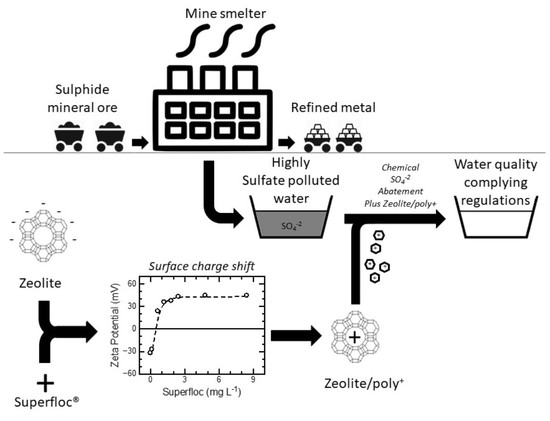
Sulfate Kinetics and Adsorption Studies on a Zeolite/Polyammonium Cation Composite for Environmental Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Modification Process
2.4. Adsorption
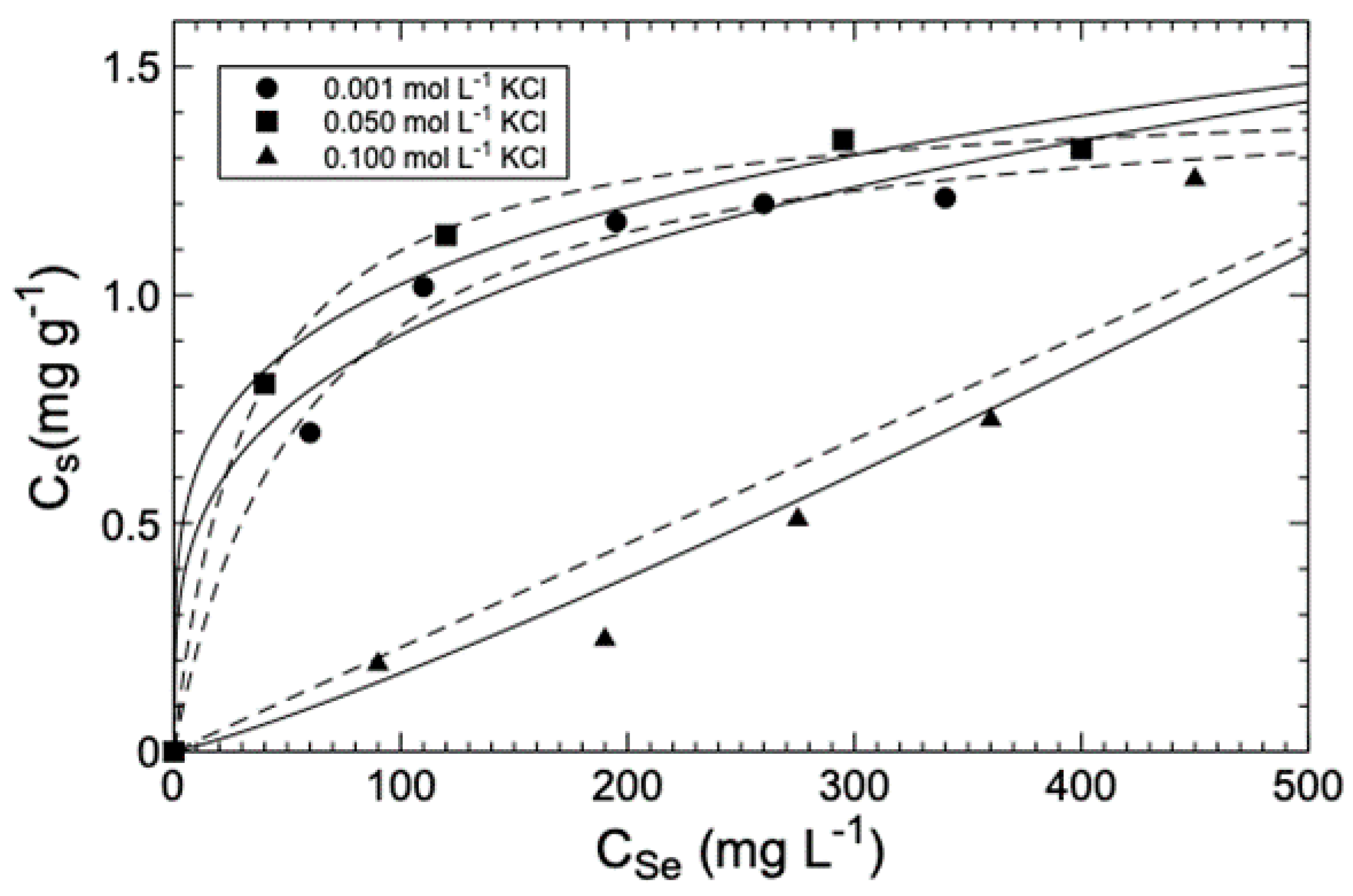
2.4.1. Ionic Strength Effect
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherms
2.4.4. Turbidimetric Analysis of Sulfate
3. Results and Discussion
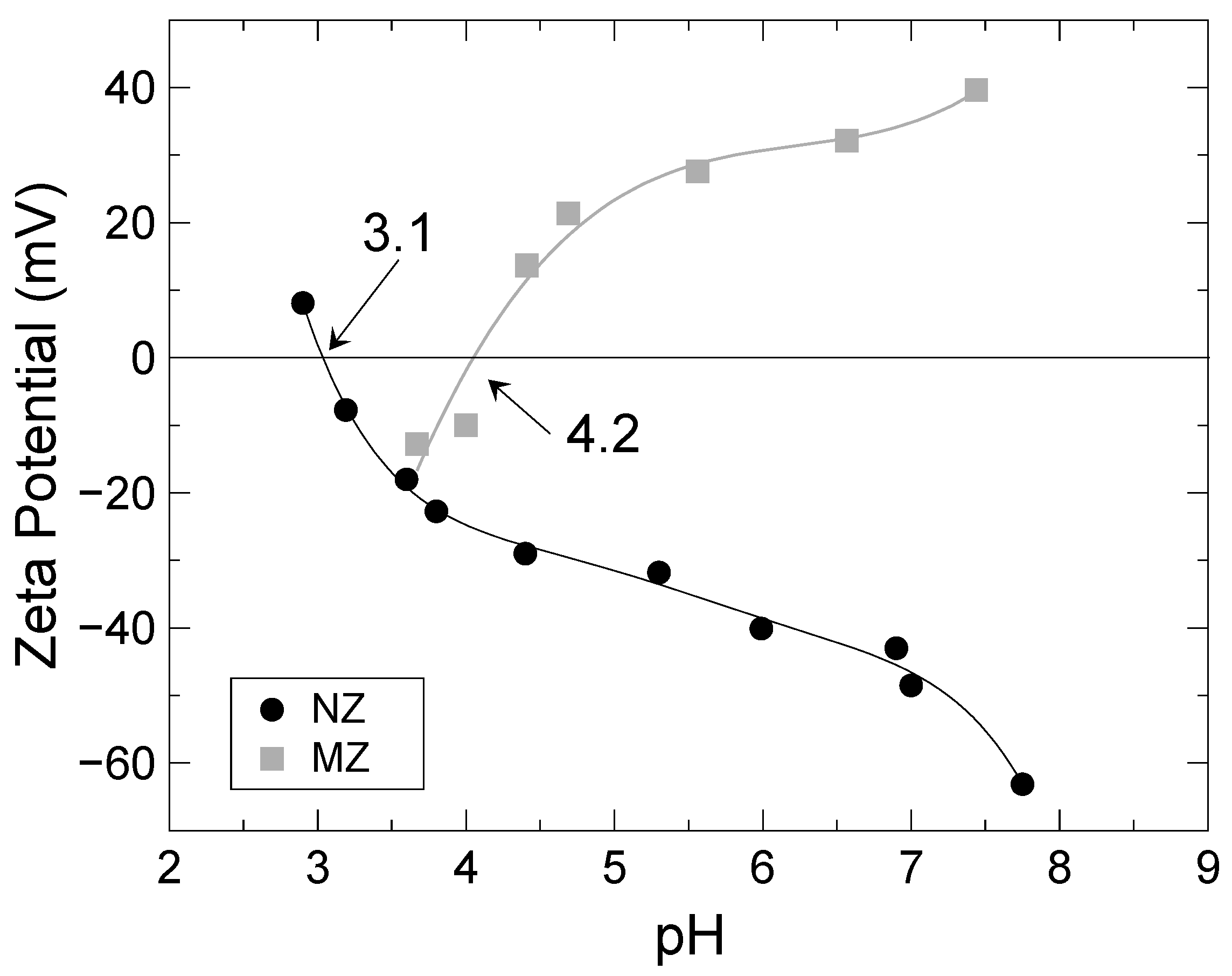
3.1. Preparation of Modified Zeolite
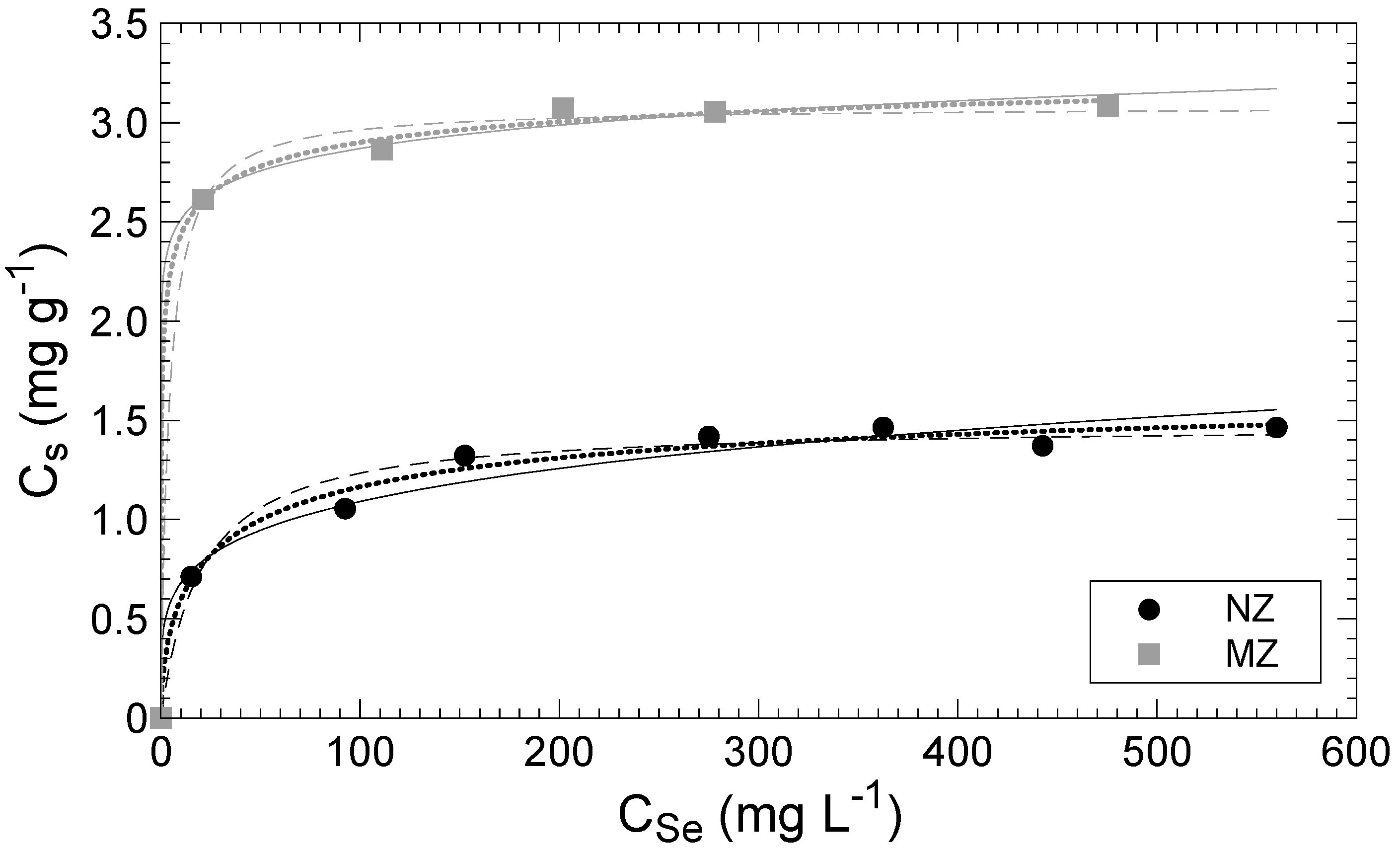
3.2. Adsorption
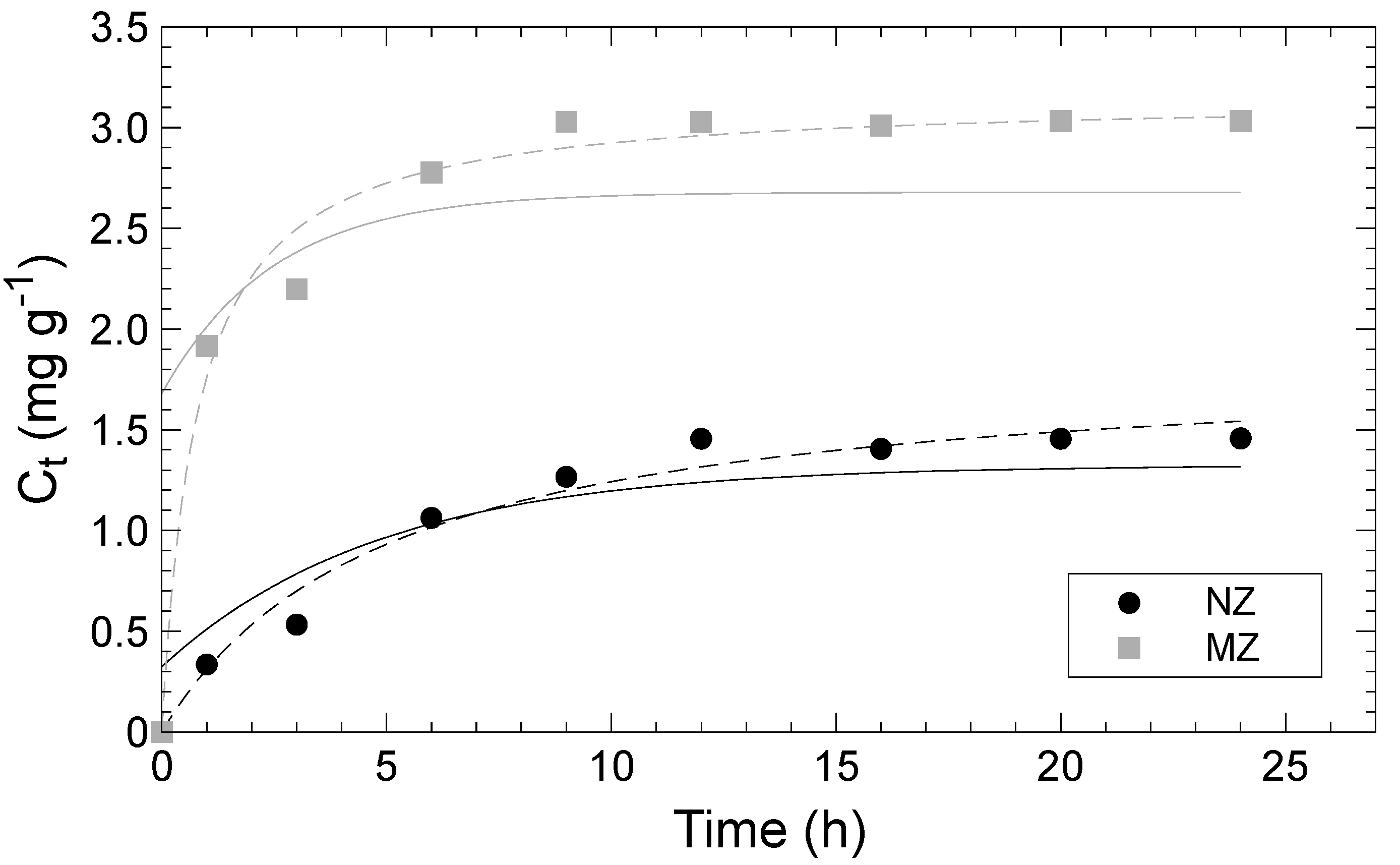
3.3. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista. Mining—Statistics & Facts. 2018. Available online: https://www.statista.com/topics/1143/mining/ (accessed on 28 October 2020).
- Harris, J.; McCartor, A. The top ten of the toxic twenty. In The World’s Worst Toxic Pollution Problems; Blacksmith Institute and Green Cross Switzerland: New York, NY, USA; Zurich, Switzerland, 2011; Available online: http://www.worstpolluted.org (accessed on 28 October 2020).
- Du, W.; Wang, X.; Chen, G.; Zhang, J.; Slaný, M. Synthesis, property and mechanism analysis of a novel polyhydroxy organic amine shale hydration inhibitor. Minerals 2020, 10, 128. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Rieger, A.; Steinberger, P.; Pelz, W.; Haseneder, R.; Härtel, G. Optimization study for treatment of acid mine drainage using membrane technology. Sep. Sci. Technol. 2010, 45, 2004–2016. [Google Scholar] [CrossRef]
- Grout, J.A.; Levings, C.D. Effects of acid mine drainage from an abandoned copper mine, Britannia Mines, Howe Sound, British Columbia, Canada, on transplanted blue mussels (Mytilus edulis). Mar. Environ. Res. 2001, 51, 265–288. [Google Scholar] [CrossRef]
- Tait, S.; Clarke, W.P.; Keller, J.; Batstone, D.J. Removal of sulfate from high-strength wastewater by crystallisation. Water Res. 2009, 43, 762–772. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A. Impact of sulfate pollution on anaerobic biogeochemical cycles in a wetland sediment. Water Res. 2012, 46, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shaheen, S.M.; Jiang, Y.; Li, R.; Slaný, M.; Abdelrahman, H.; Kwon, E.; Bolan, N.; Rinklebe, J.; Zhang, Z. Fe/Mn- and P-modified drinking water treatment residuals reduced Cu and Pb phytoavailability and uptake in a mining soil. J. Hazard. Mater. 2021, 403, 123628. [Google Scholar] [CrossRef]
- Du, W.; Slaný, M.; Wang, X.; Chen, G.; Zhang, J. The inhibition property and mechanism of a novel low molecularweight zwitterionic copolymer for improving wellbore stability. Polymers 2020, 12, 708. [Google Scholar] [CrossRef]
- Silva, A.M.; Lima, R.M.F.; Leão, V.A. Mine water treatment with limestone for sulfate removal. J. Hazard. Mater. 2012, 221, 45–55. [Google Scholar] [CrossRef]
- Gacitúa, M.A.; Muñoz, E.; González, B. Bioelectrochemical sulphate reduction on batch reactors: Effect of inoculum-type and applied potential on sulphate consumption and pH. Bioelectrochemistry 2018, 119, 26–32. [Google Scholar] [CrossRef]
- Vujaković, A.; Daković, A.; Lemić, J.; Radosavljević-Mihajlović, A.; Tomasevic-Canovic, M. Adsorption of inorganic anionic contaminants on surfactant modified minerals. J. Serbian Chem. Soc. 2003, 68, 833–841. [Google Scholar] [CrossRef]
- Barczyk, K.; Mozgawa, W.; Król, M. Studies of anions sorption on natural zeolites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Maree, J.P.; Hlabela, P.; Nengovhela, R.; Geldenhuys, A.J.; Mbhele, N.; Nevhulaudzi, T.; Waanders, F.B. Treatment of mine water for sulphate and metal removal using barium sulphide. Mine Water Environ. 2004, 23, 195–203. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Schulze, D.G. Soil Mineralogy with Environmental Applications; Soil Science Society of America Inc.: Madison, WI, USA, 2002; ISBN 0891188398. [Google Scholar]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of heavy metals from acid mine drainage by natural zeolite. Int. J. Miner. Process. 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Alshameri, A.; Ibrahim, A.; Assabri, A.M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 2014, 258, 20–31. [Google Scholar] [CrossRef]
- Guida, S.; Potter, C.; Jefferson, B.; Soares, A. Preparation and evaluation of zeolites for ammonium removal from municipal wastewater through ion exchange process. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Sun, Y.; Ma, C. Effect and mechanism of modification treatment on ammonium and phosphate removal by ferric-modified zeolite. Environ. Technol. 2019, 40, 1959–1968. [Google Scholar] [CrossRef]
- Cristiani, C.; Iannicelli-Zubiani, E.M.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Latorrata, S.; Ramis, G.; Stampino, P.G. Capture and release mechanism of La ions by new polyamine-based organoclays: A model system for rare-earths recovery in urban mining process. J. Environ. Chem. Eng. 2020, 9, 104730. [Google Scholar] [CrossRef]
- Kar, S.; Ghosh, S.; Leszczynski, J. Is clay-polycation adsorbent future of the greener society? In silico modeling approach with comprehensive virtual screening. Chemosphere 2019, 220, 1108–1117. [Google Scholar] [CrossRef]
- Gardi, I.; Nir, S.; Mishael, Y.G. Filtration of triazine herbicides by polymer-clay sorbents: Coupling an experimental mechanistic approach with empirical modeling. Water Res. 2015, 70, 64–73. [Google Scholar] [CrossRef]
- Bowman, R.S.; Sullivan, E.J.; Li, Z. Uptake of cations, anions, and nonpolar organic molecules by surfactant-modified clinoptilolite-rich tuff. In Natural Zeolites for the Third Millennium; Colella, C., y Mumpton, F.A., Eds.; De Frede Editore: Napoli, Italy, 2000; pp. 287–297. [Google Scholar]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Dickson, J.; Conroy, N.A.; Xie, Y.; Powell, B.A.; Seaman, J.C.; Boyanov, M.I.; Kemner, K.M.; Kaplan, D.I. Surfactant-modified siliceous zeolite Y for pertechnetate remediation. Chem. Eng. J. 2020, 402, 1–11. [Google Scholar] [CrossRef]
- An, J.-H.; Dultz, S. Polycation adsorption on montmorillonite: pH and T as decisive factors for the kinetics and mode of chitosan adsorption. Clay Miner. 2007, 42, 329–339. [Google Scholar] [CrossRef]
- Radian, A.; Mishael, Y. Effect of humic acid on pyrene removal from water by polycation-clay mineral composites and activated carbon. Environ. Sci. Technol. 2012, 46, 6228–6235. [Google Scholar] [CrossRef]
- Kamble, S.P.; Mangrulkar, P.A.; Bansiwal, A.K.; Rayalu, S.S. Adsorption of phenol and o-chlorophenol on surface altered fly ash based molecular sieves. Chem. Eng. J. 2008, 138, 73–83. [Google Scholar] [CrossRef]
- Kemira. Información Técnica Serie Superfloc c-500 (c-521-581). Tlaxcala, México. 2017. Available online: http://www.aniq.org.mx/pqta/pdf/Respaldo/Serie%20C500(521-581)%20(HT).pdf (accessed on 28 October 2020).
- Kemira. Superfloc Floculant and Coagulants. Product Sheet. 2017. Available online: https://www.kemira.com/products/cationic-polyacrylamides/ (accessed on 28 October 2020).
- Chen, C.L.; Wang, X.K. Influence of pH, soil humic/fulvic acid, ionic strength and foreign ions on sorption of thorium(IV) onto γ-Al2O3. Appl. Geochem. 2007, 22, 436–445. [Google Scholar] [CrossRef]
- Verbinnen, B.; Block, C.; Hannes, D.; Lievens, P.; Vaclavikova, M.; Stefusova, K.; Gallios, G.; Vandecasteele, C. Removal of Molybdate Anions from Water by Adsorption on Zeolite-Supported Magnetite. Water Environ. Res. 2012, 84, 753–760. [Google Scholar] [CrossRef]
- van Hooff, J.H.C.; Roelofsen, J.W. Introduction to Zeolite Science and Practice; Stud. Surf. Sci. Catal.; Van Bekkum, H., Flanigen, E.M., Jansen, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 58, pp. 242–282. [Google Scholar]
- Pizarro, C.; Rubio, M.A.; Escudey, M.; Albornoz, M.F.; Muńoz, D.; Denardin, J.; Fabris, J.D. Nanomagnetite-zeolite composites in the removal of arsenate from aqueous systems. J. Braz. Chem. Soc. 2015, 26, 1887–1896. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; Parallel Press, University of Wisconsin-Madison-Libraries: Madison, WI, USA, 2005; p. 101. [Google Scholar]
- Soil Survey Staff, United States Department of Agriculture. Claves Para la Taxonomía de Los Suelos, 10th ed.; Servicio de conservación de Recursos Naturales; Departamento de Agricultura de los Estados Unidos: Washington, DC, USA, 2006; pp. 77–96.
- Wu, W.; Palmarin, M.J.; Young, S. Poly(dimethylamine-co-epichlorohydrin) as an alternative to alum for the demulsification of commercial dishwasher wastewater. Sep. Purif. Technol. 2018, 195, 281–287. [Google Scholar] [CrossRef]
- Carter, D.L.; Heilman, M.D.; Gonzales, C.L. Ethylene glycol monoethyl ether for determining surface area of silicate minerals. Soil Sci. 1965, 100, 356–360. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, Advanced Course, 2nd ed.; 11th printing; Department of Soil Science, University of Wisconsin–Madison, 1525 Observatory Drive: Madison, WI, USA, 1985. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science. Principles and Applications; Academic Press: London, UK, 1983. [Google Scholar]
- Evangelou, V.P. Environmental Soil and Water Chemistry: Principles and Applications, 1st ed.; Wiley-Interscience: Lexington, KY, USA, 1998. [Google Scholar]
- Hsini, A.; Naciri, Y.; Benafqir, M.; Ajmal, Z.; Aarab, N.; Laabd, M.; Navío, J.A.; Puga, F.; Boukherroub, R.; Bakiz, B.; et al. Facile synthesis and characterization of a novel 1,2,4,5-benzene tetracarboxylic acid doped polyaniline@zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J. Colloid Interface Sci. 2021, 585, 560–573. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; El Ouardi, M.; Ajmal, Z.; Lakhmiri, R.; Boukherroub, R.; Albourine, A. Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: Equilibrium, reusability and process optimization. J. Mol. Liq. 2020, 316, 113832. [Google Scholar] [CrossRef]
- Andrade, J.D. Surface and Interfacial Aspects of Biomedical Polymer; Plenum Press: New York, NY, USA, 1995; Volume 2. [Google Scholar]
- Sharma, S.; Agarwal, G.P. Interactions of proteins with immobilized metal ions: A comparative analysis using various isotherm models. Anal. Biochem. 2001, 288, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Carvajal-Bernal, A.M.; Gómez-Granados, F.; Giraldo, L.; Moreno-Piraján, J.C. Calorimetric evaluation of activated carbons modified for phenol and 2,4-dinitrophenol adsorption. Adsorption 2016, 22, 13–21. [Google Scholar] [CrossRef]
- Rand, M.C.; Greenberg, A.E.; Taras, M.J. Standard Methods for the Examination of Water and Wastewater, 14th ed.; APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation): Washington, DC, USA, 1975; Method 427C; p. 496. [Google Scholar]
- Escudey, M.; Gil-Llambias, F. Effect of cation and anion adsorption on the electrophoretic behavior of MoO3/γ-AIO3 catalysts. J. Colloid Interface Sci. 1985, 107, 272–275. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Silva-Yumi, J.; Escudey, M. Effect of cations in the background electrolyte on the adsorption kinetics of copper and cadmium and the isoelectric point of imogolite. J. Hazard. Mater. 2015, 299, 675–684. [Google Scholar] [CrossRef]
- Smets, G.; Hesbain, A.M. Hydrolysis of polyacrylamide and acrylic acid–acrylamide copolymers. J. Polym. Sci. 1959, 40, 217–226. [Google Scholar] [CrossRef]
- Pubchem. Compound Summary for CAS Nº 42751-79-1. 2017. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/115172#section=Top (accessed on 28 October 2020).
- Mondal, P.; Tran, A.T.K.; Van der Bruggen, B. Removal of As(V) from simulated groundwater using forward osmosis: Effect of competing and coexisting solutes. Desalination 2014, 348, 33–38. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.; Yang, S.; Hu, J.; Tan, X.; Wang, X. Effect of pH, ionic strength and temperature on sorption of Pb(II) on NKF-6 zeolite studied by batch technique. Chem. Eng. J. 2011, 168, 86–93. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; Walter de Gruyter, Hubert and Co, GmbH and Co: Gottigen, Germany, 2012; ISBN 3110240238. [Google Scholar]
- Chen, W.; Liu, H.C. Adsorption of sulfate in aqueous solutions by organo-nano-clay: Adsorption equilibrium and kinetic studies. J. Cent. South Univ. 2014, 21, 1974–1981. [Google Scholar] [CrossRef]
- Matusik, J. Arsenate, orthophosphate, sulfate, and nitrate sorption equilibria and kinetics for halloysite and kaolinites with an induced positive charge. Chem. Eng. J. 2014, 246, 244–253. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y. Use of surfactant-modified zeolite to carry and slowly release sulfate. Desalin. Water Treat. 2010, 21, 73–78. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Rubio, J. New basis for adsorption of ionic pollutants onto modified zeolites. Miner. Eng. 2007, 20, 552–558. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sang, P.L.; Wang, Y.Y.; Zhang, L.Y.; Chai, L.Y.; Wang, H.Y. Effective adsorption of sulfate ions with poly(m-phenylenediamine) in aqueous solution and its adsorption mechanism. Trans. Nonferrous Met. Soc. China 2013, 23, 243–252. [Google Scholar] [CrossRef]
- Sadeghalvad, B.; Khorshidi, N.; Azadmehr, A.; Sillanpää, M. Sorption, mechanism, and behavior of sulfate on various adsorbents: A critical review. Chemosphere 2021, 263, 128064. [Google Scholar] [CrossRef]
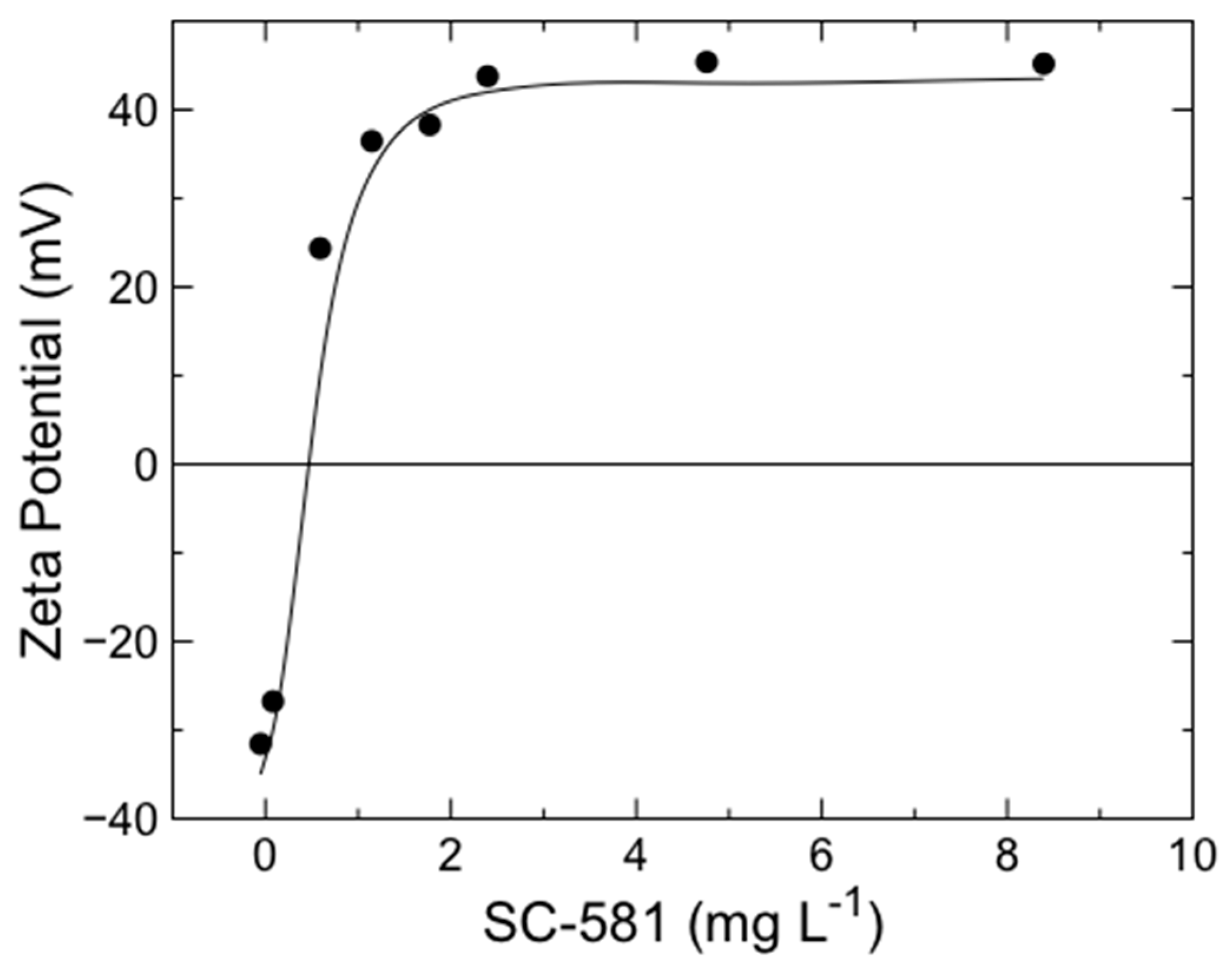
| pH (zeolite: water = 1.0:2.5) | 6.6 ± 0.1 74 ± 6 42 ± 1 2.6 × 5.7 and 6.5 × 7.5 Å |
| Total specific surface area TSSA (m2 g−1) External surface area BET (m2 g−1) Pore size | |
| Isoelectric point (IEP) | 3.1 ± 0.1 |
| General Formula (XRD analysis) | Ca3.4Al7.4Si40.6O96(H2O)31 |
| Particle size distribution | |
| Size fraction (μm) | % |
| <2 | 20.2 ± 0.1 |
| 2–20 | 54.4 ± 1.5 |
| 20–53 | 21.9 ± 1.1 |
| 53–2000 | 3.5 ± 0.3 |
| Pseudo 1st Order Model | NZ | MZ | |
| Cm | (mg g−1) | 1.32 | 2.68 |
| k1 | (h−1) | 0.21 | 0.41 |
| R2 | 0.88 | 0.57 | |
| Pseudo 2nd Order Model | NZ | MZ | |
| Cm | (mg g−1) | 1.86 | 3.16 |
| k2 | (g mg−1 h−1) | 0.11 | 0.40 |
| R2 | 0.98 | 0.98 | |
| Langmuir | NZ | MZ | |
| Cm-cal | (mg g−1) | 1.48 | 3.08 |
| KL | (L mg−1) | 0.05 | 0.25 |
| R2 | 0.98 | 1.00 | |
| Freundlich | NZ | MZ | |
| KF | (mg g−1) | 0.42 | 2.2 |
| 1/nfads | 0.21 | 0.06 | |
| R2 | 0.94 | 0.94 | |
| Langmuir–Freundlich | NZ | MZ | |
| Cm-cal | (mg g−1) | 1.74 | 3.48 |
| Kd | 7.45 | 0.93 | |
| N | 0.59 | 0.33 | |
| R2 | 0.99 | 1.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizarro, C.; Escudey, M.; Bravo, C.; Gacitua, M.; Pavez, L. Sulfate Kinetics and Adsorption Studies on a Zeolite/Polyammonium Cation Composite for Environmental Remediation. Minerals 2021, 11, 180. https://doi.org/10.3390/min11020180
Pizarro C, Escudey M, Bravo C, Gacitua M, Pavez L. Sulfate Kinetics and Adsorption Studies on a Zeolite/Polyammonium Cation Composite for Environmental Remediation. Minerals. 2021; 11(2):180. https://doi.org/10.3390/min11020180
Chicago/Turabian StylePizarro, Carmen, Mauricio Escudey, Camila Bravo, Manuel Gacitua, and Lynda Pavez. 2021. "Sulfate Kinetics and Adsorption Studies on a Zeolite/Polyammonium Cation Composite for Environmental Remediation" Minerals 11, no. 2: 180. https://doi.org/10.3390/min11020180
APA StylePizarro, C., Escudey, M., Bravo, C., Gacitua, M., & Pavez, L. (2021). Sulfate Kinetics and Adsorption Studies on a Zeolite/Polyammonium Cation Composite for Environmental Remediation. Minerals, 11(2), 180. https://doi.org/10.3390/min11020180