Single-Cell Imaging for Studies of Renal Uranium Transport and Intracellular Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Uranium Treatment
2.3. Cytotoxicity Studies
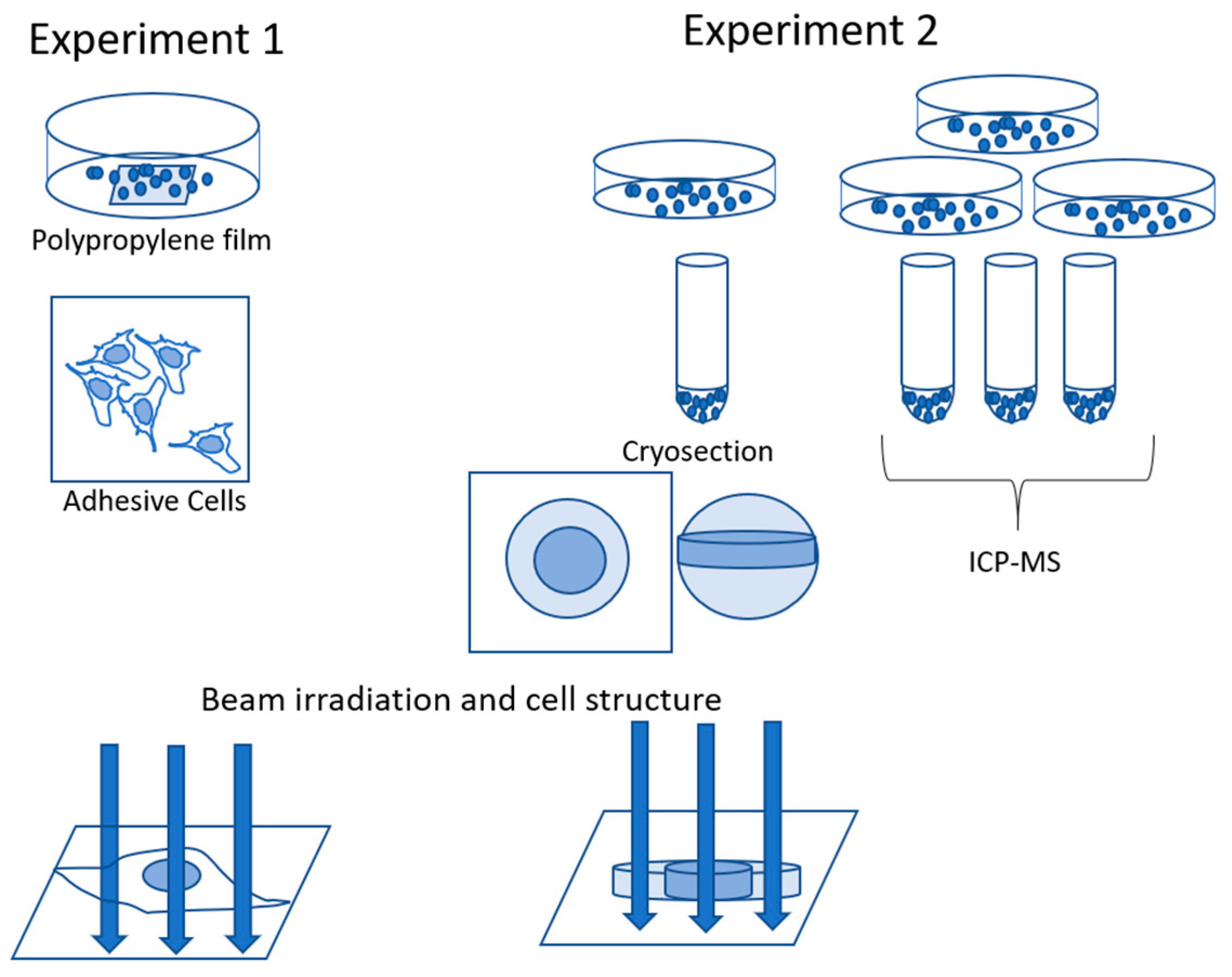
2.4. Specimen Preparation for Uranium Imaging
2.4.1. Experiment 1: Adhesive Cells
2.4.2. Experiment 2: Cryosections of Cells
2.5. Uranium Uptake Experiment
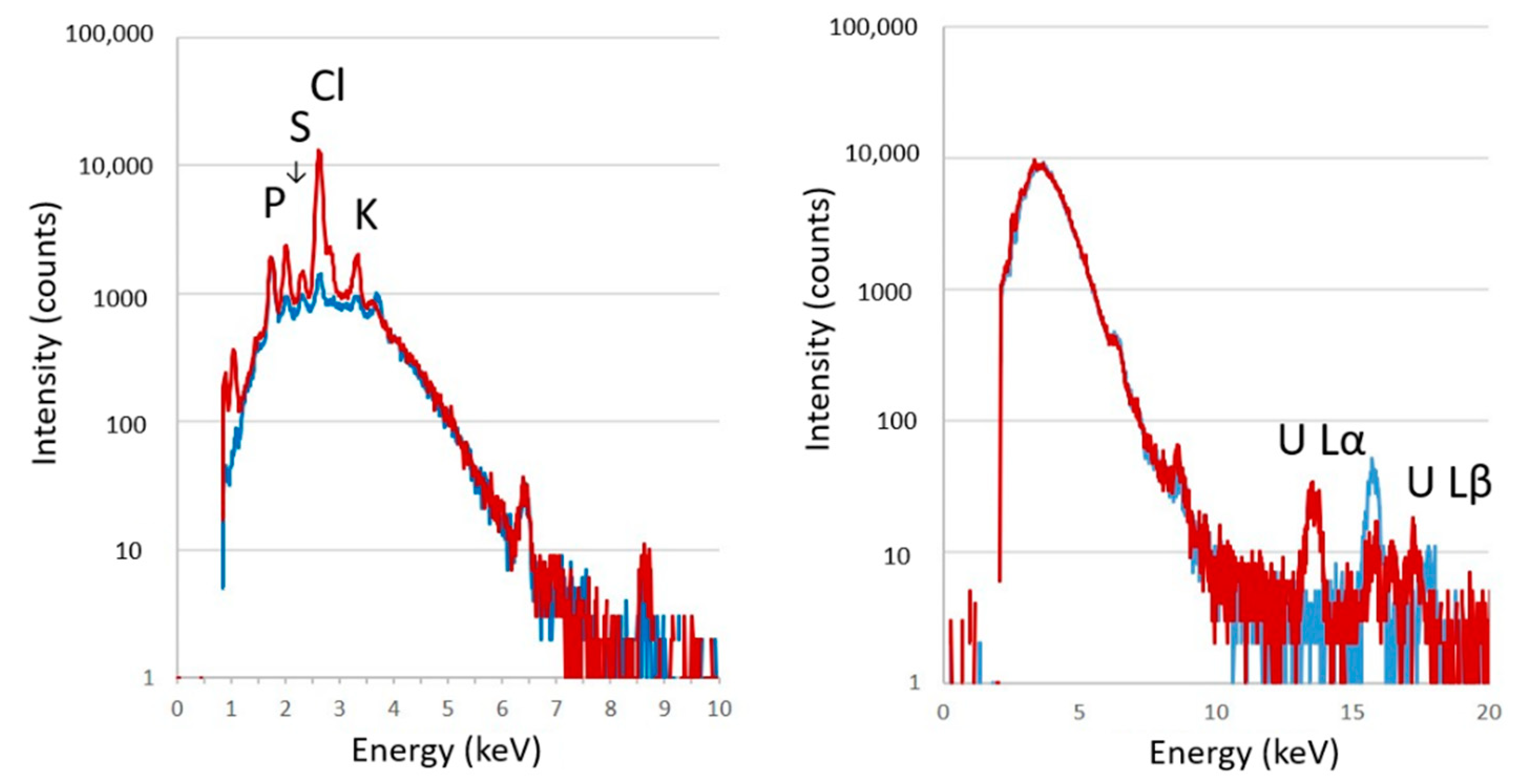
2.6. μ-PIXE Analysis
3. Results
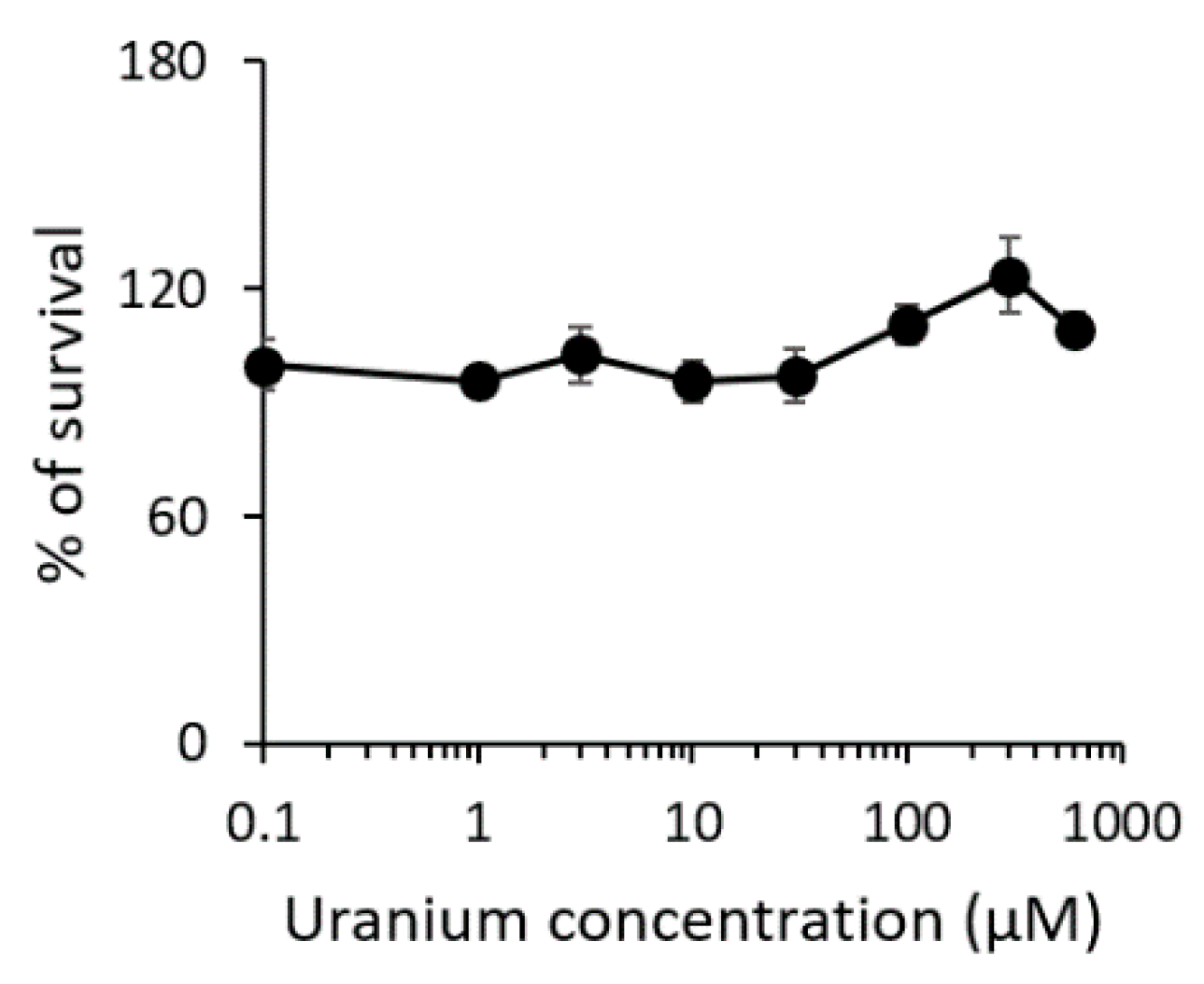
3.1. Sensitivity of S3 Cells to Uranium
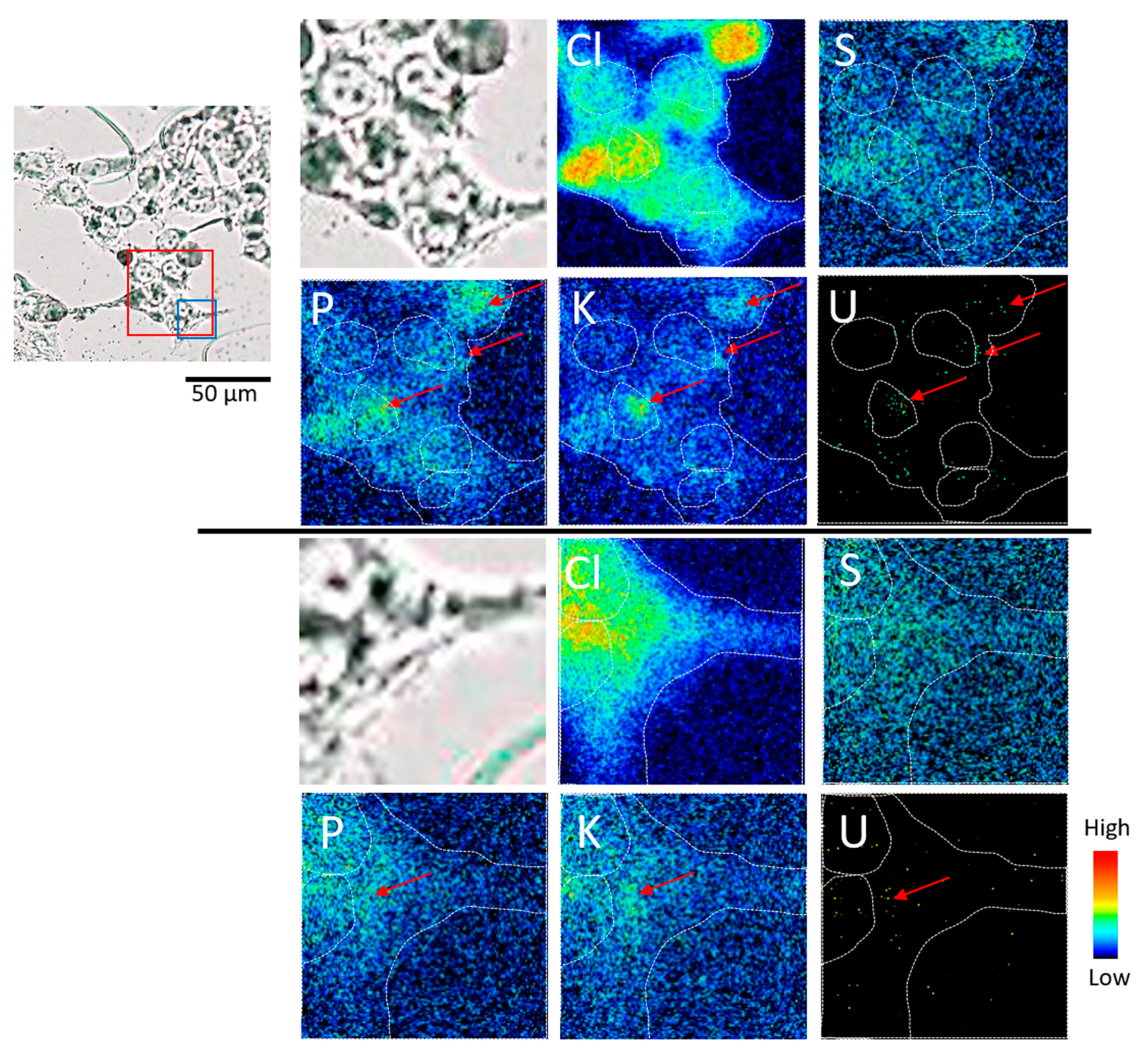
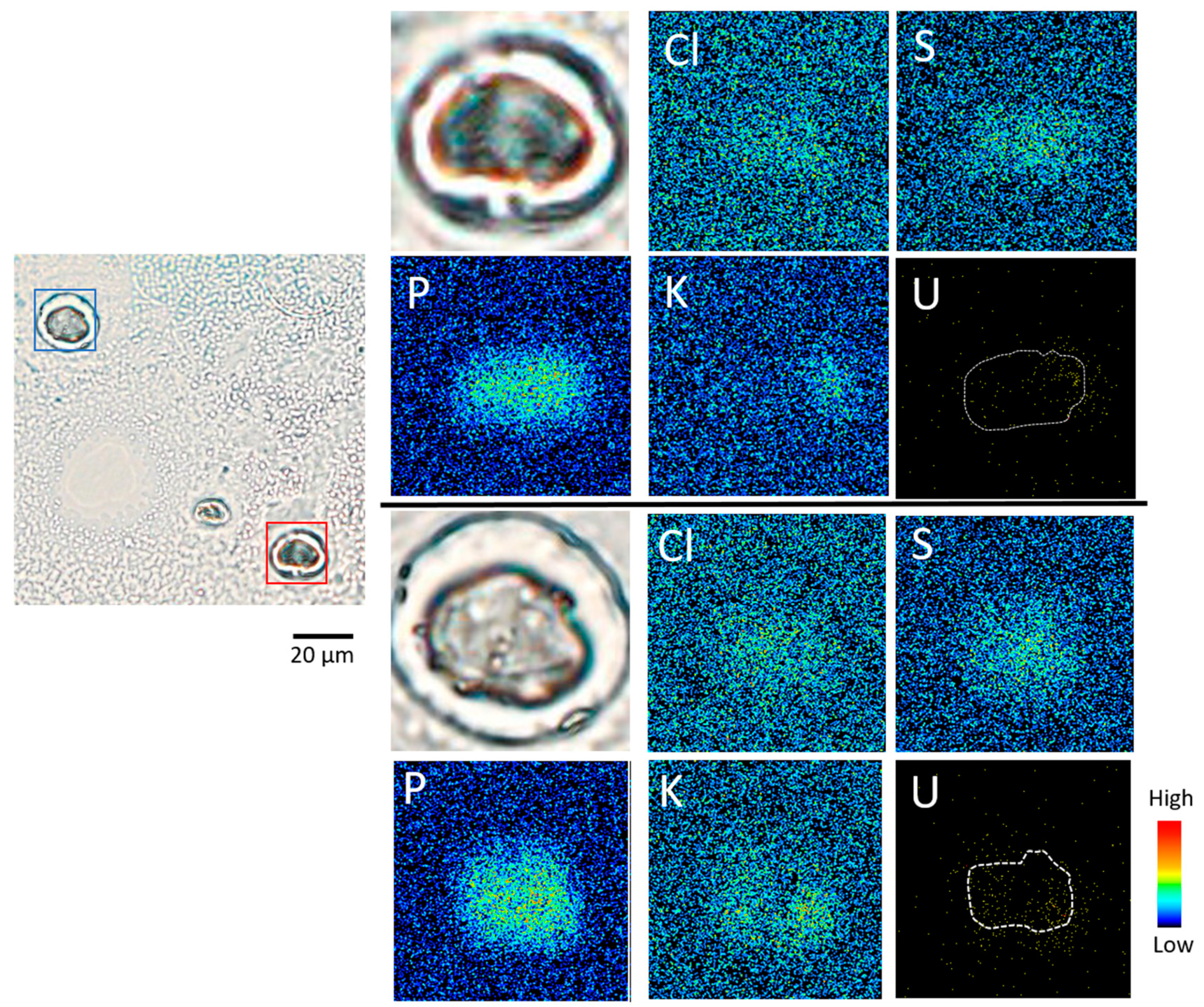
3.2. Elemental Imaging of S3 Adhesive Cells (Experiment 1)
3.3. Elemental Imaging of Cryosections of S3 Cells (Experiment 2)
3.4. Amount of Uranium Accumulated in Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiraishi, K.; Tagami, K.; Muramastu, Y.; Yamamoto, M. Contributions of 18 food categories to intakes of 232Th and 238U in Japan. Health Phys. 2000, 78, 28–36. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. IPCS Assessing Human Health Risks of Chemicals: Derivation of Guidance Values for Health-Based Exposure Limits; International Programme on Chemical Safety: Geneva, Switzerland, 1994. [Google Scholar]
- Food Safety Committee. Document on Safety of Dietary Cadmium Intake; Minster of Health, Labour and Welfare of Japan: Tokyo, Japan, 2008.
- WHO. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2011; Volume 1, pp. 430–431, 475. [Google Scholar]
- Uchida, S.T.K.; Tabei, K.; Hirai, I. Concentrations of REEs, Th and U in river waters collected in Japan. J. Alloy. Compd. 2006, 408/412, 525–528. [Google Scholar] [CrossRef]
- Kurttio, P.; Auvinen, A.; Salonen, L.; Saha, H.; Pekkanen, J.; Mäkeläinen, I.; Väisänen, S.B.; Penttilä, I.M.; Komulainen, H. Renal effects of uranium in drinking water. Environ. Health Perspect. 2002, 110, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Magdo, H.S.; Forman, J.; Graber, N.; Newman, B.; Klein, K.; Satlin, L.; Amler, R.W.; Winston, J.A.; Landrigan, P.J. Grand rounds: Nephrotoxicity in a young child exposed to uranium from contaminated well water. Environ. Health Perspect. 2007, 115, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- La Touche, Y.D.; Willis, D.L.; Dawydiak, O.I. Absorption and biokinetics of U in rats following an oral administration of uranyl nitrate solution. Health Phys. 1987, 53, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Paquet, F.; Houpert, P.; Blanchardon, E.; Delissen, O.; Maubert, C.; Dhieux, B.; Moreels, A.M.; Frelon, S.; Gourmelon, P. Accumulation and distribution of uranium in rats after chronic exposure by ingestion. Health Phys. 2006, 90, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; Quiros, Y.; Pérez-Barriocanal, F.; López-Novoa, J.M.; López-Hernández, F.J.; Morales, A.I. Nephrotoxicity of uranium: Pathophysiological, diagnostic and therapeutic perspectives. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 118, 324–347. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation: Biological Effects of Selected Internal Emitters—Uranium; United Nations (UN): New York, NY, USA, 2016. [Google Scholar]
- Leggett, R.W. The behavior and chemical toxicity of U in the kidney: A reassessment. Health Phys. 1989, 57, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, Y.; Goto, T.; Sakakima, M.; Fukasawa, H.; Miyaji, T.; Yamamoto, T.; Hishida, A. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2006, 21, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, F.Y. Nephrotoxic limit and annual limit on intake for natural U. Health Phys. 1990, 58, 619–623. [Google Scholar] [PubMed]
- Homma-Takeda, S.; Terada, Y.; Nakata, A.; Sahoo, S.K.; Yoshida, S.; Ueno, S.; Inoue, M.; Iso, H.; Ishikawa, T.; Konishi, T.; et al. Elemental imaging of kidneys of adult rats exposed to uranium acetate. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 2167–2170. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Kokubo, T.; Terada, Y.; Suzuki, K.; Ueno, S.; Hayao, T.; Inoue, T.; Kitahara, K.; Blyth, B.J.; Nishimura, M.; et al. Uranium dynamics and developmental sensitivity in rat kidney. J. Appl. Toxicol. 2013, 33, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Homma-Takeda, S.; Kitahara, K.; Suzuki, K.; Blyth, B.J.; Suya, N.; Konishi, T.; Terada, Y.; Shimada, Y. Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol. 2015, 35, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, K.; Numako, C.; Terada, Y.; Nitta, K.; Shimada, Y.; Homma-Takeda, S. Uranium XAFS analysis of kidney from rats exposed to uranium. J. Synchrotron Radiat. 2017, 24 Pt 2, 456–462. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Numako, C.; Kitahara, K.; Yoshida, T.; Oikawa, M.; Terada, Y.; Kokubo, T.; Shimada, Y. Phosphorus Localization and Its Involvement in the Formation of Concentrated Uranium in the Renal Proximal Tubules of Rats Exposed to Uranyl Acetate. Int. J. Mol. Sci. 2019, 20, 4677. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Hosoyamada, M.; Shirato, I.; Obinata, M.; Suzuki, M.; Endou, H. Establishment of vasopressin-responsive early proximal tubular cell lines derived from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Biochem. Mol. Biol. Int. 1995, 37, 507–515. [Google Scholar] [PubMed]
- Takeda, M.; Kobayashi, M.; Endou, H. Establishment of a mouse clonal early proximal tubule cell line and outer medullary collecting duct cells expressing P2 purinoceptors. Biochem. Mol. Biol. Int. 1998, 44, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, M.; Obinata, M.; Suzuki, M.; Endou, H. Cisplatin-induced toxicity in immortalized renal cell lines established from transgenic mice harboring temperature sensitive SV40 large T-antigen gene. Arch. Toxicol. 1996, 70, 284–292. [Google Scholar] [CrossRef]
- Fujishiro, H.H.S. Gene expression profiles of immortalized S1, S2, and S3 cells derived from each segment of mouse kidney proximal tubules. Fund. Toxicol. Sci. 2019, 6, 117–123. [Google Scholar] [CrossRef]
- Fujishiro, H.; Hamao, S.; Isawa, M.; Himeno, S. Segment-specific and direction-dependent transport of cadmium and manganese in immortalized S1, S2, and S3 cells derived from mouse kidney proximal tubules. J. Toxicol. Sci. 2019, 44, 611–619. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yamamoto, H.; Otera, N.; Oka, N.; Jinno, M.; Himeno, S. In Vitro Evaluation of the Effects of Cadmium on Endocytic Uptakes of Proteins into Cultured Proximal Tubule Epithelial Cells. Toxics 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H.; Okugaki, S.; Kubota, K.; Fujiyama, T.; Miyataka, H.; Himeno, S. The role of ZIP8 down-regulation in cadmium-resistant metallothionein-null cells. J. Appl. Toxicol. 2009, 29, 367–373. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Iso, H.; Ito, M.; Suzuki, K.; Harumoto, K.; Yoshitomi, T.; Ishikawa, T.; Oikawa, M.; Suya, N.; Konishi, T.; et al. Evaluation of Pressed Powders and Thin Section Standards for Multi-Elemental Analysis by Conventional and Micro-PIXE Analysis. Int. J. PIXE 2010, 20, 21–28. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Terada, Y.; Iso, H.; Ishikawa, T.; Oikawa, M.; Konishi, T.; Imaseki, H.; Shimada, Y. Rubidium distribution in kidneys of rimmature ats. Int. J. PIXE 2009, 19, 39–45. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Nishimura, Y.; Iso, H.; Ishikawa, T.; Imaseki, H.; Yukawa, M. A new approach for standard preparation in microbeam analysis: Development and validation. J. Radioanal. Nucl. Chem. 2008, 279, 627–631. [Google Scholar] [CrossRef]
- Sato, C.; Sato, M.; Ogawa, S. Imaging of immunogold labeling in cells and tissues by helium ion microscopy. Int. J. Mol. Med. 2018, 42, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Roudeau, S.; Carmona, A.; Perrin, L.; Ortega, R. Correlative organelle fluorescence microscopy and synchrotron X-ray chemical element imaging in single cells. Anal. Bioanal. Chem. 2014, 406, 6979–6991. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Malard, V.; Avazeri, E.; Roudeau, S.; Porcaro, F.; Paredes, E.; Vidaud, C.; Bresson, C.; Ortega, R. Uranium exposure of human dopaminergic cells results in low cytotoxicity, accumulation within sub-cytoplasmic regions, and down regulation of MAO-B. Neurotoxicology 2018, 68, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Suhard, D.; Tessier, C.; Manens, L.; Rebière, F.; Tack, K.; Agarande, M.; Guéguen, Y. Intracellular uranium distribution: Comparison of cryogenic fixation versus chemical fixation methods for SIMS analysis. Microsc. Res. Tech. 2018, 81, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Guéguen, Y.; Suhard, D.; Poisson, C.; Manens, L.; Elie, C.; Landon, G.; Bouvier-Capely, C.; Rouas, C.; Benderitter, M.; Tessier, C. Low-concentration uranium enters the HepG2 cell nucleus rapidly and induces cell stress response. Toxicol. In Vitro Int. J. Publ. Assoc. Bibra 2015, 30, 552–560. [Google Scholar] [CrossRef]
- Ghadially, F.N.; Lalonde, J.M.; Yang-Steppuhn, S. Uraniosomes produced in cultured rabbit kidney cells by uranyl acetate. Virchows Arch. Bcell Pathol. Incl. Mol. Pathol. 1982, 39, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mirto, H.; Henge-Napoli, M.H.; Gibert, R.; Ansoborlo, E.; Fournier, M.; Cambar, J. Intracellular behaviour of uranium(VI) on renal epithelial cell in culture (LLC-PK1): Influence of uranium speciation. Toxicol. Lett. 1999, 104, 249–256. [Google Scholar] [CrossRef]
- Milgram, S.; Carriere, M.; Malaval, L.; Gouget, B. Cellular accumulation and distribution of uranium and lead in osteoblastic cells as a function of their speciation. Toxicology 2008, 252, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Basset, C.; Averseng, O.; Quemeneur, E.; Hagege, A.; Vidaud, C. Characterization of UO2(2+) binding to osteopontin, a highly phosphorylated protein: Insights into potential mechanisms of uranyl accumulation in bones. Met. Integr. Biometal Sci. 2014, 6, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Raff, J.; Barkleit, A.; Bernhard, G.; Foerstendorf, H. Complexation of U(VI) with highly phosphorylated protein, phosvitin A vibrational spectroscopic approach. J. Inorg. Biochem. 2010, 104, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Pible, O.; Vidaud, C.; Plantevin, S.; Pellequer, J.L.; Quemeneur, E. Predicting the disruption by UO2(2+) of a protein-ligand interaction. Protein Sci. Publ. Protein Soc. 2010, 19, 2219–2230. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homma-Takeda, S.; Fujishiro, H.; Tanaka, I.; Yakumaru, H.; Ayama, K.; Uehara, A.; Oikawa, M.; Himeno, S.; Ishihara, H. Single-Cell Imaging for Studies of Renal Uranium Transport and Intracellular Behavior. Minerals 2021, 11, 191. https://doi.org/10.3390/min11020191
Homma-Takeda S, Fujishiro H, Tanaka I, Yakumaru H, Ayama K, Uehara A, Oikawa M, Himeno S, Ishihara H. Single-Cell Imaging for Studies of Renal Uranium Transport and Intracellular Behavior. Minerals. 2021; 11(2):191. https://doi.org/10.3390/min11020191
Chicago/Turabian StyleHomma-Takeda, Shino, Hitomi Fujishiro, Izumi Tanaka, Haruko Yakumaru, Kyoko Ayama, Akihiro Uehara, Masakazu Oikawa, Seiichiro Himeno, and Hiroshi Ishihara. 2021. "Single-Cell Imaging for Studies of Renal Uranium Transport and Intracellular Behavior" Minerals 11, no. 2: 191. https://doi.org/10.3390/min11020191
APA StyleHomma-Takeda, S., Fujishiro, H., Tanaka, I., Yakumaru, H., Ayama, K., Uehara, A., Oikawa, M., Himeno, S., & Ishihara, H. (2021). Single-Cell Imaging for Studies of Renal Uranium Transport and Intracellular Behavior. Minerals, 11(2), 191. https://doi.org/10.3390/min11020191





