High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland
Abstract
:1. Introduction
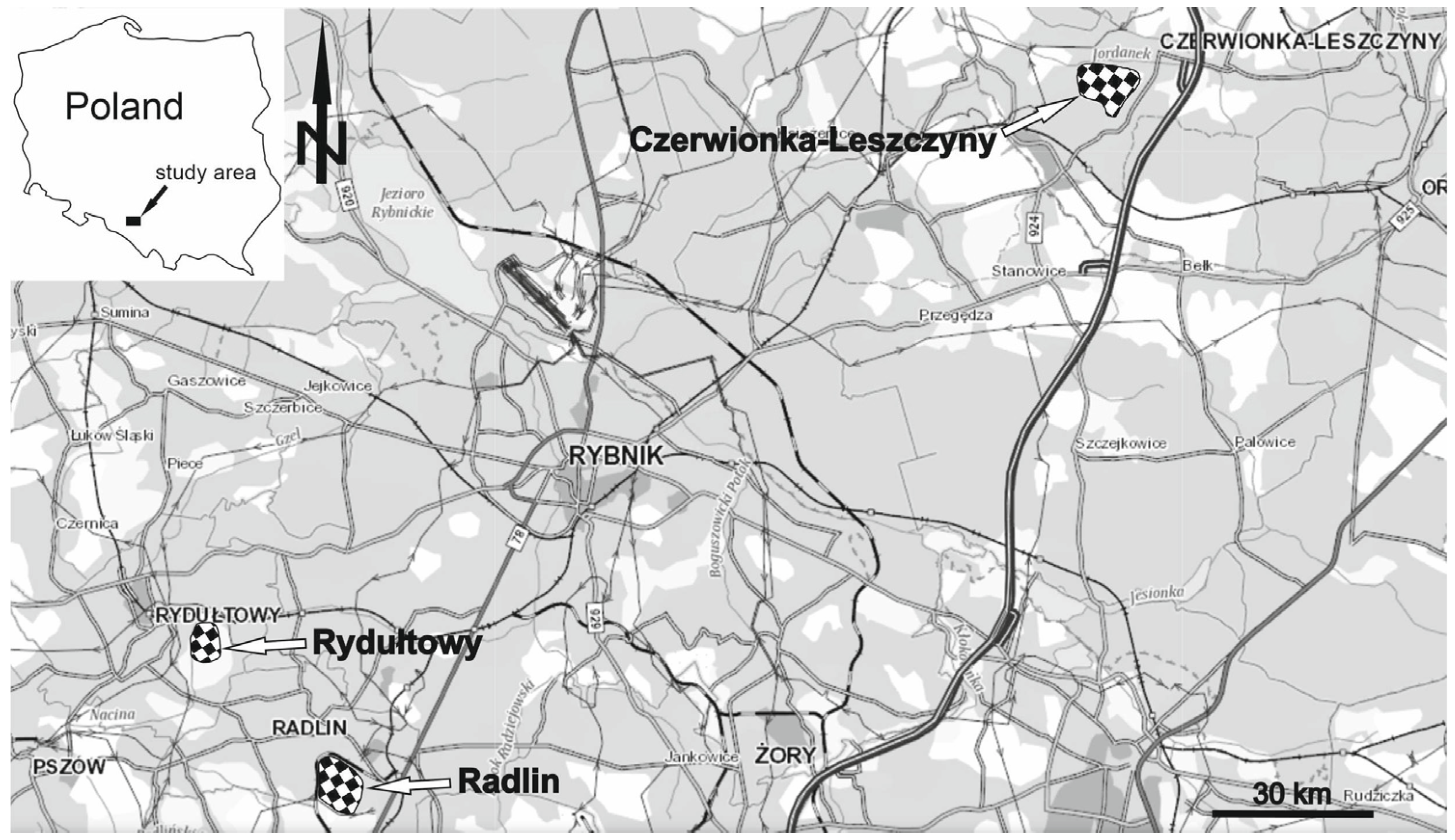
2. Materials and Methods
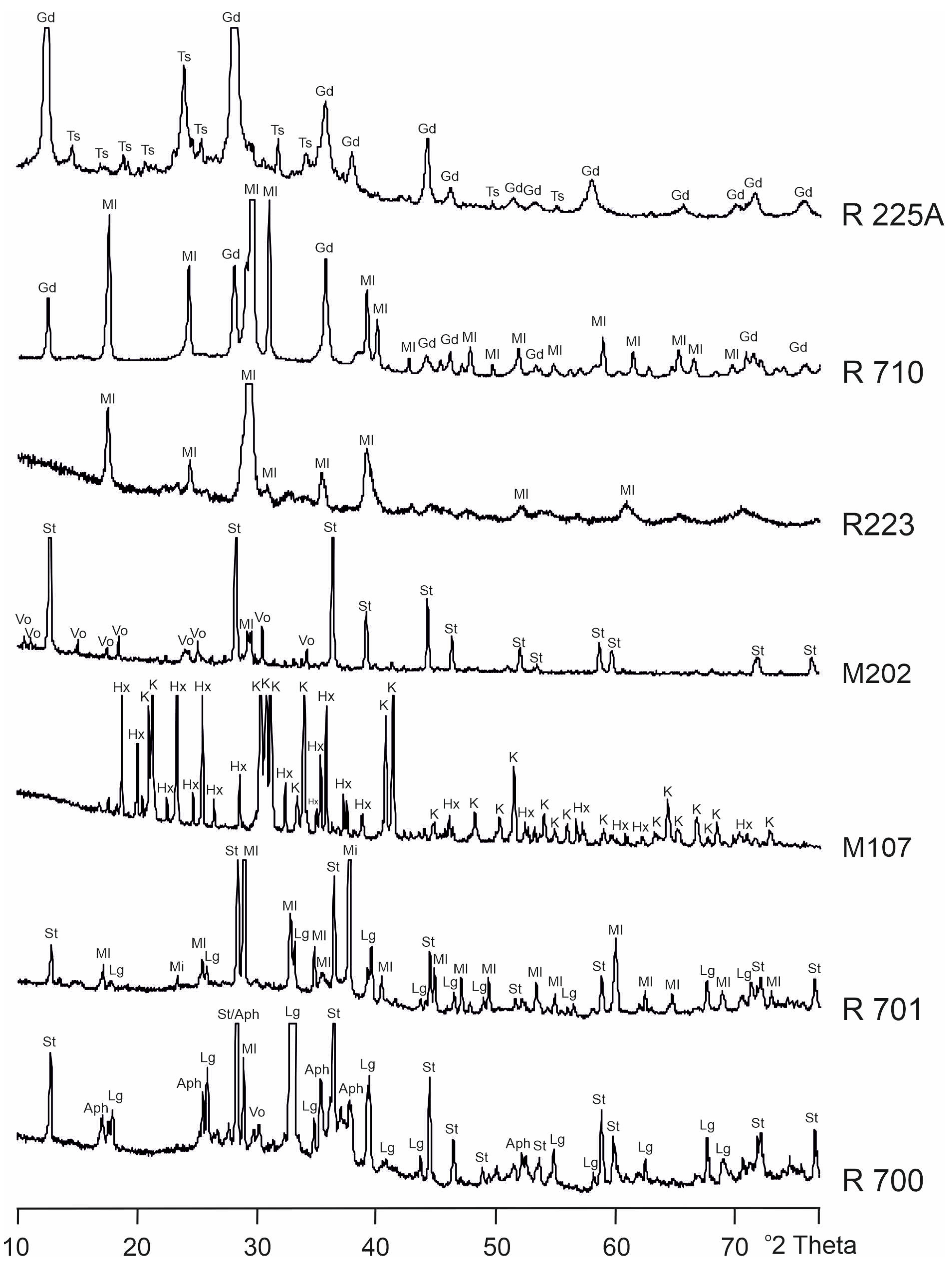
3. Anhydrous Sulfate Minerals from the Burning Coal-Dumps
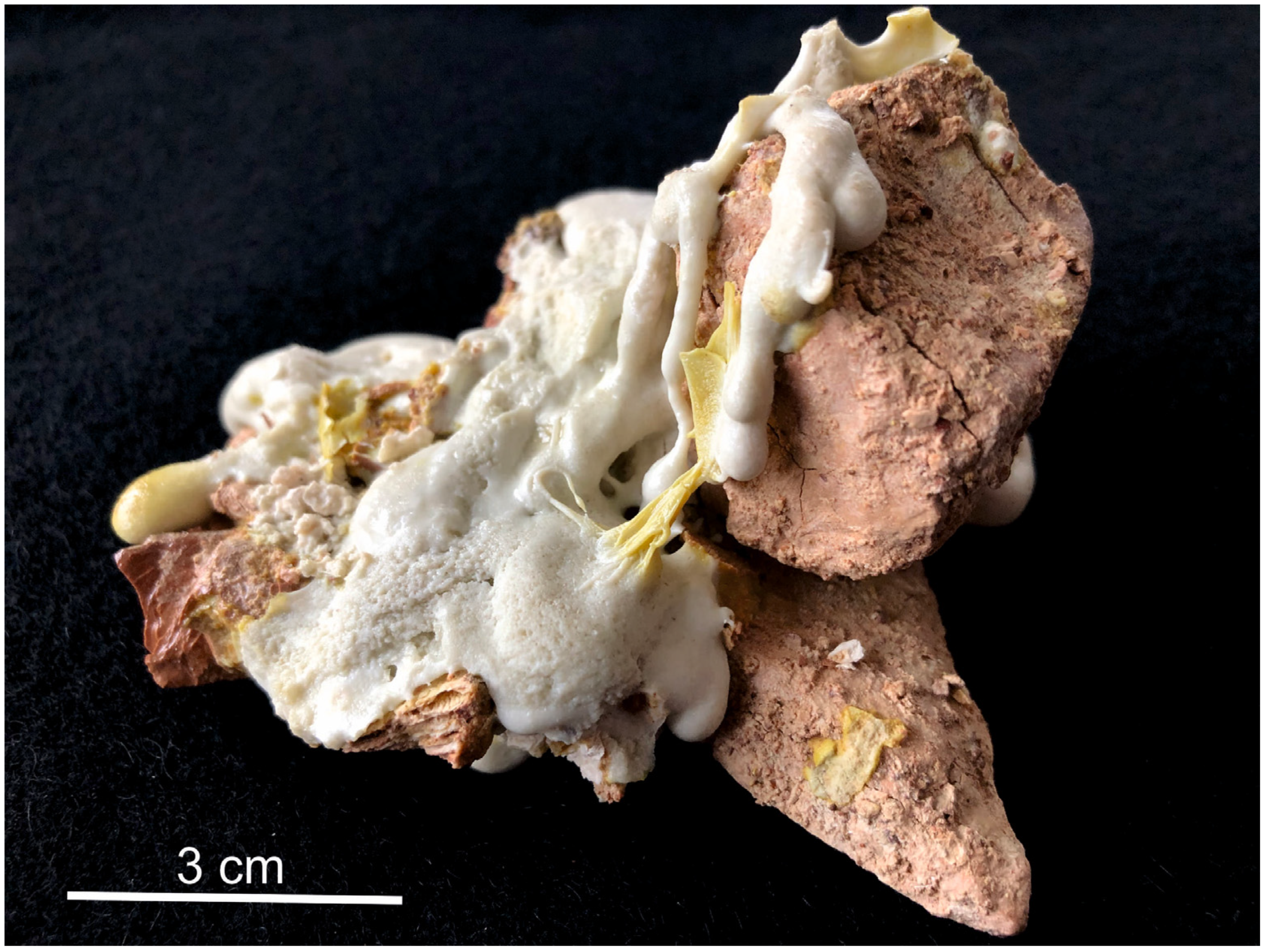
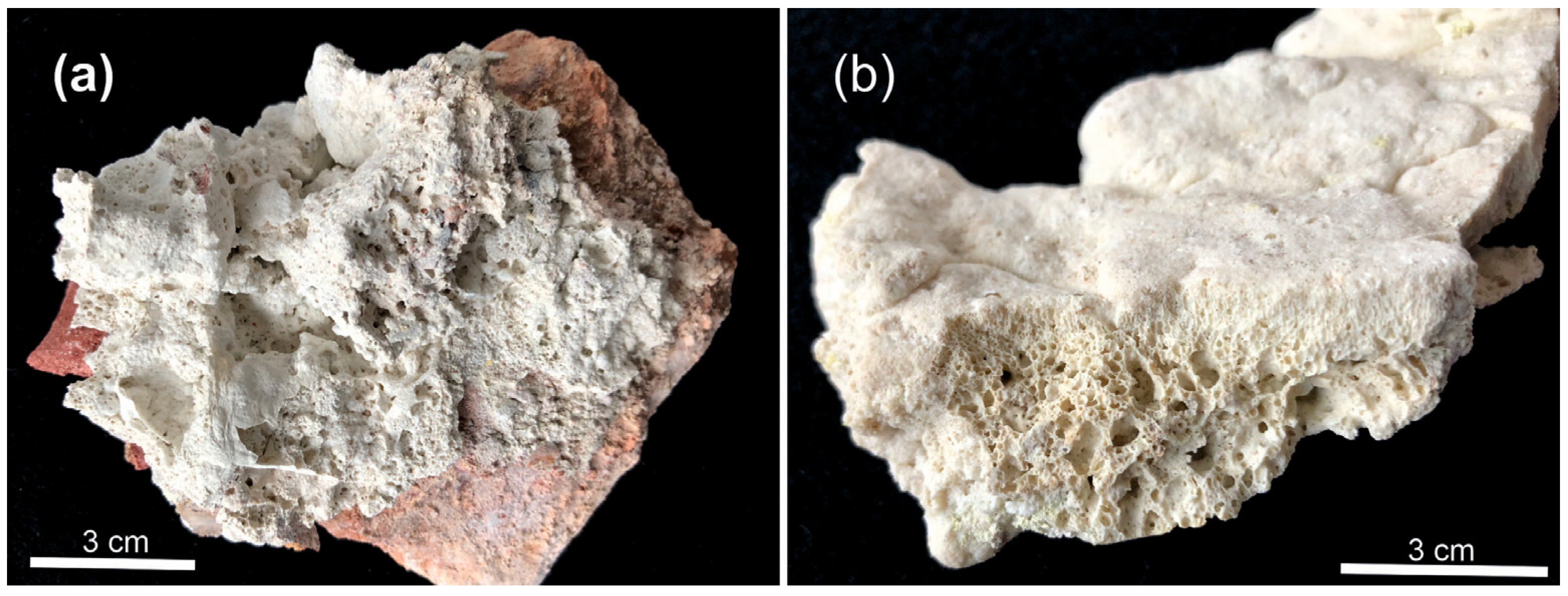
3.1. Godovikovite NH4Al(SO4)2
3.2. Millosevichite Al2(SO4)3
3.3. Steklite KAl(SO4)2
3.4. Unnamed Anhydrous Magnesium Sulfate MgSO4
3.5. Other Anhydrous Sulfates
4. Conditions for the Crystallization of Assemblage of High Temperature Sulfates
5. A Separate Genetic Type of Sulfate Minerals
Author Contributions
Funding
Conflicts of Interest
References
- Stracher, G.B.; Prakash, A.; Sokol, E.V. Volume 3: Case Studies—Coal Fires. In Coal and Peat Fires: A Global Perspective; Elsevier Science: Amsterdam, The Netherlands, 2014; 816p. [Google Scholar]
- Parafiniuk, J.; Hatert, F. New IMA CNMNC guidelines on combustion products from burning coal dumps. Eur. J. Mineral. 2020, 32, 215–217. [Google Scholar] [CrossRef] [Green Version]
- Shcherbakova, Y.P.; Bazhenova, L.F.; Chesnokov, B.V. Godovikovite–NH4(Al,Fe)(SO4)2, a new ammonium-bearing sulfate. Zap. Vsesoyuznogo Mineral. Obs. 1988, 117, 208–211. (In Russian) [Google Scholar]
- Žáček, V.; Škoda, R.; Laufek, F.; Košek, F.; Jehlička, J. Complementing knowledge about rare sulphates lonecreekite, NH4Fe3+(SO4)2·12H2O and sabieite NH4Fe3+(SO4)2: Chemical composition, XRD and RAMAN spectroscopy (Libušin near Kladno, the Czech Republic). J. Geosci. 2019, 64, 149–159. [Google Scholar] [CrossRef]
- Žáček, V.; Ondruš, P. Mineralogy of recently formed sublimates from Kateřina colliery in Radvanice, Eastern Bohemia, Czech Republic. Věstník Českého Geol. Ust. 1997, 72, 289–302. [Google Scholar]
- Sejkora, J.; Kotrlý, M. Sulfáty vysokoteplotní oxidační minerální asociace; hořící odval dolu Kateřina v Radvanicích v Čechách. Bull. Mineral. Petrogr. Oddělení Národního Muz. Praze 2001, 9, 261–267. (In Czech) [Google Scholar]
- Sindern, S.; Warnsloh, J.; Witzke, T.; Havenith, V.; Neef, R.; Etoundi, Y. Mineralogy and geochemistry of vents formed on the burning coal mining waste dump Anna I, Alsdorf, Germany. Eur. J. Mineral. 2005, 17, 130. [Google Scholar]
- Szakáll, S.; Kristály, F. Ammonium sulphates from burning coal dumps at Komló and Pécs-Vasas, Mecsek Mts., South Hungary. Mineral. Spec. Pap. 2008, 32, 154. [Google Scholar]
- Stracher, G.B.; Prakash, A.; Schroeder, P.; McCormack, J.; Zhang, X.; Van Dijk, P.; Blake, D. New mineral occurrences and mineralization processes: Wuda coal-fire gas vents of Inner Mongolia. Am. Mineral. 2005, 90, 1729–1739. [Google Scholar] [CrossRef]
- Belakovski, D. The minerals of the burning coal seams of Ravat in Tajikistan. Lapis 1990, 15, 21–26. (In German) [Google Scholar]
- Parafiniuk, J.; Kruszewski, Ł. Ammonium minerals from burning coal-dumps of the Upper Silesian Coal Basin (Poland). Geol. Q. 2009, 53, 341–356. [Google Scholar]
- Panichi, U. Millosevichite, Nuovo Minerale del Faraglione di Levante nell’Isola di Vulcano; Accademia Nazionale del Lincei, Classe di Scienze Fische, Matematiche e Naturali: Roma, Italy, 1913; Volume 22, p. 303. [Google Scholar]
- Lapham, M.D.; Barnes, J.H.; Downey, W.F., Jr.; Finkelman, R.B. Mineralogy Associated with Burning Anthracite Deposits of Eastern Pennsylvania; Mineral Resource Report 78; Pennsylvania Topographic and Geologic Survey: Harrisburg, PA, USA, 1980; pp. 1–788.
- Chesnokov, B.V.; Bazhenova, L.F.; Bushmakin, A.F. New minerals from burnt dumps of the Chelyabinsk coal basin (the 7th Report). Ural. Mineral. Sb. 1995, 4, 3–28. (In Russian) [Google Scholar]
- Pekov, I.; Krivovichev, S.; Chernyatyeva, A.; Yapaskurt, V.; Zadov, A.; Zelensky, M. Steklite, KAl(SO4)2: A finding at the Tolbachik Volcano, Kamchatka, Russia, validating its status as a mineral species and crystal structure. Geol. Ore Depos. 2013, 55, 594–600. [Google Scholar] [CrossRef]
- Košek, F.; Culka, A.; Jehlička, J. Raman spectroscopic study of six synthetic anhydrous sulfates relevant to the mineralogy of fumaroles. J. Raman Spectrosc. 2018, 49, 1205–1216. [Google Scholar] [CrossRef]
- Cesnokov, B.; Kotrly, M.; Nisanbajev, T. Brennende Abraumhalden und Aufschlüsse im Tscheljabinsker Kohlenbecken—Eine reiche Mineralienküche. Miner. Welt 1998, 9, 54–63. (In German) [Google Scholar]
- Van Essen, V.M.; Zondag, H.A.; Gores, J.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; Van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J. Sol. Energy Eng. 2009, 131, 041014. [Google Scholar] [CrossRef]
- Hršelová, P.; Cempírek, J.; Houzar, S.; Sejkora, J. S,F,Cl-rich mineral assemblages from burned spoil heaps in the Rosice-Oslovany Coalfield, Czech Republic. Can. Mineral. 2013, 51, 171–188. [Google Scholar] [CrossRef]
- Martini, J.E.J. Lonecreekite, sabieite and claraite, new secondary ammonium ferric-iron sulphates from Lone Creek Fall Cave, near Sabie, Eastern Transvaal. Ann. Geol. Surv. S. Afr. 1983, 17, 29–34. [Google Scholar]
- Witzke, T.; de Wit, F.; Kolitsch, U.; Blaß, G. Mineralogy of the Burning Anna I Coal Mine Dump, Alsdorf, Germany. In Coal and Peat Fires: A Global Perspective, 1st ed.; Stracher, G.B., Prakash, A., Sokol, E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 203–240. [Google Scholar] [CrossRef]
- Kampf, A.; Richards, R.; Nash, B. The 2H and 3R polytypes of sabieite, NH4Fe3+(SO4)2 from a natural fire in an oil-bearing shale near Milan, Ohio. Am. Mineral. 2014, 99, 1500–1506. [Google Scholar] [CrossRef]
- Shcherbakova, Y.P.; Bazhenova, L.F. Efremovite (NH4)2Mg2(SO4)3—ammonium analogue of langbeinite—A new mineral. Zap. Vsesoyuznogo Mineral. Obs. 1989, 118, 84–87. (In Russian) [Google Scholar]
- Binnewies, M.; Glaum, R.; Schmidt, M.; Schmidt, P. Chemical Vapor Transport Reactions; Walter de Gruyter: Berlin, Germany; GmbH and Co. KG: Boston, MA, USA, 2012; 614p. [Google Scholar]
- López-Beceiro, J.; Pascual-Cosp, J.; Artiaga, R.; Tarrío-Saavedra, J.; Salvador, N. Thermal characterization of ammonium alum. J. Therm. Anal. Calorim. 2011, 104, 127–130. [Google Scholar] [CrossRef]
- Miura, H.; Niida, K.; Hirama, T. Mikasaite, (Fe3+,Al)2(SO4)3, a new ferric sulphate mineral from Mikasa city, Hokkaido, Japan. Mineral. Mag. 1994, 58, 649–653. [Google Scholar] [CrossRef]
- Ventruti, G.; Ventura, G.D.; Gomez, M.A.; Capitani, G.; Sbroscia, M.; Sodo, A. High-temperature study of basic ferric sulfate, FeOHSO4. Phys. Chem. Miner. 2020, 47, 43. [Google Scholar] [CrossRef]
- Africano, F.; Van Rompaey, G.; Bernard, A.; Le Guern, F. Deposition of trace elements from high temperature gases of Satsuma-Iwojima volcano. Earth Planets Space 2002, 54, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Hansteen, T.H.; Burke, F.A. Aphthitalite in high temperature fluid inclusion in quartz from the Eikeren-Skrim granite complex, the Oslo paleorift. Nor. Geol. Tidsskr. 1994, 74, 238–240. [Google Scholar]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Kruszewski, Ł.; Fabiańska, M.; Ciesielczuk, J.; Segit, T.; Orłowski, R.; Motyliński, R.; Kusy, D.; Moszumańska, I. First multi-tool exploration of a gas-condensate-pyrolisate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials. Sci. Total Environ. 2018, 640–641, 1044–1071. [Google Scholar] [CrossRef] [PubMed]



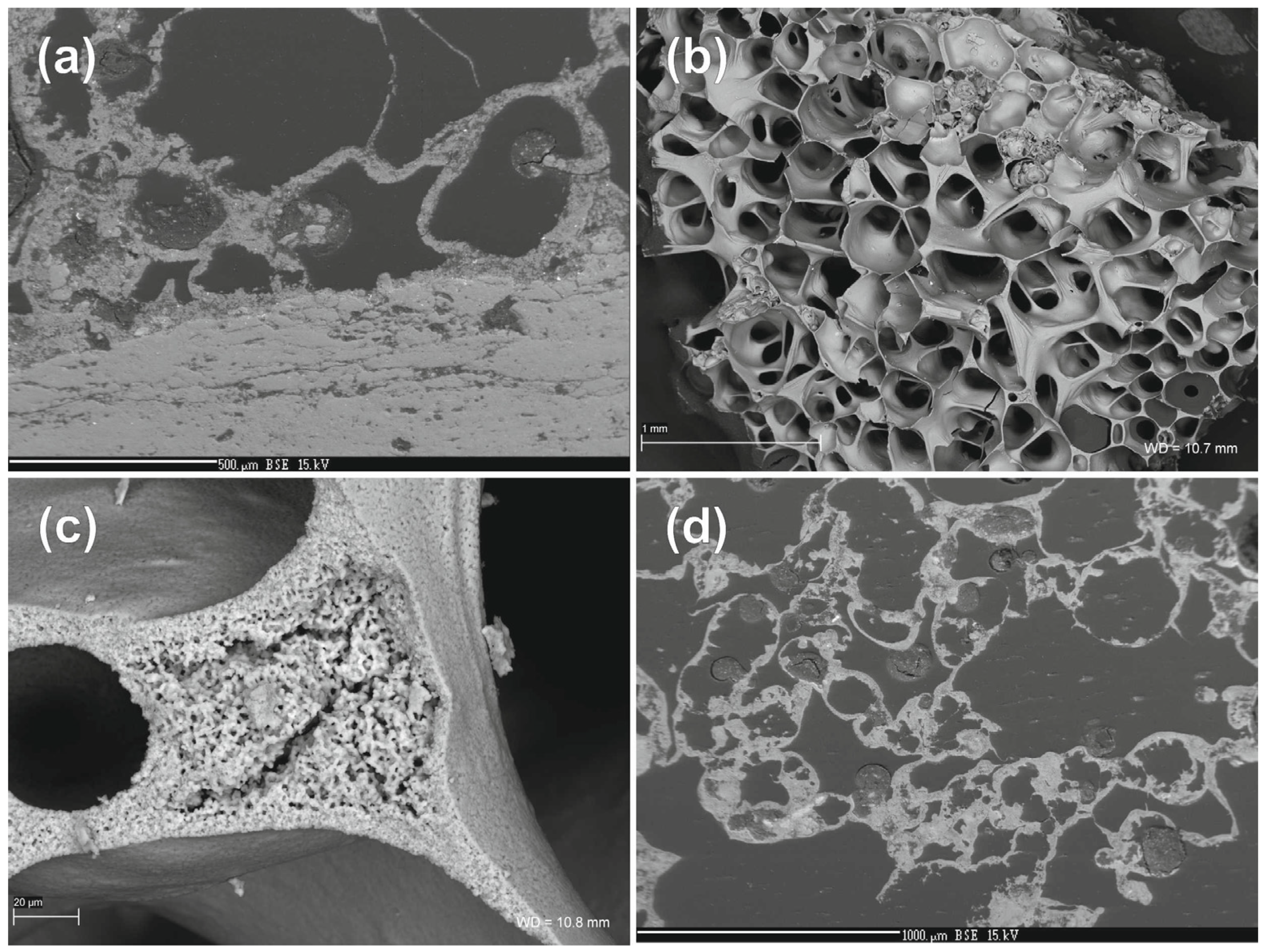
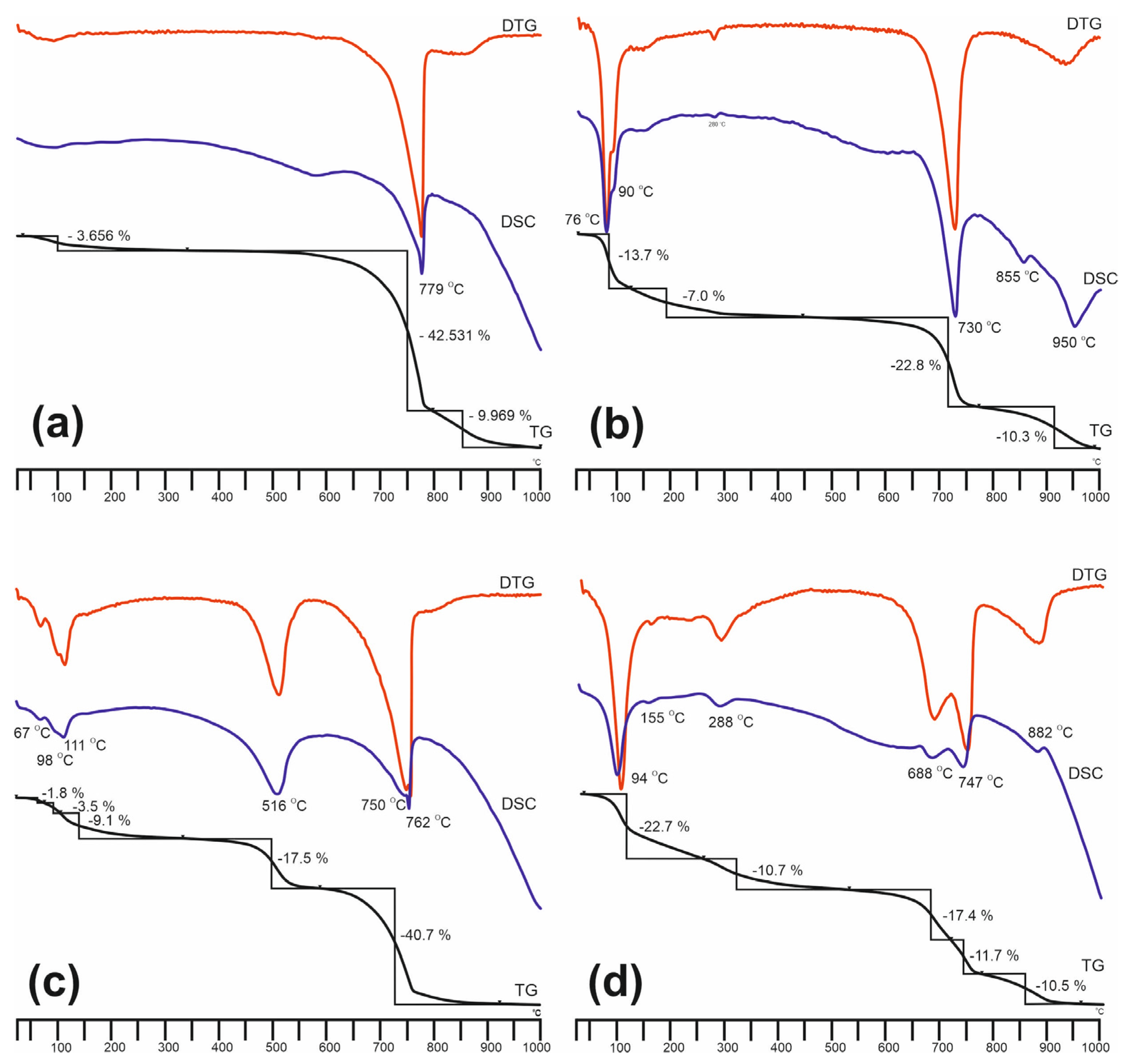
| Sample Type | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | SO3 | P2O5 | SiO2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Godovikovite with traces of millosevichite (sample 225A) | 22.59 | 4.69 | 1.55 | 0.36 | 0.25 | 1.94 | 0.46 | 56.26 | 0.15 | 3.74 | 92.00 * |
| 24.95 | 4.63 | 1.61 | 0.46 | 0.25 | 0.99 | 0,55 | 53.33 | 0.23 | 3.93 | 90.92 * | |
| 20.87 | 5.98 | 1.27 | 1.33 | 0.22 | 1.53 | 0,55 | 57.20 | 0.19 | 0.72 | 89.84 * | |
| 21.71 | 6.11 | 1.35 | 0.43 | 0.22 | 1.04 | 0.53 | 55.11 | 0.16 | 0.28 | 86.93 * | |
| 23.72 | 4.46 | 1.02 | 0.41 | 0.19 | 0.94 | 0.58 | 54.61 | 0.20 | 0.36 | 86.50 * | |
| 23.27 | 5.74 | 1.47 | 0.40 | 0.20 | 0.95 | 0.53 | 58.68 | 0.18 | 0.54 | 91.96 * | |
| Millosevichite with MgSO4 (sample R743) | 29.01 | 1.09 | 4.21 | 0.49 | 1.40 | 0.29 | 0.21 | 60.69 | 0.34 | 0.10 | 97.83 |
| 29.06 | 1.19 | 4.33 | 0.23 | 0.97 | 0.27 | 0.19 | 63.02 | 0.35 | 0.38 | 99.99 | |
| 30.75 | 1.14 | 5.00 | 0.24 | 1.52 | 0.34 | 0.18 | 57.73 | 0.39 | 0.01 | 97.29 | |
| 28.84 | 1.13 | 4.44 | 0.29 | 0.85 | 0.22 | 0.20 | 63.25 | 0.34 | 0.05 | 99.59 | |
| 28.23 | 1.90 | 4.29 | 0.26 | 1.33 | 0.28 | 0.20 | 61.59 | 0.34 | 0.24 | 98.67 | |
| 27.98 | 1.28 | 4.14 | 0.21 | 0.61 | 0.26 | 0.20 | 61.84 | 0.37 | 3.58 | 100.49 | |
| 30.12 | 1.22 | 4.46 | 0.22 | 0.69 | 0.19 | 0.19 | 60.60 | 0.35 | 0.15 | 98.19 | |
| 23.94 | 3.33 | 4.99 | 0.71 | 1.83 | 2.02 | 0.20 | 54.96 | 0.41 | 3.85 | 96.26 | |
| 23.14 | 7.48 | 3.65 | 0.56 | 1.09 | 0.73 | 0.33 | 58.07 | 0.21 | 0.15 | 95.42 | |
| Steklite (sample R741) | 14.22 | 4.14 | 1.95 | 2.69 | 1.31 | 11.29 | 0.00 | 48.49 | 0.31 | 0.55 | 84.96 |
| 20.33 | 4.79 | 2.64 | 2.32 | 0.76 | 9.50 | 0.00 | 57.78 | 0.39 | 0.39 | 98.91 | |
| 19.71 | 3.70 | 1.92 | 4.17 | 0.89 | 9.07 | 0.00 | 53.05 | 0.32 | 0.71 | 93.54 | |
| 16.77 | 4.32 | 2.29 | 3.83 | 1.08 | 10.78 | 0.00 | 55.78 | 0.36 | 0.72 | 95.92 | |
| 18.94 | 4.83 | 3.03 | 1.83 | 1.35 | 10.47 | 0.00 | 54.59 | 0.42 | 0.79 | 96.24 | |
| 23.16 | 4.46 | 1.54 | 2.41 | 0.56 | 7.68 | 0.00 | 54.39 | 0.39 | 0.20 | 94.81 | |
| Steklite with MgSO4 (sample R700A) | 14.32 | 4.28 | 9.55 | 0.90 | 1.06 | 9.71 | 0.00 | 58.11 | 0.45 | 0.92 | 99.31 |
| 14.71 | 4.23 | 11.11 | 0.49 | 1.02 | 7.62 | 0.00 | 58.47 | 0.38 | 0.98 | 98.99 | |
| 13.80 | 4.13 | 10.41 | 0.75 | 1.02 | 8.94 | 0.00 | 58.03 | 0.32 | 0.64 | 98.04 | |
| 13.80 | 4.58 | 11.92 | 0.47 | 1.14 | 6.44 | 0.00 | 60.24 | 0.27 | 0.49 | 99.35 | |
| 11.50 | 4.41 | 10.39 | 0.37 | 2.69 | 9.13 | 0.00 | 58.54 | 0.30 | 0.62 | 97.95 | |
| 12.42 | 4.17 | 8.82 | 0.68 | 1.63 | 11.15 | 0.00 | 58.52 | 0.29 | 0.85 | 98.53 | |
| 12.13 | 5.16 | 9.90 | 0.36 | 2.18 | 7.96 | 0.00 | 58.59 | 0.58 | 0.33 | 97.18 | |
| 4.84 | 10.58 | 8.95 | 1.24 | 1.27 | 9.67 | 0.00 | 56.55 | 0.17 | 0.30 | 93.57 | |
| 4.42 | 10.10 | 8.77 | 1.26 | 1.36 | 9.83 | 0.00 | 57.14 | 0.24 | 0.53 | 93.65 | |
| 4.66 | 9.87 | 8.77 | 1.34 | 1.44 | 10.38 | 0.00 | 53.96 | 0.14 | 0.07 | 90.63 | |
| 5.89 | 8.94 | 7.40 | 1.14 | 2.05 | 12.12 | 0.00 | 55.83 | 0.31 | 0.07 | 93.75 |
| Mineral | Crystallographic System | Crystallization Temperature Range (°C) | References |
|---|---|---|---|
| Steklite KAl(SO4)2 | trigonal | 510–650 | this work |
| Millosevichite Al2(SO4)3 | trigonal | 510–650 | this work |
| Mikasaite Fe2(SO4)3 | trigonal | 300 | [2] |
| Godovikovite NH4Al(SO4)2 | trigonal | 280–450 (546) | this work [25] |
| Sabieite NH4Fe(SO4)2 | trigonal | 115–350 | [3] |
| Unnamed MgSO4 | amorphous | 510–600 | this work |
| Efremovite (NH4)2Mg2(SO4)3 | cubic | 180–400 | [4] |
| Langbeinite K2Mg2(SO4)3 | cubic | ||
| Aphthitalite (K,Na)3Na(SO4)2 | trigonal | 400–800 | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parafiniuk, J.; Siuda, R. High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland. Minerals 2021, 11, 228. https://doi.org/10.3390/min11020228
Parafiniuk J, Siuda R. High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland. Minerals. 2021; 11(2):228. https://doi.org/10.3390/min11020228
Chicago/Turabian StyleParafiniuk, Jan, and Rafał Siuda. 2021. "High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland" Minerals 11, no. 2: 228. https://doi.org/10.3390/min11020228
APA StyleParafiniuk, J., & Siuda, R. (2021). High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland. Minerals, 11(2), 228. https://doi.org/10.3390/min11020228





