novel Fe(III)-reducing Geothrix fermentans strain HradG1 isolated from a hydrocarbon-contaminated sediment with increased magnetic susceptibility. Geomicrobiol. J. 2013, 30, 863–873. [Google Scholar] [CrossRef]
- Li, C.; Yi, X.; Dang, Z.; Yu, H.; Zeng, T.; Wei, C.; Feng, C. Fate of Fe and Cd upon microbial reduction of Cd-loaded polyferric flocs by Shewanella oneidensis MR-1. Chemosphere 2016, 144, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Sebae, G.E.; Jung, J.-H.; Jung, D.-H.; Park, C.-S.; Holden, J.F. Pyrodictium delaneyi sp. nov., a hyperthermophilic autotrophic archaeon that reduces Fe(III) oxide and nitrate. Int. J. Syst. Evol. Microbiol. 2016, 66, 3372–3376. [Google Scholar] [CrossRef] [PubMed]
- Markovski, C.; Byrne, J.M.; Lalla, E.; Lozano-Gorrín, A.D.; Klingelhöfer, G.; Rull, F.; Kappler, A.; Hoffmann, T.; Schröder, C. Abiotic versus biotic iron mineral transformation studied by a miniaturized backscattering Mössbauer spectrometer (MIMOS II), X-ray diffraction and Raman spectroscopy. Icarus 2017, 296, 49–58. [Google Scholar] [CrossRef]
- Zachara, J.M.; Kukkadapu, R.K.; Fredrickson, J.K.; Gorby, Y.A.; Smith, S.C. Biomineralization of poorly crystalline Fe(III) oxides by dissimilatory metal reducing bacteria (DMRB). Geomicrobiol. J. 2002, 19, 179–207. [Google Scholar] [CrossRef]
- Parmar, N.; Gorby, Y.A.; Beveridge, T.J.; Ferris, F.G. Formation of green rust and immobilization of nickel in response to bacterial reduction of hydrous ferric oxide. Geomicrobiol. J. 2001, 18, 375–385. [Google Scholar] [CrossRef]
- Revesz, E.; Fortin, D.; Paktunc, D. Reductive dissolution of arsenical ferrihydrite by bacteria. Appl. Geochem. 2016, 66, 129–139. [Google Scholar] [CrossRef]
- Revesz, E.; Fortin, D.; Paktunc, D. Reductive dissolution of scorodite in the presence of Shewanella sp. CN32 and Shewanella sp. ANA-3. Appl. Geochem. 2015, 63, 347–356. [Google Scholar] [CrossRef]
- Zachara, J.M.; Fredrickson, J.K.; Smith, S.C.; Gassman, P.L. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim. Cosmochim. Acta 2001, 65, 75–93. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Kumar, R.; Lee, S.H.; Park, H.S.; Jeon, B.H. Impact of Shewanella oneidensis on heavy metals remobilization under reductive conditions in soil of Guilan Province, Iran. Geosci. J. 2018, 22, 423–432. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Albrecht-Schmitt, T.E. Microbial dissolution and reduction of uranyl crystals by Shewanella oneidensis MR-1. Chem. Geol. 2014, 387, 59–65. [Google Scholar] [CrossRef]
- Um Ano Do Rompimento de Fundão. Available online: https://www.samarco.com/wp-content/uploads/2020/12/Book-Samarco_final_baixa.pdf (accessed on 2 January 2021).
- Morgenstern, N.R.; Vick, S.G.; Viotti, C.B.; Watts, B.D. Fundão Tailings Dam Review Panel—Report on the Immediate Causes of the Failure of Fundão Dam. Available online: http://fundaoinvestigation.com/wp-content/uploads/general/PR/en/FinalReport.pdf (accessed on 2 January 2021).
- Carmo, F.F.; Kamino, L.H.Y.; Tobias Junior, R.; Campos, I.C.; Carmo, F.F.; Silvino, G.; Castro, K.J.S.X.; Mauro, M.L.; Rodrigues, N.U.A.; Miranda, M.P.S.; et al. Fundão tailings dam failures: The environment tragedy of the largest technological disaster of Brazilian mining in global context. Persp. Ecol. Cons. 2017, 15, 145–151. [Google Scholar] [CrossRef]
- Valeriano, C.M.; Neumann, R.; Alkmim, A.R.; Evangelista, H.; Heilbron, M.; Aguiar Neto, C.C.; Souza, G.P. Sm-Nd and Sr isotope fingerprinting of iron mining tailing deposits spilled from the failed SAMARCO Fundão dam 2015 accident at Mariana, SE-Brazil. Appl. Geochem. 2019, 106, 34–44. [Google Scholar] [CrossRef]
- Reis, D.A.; Nascimento, L.P.; Abreu, A.T.; Nalini Júnior, H.A.; Roeser, H.M.P.; Santiago, A.F. Geochemical evaluation of bottom sediments affected by historic mining and the rupture of the Fundão dam, Brazil. Environ. Sci. Pol. Res. 2020, 27, 4365–4375. [Google Scholar] [CrossRef] [PubMed]
- Schettini, C.A.F.; Hatje, V. The suspended sediment and metals load from the Mariana’s tailing dam failure to the coastal sea. Integr. Environ. Assessm. Manag. 2020, 16, 661–668. [Google Scholar] [CrossRef]
- Viana, L.M.S.; Pestana, I.A.; Carvalho, C.E.V.; Salomão, M.S.M.B. Doce River estuary: Geochemical changes following the largest tailing spill in South America. Arch. Environ. Contam. Toxicol. 2020, 79, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.; Oliveira, A.F.; Pacheco, A.A.; Lopes, R.P.; Neves, A.A.; Queiroz, M.E.L.R. Characterization and evaluation of sorption potential of the iron mine waste after Samarco dam disaster in Doce River basin—Brazil. Chemosphere 2018, 209, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, H.M.; Nóbrega, G.N.; Ferreira, T.O.; Almeida, L.S.; Romero, T.B.; Santaella, S.T.; Bernardino, A.F.; Otero, X.L. The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci. Total Environ. 2018, 637–638, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Jardim, F.A.; von Sperling, E.; Jardim, B.F.M.; Almeida, K.C.B. Fatores determinantes das florações de cianobactérias na água do Rio Doce, Minas Gerais, Brasil. Eng. Sanit. Ambient. 2014, 19, 207–218. [Google Scholar] [CrossRef]
- Richard, E.C.; Duarte, H.A., Jr.; Estrada, G.C.D.; Bechtold, J.-P.; Maioli, B.G.; Freitas, A.H.A.; Warner, K.E.; Figueiredo, L.H.M. Influence of Fundão tailings dam breach on water quality in the Doce River watershed. Integr. Environ. Assessm. Manag. 2020, 16, 585–595. [Google Scholar] [CrossRef]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of methane by bacterial extracts. J. Biol. Chem. 1963, 238, 2882–2886. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Ryden, J.C.; Skyers, J.K. Sorption of inorganic phosphate by iron- and aluminum-containing components. Eur. J. Soil Sci. 1981, 32, 365–378. [Google Scholar] [CrossRef]
- Viollier, E.; Inglett, P.W.; Hunter, K.; Roychoudhury, A.N.; Van Cappellen, P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 2000, 15, 785–790. [Google Scholar] [CrossRef]
- Jurelevicius, D.; Alvarez, V.M.; Marques, J.M.; Lima, L.R.F.S.; Dias, F.A.; Seldin, L. Bacterial community response to petroleum hydrocarbon amendments in freshwater, marine, and hypersaline water-containing microcosms. Appl. Environ. Microbiol. 2013, 79, 5927–5935. [Google Scholar] [CrossRef]
- Pitcher, D.G.; Saunders, N.A.; Owen, R.J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Let. Appl. Microbiol. 1989, 8, 151–156. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Tintelnot, M.; Brichta, A.; Morais, J.O. Clay Mineralogy of River Sediments on the Brazilian Coast. In Proceedings of the Berichte der DTTG e.V.—Band 6, Jahrestagung der DTTG, Greifswald, Germany, 3–5 September 1998; Available online: http://www.dttg.ethz.ch/cd_dttg1998/tintelnot/tintelnot.htm (accessed on 23 December 2020).
- Sparks, N.H.C.; Mann, S.; Bazylinski, D.A.; Lovley, D.R.; Jannasch, H.W.; Frankel, R.B. Structure and morphology of magnetite anaerobically-produced by a marine magnetotactic bacterium and a dissimilatory iron-reducing bacterium. Earth Planet. Sci. Let. 1990, 98, 14–22. [Google Scholar] [CrossRef][Green Version]
- Lagroix, F.; Guyodo, Y. A new tool for separating the magnetic mineralogy of complex mineral assemblages from low temperature magnetic behavior. Front. Earth Sci. 2017, 5, 61. [Google Scholar] [CrossRef]
- Dormann, J.L.; Fiorani, D.; Tronc, E. Magnetic relaxation in fine-particle system. Adv. Chem. Phys. 1997, 98, 283. [Google Scholar]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.D.P.; Serna, C.J.; Batlle, X. Surfactant effects in magnetite nanoparticles of controlled size. J. Magn. Magn. Mater. 2007, 316, e756–e759. [Google Scholar] [CrossRef]
- Rondinone, A.J.; Samia, A.C.S.; Zhang, Z.J. Superparamagnetic relaxation and magnetic anisotropy energy distribution in CoFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 1999, 103, 6876. [Google Scholar] [CrossRef]
- Granitzer, P.; Rumpf, K.; Venkatesan, M.; Roca, A.G.; Cabrera, L.; Morales, M.P.; Poelt, P.; Albu, M. Magnetic study of Fe3O4 nanoparticles incorporated within mesoporous silicon. J. Electrochem. Soc. 2010, 157, K145–K151. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J., Jr.; Foner, S. Surface spin disorder in ferrite nanoparticles. J. Appl. Phys. 1997, 81, 5552–5557. [Google Scholar] [CrossRef]
- Muscas, G.; Concas, G.; Cannas, C.; Musinu, A.N.N.A.; Ardu, A.; Orrù, F.; Peddis, D. Magnetic properties of small magnetite nanocrystals. J. Phys. Chem. C 2013, 117, 23378–23384. [Google Scholar] [CrossRef]
- Zysler, R.D.; Fiorani, D.; Testa, A.M. Investigation of magnetic properties of interacting Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2001, 224, 5–11. [Google Scholar] [CrossRef]
- Gittleman, J.I.; Abeles, B.; Bozowski, S. Superparamagnetism and relaxation effects in granular Ni-SiO2 and Ni-Al2O3 films. Phys. Rev. B 1974, 9, 3891. [Google Scholar] [CrossRef]
- Nunes, W.C.; Socolovsky, L.M.; Denardin, J.C.; Cebollada, F.; Brandl, A.L.; Knobel, M. Role of magnetic interparticle coupling on the field dependence of the superparamagnetic relaxation time. Phys. Rev. B 2005, 72, 212413. [Google Scholar] [CrossRef]
- Cullity, B.D. Introduction to Magnetic Materials; Addison-Wesley: Reading, MA, USA, 1972. [Google Scholar]
- Lima, E.; Brandl, A.L.; Arelaro, A.D.; Goya, G.F. Spin disorder and magnetic anisotropy in Fe3O4 nanoparticles. J. Appl. Phys. 2006, 99, 083908. [Google Scholar] [CrossRef]
- Mamiya, H.; Fukumoto, H.; Cuya Huaman, J.L.; Suzuki, K.; Miyamura, H.; Balachandran, J. Estimation of magnetic anisotropy of individual magnetite nanoparticles for magnetic hyperthermia. ACS Nano 2020, 14, 8421–8432. [Google Scholar] [CrossRef] [PubMed]
- Stoner, E.C.; Wohlfarth, E.P. A mechanism of magnetic hysteresis in heterogeneous alloys. IEEE Trans. Magn. 1991, 27, 3475–3518. [Google Scholar] [CrossRef]
- Hadjipanayis, G.; Sellmyer, D.J.; Brandt, B. Rare-earth-rich metallic glasses. I. Magnetic hysteresis. Phys. Rev. B 1981, 23, 3349–3354. [Google Scholar] [CrossRef]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous iron (III) oxide a review. J. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar] [CrossRef]
- Bell, P.E.; Mills, A.L.; Herman, J.S. Biogeochemical conditions favoring magnetite formation during anaerobic iron reduction. Appl. Environ. Microbiol. 1987, 53, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.; Moon, H.-S. Iron reduction by a psychrotolerant Fe(III)-reducing bacterium isolated from ocean sediment. Geosci. J. 2001, 5, 183–190. [Google Scholar] [CrossRef]
- Winklhofer, M.; Petersen, N. Paleomagnetism and magnetic bacteria. In Magnetoreception and Magnetosomes in Bacteria; Schüler, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 255–273. [Google Scholar] [CrossRef]
- Frankel, R.B. Anaerobes pumping iron. Nature 1987, 330, 208. [Google Scholar] [CrossRef]
- Berny, C.; Le Fèvre, R.; Guyot, F.; Blondeau, K.; Guizonne, C.; Rousseau, E.; Bayan, N.; Alphandéry, E. A method for producing highly pure magnetosomes in large quantity for medical applications using Magnetospirillum gryphiswaldense MSR-1 magnetotactic bacteria amplified in minimal growth media. Front. Bioeng. Biotechnol. 2020, 8, 16. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.H.; Yang, H.-M.; Lee, J.W.; Kim, J.-D. Formation pathways of magnetite nanoparticles by coprecipitation method. J. Phys. Chem. 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Yan, W.; Zhou, J.; Liu, H.; Chen, H.; Zhang, Y.; Wei, Y. Formation of goethite and magnetite rust via reaction with Fe(II). J. Electrochem. Soc. 2016, 163, C289–C295. [Google Scholar] [CrossRef]
- Giongo, A.; Borges, L.G.A.; Marconatto, L.; Palhano, P.L.; Serbent, M.P.; Moreira-Silva, E.; Siqueira, T.A.; Martinho, C.T.; Barili, R.; Paz, L.V.; et al. Adaption of microbial communities to the hostile environment in the Doce River after the collapse of two iron ore tailing dams. Heliyon 2020, 6, e04778. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.P.; Suhadolnik, M.L.S.; Dias, M.F.; Ávila, M.P.; Motta, A.M.; Barbosa, F.A.R.; Nascimento, A.M.A. Characterizing a riverine microbiome impacted by extreme disturbance caused by a mining sludge tsunami. Chemosphere 2020, 253, 126584. [Google Scholar] [CrossRef]
- Postma, D. Formation of siderite and vivianite and the pore-water composition of a recent bog sediment in Denmark. Chem. Geol. 1981, 31, 225–244. [Google Scholar] [CrossRef]
- Aller, R.C.; Mackin, J.E.; Cox, R.T. Diagenesis of Fe and S in Amazon inner shelf muds: Apparent dominance of Fe reduction and implications for the genesis of ironstones. Cont. Shelf Res. 1986, 6, 263–289. [Google Scholar] [CrossRef]
- Karlin, R.; Lyle, M.; Heath, G. Authigenic magnetite formation in suboxic marine sediments. Nature 1987, 326, 490–493. [Google Scholar] [CrossRef]
- Blöthe, M.; Roden, E.E. Microbial iron redox cycling in a circumneutral-pH groundwater seep. Appl. Environ. Microbiol. 2009, 75, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Vuillemin, A.; Friese, A.; Wirth, R.; Schuessler, J.A.; Schleicher, A.M.; Kemnitz, H.; Lücke, A.; Bauer, K.W.; Nomosatryo, S.; von Blanckenburg, F.; et al. Vivianite formation in ferruginous sediments from Lake Towuti, Indonesia. Biogeosciences 2020, 17, 1955–1973. [Google Scholar] [CrossRef]
- Stumm, W.; Sulzberger, B. The cycling of iron in natural environments: Considerations based on laboratory studies of heterogeneous redox processes. Geochim. Cosmochim. Acta 1992, 56, 3233–3257. [Google Scholar] [CrossRef]
- Lynch, S.F.L.; Batty, L.C.; Byrne, P. Environmental risk of metal mining contaminated river bank sediment at redox-transitional zones. Minerals 2014, 4, 52–73. [Google Scholar] [CrossRef]
- Fortin, D.; Leppard, G.G.; Tessier, A. Characteristics of lacustrine diagenetic iron oxyhydroxides. Geochim. Cosmochim. Acta 1993, 57, 4391–4404. [Google Scholar] [CrossRef]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Schmidt, C.; Kappler, A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Emerson, D.; Revsbech, N.P. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: Field studies. Appl. Environ. Microbiol. 1994, 60, 4022–4031. [Google Scholar] [CrossRef]
- Dekov, V.M.; Vanlierde, E.; Billström, K.; Garbe-Schönberg, C.-D.; Weiss, D.J.; Gatto Rotondo, G.; Van Meel, K.; Kuzmann, E.; Fortin, D.; Darchuk, L.; et al. Ferrihydrite precipitation in groundwater-fed river systems (Nete and Demer river basins, Belgium): Insights from a combined Fe-Zn-Sr-Nd-Pb-isotope study. Chem. Geol. 2014, 386, 1–15. [Google Scholar] [CrossRef]
- Johnston, S.G.; Rose, A.L.; Burton, E.D.; Webster-Brown, J. Landslide-induced iron mobilisation shapes benthic accumulation of nutrients, trace metals and REE fractionation in an oligotrophic alpine stream. Geochim. Cosmochim. Acta 2015, 148, 1–22. [Google Scholar] [CrossRef]
- Posth, N.R.; Canfield, D.E.; Kappler, A. Biogenic Fe(III) minerals: From formation to diagenesis and preservation in the rock record. Earth-Sci. Rev. 2014, 135, 103–121. [Google Scholar] [CrossRef]
- Baken, S.; Salaets, P.; Desmet, N.; Seuntjens, P.; Vanlierde, E.; Smolders, E. Oxidation of iron causes removal of phosphorus and arsenic from streamwater in groundwater-fed lowland catchments. Environ. Sci. Technol. 2015, 49, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- De Vitri, R.; Belzile, N.; Tessier, A. Speciation and adsorption of arsenic on diagenetic iron oxyhydroxides. Limnol. Oceanogr. 1991, 36, 1480–1485. [Google Scholar] [CrossRef]
- Lienemann, C.-P.; Monnerat, M.; Dominik, J.; Perret, D. Identification of stoichiometric iron-phosphorus colloids produced in a eutrophic lake. Aquat. Sci. 1999, 61, 133–149. [Google Scholar] [CrossRef]
- Gounot, A.M. Microbial oxidation and reduction of manganese: Consequences in groundwater and applications. FEMS Microbiol. Rev. 1994, 14, 339–350. [Google Scholar] [CrossRef]
- Fraga, M.S.; Reis, G.B.; Silva, D.D.; Guedes, H.A.S.; Elesbon, A.A.A. Use of multivariate statistical methods to analyze the monitoring of surface water quality in the Doce River basin, Minas Gerais, Brazil. Environ. Sci. Pol. Res. 2020, 27, 35303–35318. [Google Scholar] [CrossRef] [PubMed]
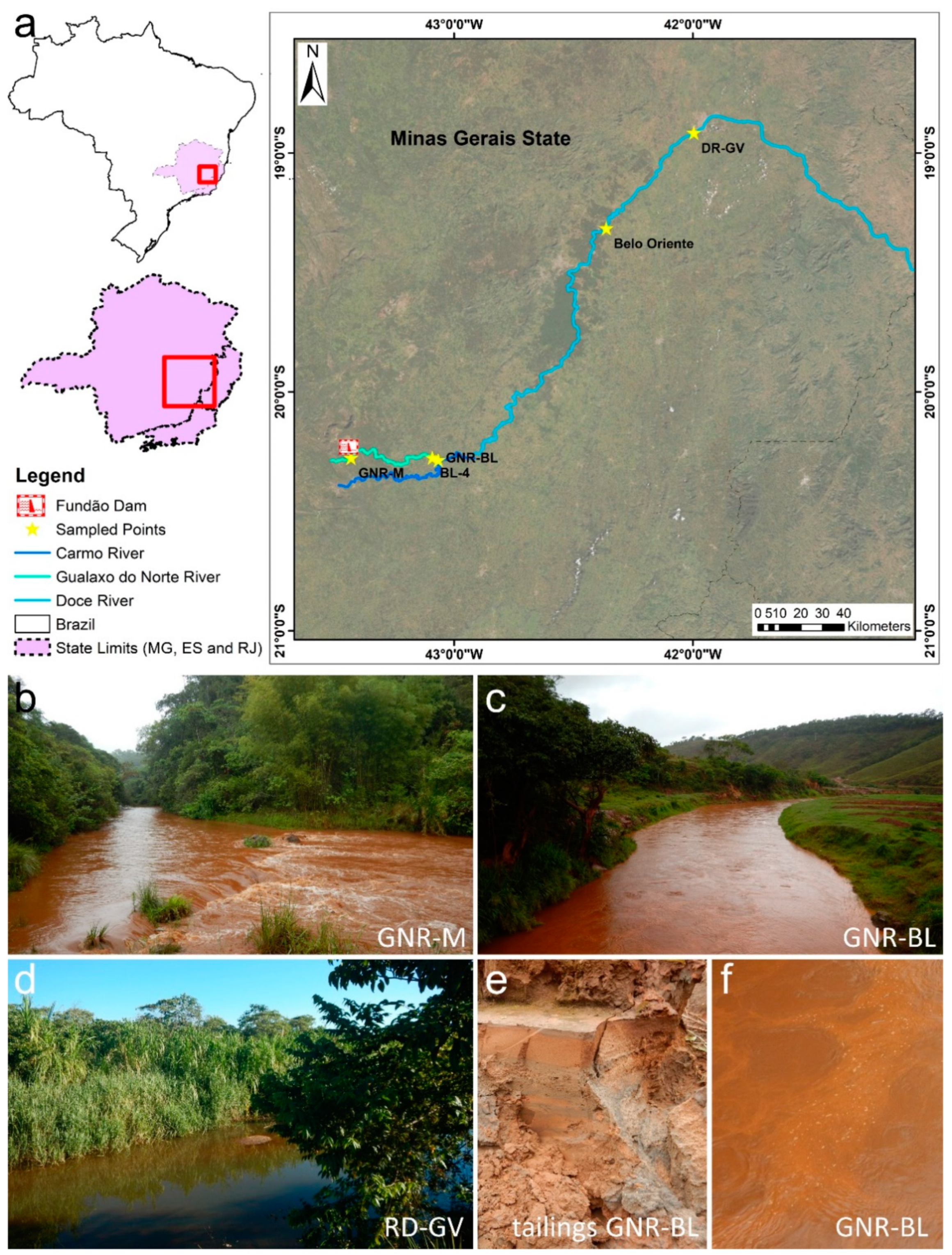


| Length of the Experiment | Fe(III) Source | Presence of Tailings in the Inoculum | Replicates | Code * | Appearance of the Solids at the End of Incubation | Analytical Methods | |
|---|---|---|---|---|---|---|---|
| Color | Interaction with Magnets | ||||||
| Short term (0, 7, 14, 21, and 28 days) | Synthetic | − | 6 | GNR-M | Rust | Weak | Fe(II) quantification in the liquid phase using the ferrozine assay |
| ++ | 6 | GNR-BL | Rust | Weak | |||
| + | 6 | DR-GV | Rust | Weak | |||
| Tailings | − | 6 | GNR-M | Gray | Weak | ||
| ++ | 6 | GNR-BL | Gray | Weak | |||
| + | 6 | DR-GV | Gray | Weak | |||
| Long term (170 days) | Synthetic | − | A | sGNR-M-A | Rust | Weak | DGGE |
| B | (s)GNR-M-B | Black | Strong | DGGE, XRD, TEM, SQUID | |||
| C | (s)GNR-M-C | Black | Strong | ||||
| ++ | A | (s)GNR-BL-A | Black | Strong | |||
| B | sGNR-BL-B | Rust | Weak | DGGE | |||
| C | sGNR-BL-C | Rust | Weak | ||||
| Tailings | − | 3 | tGNR-M | Gray | Weak | DGGE | |
| ++ | 3 | tGNR-BL | Gray | Weak | |||
| Wt % Rietveld | ||
|---|---|---|
| Phase Name | GNR-M-B * | GNR-BL-A * |
| Magnetite | 52.9 | 57.1 |
| Goethite | 7.1 | 6.1 |
| Hematite | 2.1 | 2.6 |
| Quartz | 16.3 | 24.8 |
| Muscovite | 8.4 | 7.5 |
| Kaolinite | 0.9 | 1.9 |
| Illite | 12.0 | 0.0 |
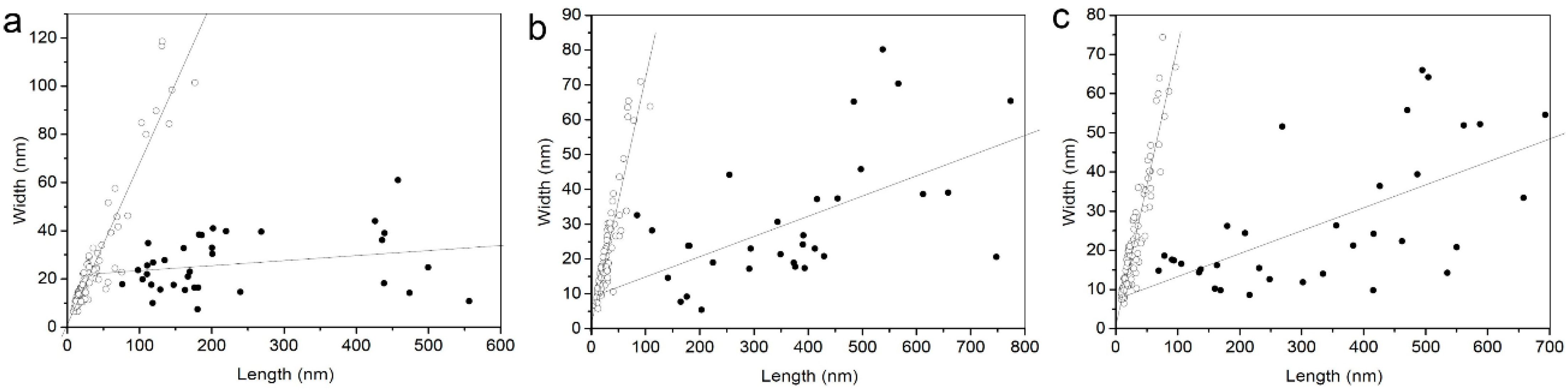
| Crystal Shape | Sample | Length (nm) (Average ± SD) | Length Range (nm) | Width (nm) (Average ± SD) | Width Range (nm) | Width/Length | N |
|---|---|---|---|---|---|---|---|
| Isotropic (octahedral, prismatic, or rounded) | GNR-M-B * | 35 ± 34 | 9–177 | 24 ± 24 | 7–119 | 0.74 ± 0.17 | 100 |
| GNR-M-C * | 28 ± 18 | 7–108 | 21 ± 14 | 7–71 | 0.78 ± 0.15 | 100 | |
| GNR-BL-A * | 29 ± 19 | 9–96 | 22 ± 14 | 8–74 | 0.76 ± 0.16 | 112 | |
| Elongated (lath shaped) | GNR-M-B * | 229 ± 139 | 76–556 | 26 ± 12 | 18–61 | 0.14 ± 0.07 | 35 |
| GNR-M-C * | 371 ± 181 | 84–774 | 31 ± 19 | 33–80 | 0.10 ± 0.07 | 31 | |
| GNR-BL-A * | 329 ± 184 | 69–693 | 26 ± 17 | 15–64 | 0.10 ± 0.06 | 34 |
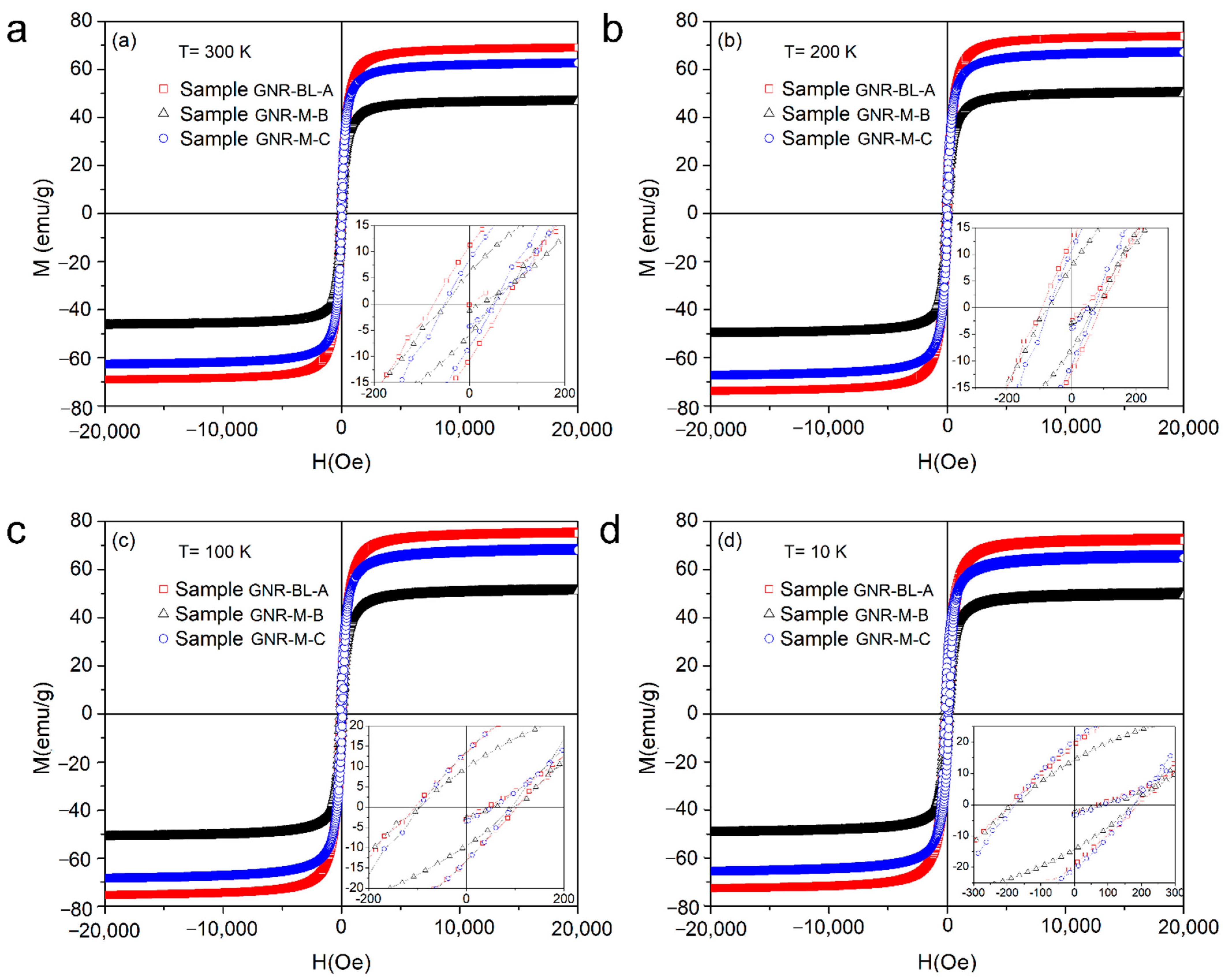
| Sample | T (K) | Hc (Oe) | Mr (emu/g) | Ms (emu/g) | Hcr (Oe) |
|---|---|---|---|---|---|
| GNR-BL-A * | 300 | 75 (10) | 11.2 | 69 | 160 (10) |
| 200 | 91 (10) | 12.6 | 74 | 200 (10) | |
| 100 | 105 (10 | 13.3 | 75 | 204 (10) | |
| 10 | 190 (10) | 19.7 | 73 | 420 (10) | |
| GNR-M-B * | 300 | 50 (10) | 5.9 | 46 | 115 (10) |
| 200 | 73 (10) | 7.7 | 50 | 140 (10) | |
| 100 | 96 (10) | 9.5 | 51 | 180 (10) | |
| 10 | 180 (10 | 14.3 | 50 | 320 (10) | |
| GNR-M-C * | 300 | 33 (10) | 8.3 | 62 | 100 (10) |
| 200 | 53 (10 | 10.7 | 67 | 140 (10) | |
| 100 | 71 (10) | 13.6 | 69 | 183 (10) | |
| 10 | 177 (10) | 20.5 | 66 | 370 (10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keim, C.N.; Serna, J.D.P.; Acosta-Avalos, D.; Neumann, R.; Silva, A.S.; Jurelevicius, D.A.; Pereira, R.S.; de Souza, P.M.; Seldin, L.; Farina, M. Dissimilatory Iron-Reducing Microorganisms Are Present and Active in the Sediments of the Doce River and Tributaries Impacted by Iron Mine Tailings from the Collapsed Fundão Dam (Mariana, MG, Brazil). Minerals 2021, 11, 244. https://doi.org/10.3390/min11030244
Keim CN, Serna JDP, Acosta-Avalos D, Neumann R, Silva AS, Jurelevicius DA, Pereira RS, de Souza PM, Seldin L, Farina M. Dissimilatory Iron-Reducing Microorganisms Are Present and Active in the Sediments of the Doce River and Tributaries Impacted by Iron Mine Tailings from the Collapsed Fundão Dam (Mariana, MG, Brazil). Minerals. 2021; 11(3):244. https://doi.org/10.3390/min11030244
Chicago/Turabian StyleKeim, Carolina N., Jilder D. P. Serna, Daniel Acosta-Avalos, Reiner Neumann, Alex S. Silva, Diogo A. Jurelevicius, Raphael S. Pereira, Pamella M. de Souza, Lucy Seldin, and Marcos Farina. 2021. "Dissimilatory Iron-Reducing Microorganisms Are Present and Active in the Sediments of the Doce River and Tributaries Impacted by Iron Mine Tailings from the Collapsed Fundão Dam (Mariana, MG, Brazil)" Minerals 11, no. 3: 244. https://doi.org/10.3390/min11030244
APA StyleKeim, C. N., Serna, J. D. P., Acosta-Avalos, D., Neumann, R., Silva, A. S., Jurelevicius, D. A., Pereira, R. S., de Souza, P. M., Seldin, L., & Farina, M. (2021). Dissimilatory Iron-Reducing Microorganisms Are Present and Active in the Sediments of the Doce River and Tributaries Impacted by Iron Mine Tailings from the Collapsed Fundão Dam (Mariana, MG, Brazil). Minerals, 11(3), 244. https://doi.org/10.3390/min11030244







