Stepwise Utilization Process to Recover Valuable Components from Copper Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
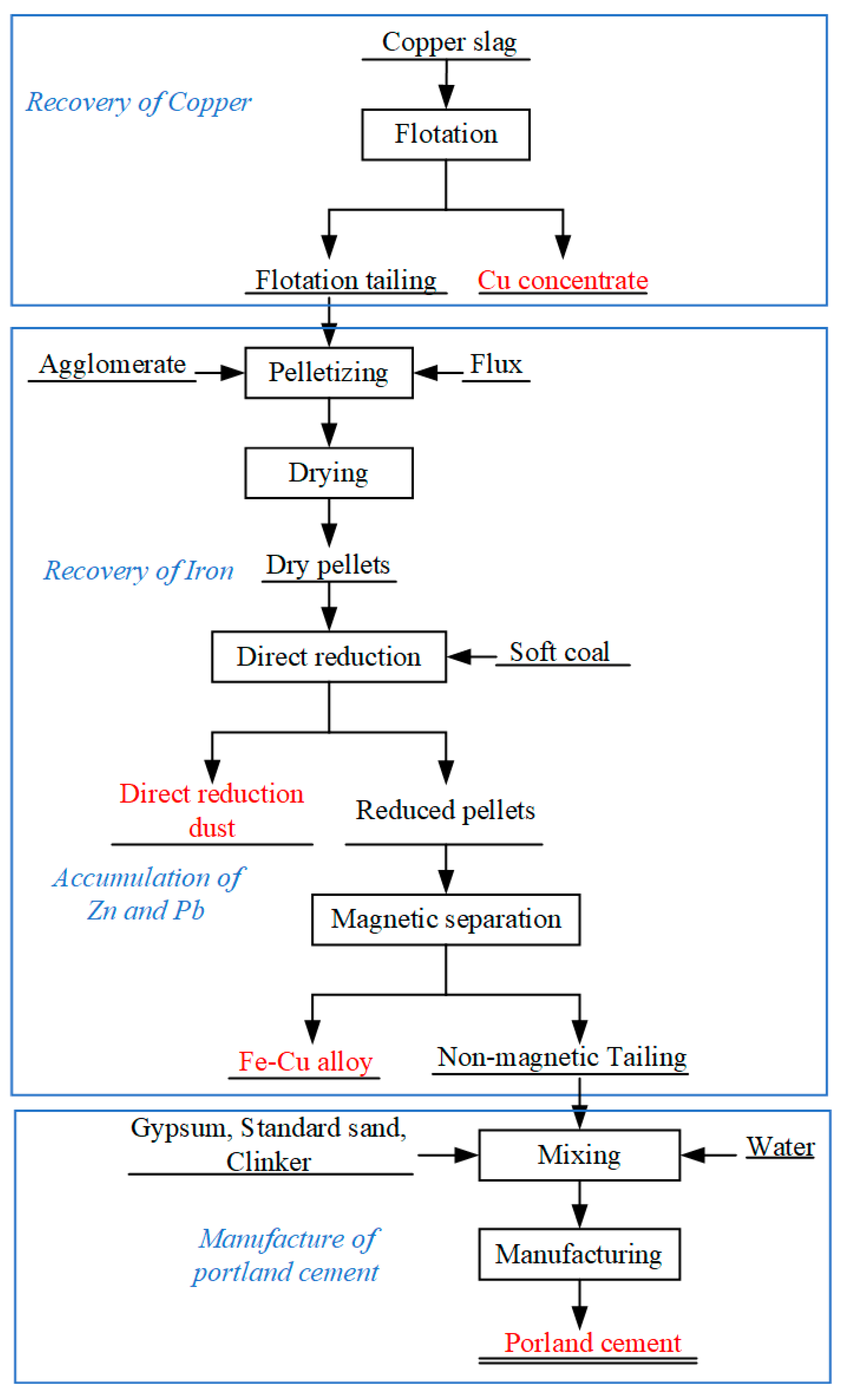
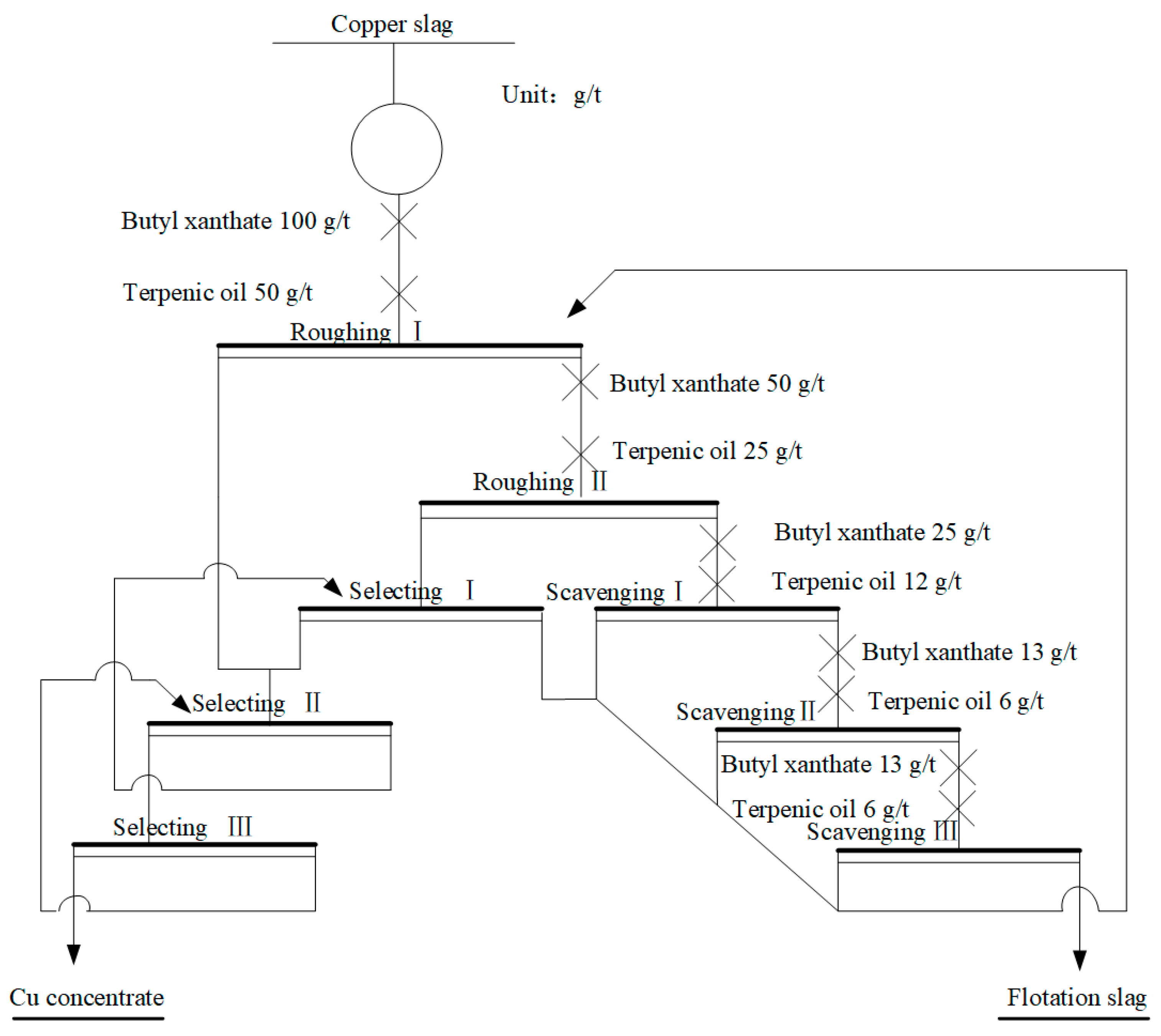
2.2.1. Flotation Process to Recover Cu
2.2.2. Preparation of Crude Fe-Cu Alloy by Direct Reduction–Magnetic Separation
2.2.3. Production of Common Portland Cement
2.3. Characterization of Raw Materials and Products
3. Results and Discussion
3.1. Flotation Process to Enrich Cu in Waste Copper Slag
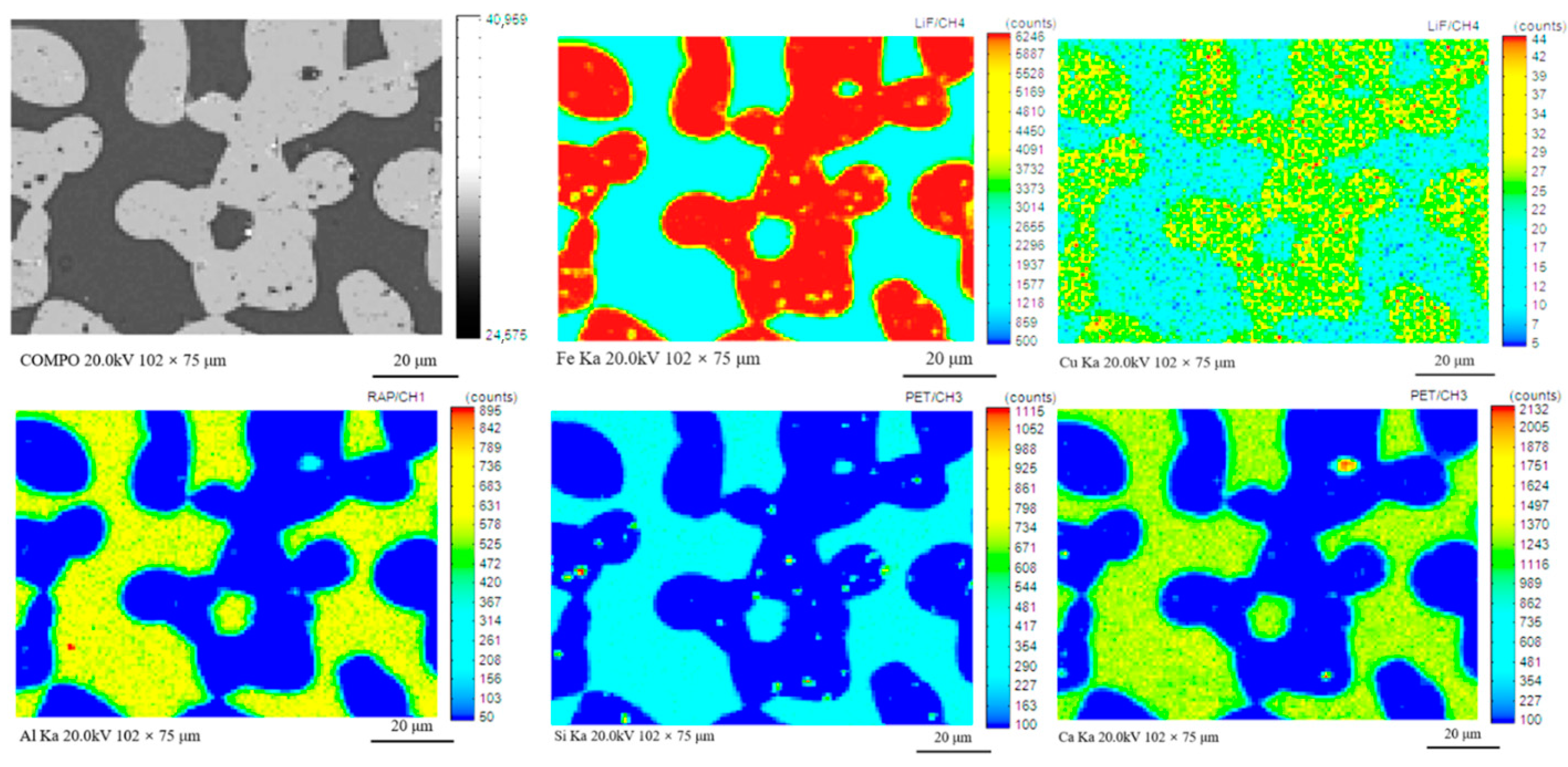
3.2. Direct Reduction–Magnetic Process to Recover Fe, Cu, Pb and Zn
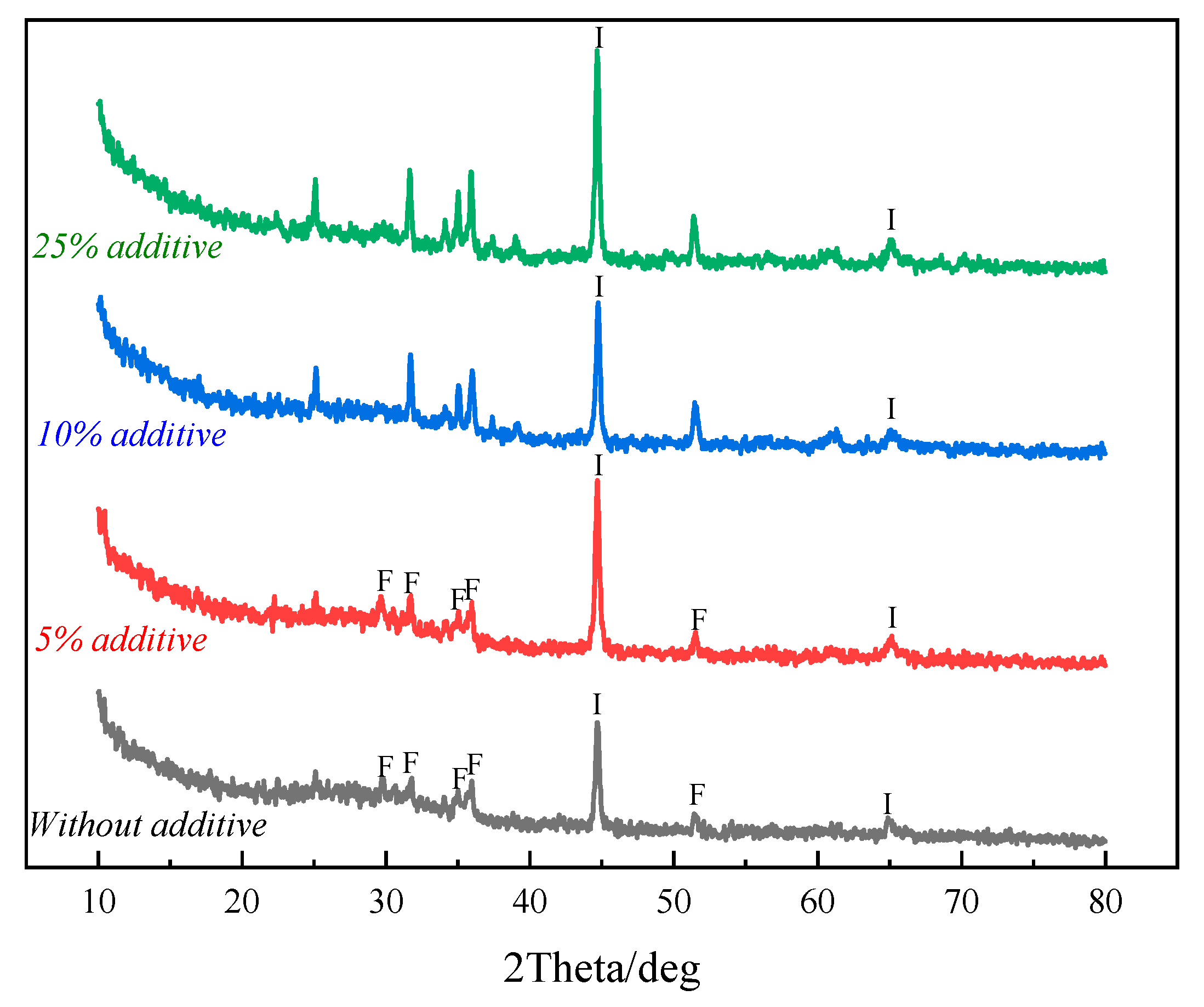
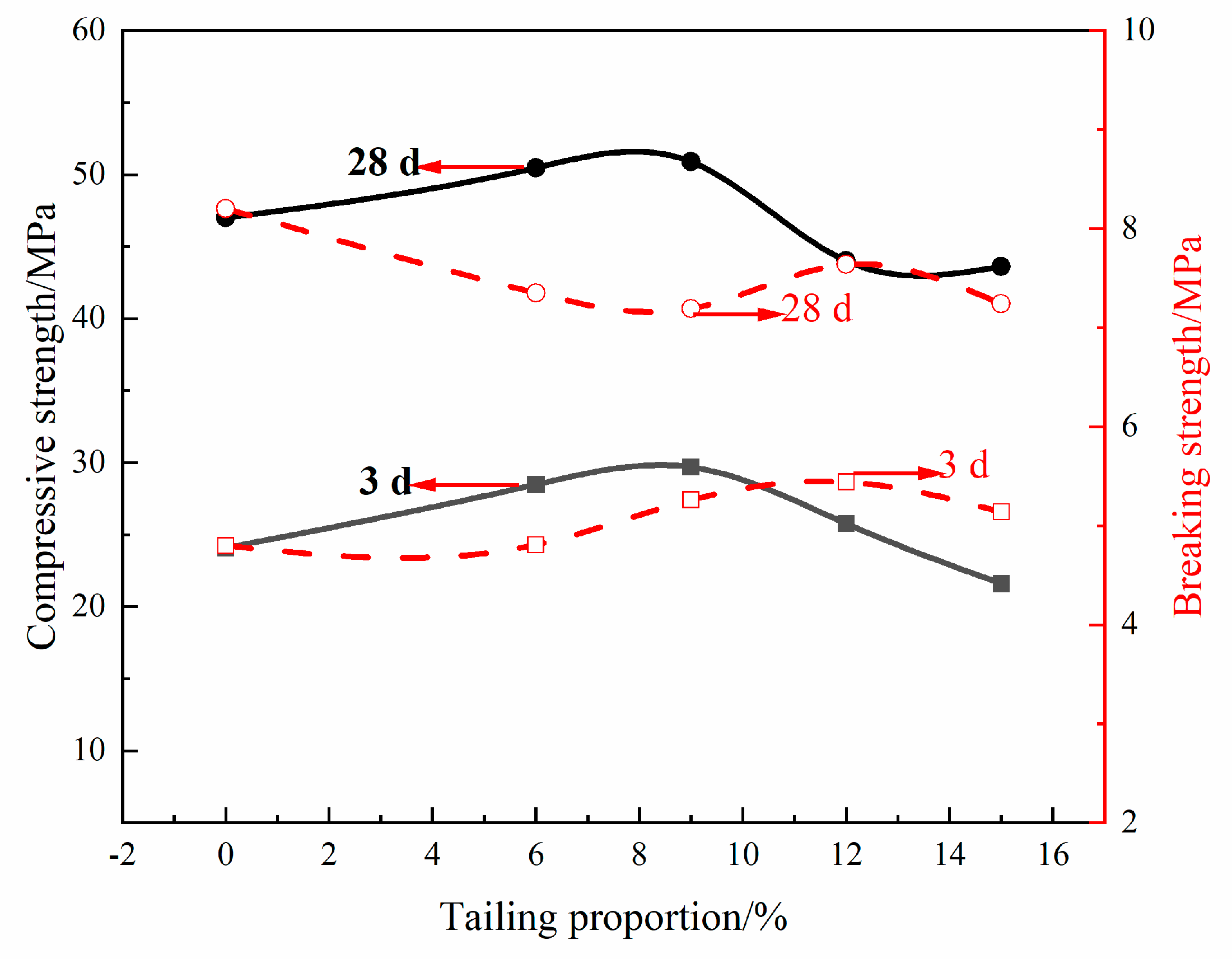
3.3. Preparation of Portland Cement from Non-Magnetic Tailings
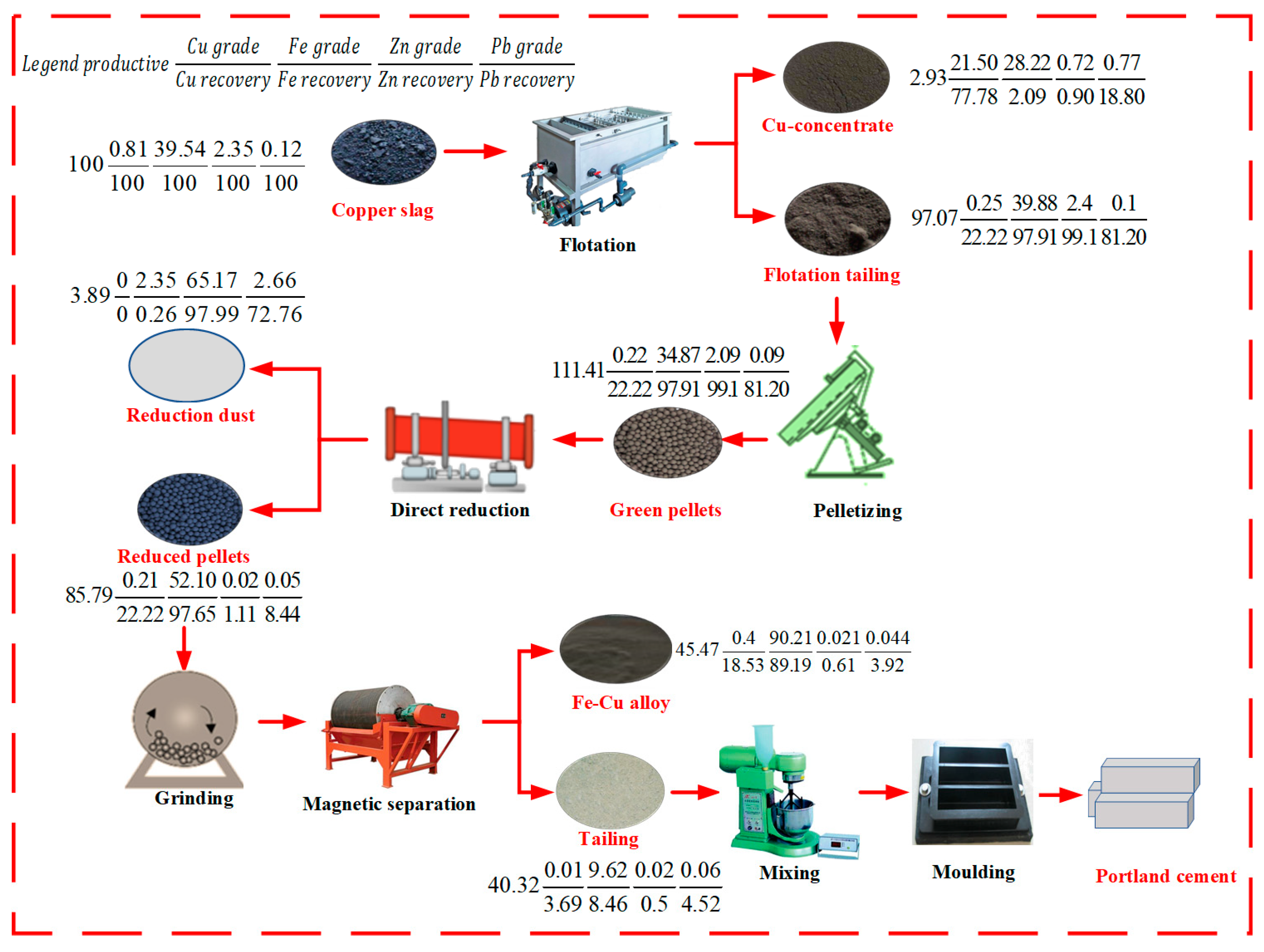
3.4. Balance of Elements in the Proposed Flow Chart
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarfo, P.; Das, A.; Wyss, G.; Young, C. Recovery of metal values from copper slag and reuse of residual secondary slag. Waste Manag. 2017, 70, 272–281. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, B.S.; Park, J.H. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon. Metall. Mater. Trans. B 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Heo, J.H.; Chung, Y.S.; Park, J.H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process. J. Clean. Prod. 2016, 137, 777–787. [Google Scholar] [CrossRef]
- Karwowska, E.; Wojtkowska, M.; Andrzejewska, D. The influence of metal speciation in combustion waste on the efficiency of Cu, Pb, Zn, Cd, Ni and Cr bioleaching in a mixed culture of sulfur-oxidizing and biosurfactant-producing bacteria. J. Hazard Mater. 2015, 299, 35–41. [Google Scholar] [CrossRef]
- Saffarzadeh, A.; Shimaoka, T.; Motomura, Y.; Watanabe, K. Characterization study of heavy metal-bearing phases in MSW slag. J. Hazard Mater. 2009, 164, 829–834. [Google Scholar] [CrossRef]
- Alp, I.; Deveci, H.; Süngün, H. Utilization of flotation wastes of copper slag as raw material in cement production. J. Hazard Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef]
- Durinck, D.; Engstrom, F.; Arnout, S.; Heulens, J.; Jones, P.T.; Bj€orkman, B.; Blanpain, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121–1131. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Zhang, F. Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Chen, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Liu, Y.; Xu, P.; Zhang, C.; Wang, J.; Liu, Y.; et al. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kalusuraman, G.; Kumaran, S.; Aslan, M.; Küçükömerog˘luc, T.; Siva, I. Use of waste copper slag filled jute fiber reinforced composites for effective erosion prevention. Measurement 2019, 148, 106950. [Google Scholar] [CrossRef]
- Potysz, A.; Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.; Guibaud, G. Copper metallurgical slags—Current knowledge and fate: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Murari, K.; Siddique, R.; Jain, K. Use of waste copper slag, a sustainable material. J. Mater. Cycles Waste Manag. 2015, 17, 13–26. [Google Scholar] [CrossRef]
- Roy, S.; Datta, A.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Wu, T.J.; Zhang, F. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag. Metals 2016, 6, 86. [Google Scholar] [CrossRef]
- Gargul, K.; Boryczko, B.; Bukowska, A.; Jarosz, P.; Małecki, S. Leaching of lead and copper from flash smelting slag by citric acid. Arch. Civ. Mech. Eng. 2019, 19, 648–656. [Google Scholar] [CrossRef]
- Muhammad, K.K.; Joseph, H.; Vivek, A.; Jouni, P.; Mika, H.; Mari, L. Sulfuric acid leaching for capturing value from copper rich converter slag. J. Clean. Prod. 2019, 25, 1005–1013. [Google Scholar]
- Nadirov, R.K.; Syzdykova, L.I.; Zhussupova, A.K.; Usserbaev, M.T. Recovery of value metals from copper smelter slag by ammonium chloride treatment. Int. J. Miner. Process 2013, 124, 145–149. [Google Scholar] [CrossRef]
- Mikoda, B.; Potysz, A.; Kmiecik, E. Bacterial leaching of critical metal values from polish copper metallurgical slags using acidithiobacillus thiooxidans. J. Environ. Manag. 2019, 236, 436–445. [Google Scholar] [CrossRef]
- Li, S.W.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Xu, J.W.; Chou, J.L. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Zhou, S.W.; Wei, Y.G.; Li, B.; Wang, H. Cleaner recycling of iron from waste copper slag by using walnut shell char as green reductant. J. Clean. Prod. 2019, 217, 423–431. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Pan, J.; Zhu, D.Q.; Zhang, F. Green and efficient utilization of waste ferric-oxide desulfurizer to clean waste copper slag by the smelting reduction-sulfurizing process. J. Clean. Prod. 2018, 199, 891–899. [Google Scholar] [CrossRef]
- Keiran, H.; Hürman, E.R.; Pekka, T.; Ari, J. Upgrading copper slag cleaning tailings for re-use. Miner. Eng. 2019, 133, 35–42. [Google Scholar]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Wu, T.J. Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ 2015, 55, 1347–1352. [Google Scholar]
- Zhu, D.Q.; Xu, J.W.; Guo, Z.Q.; Pan, J.; Li, S.W.; Pan, L.T.; Yang, C.C. Synergetic utilization of copper slag and ferruginous manganese ore via co-reduction followed by magnetic separation process. J. Clean. Prod. 2020, 250, 119462. [Google Scholar] [CrossRef]
- Shaker, F.; Rashad, A.; Allam, M. Properties of concrete incorporating locally produced Portland limestone cement. Ain Shams Eng. J. 2018, 9, 2301–2309. [Google Scholar] [CrossRef]
- Huang, Y.K.; Geng, Y.B.; Han, G.H.; Cao, Y.J.; Peng, W.J.; Zhu, X.F.; Zhang, T.A.; Dou, Z.H. A perspective of stepwise utilization of hazardous zinc plant purification residue based in selective alkaline leaching of zinc. J. Hazard Mater. 2020, 389, 122090. [Google Scholar] [CrossRef]
- Zhang, H.X.; Yang, Y.T.; Liu, X.J. Study of cadmium-doped zinc oxide nanocrystals with composition and size dependent band gaps. Chin. J. Chem. Phys. 2018, 31, 197–202. [Google Scholar] [CrossRef]
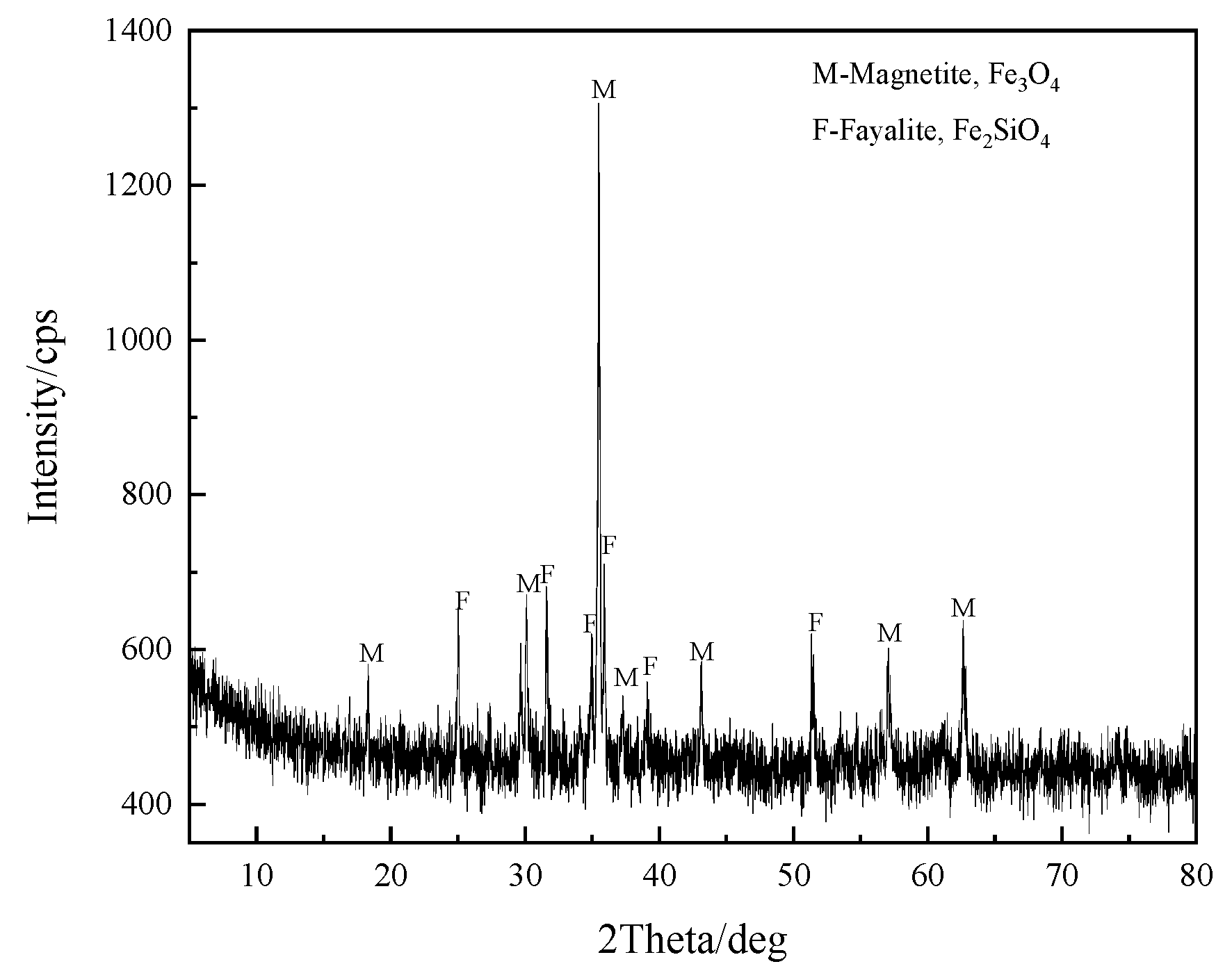
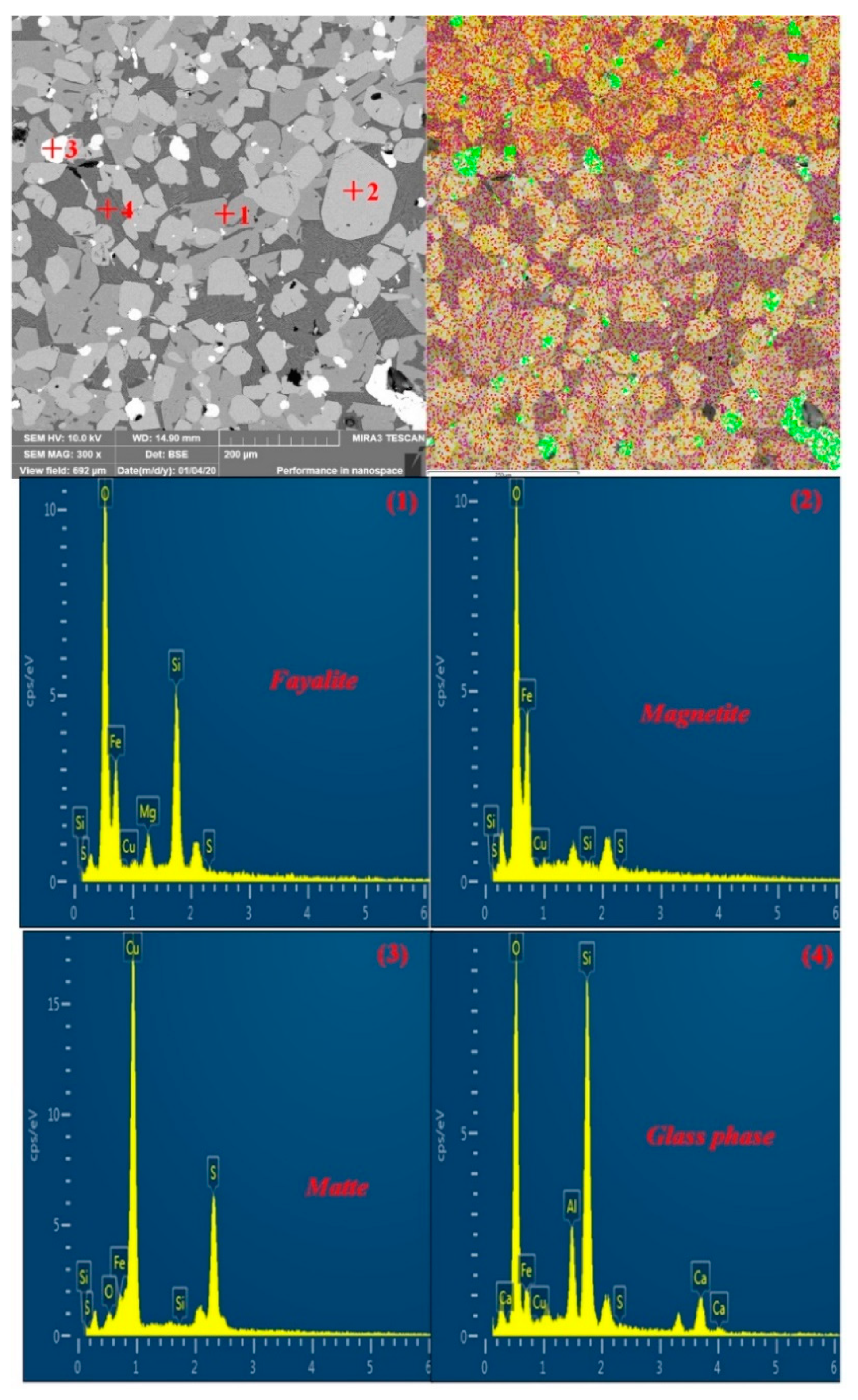
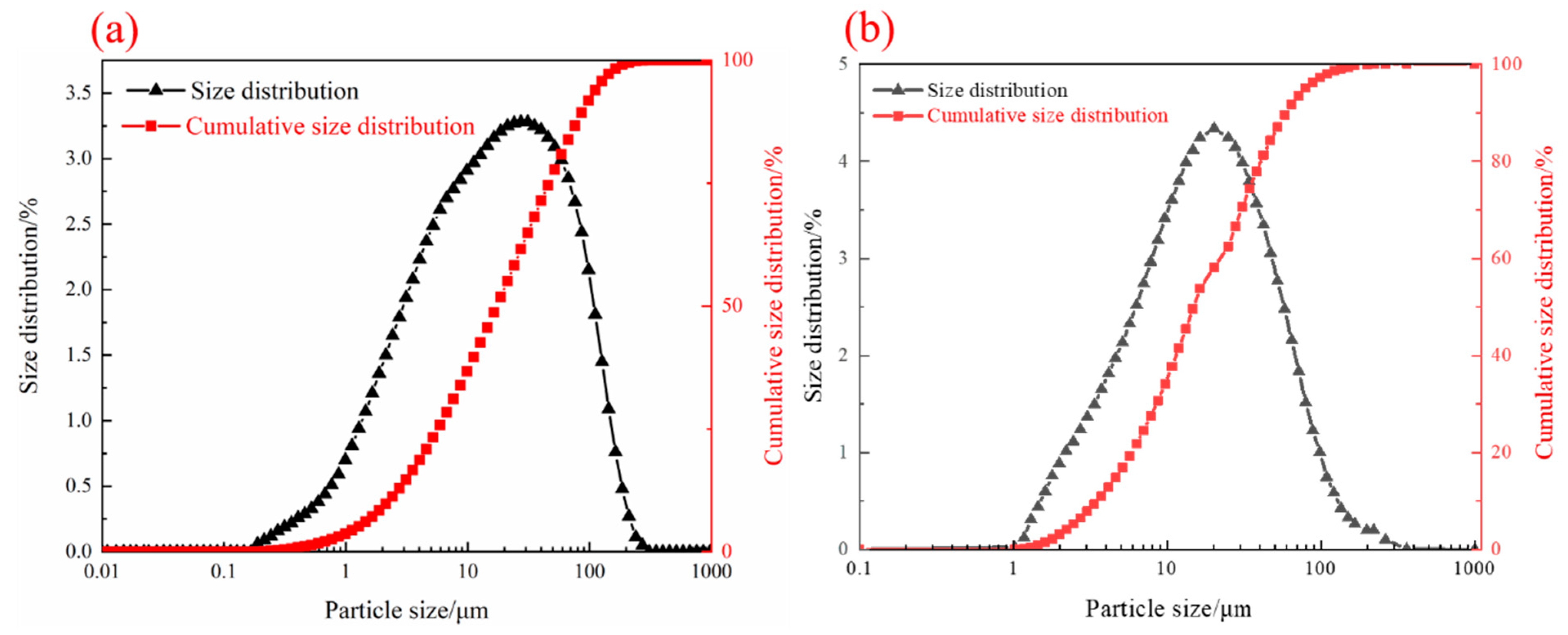
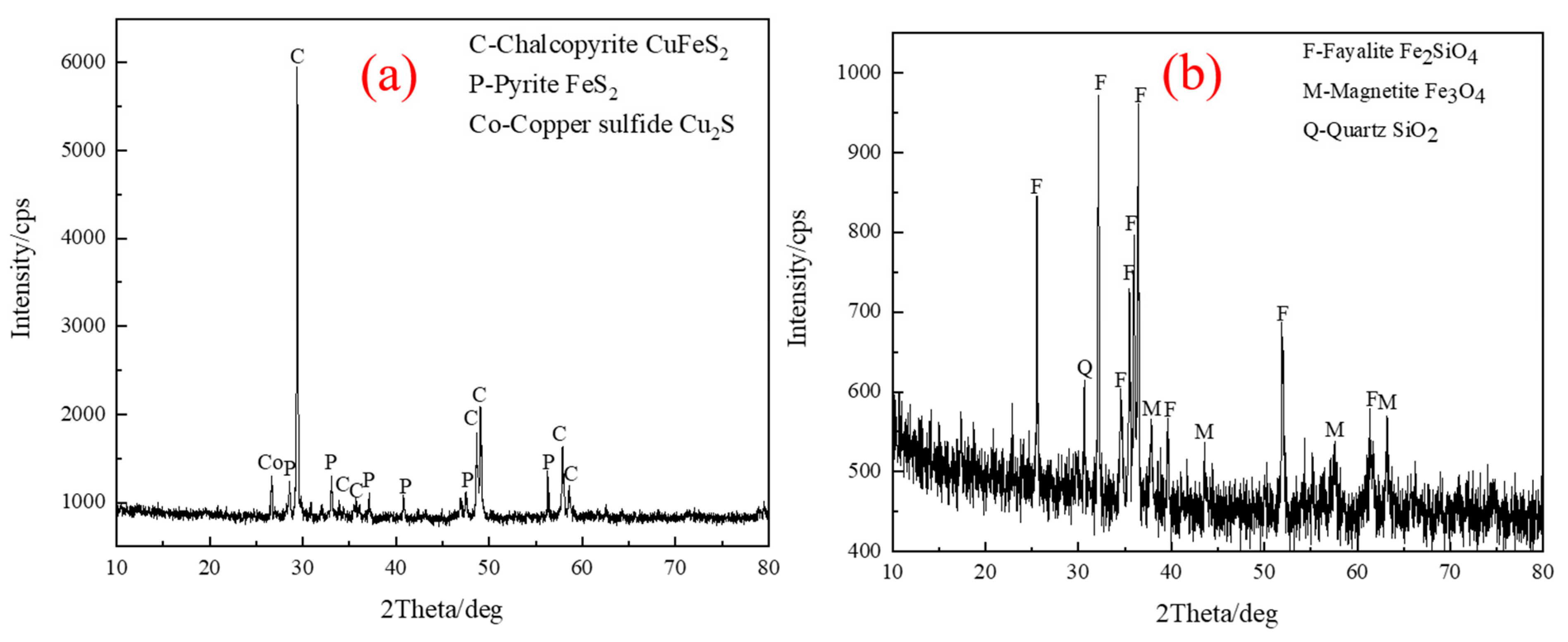
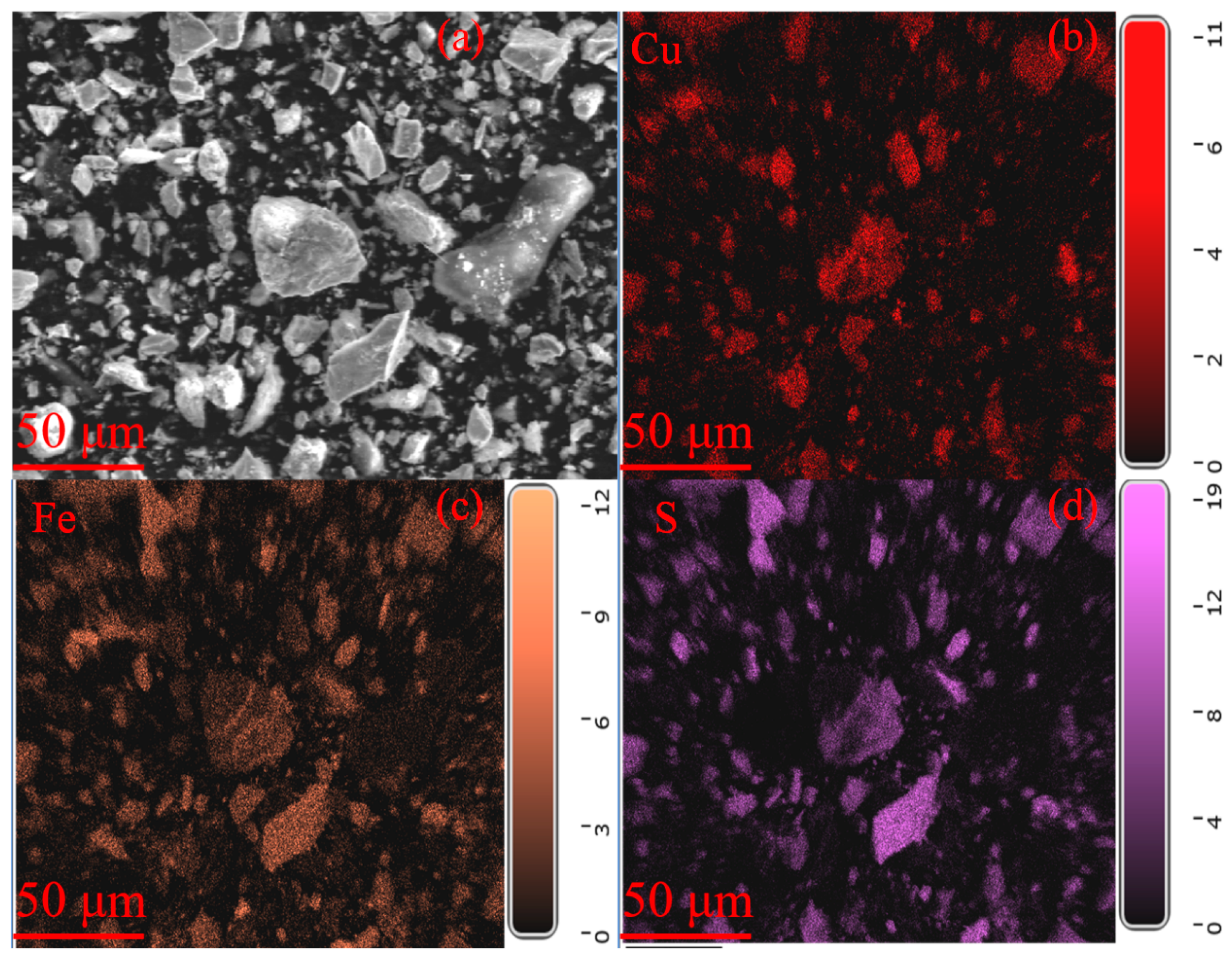
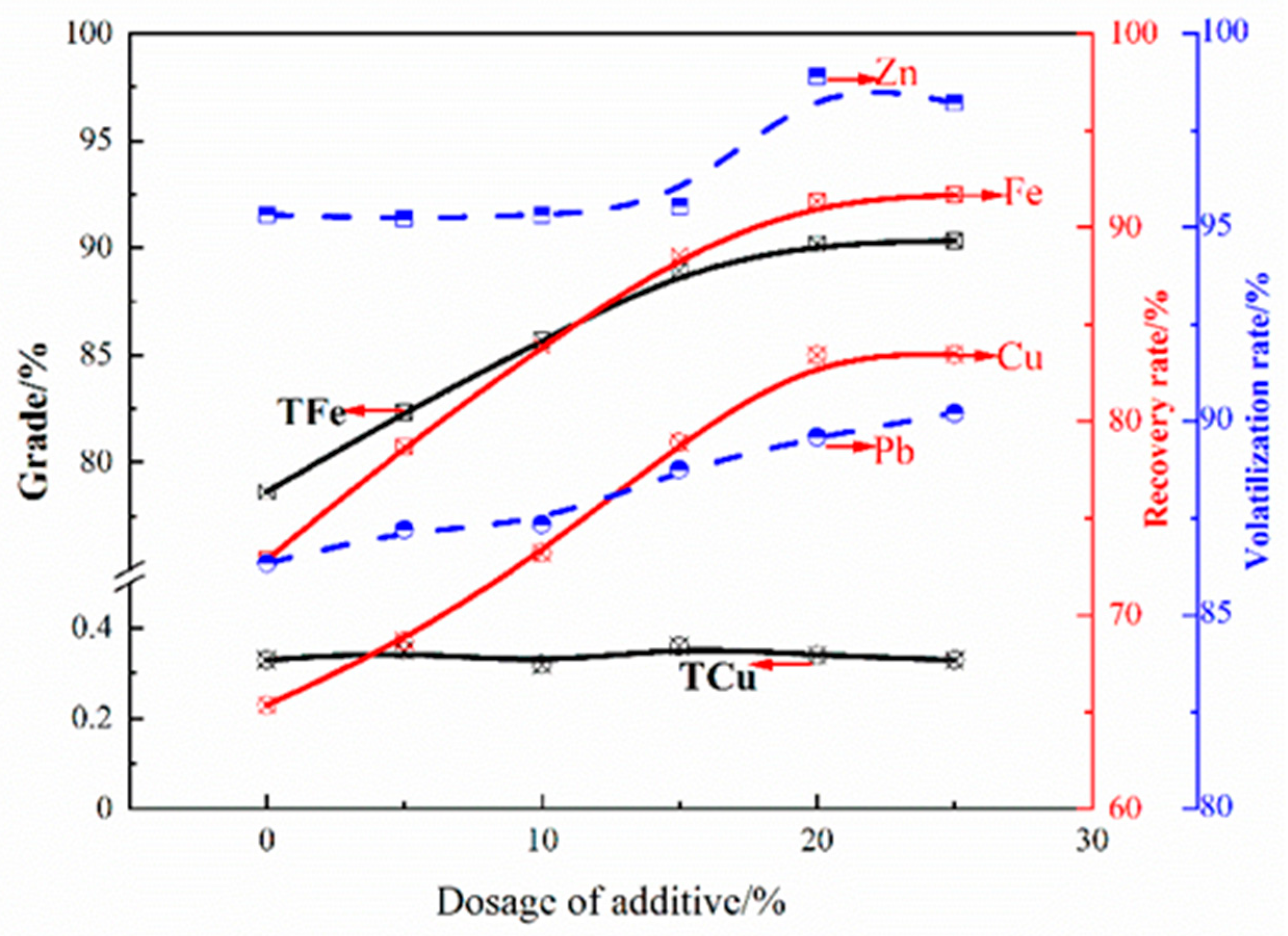

| TFe | FeO | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | Zn | S | P | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 39.54 | 40.96 | 32.11 | 2.78 | 1.65 | 3.34 | 0.81 | 0.12 | 2.35 | 0.48 | 0.11 | 1.02 |
| Point | Element/wt.% | Minerals | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Cu | S | Si | O | Mg | Al | Ca | Na | ||
| 1 | 49.15 | 0 | 0.25 | 16.18 | 31.55 | 2.87 | 0 | 0 | 0 | Fayalite |
| 2 | 70.32 | 0 | 0.05 | 0.59 | 29.04 | 0 | 0 | 0 | 0 | Magnetite |
| 3 | 11.81 | 62.92 | 22.31 | 0.07 | 2.88 | 0 | 0 | 0 | 0 | Matte |
| 4 | 14.14 | 0 | 0.38 | 29.34 | 42.61 | 0 | 6.35 | 7.18 | 0 | Glass phase |
| Sample | Non-Magnetic Tailing | Gypsum | Clinker | Standard Sand/Cement Ratio | Water/Cement Ratio |
|---|---|---|---|---|---|
| PC0 | 0 | 5 | 95 | 9 | 0.5 |
| PC6 | 6 | 5 | 89 | 9 | 0.5 |
| PC9 | 9 | 5 | 86 | 9 | 0.5 |
| PC12 | 12 | 5 | 83 | 9 | 0.5 |
| PC15 | 15 | 5 | 80 | 9 | 0.5 |
| Sample | TFe | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | Zn | S |
|---|---|---|---|---|---|---|---|---|---|
| Raw WCS | 39.54 | 32.11 | 2.78 | 1.65 | 3.34 | 0.81 | 0.12 | 2.35 | 0.48 |
| Concentrate | 28.22 | 21.33 | 2.34 | 1.02 | 1.71 | 21.50 | 0.77 | 0.72 | 10.15 |
| Tailing | 39.88 | 32.44 | 2.79 | 1.67 | 3.39 | 0.25 | 0.1 | 2.4 | 0.19 |
| Sample | TFe | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | Zn | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe-Cu alloy | 90.21 | 3.32 | 0.89 | 0.24 | 0.32 | 0.4 | 0.044 | 0.021 | 0.032 | 0.025 |
| Sample | ZnO | PbO | C | SiO2 | CaO | MgO | Al2O3 | Na2O | S |
|---|---|---|---|---|---|---|---|---|---|
| Dust | 65.17 | 2.66 | 8.99 | 1.78 | 0.09 | 0.14 | 0.22 | 0.56 | 2.19 |
| Sample | TFe | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | Zn | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Tailing | 9.62 | 55.64 | 16.83 | 2.91 | 5.99 | 0.01 | 0.06 | 0.02 | 0.15 | 0.18 |
| Elements | Cu | Pb | Zn |
|---|---|---|---|
| Tailing | 0.021 | 0.001 | 0.046 |
| Toxicity thresholds | 100 | 5 | 100 |
| Particle Size/μm | Original Sample | Ball Milling 1 h | Ball Milling 2 h | Ball Milling 3 h | Ball Milling 4 h |
|---|---|---|---|---|---|
| D10 | 2.66 | 0.87 | 0.85 | 0.78 | 0.75 |
| D50 | 21 | 7.67 | 6.52 | 5.22 | 5.10 |
| D90 | 106.5 | 29.68 | 24.05 | 23.58 | 29.7 |
| SSA/g/cm2 | 1.03 | 2.22 | 2.34 | 2.63 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Guo, Z.; Pan, J.; Zhu, D.; Dong, T.; Lu, S. Stepwise Utilization Process to Recover Valuable Components from Copper Slag. Minerals 2021, 11, 211. https://doi.org/10.3390/min11020211
Li S, Guo Z, Pan J, Zhu D, Dong T, Lu S. Stepwise Utilization Process to Recover Valuable Components from Copper Slag. Minerals. 2021; 11(2):211. https://doi.org/10.3390/min11020211
Chicago/Turabian StyleLi, Siwei, Zhengqi Guo, Jian Pan, Deqing Zhu, Tao Dong, and Shenghu Lu. 2021. "Stepwise Utilization Process to Recover Valuable Components from Copper Slag" Minerals 11, no. 2: 211. https://doi.org/10.3390/min11020211
APA StyleLi, S., Guo, Z., Pan, J., Zhu, D., Dong, T., & Lu, S. (2021). Stepwise Utilization Process to Recover Valuable Components from Copper Slag. Minerals, 11(2), 211. https://doi.org/10.3390/min11020211






