Quantitative Analyses of Urinary Uranium by µ-PIXE
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation for Uranium Specimens Using 0.1 M Nitric Acid
2.2. Preparation for Uranium Specimens Using Mixed Solutions of Urine with Nitric Acid
2.3. Animal Experiments
2.4. µ-PIXE Measurement
3. Results and Discussion
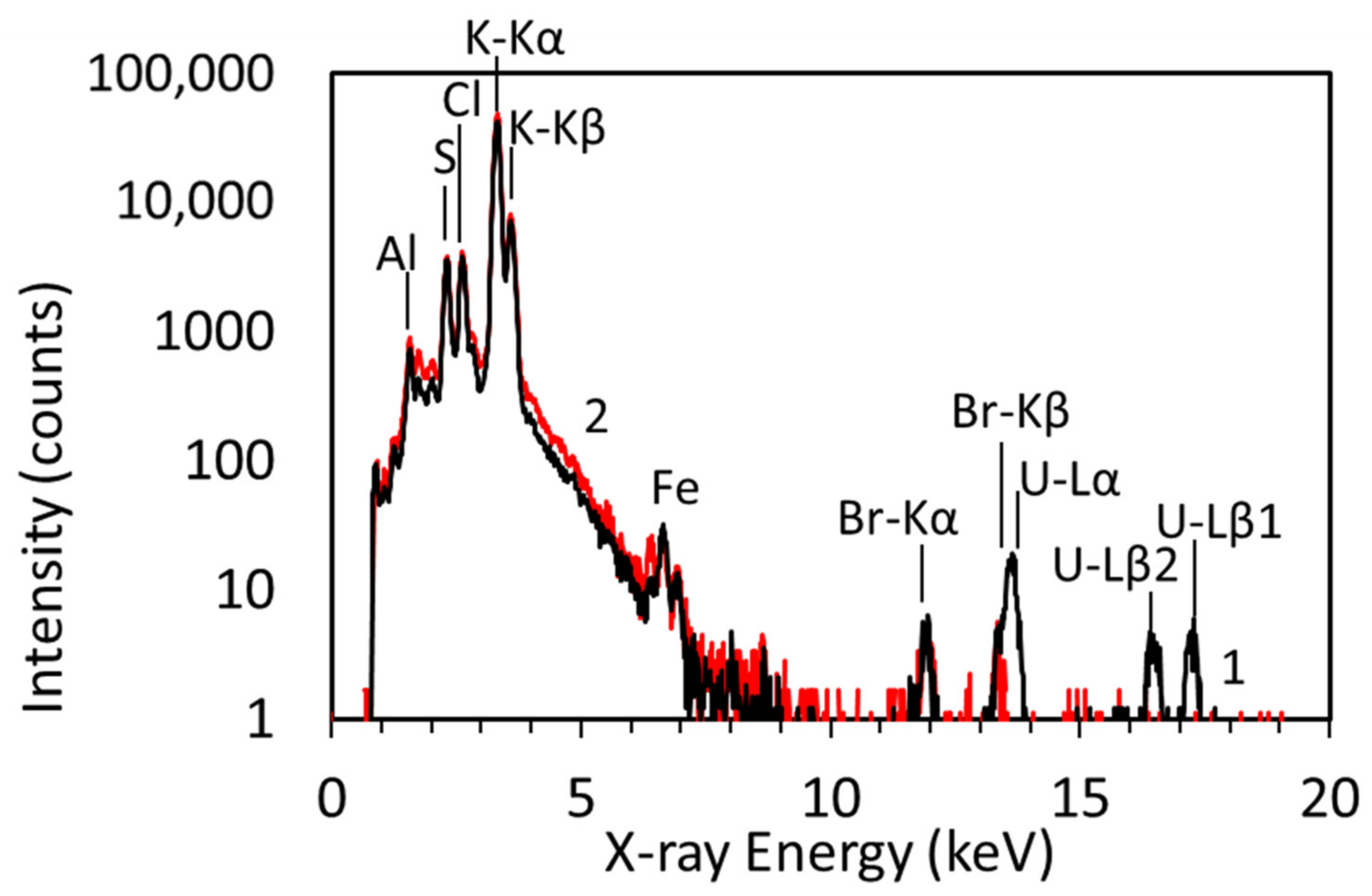
3.1. Uranium Distribution and Concentration Dependence of U Specimen Prepared Using HNO3
3.2. Quantification of U in Urine Collected from U Injected Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Thakur, P.; Ward, A.L. An overview of analytical methods for in vitro bioassay of actinides. Health Phys. 2019, 116, 694–714. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W. The behavior and chemical toxicity of U in the kidney: A reassessment. Health Phys. 1989, 57, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Vidaud, C.; Bourgeois, D.; Meyer, D. Bone as target organ for metals: The case of f-elements. Chem. Res. Toxicol. 2012, 25, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Biological Effects of Selected Internal Emitters-Uranium. ANNEX D. 2016. Available online: https://www.unscear.org/unscear/en/publications/2016.html (accessed on 31 January 2021).
- Ivanenko, N.B.; Ganeev, A.A.; Solovyev, N.D.; Moskvin, L.N. Determination of trace elements in biological fluids. J. Anal. Chem. 2011, 66, 784–799. [Google Scholar] [CrossRef]
- Israelsson, A.; Pettersson, H. Measurements of (234)U and (238)U in hair, urine, and drinking water among drilled bedrock well water users for the evaluation of hair as a biomonitor of uranium intake. Health Phys. 2014, 107, 143–149. [Google Scholar] [CrossRef]
- Byrne, A.R.; Benedik, L. Uranium content of blood, urine and hair of exposed and non-exposed persons determined by radiochemical neutron-activation analysis, with emphasis on quality-control. Sci. Total. Environ. 1991, 107, 143–157. [Google Scholar] [CrossRef]
- Moorthy, A.R.; Schopfer, C.J.; Banerjee, S. Plutonium from atmospheric weapons testing-fission-track analysis of urine samples. Anal. Chem. 1988, 60, A857–A860. [Google Scholar] [CrossRef]
- Zarkadas, C.; Karydas, A.G.; Paradellis, T. Determination of uranium in human urine by total reflection X-ray fluorescence. Spectrochim Acta B 2001, 56, 2505–2511. [Google Scholar] [CrossRef]
- Karpas, Z.; Halicz, L.; Roiz, J.; Marko, R.; Katorza, E.; Lorber, A.; Goldbart, Z. Inductively coupled plasma mass spectrometry as a simple, rapid, and inexpensive method for determination of uranium in urine and fresh water: Comparison with LIF. Health Phys. 1996, 71, 879–885. [Google Scholar] [CrossRef]
- Karpas, Z.; Paz-Tal, O.; Lorber, A.; Salonen, L.; Komulainen, H.; Auvinen, A.; Saha, H.; Kurttio, P. Urine, hair, and nails as indicators for ingestion of uranium in drinking water. Health Phys. 2005, 88, 229–242. [Google Scholar] [CrossRef]
- Muikku, M.; Puhakainen, M.; Heikkinen, T.; Ilus, T. The mean concentration of uranium in drinking water, urine, and hair of the occupationally unexposed Finnish working population. Health Phys. 2009, 96, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Kristsananuwat, R.; Fukushi, M. Actinide analysis in biological materials. Radiat. Emerg. Med. 2012, 1, 22–26. [Google Scholar]
- Lee, Y.K.; Bakhtiar, S.N.; Akbarzadeh, M.; Lee, J.S. Sequential isotopic determination of strontium, thorium, plutonium, uranium, and americium in bioassay samples. J. Radioanal Nucl. Chem. 2000, 243, 525–533. [Google Scholar] [CrossRef]
- Oeh, U.; Andrasi, A.; Bouvier-Capely, C.; de Carlan, L.; Fischer, H.; Franck, D.; Hollriegl, V.; Li, W.B.; Ritt, J.; Roth, P.; et al. Implementation of bioassay methods to improve assessment of incorporated radionuclides. Radiat. Prot. Dosim. 2007, 125, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Futatsugawa, S.; Saitoh, Y.; Ojima, F.; Sera, K. Application of a powdered-internal-standard method to plant and seaweeed samples. Int. J. PIXE 2005, 15, 27–39. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Yaginuma, N.; Iso, H.; Ishikawa, T.; Imaseki, H.; Homma-Takeda, S. Mercury distribution by micro pixe analysis in stenopsyche marmorata exposed to mercuric chloride. Int. J. PIXE 2008, 18, 69–75. [Google Scholar] [CrossRef]
- Yukawa, M.; Aoki, K.; Iso, H.; Kodama, K.; Imaseki, H.; Ishikawa, Y. Determination of the metal balance shift induced in small fresh water fish by X-ray irradiation using PIXE analysis. J. Radioanal Nucl. Chem. 2007, 272, 345–352. [Google Scholar] [CrossRef]
- Sera, K.; Miura, Y.; Futatsugawa, S. Application of a standard-free method to quantitative analysis of urine samples. Int. J. PIXE 2001, 11, 149–158. [Google Scholar] [CrossRef]
- Kennedy, V.J.; Augusthy, A.; Varier, K.M.; Magudapathy, P.; Panchapakesan, S.; Nair, G.M. Trace element analysis of mineral water samples by PIXE and ICP-MS. Int. J. PIXE 1998, 8, 11–18. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Iso, H.; Ito, M.; Suzuki, K.; Harumoto, K.; Yoshitomi, T. Evaluation of pressed powders and thin section standards for multi-elemental analysis by conventional and micro-pixe analysis. Int. J. PIXE 2010, 20, 21–28. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Suzuki, K.; Harumoto, K.; Yoshitomi, T.; Iso, H.; Ishikawa, T.; Konishi, T.; Oikawa, M. Evaluation of thin section standards for local analysis of light elements by micro-pixe analysis. Int. J. PIXE 2011, 21, 25–30. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawasaki, T.; Takao, K.; Harada, M.; Nogami, M.; Ikeda, Y. A study on selective precipitation ability of cyclic urea to U (VI) for developing reprocessing system based on precipitation method. J. Nucl. Sci. Technol. 2012, 49, 1010–1017. [Google Scholar] [CrossRef][Green Version]
- Homma-Takeda, S.; Numako, C.; Kitahara, K.; Yoshida, T.; Oikawa, M.; Terada, Y.; Kokubo, T.; Shimada, Y. Phosphorus localization and its involvement in the formation of concentrated uranium in the renal proximal tubules of rats exposed to uranyl acetate. Int. J. Mol. Sci. 2019, 20, 4677. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Oikawa, M.; Tanaka, I.; Ishihara, H.; Homma-Takeda, S. Sample preparation for the elemental analysis in biological fluids by micro-PIXE. Adv. X-Ray Chem. Anal. 2020, 51, 81–90. [Google Scholar]
- Homma-Takeda, S.; Terada, Y.; Nakata, A.; Sahoo, S.K.; Yoshida, S.; Ueno, S.; Inoue, M.; Iso, H.; Ishikawa, T.; Konishi, T.; et al. Elemental imaging of kidneys of adult rats exposed to uranium acetate. Nucl. Instrum. Meth. B 2009, 267, 2167–2170. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Kitahara, K.; Suzuki, K.; Blyth, B.J.; Suya, N.; Konishi, T.; Terada, Y.; Shimada, Y. Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol. 2015, 35, 1594–1600. [Google Scholar] [CrossRef]
- Oikawa, M.; Suya, N.; Konishi, T.; Ishikawa, T.; Hamano, T.; Homma-Takeda, S. Micro-PIXE analysis system at NIRS-electrostatic accelerator facility for various applications. Int. J. PIXE 2015, 25, 217–225. [Google Scholar] [CrossRef]
- Abuku, S.; Tanaka, S.; Seki, Y.; Imamiya, S. Determination of bromide ions in total blood, plasma and urine by ion chromatography with amperometric detection. Jpn. J. Hyg. 1989, 44, 945–952. [Google Scholar] [CrossRef]
- Goulle, J.P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Laine, G.; Bouige, D.; Lacroix, C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: Reference values. Forensic. Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef]
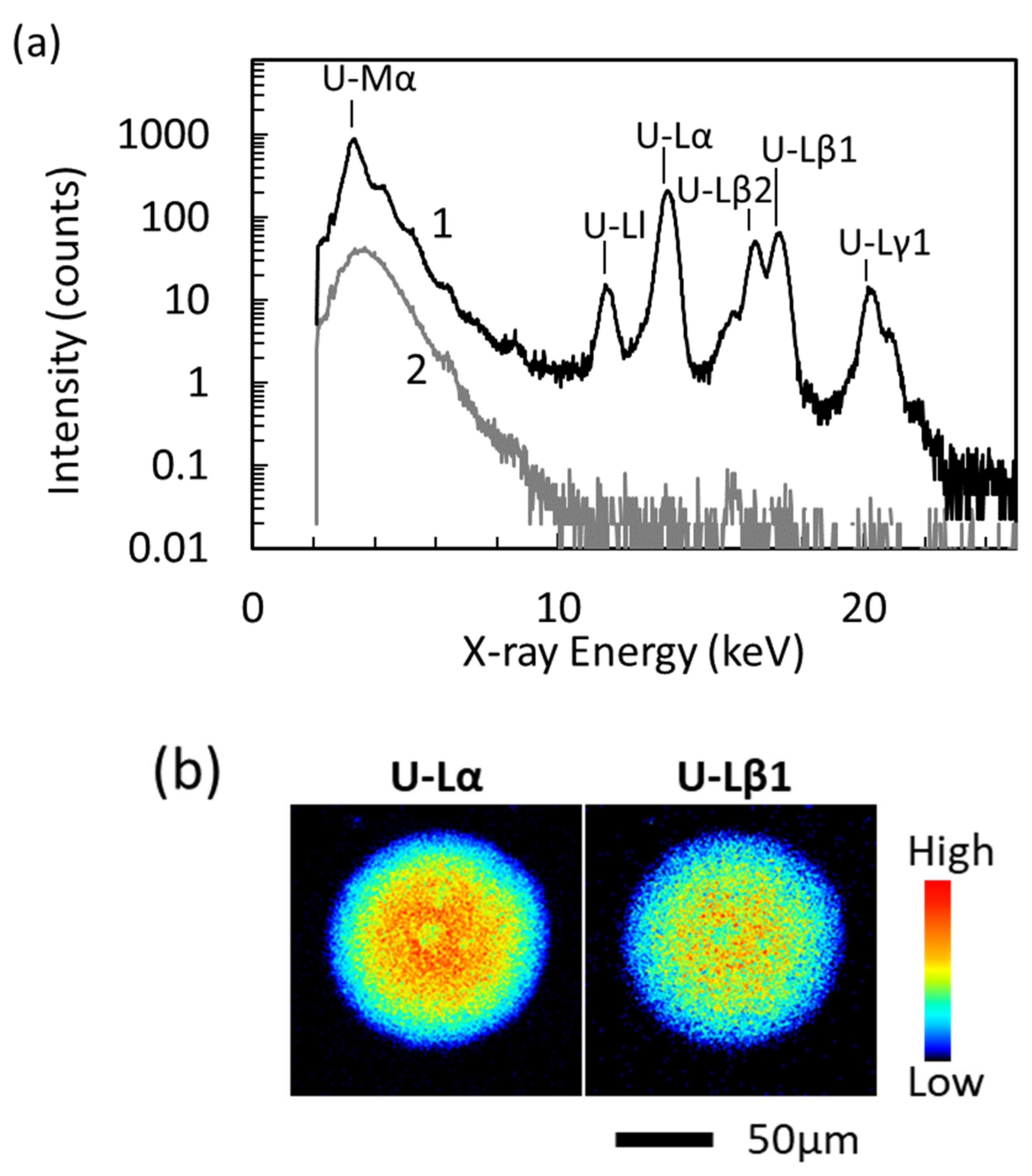
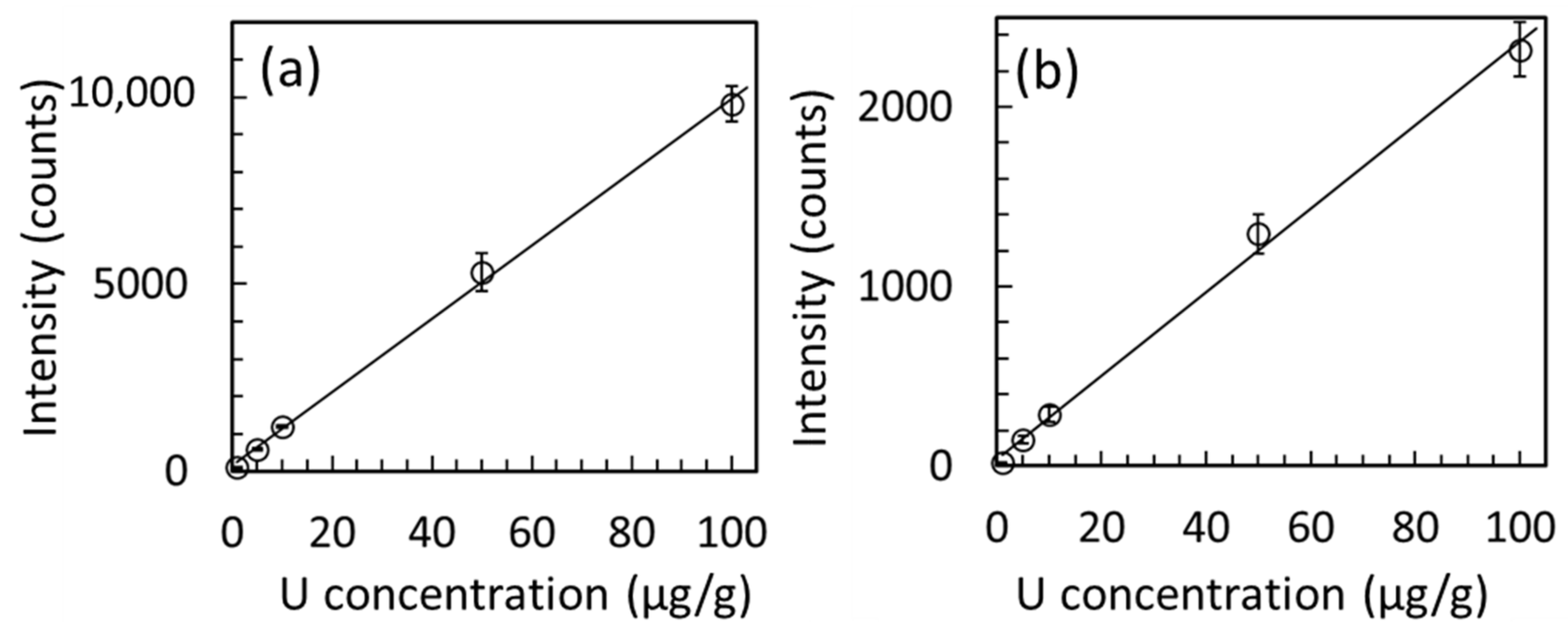
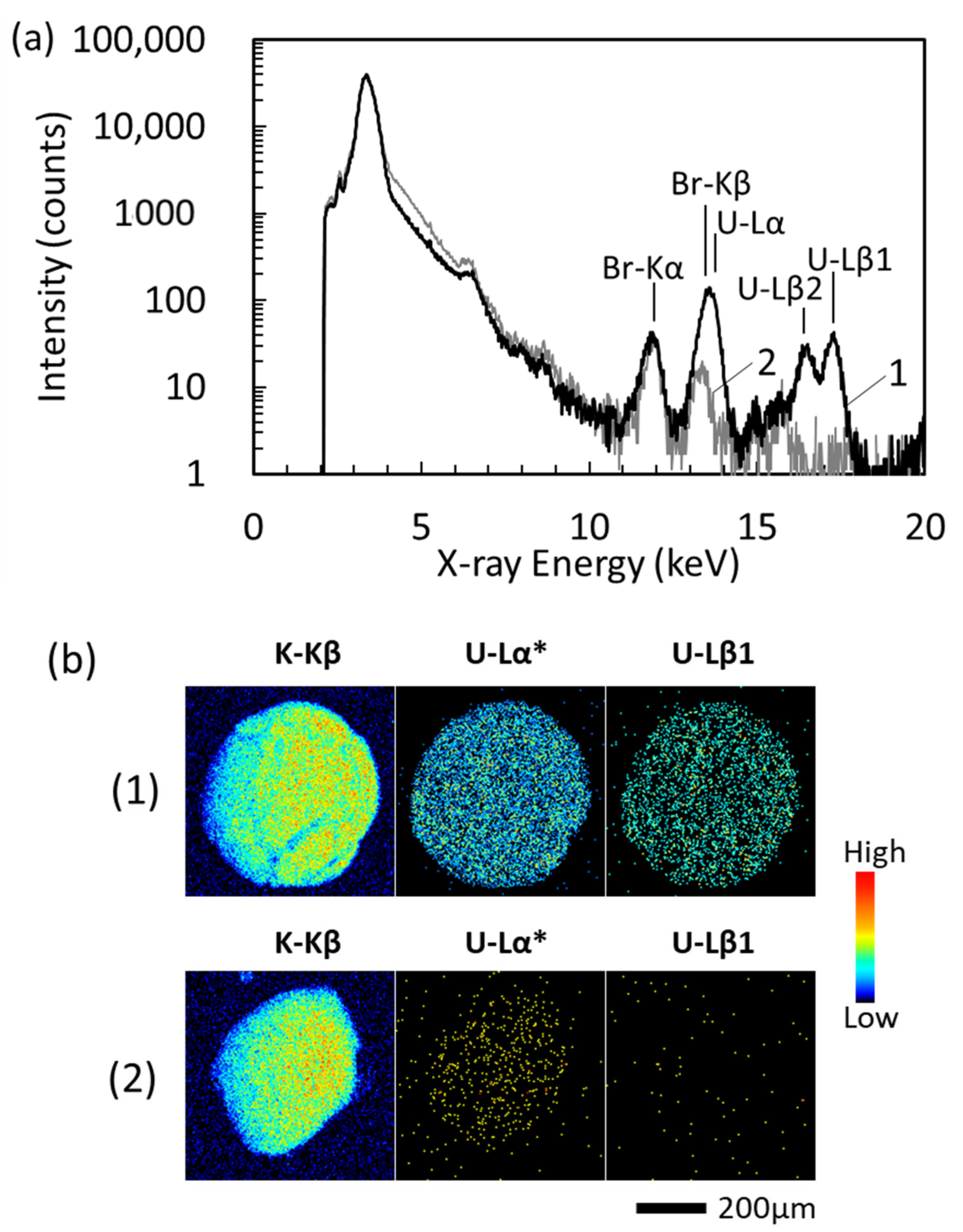

| U Concentration * (µg/g) | U-Lα line | U-Lβ1 line | ||
|---|---|---|---|---|
| Intensity (Counts ± SD) | RSD (%) | Intensity (Counts ± SD) | RSD (%) | |
| 1 | 103 ± 5 | 5 | 19 ± 4 | 21 |
| 5 | 579 ± 40 | 7 | 148 ± 21 | 14 |
| 10 | 1191 ± 47 | 4 | 286 ± 44 | 15 |
| 50 | 5322 ± 515 | 10 | 1294 ± 110 | 9 |
| 100 | 9811 ± 482 | 5 | 2321 ± 151 | 7 |
| U Concentration * (µg/g) | U-Lα line | U-Lβ1 line | ||
|---|---|---|---|---|
| Intensity (Counts ± SD) | RSD (%) | Intensity (Counts ± SD) | RSD (%) | |
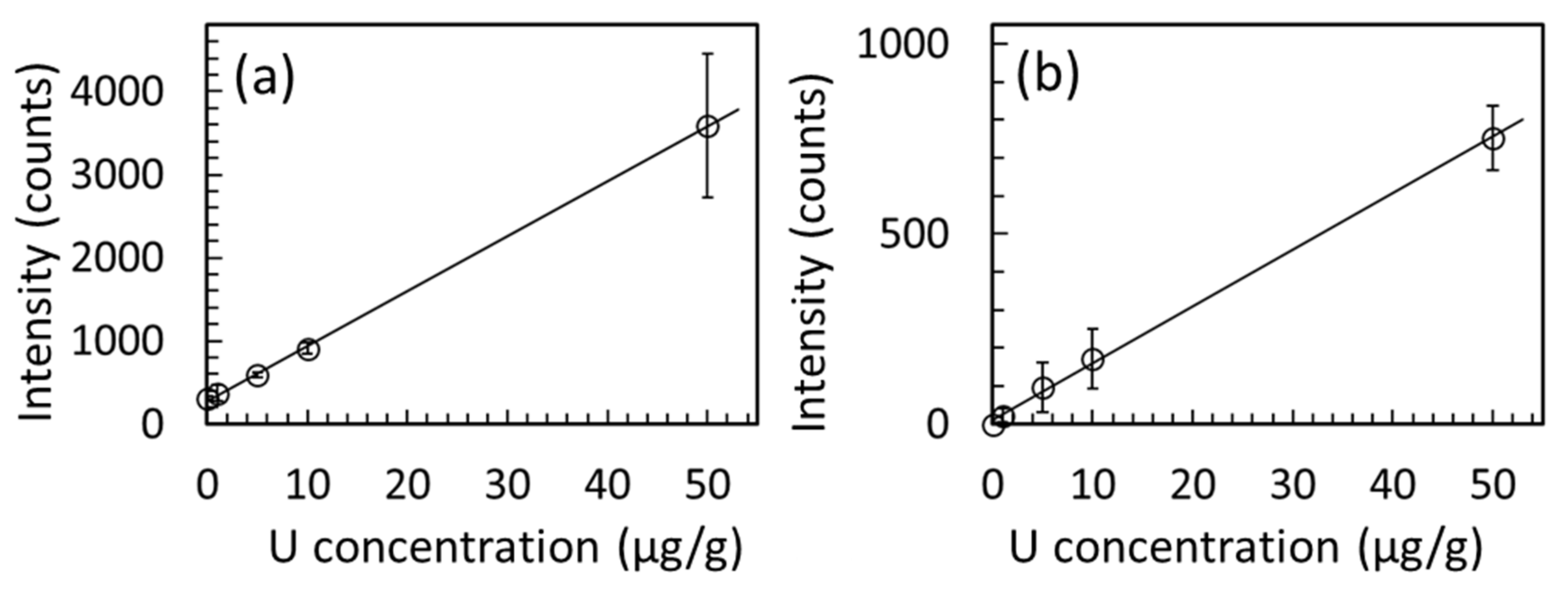
| 0 | 311 ± 21 | 7 | 0 | 0 |
| 1 | 375 ± 95 | 25 | 23 ± 18 | 78 |
| 5 | 589 ± 31 | 5 | 97 ± 64 | 66 |
| 10 | 913 ± 62 | 7 | 172 ± 79 | 46 |
| 50 | 3589 ± 869 | 24 | 753 ± 85 | 11 |
| Administration U (mg/kg) | U Concentration (µg/g) | |
|---|---|---|
| U-Lβ1 Line * | ICP-MS | |
| 0.5 | 18.5 ± 12.5 | 14.1 ** |
| 4 | 50.7 ± 9.0 | 53.9 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, A.; Oikawa, M.; Tanaka, I.; Ishihara, H.; Homma-Takeda, S. Quantitative Analyses of Urinary Uranium by µ-PIXE. Minerals 2021, 11, 147. https://doi.org/10.3390/min11020147
Uehara A, Oikawa M, Tanaka I, Ishihara H, Homma-Takeda S. Quantitative Analyses of Urinary Uranium by µ-PIXE. Minerals. 2021; 11(2):147. https://doi.org/10.3390/min11020147
Chicago/Turabian StyleUehara, Akihiro, Masakazu Oikawa, Izumi Tanaka, Hiroshi Ishihara, and Shino Homma-Takeda. 2021. "Quantitative Analyses of Urinary Uranium by µ-PIXE" Minerals 11, no. 2: 147. https://doi.org/10.3390/min11020147
APA StyleUehara, A., Oikawa, M., Tanaka, I., Ishihara, H., & Homma-Takeda, S. (2021). Quantitative Analyses of Urinary Uranium by µ-PIXE. Minerals, 11(2), 147. https://doi.org/10.3390/min11020147






