Influence of Mineralogy on the Dry Magnetic Separation of Ferruginous Manganese Ore—A Comparative Study
Abstract
1. Introduction
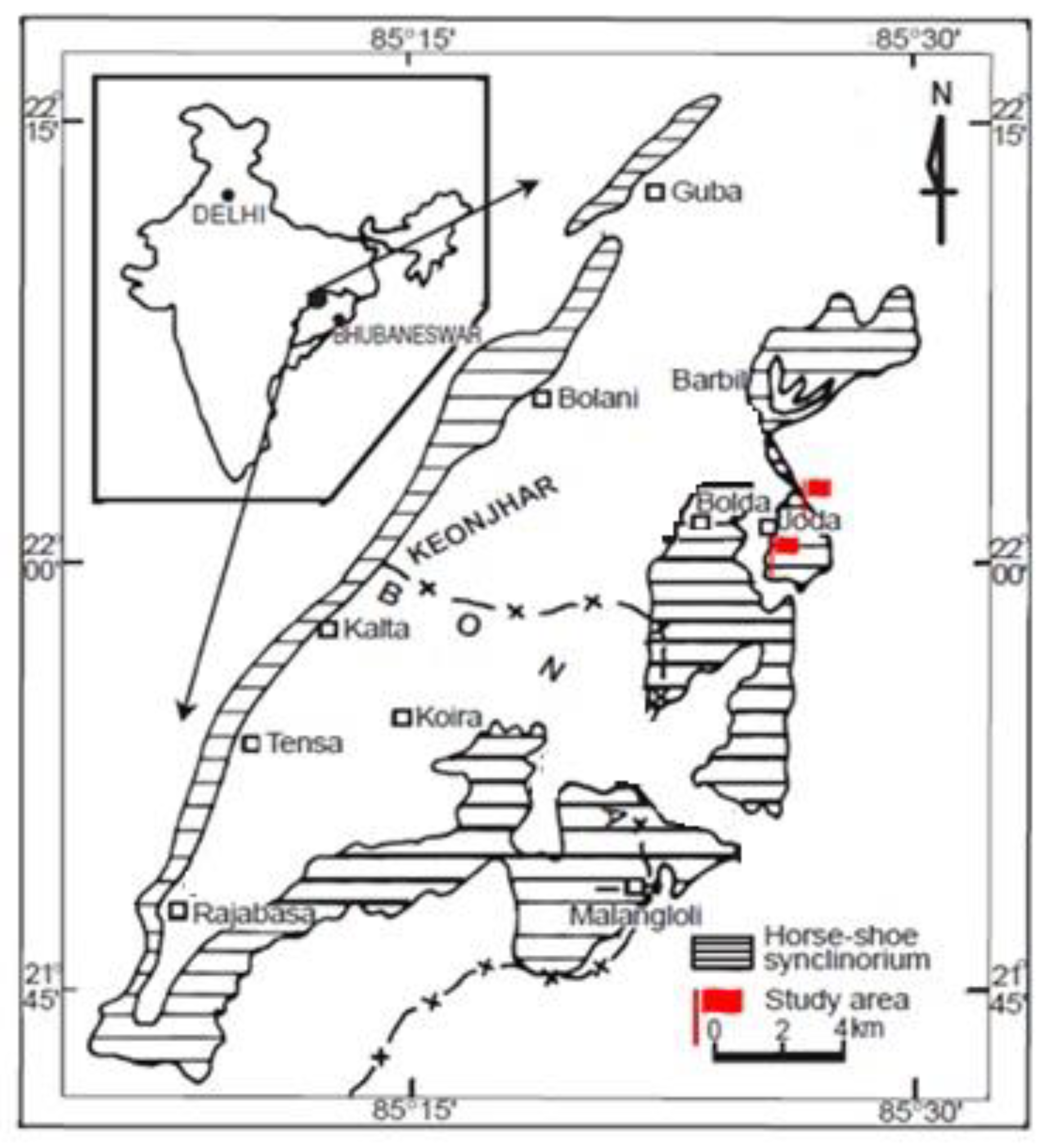
2. Geology of the Deposit
3. Materials and Methods
3.1. Manganese Ore
3.2. Characterization Studies
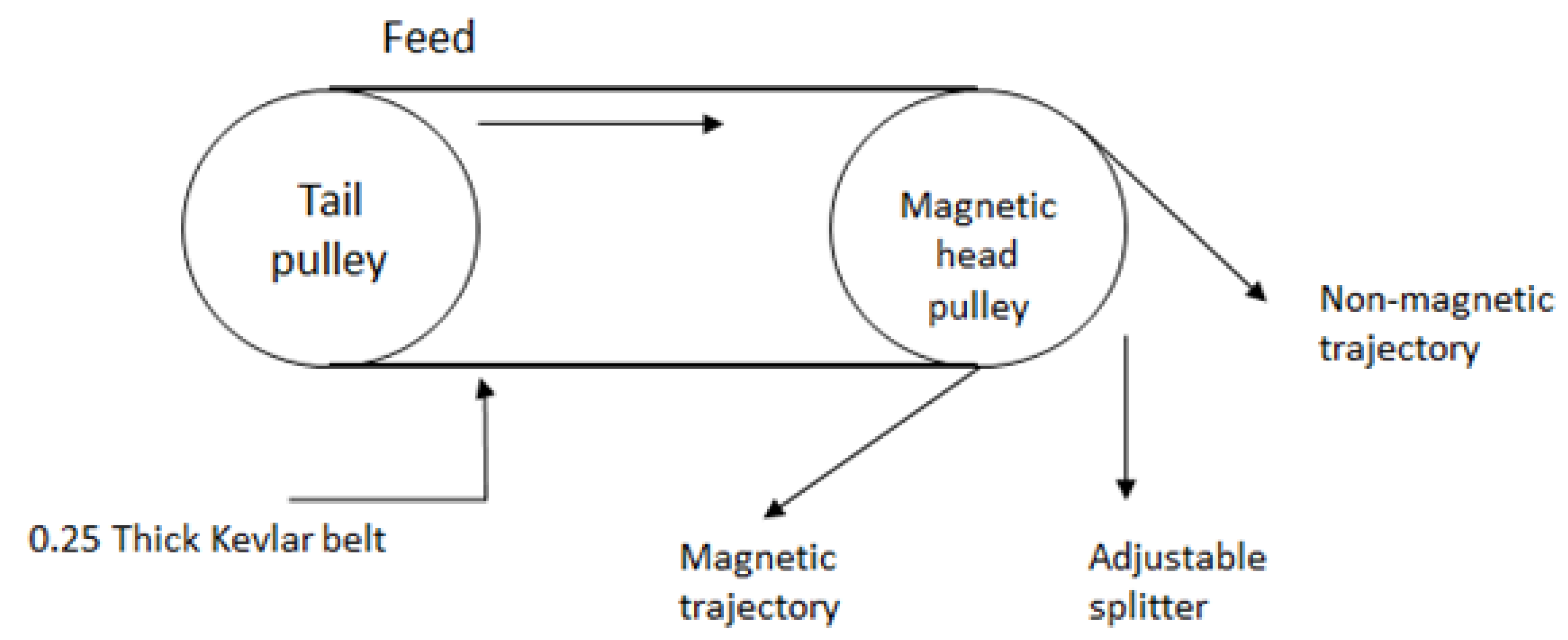
3.3. Magnetic Separation Studies
4. Results and Discussion
4.1. Characterization Results
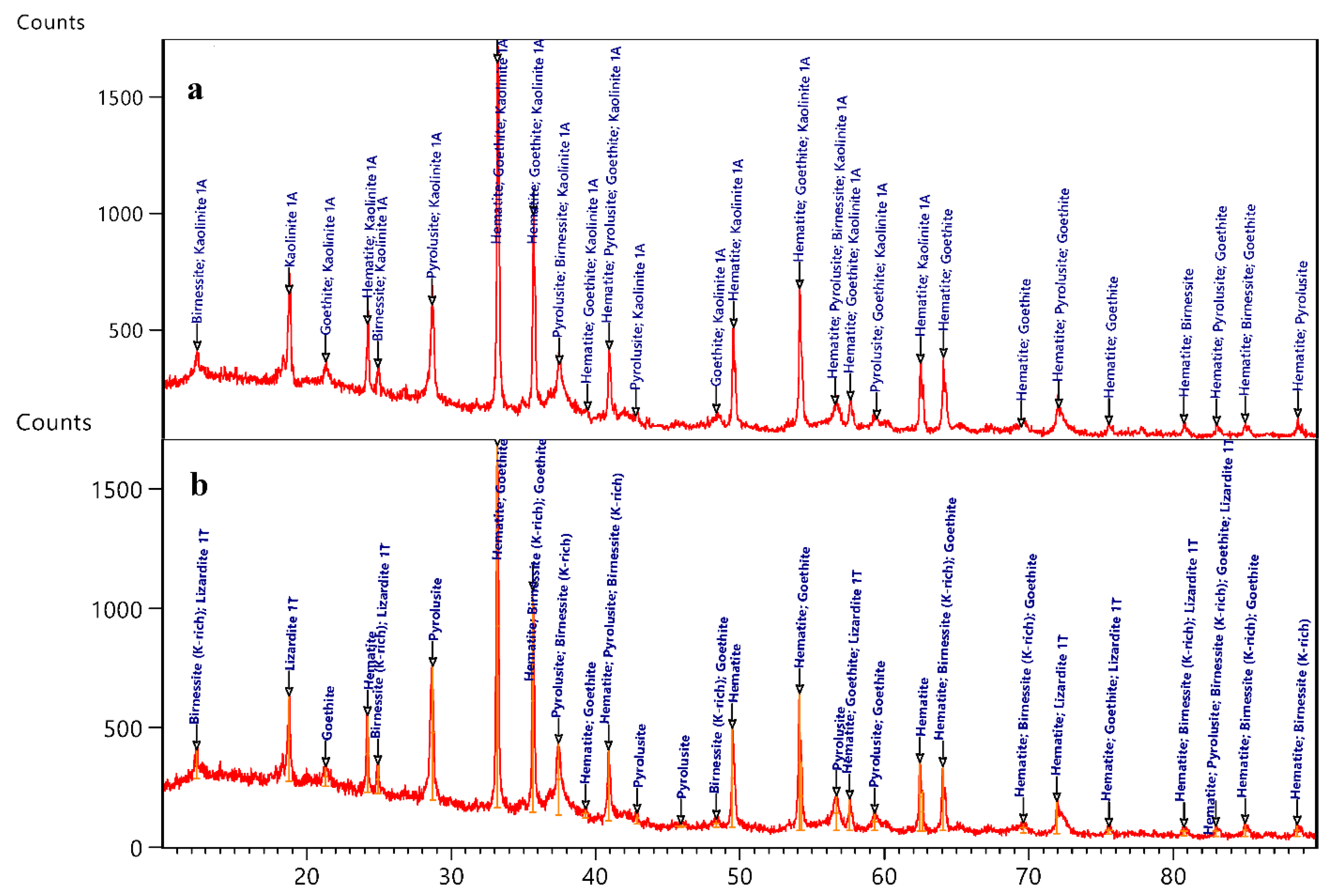
4.1.1. XRD Studies
4.1.2. Mineralogical Studies
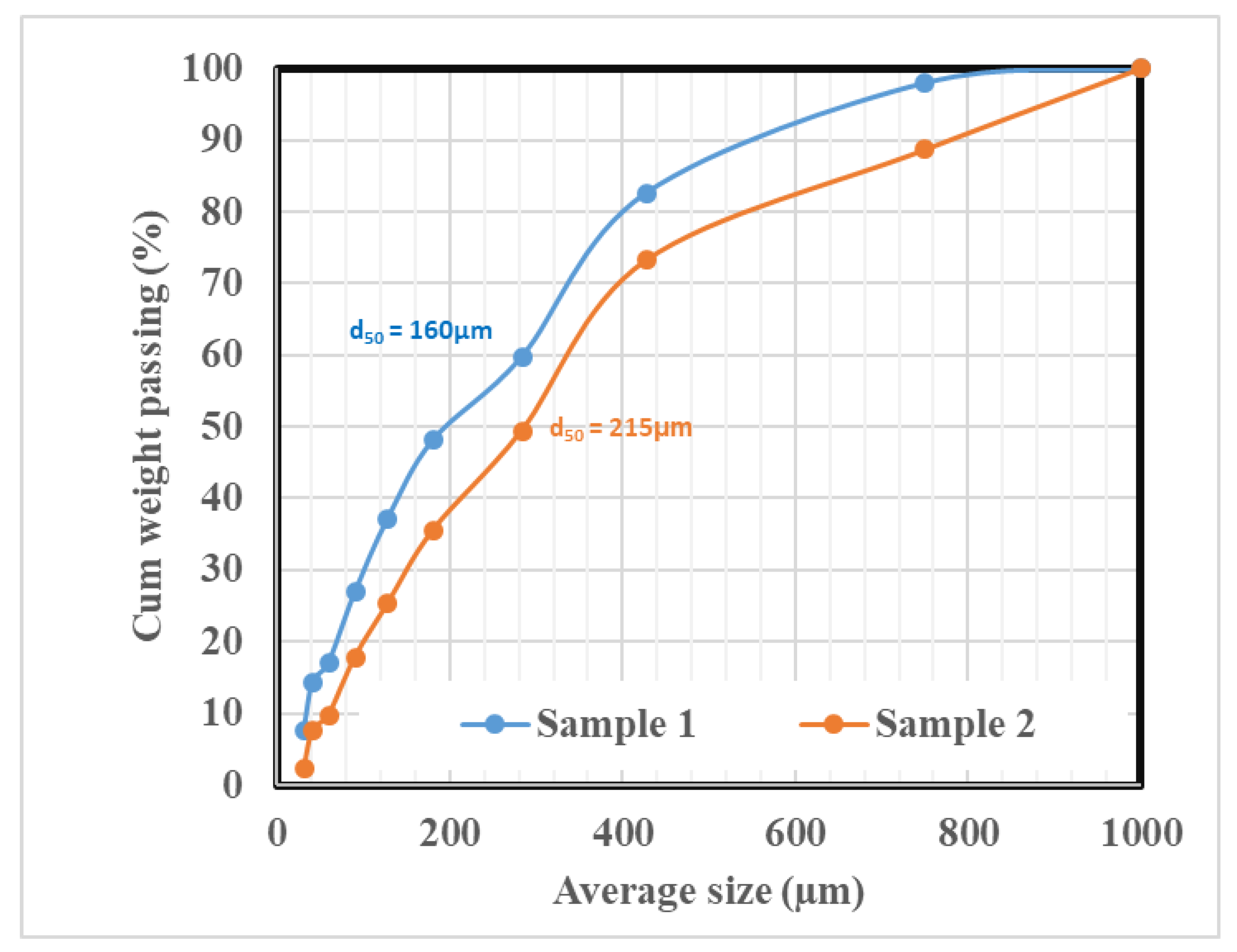
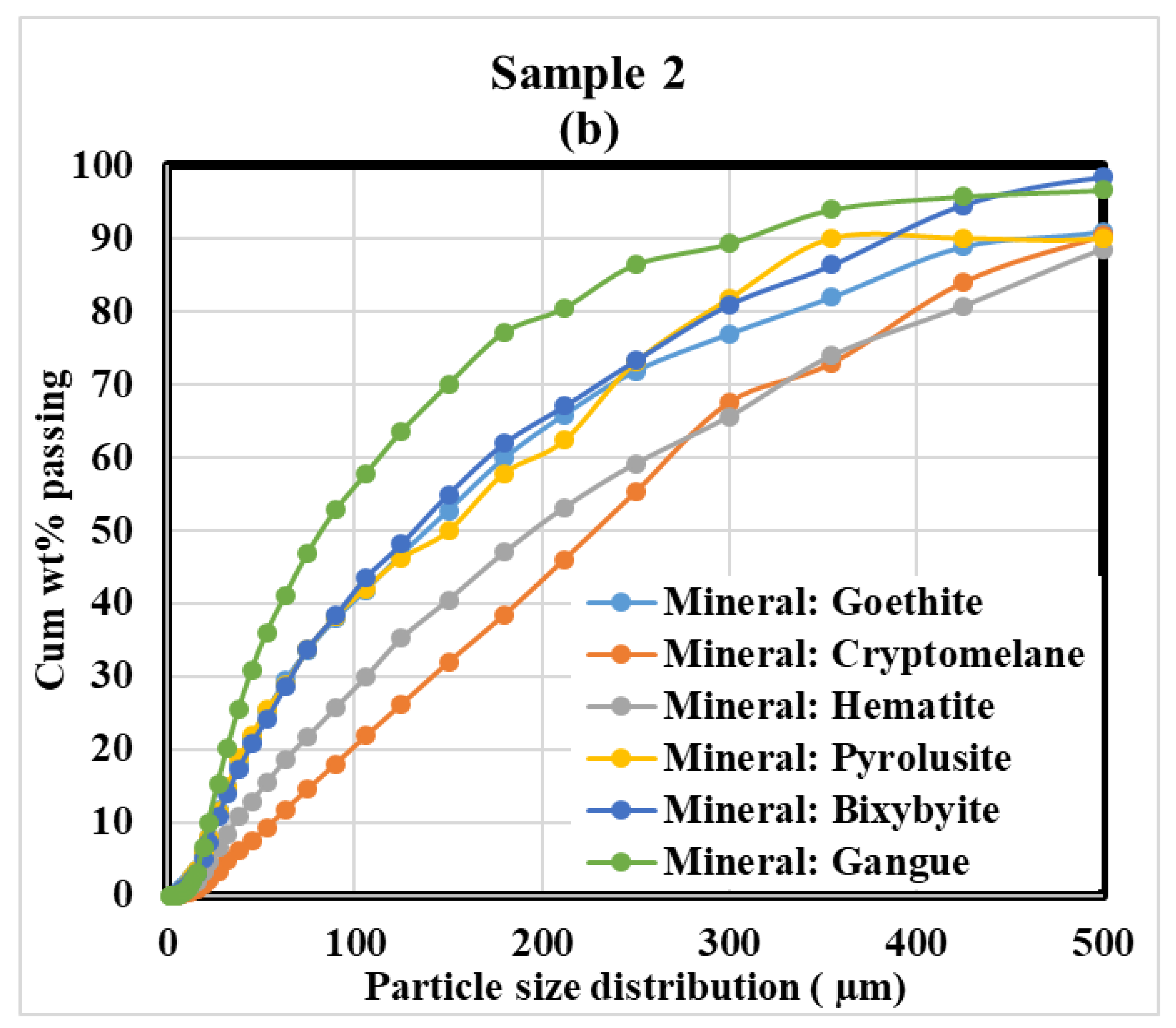
Mineral Grain Size Distribution
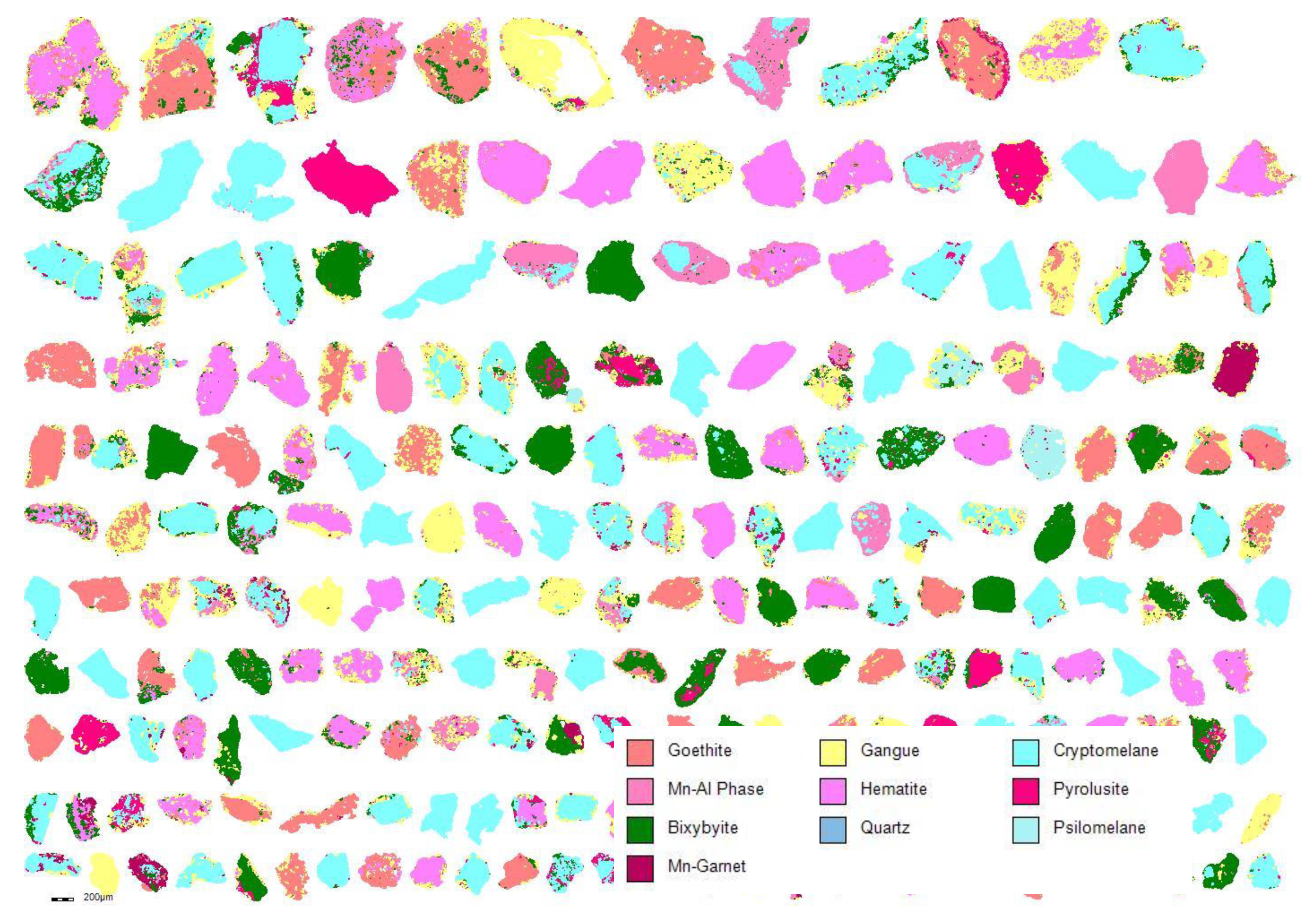
Particle Mapping Using MLA
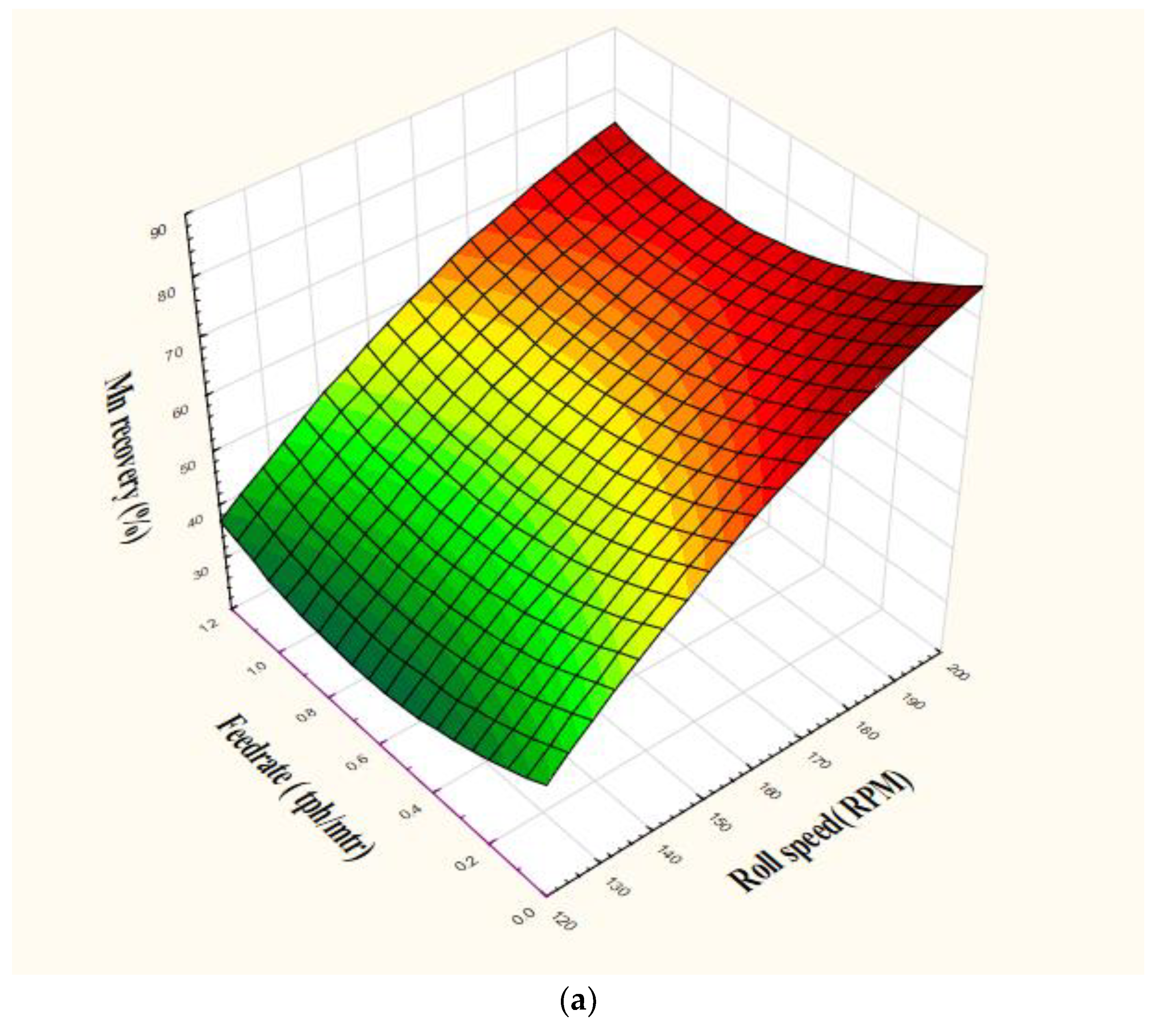
4.2. Magnetic Separation Results
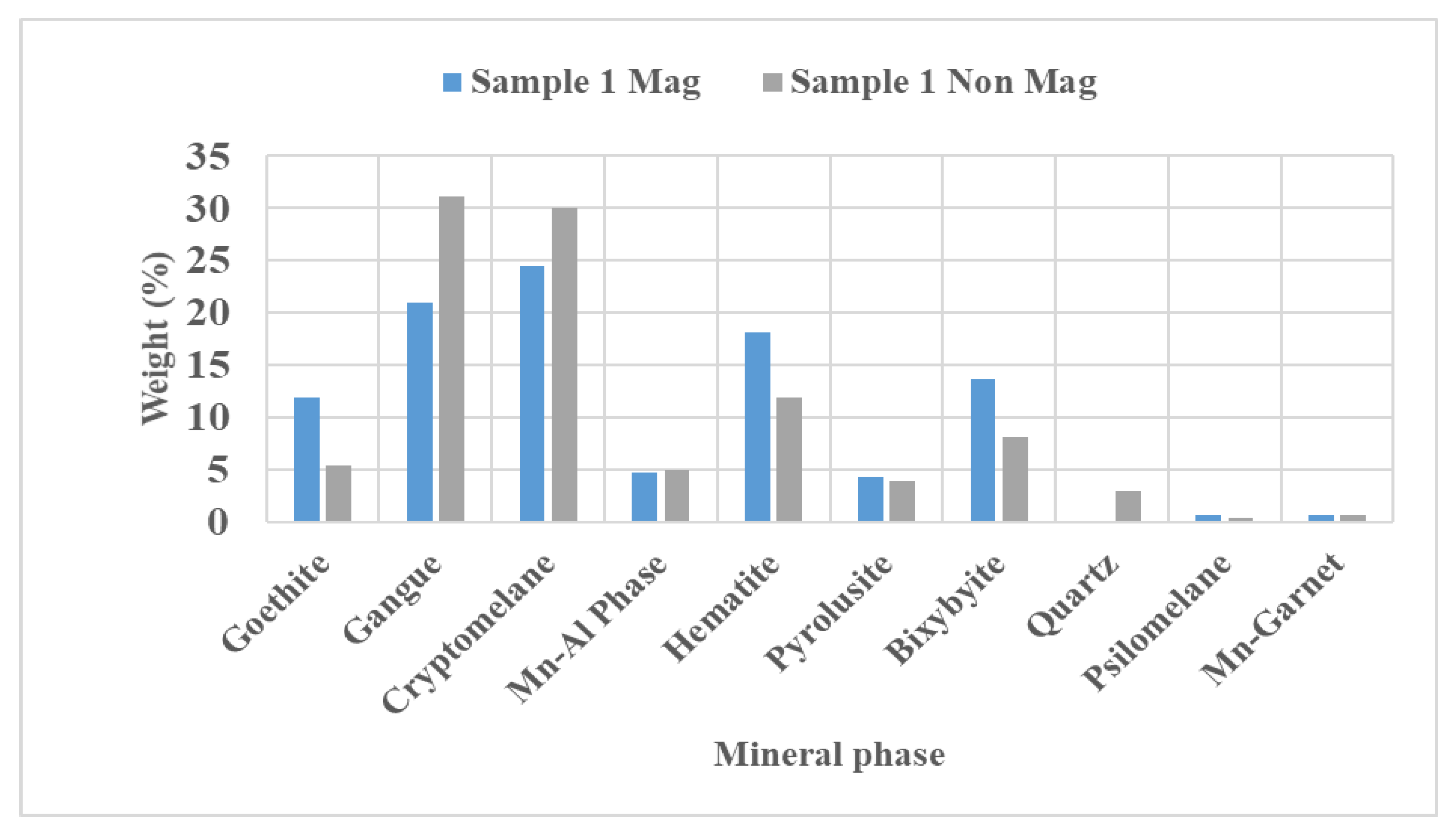
4.3. Mineralogy Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, A.C.; Amarante, S.C.; Souza, C.C.; Silva, R.R.R. Ore mineralogy and its relevance for selection of concentration methods in processing of Brazilian iron ores. Miner. Process. Extr. Metall. 2003, 112, 54–64. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Roy, S.; Venkatesh, A.S.; Rao, M.V.S.; Banerjee, P.K. Relevance of geological aspects and ore mineralogy in selecting beneficiation methods for processing of eastern Indian iron ores. Miner. Process. Extr. Metall. 2009, 118, 49–59. [Google Scholar] [CrossRef]
- Dwivedy, K.K. The role of process mineralogy in mineral beneficiation. Met. Mater. Process. 1998, 10, 1–12. [Google Scholar]
- Mishra, S.; Mohapatra, B.K.; Dash, N.; Rao, D.S. Ore petrography of low-grade siliceous manganese ores from the Bonai-Keonjhar belt, Orissa, India: The influence of mineral-fabric on their beneficiation. Min. Sci. Technol. China 2011, 21, 803–808. [Google Scholar] [CrossRef]
- Ahmed, Y. Role of ore mineralogy in selecting beneficiation route for magnesite-dolomite separation. Phys. Chem. Probl. Miner. Process. 2013, 49, 525–534. [Google Scholar]
- Bradshaw, D. The role of “process mineralogy”. Improving the process performance of complex sulphide ores. In Proceedings of the XXVII International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Celik, I.B.; Can, N.M.; Sherazadishvili, J. Influence of process mineralogy on improving metallurgical performance of a flotation plant. Miner. Process. Extr. Metall. Rev. 2010, 32, 30–46. [Google Scholar] [CrossRef]
- Evans, C.L.; Wightman, E.M.; Manlapig, E.V.; Coulter, B.L. Application of process mineralogy as a tool in sustainable processing. Spec. Issue Process Mineral. 2011, 24, 1242–1248. [Google Scholar] [CrossRef]
- Lotter, N.O. Modern process mineralogy: An integrated multi-disciplined approach to flowsheeting. Spec. Issue Process Mineral. 2011, 24, 1229–1237. [Google Scholar] [CrossRef]
- Can, N.M.; Celik, I.B.; Bicak, O.; Altun, O. Mass balance and quantitative mineralogy studies for circuit modification. Miner. Process. Extr. Metall. Rev. 2013, 34, 348–365. [Google Scholar] [CrossRef]
- Mohanan, S.; Bhoja, S.K.; Kumar, C.R.; Kumar, A.; Venugopalan, T. Estimation of ore mineralogy from analytical analysis of iron ore. Miner. Metall. Process. 2015, 32, 97–101. [Google Scholar] [CrossRef]
- Grammatikopoulos, T.; Downing, S. The disruptive role of process mineralogy in geology and mineral processing industry. Asp. Min. Miner. Sci. 2020, 5, 571–579. [Google Scholar] [CrossRef]
- Roskill Information Services. Manganese: Global Industry Markets & Outlook to 2020; Roskill Information Services: London, UK, 2015. [Google Scholar]
- Hund, K.; Porta, D.L.; Fabregas, T.P.; Laing, T.; Drexhage, J. The Mineral Intensity of the Clean Energy Transition; The World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Grieco, G.; Kastrati, S.; Pedrotti, M. Magnetic enrichment of braunite-rich manganese ore at different grain sizes. Miner. Process. Extr. Metall. Rev. 2014, 35, 257–265. [Google Scholar] [CrossRef]
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of low grade manganese ore upgradation processes. Miner. Process. Extr. Metall. Rev. 2020, 41, 417–438. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N.; Murthy, Y.R.; Singh, V. Dry high-intensity magnetic separation in mineral industry—A review of present status and future prospects. Miner. Process. Extr. Metall. Rev. 2017, 38, 339–365. [Google Scholar] [CrossRef]
- Nunna, V.; Hapugoda, S.; Pownceby, M.I. Dry Beneficiation of Low-Grade Goethite-Rich Iron Ore Fines by Air Classification, Reduction Roasting and Magnetic Separation; Institute of Mining Engineers: Perth, WA, Australia, 2019. [Google Scholar]
- Gill, C.B. Magnetic separation. In Materials Beneficiation; Materials Research and Engineering; Gill, C.B., Ed.; Springer: New York, NY, USA, 1991; pp. 128–140. ISBN 978-1-4612-3020-5. [Google Scholar]
- Elliott, R.; Barati, M. A review of the beneficiation of low-grade manganese ores by magnetic separation. Can. Metall. Q. 2020, 59, 1–16. [Google Scholar] [CrossRef]
- Singh, V.; Ghosh, T.K.; Ramamurthy, Y.; Tathavadkar, V. Beneficiation and agglomeration process to utilize low-grade ferruginous manganese ore fines. Int. J. Miner. Process. 2011, 99, 84–86. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N. Effect of desliming on the magnetic separation of low-grade ferruginous manganese ore. Int. J. Miner. Metall. Mater. 2015, 22, 661. [Google Scholar] [CrossRef]
- Sharath, K.B.; Sunil, K.T.; Veerender, S.; Raghu, K.C.; Chakraborty, D.P. Beneficiation Studies of Manganese ore Fines Using Dry Magnetic Separation; IIT-Indian School of Mines: Dhanbad, India, 2018. [Google Scholar]
- Liu, B.; Zhang, Y.; Lu, M.; Su, Z.; Li, G.; Jiang, T. Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner. Eng. 2019, 131, 286–303. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Z.; Chu, M.; Wang, R.; Wang, Z.; Feng, C. Upgrading of low-grade manganese ore based on reduction roasting and magnetic separation technique. Sep. Sci. Technol. 2019, 54, 195–206. [Google Scholar] [CrossRef]
- Mohapatra, B.K.; Rao, D.S.; Sahu, R.K. Characterisation and magnetic separation studies on chikla manganese ore, Maharastra. Ind. Miner. Eng. 1995, 3, 37–41. [Google Scholar]
- Rao, G.V.; Acharya, B.C.; Murty, B.V.R.; Mohanty, J.N.; Swamy, Y.V.; Chattopadhay, P.; Tripathy, A.K. Removal of phosphorus and enrichment of manganese from a complex ferruginous manganese ore. Magn. Electr. Sep. 1998, 9, 095128. [Google Scholar] [CrossRef]
- Mishra, P.; Mohapatra, B.K.; Pradeep, K.M.; Khageswar, M. Influence of microstructure on beneficiation of low-grade siliceous manganese ore from Orissa, India. J. Miner. Mater. Charact. Eng. 2013, 1, 80–84. [Google Scholar] [CrossRef][Green Version]
- Mishra, P.; Mohapatra, B.; Mahanta, K. Upgradation of low-grade siliceous manganese ore from Bonai-Keonjhar Belt, Orissa, India. J. Miner. Mater. Charact. Eng. 2009, 8, 47–56. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Mishra, S.K.; Biswal, D. Beneficiation of low-grade, high-phosphorus manganese ores of Andhra Pradesh, India, by wet high-intensity magnetic separation. Min. Met. Explor. 2000, 17, 181–185. [Google Scholar] [CrossRef]
- Binta, H.; Yaro, S.A.; Thomas, D.; Dodo, R. Beneficiation of low grade manganese ore from Wasagu, Kebbi State, Nigeria. J. Raw Mater. Res. Raw Mater. Res. Dev. Counc. Niger. 2016, 10, 63–73. [Google Scholar]
- Güven, O.; Burat, F.; Kangal, O.; Acarkan, N. Beneficiation of low-grade Turkish manganese ore. In Proceedings of the XXVI nternational Mineral Processing Congress, New Delhi, India, 24–28 September 2012. [Google Scholar]
- Mehdilo, A.; Irannajad, M.; Hojjati-Rad, M.R. Characterization and beneficiation of Iranian low-grade manganese ore. Physicochem. Probl. Miner. Process. 2013, 49, 725–741. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, B.; Ge, W.; Yan, C.J.; Yang, X. Magnetic separation and magnetic properties of low-grade manganese carbonate ore. JOM 2015, 67, 361–368. [Google Scholar] [CrossRef]
- Mpho, M.; Samson, B.; Ayo, A. Evaluation of reduction roasting and magnetic separation for upgrading Mn/Fe ratio of fine ferromanganese. Int. J. Min. Sci. Technol. 2013, 23, 537–541. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Suresh, N. Influence of particle size on dry high-intensity magnetic separation of paramagnetic mineral. Adv. Powder Technol. 2017, 28, 1092–1102. [Google Scholar] [CrossRef]
- Turkenich, A.M. A continuous barrier magnetic separator for the treatment of weakly magnetic ores. Magn. Electr. Sep. 1900, 10, 052860. [Google Scholar] [CrossRef]
- Svoboda, J. Magnetic Techniques for the Treatment of Materials; Kluwer Academic Publishers: New York, NY, USA, 2004; ISBN 1-4020-2038-4. [Google Scholar]
- Zhang, X.; Tan, X.; Yi, Y.; Liu, W.; Li, C. Recovery of manganese ore tailings by high-gradient magnetic separation and hydrometallurgical method. JOM 2017, 69, 2352–2357. [Google Scholar] [CrossRef]
- Supriya, R. Ancient manganese deposits. In Handbook of Strata-Bound and Stratiform Ore Deposits; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Mishra, P.; Mvohapatra, B.K.; Singh, P.P. Mode of occurrence and characteristics of Mn-ore bodies in iron ore group of rocks, NORTH Orissa, India and its significance in resource evaluation. Resour. Geol. 2006, 56, 55–64. [Google Scholar] [CrossRef]
- Devasahayam, S. Predicting the liberation of sulfide minerals using the breakage distribution function. Miner. Process. Extr. Metall. Rev. 2015, 36, 136–144. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Singh, V.; Murthy, Y.R.; Banerjee, P.K.; Suresh, N. Influence of process parameters of dry high intensity magnetic separators on separation of hematite. Int. J. Miner. Process. 2017, 160, 16–31. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Murthy, Y.R.; Singh, V.; Suresh, N. Processing of ferruginous chromite ore by dry high- intensity magnetic separation. Miner. Process. Extr. Metall. Rev. 2016, 37, 196–210. [Google Scholar] [CrossRef]
- Singh, V.; Biswas, A. Physicochemical processing of low grade ferruginous manganese ores. Int. J. Miner. Process. 2017, 158, 35–44. [Google Scholar] [CrossRef]







| Mineral Name | Elemental Composition | Density (Kg/m3) | Specific Magnetic Susceptibility (10−8 m3/Kg) | Relative Magnetism |
|---|---|---|---|---|
| Major manganese minerals | ||||
| Birnessite | (NaO0.3CaO0.1KO0.1) (Mn, Fe)2O4 1.5H2O | 3 | 10 | P |
| Bixbyite | (Mn, Fe)2O3 | 5.12 | 0.0002 | P |
| Braunite | 3(Mn, Fe)2O3·MnSiO3 | 4.72 | 0.01 | P |
| Cryptomelene | KMn8O16 | 4.11 | 0.004 | P |
| Hausmanite | (Mn, Fe)3O4 | 4.76 | 0.006 | P |
| Jacobsite | MnFe2O4 | 4.99 | 500 | F |
| Manganite | MnO·OH | 4.38 | 0.003 | P |
| Psilomelane | BaMn8O16(OH)4 | 5.14 | 0.004 | P |
| Pyrolusite | MnO2 | 4.73 | 0.004 | P |
| Rhodochrosite | MnCO3 | 3.69 | 100 | P |
| Todorokite | (Na, Ca, K, Ba, Sr) (Mn, Mg, Al)6O12.·3H2O | 3.66 | 0.003 | P |
| Major gangue bearing minerals | ||||
| Alumina | Al2O3 | 3.95 | −0.04 | d |
| Calcite | CaCO3 | 2.83 | −0.3–1.4 | d/p |
| Goethite | FeO·OH | 4.27 | 26–280 | F |
| Hematite | Fe2O3 | 5.26 | 10–760 | F |
| Kaolinite | Al2(Si2O5) (OH)4 | 2.65 | −2 | d |
| Limonite | FeO·OH nH2O | 4.2 | 66–74 | P |
| Quartz | SiO2 | 2.65 | −0.5–0.6 | D |
| Ore Deposit Name | Ore Type | Dry/Wet | Type of Separator | Process Condition | Feed Quality | Product Quality and Performance | References |
|---|---|---|---|---|---|---|---|
| Chikla, Maharastra India | Siliceous | D | WHIMS | Mfi:1.2–1.8 tesla | Mn:44% Fe: 7.8%, | Mn: 51%, Mn recovery: 95% | [18,19] |
| Bonai-Keonjhar, Odisha, India | Siliceous | D | DBMS | Mfi: 0.73–1.2 tesla | Mn: 26%, SiO2: 32–34%, Fe: 2.98–7.7% | Mn: 49%, Mn Recovery: 69% | [20] |
| Bonai-Keonjhar, Odisha, India | Siliceous | W | WHIMS | Mfi: 0.73–1.2 tesla | Mn: 25%, SiO2: 34%, Fe: 3–8% | Mn: 42%, Mn recovery: 56% | [21] |
| Garividi-Garnham, Andraparadesh, India | Siliceous | W | WHIMS | Mfi:1.3–1.75 tesla, Size −200 µm | Mn: 26.71%, SiO2: 18.3%, Fe: 12.2% | Mn: 33.71, Mn Recovery: ~52.74–80.94% | [22] |
| Wasagu, Kebbi, Nigeria | - | D | DBMS | Size 80 % passing −355 + 250 μm, | Mn: 38% | Mn: 52%, Mn Recovery: 77% | [23] |
| Kilis region, Turkey | Siliceous | D | IRMS | Size: −9 + 2 mm | Mn: 13.8%, SiO2: 70.59%, Fe: 0.98 | Mn: 49.51%, Mn recovery: 68.5% | [24] |
| Charagah, Tabriz, Iran | Calcitic | W | WHIMS | Mfi: 1.2 to 1.7 tesla, | Mn: 15.4%, SiO2: 3%, CaO: 46% | Mn: 44%, Mn Recovery: 52.2% | [25] |
| Xialei, Guangxi, China | Siliceous | W | HGMS | Mfi: 1.25 tesla | Mn: 21.53%, SiO2: 33.47%, Fe: 8.87% | Mn: 30%, Mn recovery; 64% | [26] |
| Marganetzk, Russia | Siliceous | W | IRMS | Mfi: 0.85 tesla, −10 + 6 mm | Mn: 33.1%, SiO2: 31.9% | Mn: 42.3%, Mn recovery: 97.6% | [27] |
| Eskişehir, Turkey | Siliceous | D | RERMS | Mfi: 0.9 tesla | Mn: 41.12%, SiO2: 36.4%, Fe: 3.75 | Mn: 46.7%, Mn recovery: 69.2% | [13] |
| Joda, Odisha, India | Ferruginous | D | IRMS | Mfi: 0.7–1.9 tesla, −3 mm | Mn: 32.4%, SiO2: 20.7%, Mn: Fe: 2.3 | Mn: 42%, Mn recovery: 47–49% | [28] |
| Joda, Odisha, India | Ferruginous | D | IRMS | Mfi: 0.9–1.6 tesla, | Mn: 22.4%, SiO2: 35.9%, Fe: 14.5% | Mn: 27%, | [29] |
| Hunan province, China | Carbonate | D | HIMS | Mfi: 0.3–1.6 tesla | Mn: 10%, SiO2: 39.07%, Fe: 2% | Mn: 25.4%, Mn recovery: 55.6% | [30] |
| Northern Cape, South Africa | Ferruginous | D | IRMS | Mfi: 1.1–1.6 tesla | Mn: 29.8%, Fe: 19.22, Mn: Fe: 1.55 | Mn: 29.7%, Mn recovery: 59.9% | [31] |
| Parameters | Coded Levels | Actual Levels | ||||
|---|---|---|---|---|---|---|
| Roll speed (rpm) | 1 | 0 | −1 | 195 | 165 | 132 |
| Feed rate (tph/m) | 1 | 0 | −1 | 1.1 | 0.43 | 0.11 |
| Splitter position (deg) | 1 | 0 | −1 | 45° | 90° | 135° |
| Sample | Assay Value (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Fe | SiO2 | Al2O3 | P | K2O | MgO | CaO | TiO2% | S | LOI | Mn:Fe Ratio | |
| Sample 1 | 27.33 | 24.62 | 5.55 | 7.85 | 0.097 | 0.76 | 0.43 | 3.31 | 0.82 | 0.01 | 2.78 | 1.11 |
| Sample 2 | 26.66 | 25.39 | 4.34 | 10.45 | 0.09 | 0.21 | 0.21 | 1.2 | 0.23 | 0.07 | 5.87 | 1.05 |
| Mineral | Weight Fraction (%) | ||
|---|---|---|---|
| Chemical Formula | Sample 1 | Sample 2 | |
| Cryptomelene | (K(Mn4+, Mn2+)8O16 | 11.4 | 13.8 |
| Bixybyite | (Mn, Fe)2O3 | 12.3 | 13.2 |
| Hematite | Fe2O3 | 16.8 | 16.4 |
| Goethite | FeO·OH | 29.0 | 18.9 |
| Gangue | Al-silicates | 5.7 | 5.5 |
| Pyrolusite | MnO2 | 4.1 | 5.5 |
| Mn-Al Phase | Mn spinel’s | 1.5 | 0.1 |
| Quartz | SiO2 | 0.3 | 0.7 |
| Psilomelane | BaMn8O16(OH)4 | 0.9 | 1.2 |
| Others | 11.4 | 13.8 | |
| Test | Process Parameters | Sample 1 | Sample 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roll Speed (rpm) | Feed Rate (tph/mtr) | Splitter Position (deg) | Mn Grade (%) | Mn Recovery (%) | Mn: Fe Ratio | Mn Grade (%) | Mn Recovery (%) | Mn: Fe Ratio | |
| 1 | 132 | 0.11 | 90 | 34.18 | 47.6 | 1.52 | 32.3 | 52.9 | 1.43 |
| 2 | 195 | 0.11 | 90 | 31.71 | 81.8 | 1.4 | 30.5 | 80.7 | 1.32 |
| 3 | 132 | 1.1 | 90 | 36.36 | 43.2 | 1.91 | 30.0 | 45.7 | 1.23 |
| 4 | 195 | 1.1 | 90 | 33.3 | 72.0 | 1.56 | 28.6 | 72.5 | 1.17 |
| 5 | 132 | 0.43 | 45 | 35.13 | 44.7 | 1.79 | 33.8 | 44.2 | 1.66 |
| 6 | 195 | 0.43 | −45 | 31.41 | 71.1 | 1.43 | 27.6 | 71.0 | 1.2 |
| 7 | 132 | 0.43 | 135 | 33.82 | 38.4 | 1.66 | 31.4 | 40.8 | 1.72 |
| 8 | 195 | 0.43 | 135 | 32.54 | 72.1 | 1.52 | 27.5 | 71.2 | 1.35 |
| 9 | 165 | 0.11 | 45 | 32.57 | 64.6 | 1.51 | 29.6 | 67.9 | 1.46 |
| 10 | 165 | 1.1 | 45 | 33.56 | 62.1 | 1.58 | 29.6 | 59.4 | 1.48 |
| 11 | 165 | 0.11 | 135 | 35.52 | 69.2 | 1.77 | 30.1 | 64.5 | 1.46 |
| 12 | 165 | 1.1 | 135 | 34.14 | 60.1 | 1.67 | 30.0 | 61.5 | 1.39 |
| 13 | 165 | 0.43 | 90 | 34.17 | 61.8 | 1.68 | 30.6 | 65.5 | 1.44 |
| 14 | 165 | 0.43 | 90 | 33.81 | 60.9 | 1.61 | 29.1 | 64.1 | 1.39 |
| 15 | 165 | 0.43 | 90 | 34.91 | 62.2 | 1.71 | 31.1 | 65.8 | 1.49 |
| Sample | Sample 1 | Sample 2 | ||
|---|---|---|---|---|
| Response | Primary Effect | Binary Interactive Effect | Ternary Interactive Effect | Primary Effect |
| Grade (%) | RS > FR | - | - | RS |
| Recovery (%) | RS | RS × SP > FR × SP | RS × FR × SP | RS |
| Mn: Fe ratio | RS > FR | - | - | RS |
| Single-Stage Separation | Non-Magnetic Fraction Recleaning | ||||||||
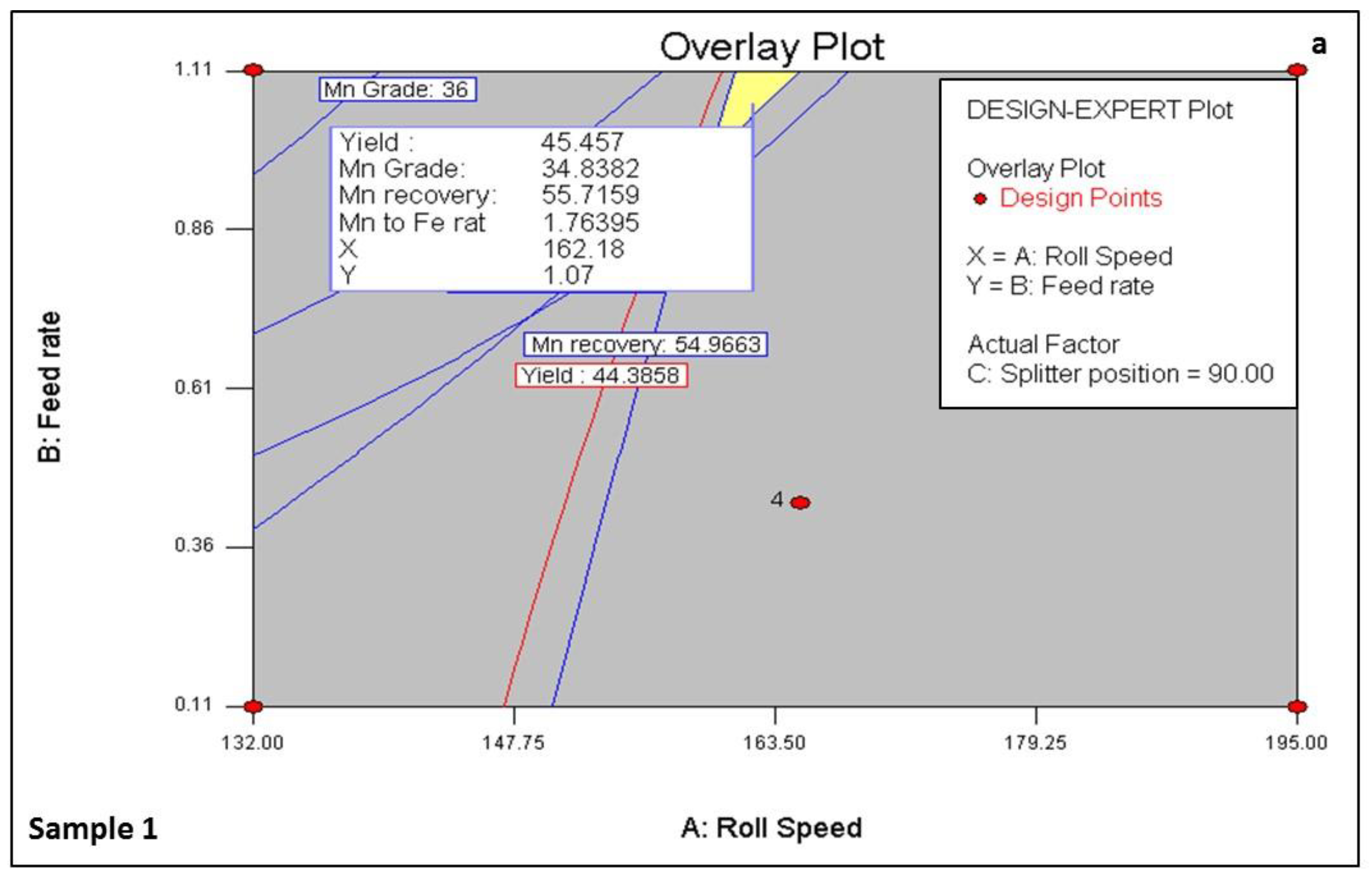
| Sample 1 Non-magnetic cleaning | Test No. | Mn Grade (%) | Mn: Fe Ratio | Yield (%) | Mn Recovery (%) | Mn Grade (%) | Mn: Fe Ratio | Yield (%) | Mn Recovery (%) |
| Exp 2 | 31.71 | 1.4 | 71.4 | 82 | 34.92 | 1.69 | 29 | 36 | |
| Exp 4 | 33.3 | 1.56 | 60.1 | 71 | 36.8 | 1.8 | 20 | 27 | |
| Exp 8 | 32.54 | 1.52 | 72 | 72 | 33.65 | 1.87 | 36 | 44 | |
| Roll 1 | Roll 2 | ||||||||
| Sample 2 Non-magnetic cleaning | Mn Grade (%) | Mn: Fe | Yield (%) | Mn Recovery (%) | Mn Grade (%) | Mn: Fe | Yield (%) | Mn Recovery (%) | |
| Exp 3 | 30.01 | 1.23 | 40.9 | 46 | 29.98 | 1.24 | 42 | 49 | |
| Exp 5 | 33.75 | 1.66 | 36 | 44 | 32.52 | 1.59 | 50.1 | 60 | |
| Exp 7 | 31.37 | 1.71 | 33.7 | 41 | 29.63 | 1.43 | 65 | 74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhoja, S.K.; Tripathy, S.K.; Murthy, Y.R.; Ghosh, T.K.; Kumar, C.R.; Chakraborty, D.P. Influence of Mineralogy on the Dry Magnetic Separation of Ferruginous Manganese Ore—A Comparative Study. Minerals 2021, 11, 150. https://doi.org/10.3390/min11020150
Bhoja SK, Tripathy SK, Murthy YR, Ghosh TK, Kumar CR, Chakraborty DP. Influence of Mineralogy on the Dry Magnetic Separation of Ferruginous Manganese Ore—A Comparative Study. Minerals. 2021; 11(2):150. https://doi.org/10.3390/min11020150
Chicago/Turabian StyleBhoja, Sharath Kumar, Sunil Kumar Tripathy, Yanamandra Rama Murthy, Tamal Kanti Ghosh, C. Raghu Kumar, and Deba Prasad Chakraborty. 2021. "Influence of Mineralogy on the Dry Magnetic Separation of Ferruginous Manganese Ore—A Comparative Study" Minerals 11, no. 2: 150. https://doi.org/10.3390/min11020150
APA StyleBhoja, S. K., Tripathy, S. K., Murthy, Y. R., Ghosh, T. K., Kumar, C. R., & Chakraborty, D. P. (2021). Influence of Mineralogy on the Dry Magnetic Separation of Ferruginous Manganese Ore—A Comparative Study. Minerals, 11(2), 150. https://doi.org/10.3390/min11020150







