Algae in Acid Mine Drainage and Relationships with Pollutants in a Degraded Mining Ecosystem
Abstract
1. Introduction
2. Materials and Methods
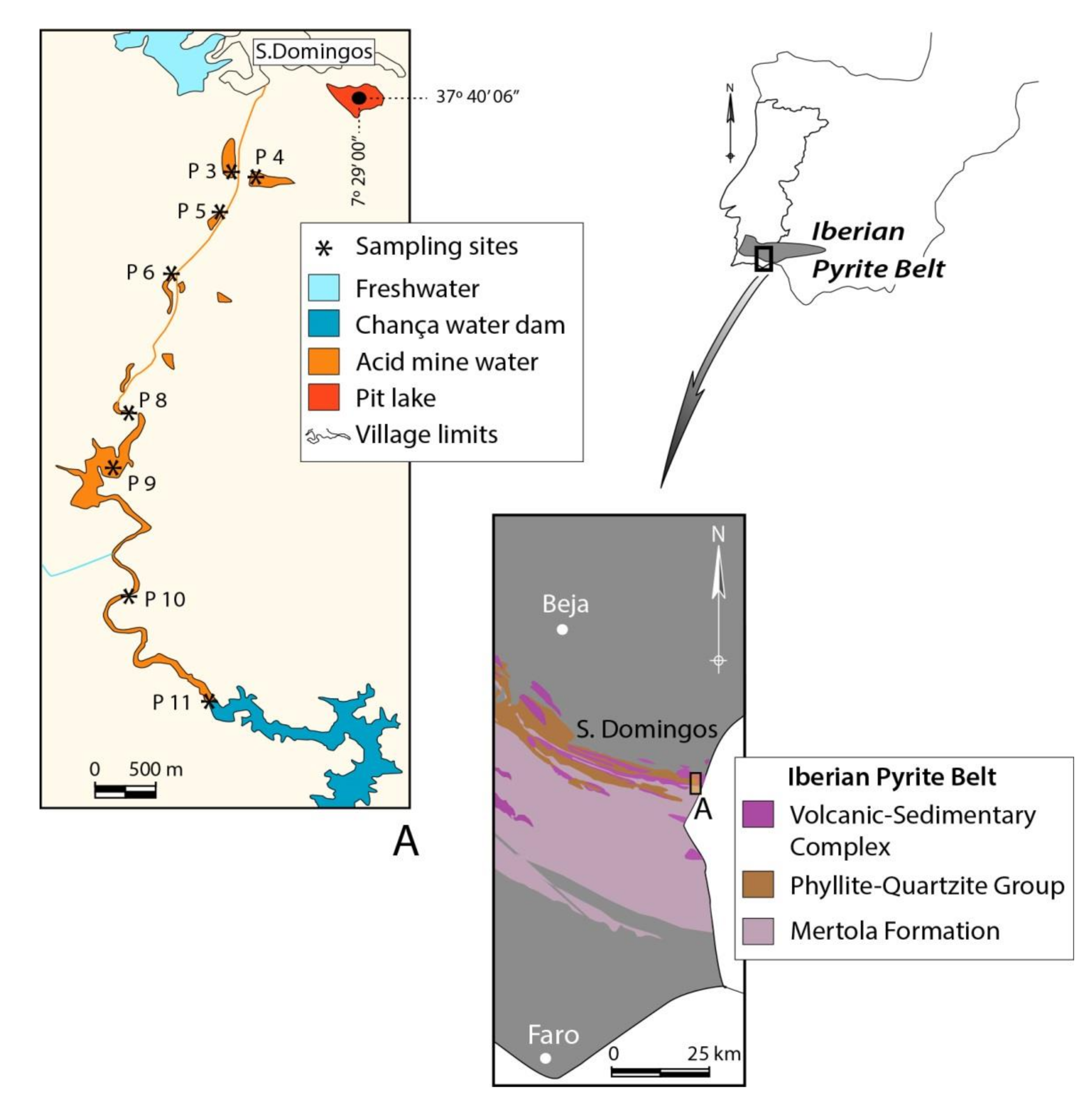
2.1. Study Area
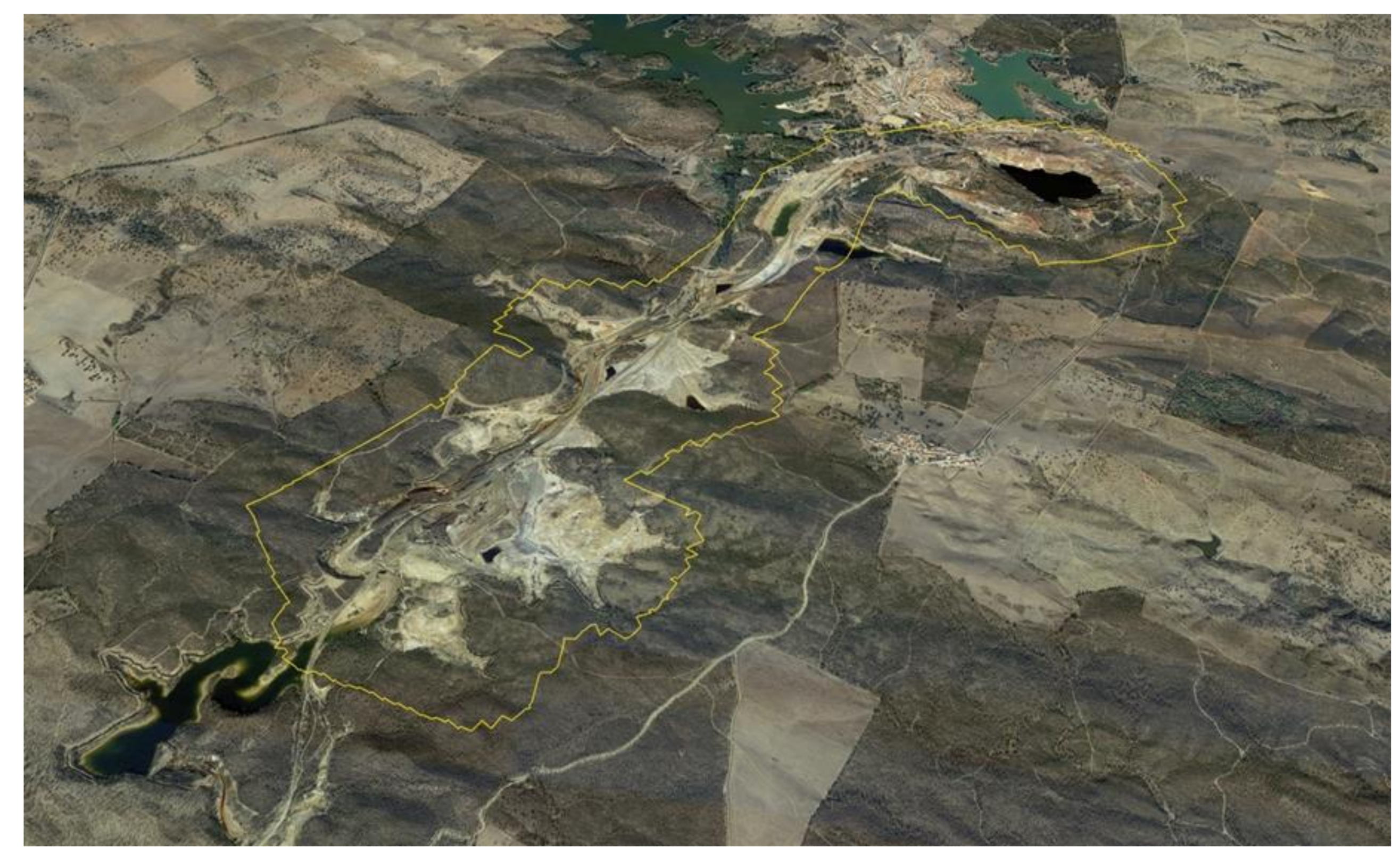
2.2. Geomorphological Characterization
2.3. Water Sampling and Analytical Methods
2.4. Algae Community—Sampling and Identification
2.5. Statistical Analysis
3. Results and Discussion
3.1. Photogrammetric Products
3.2. Water Physical-Chemical Characterization
3.3. Algae Community Study
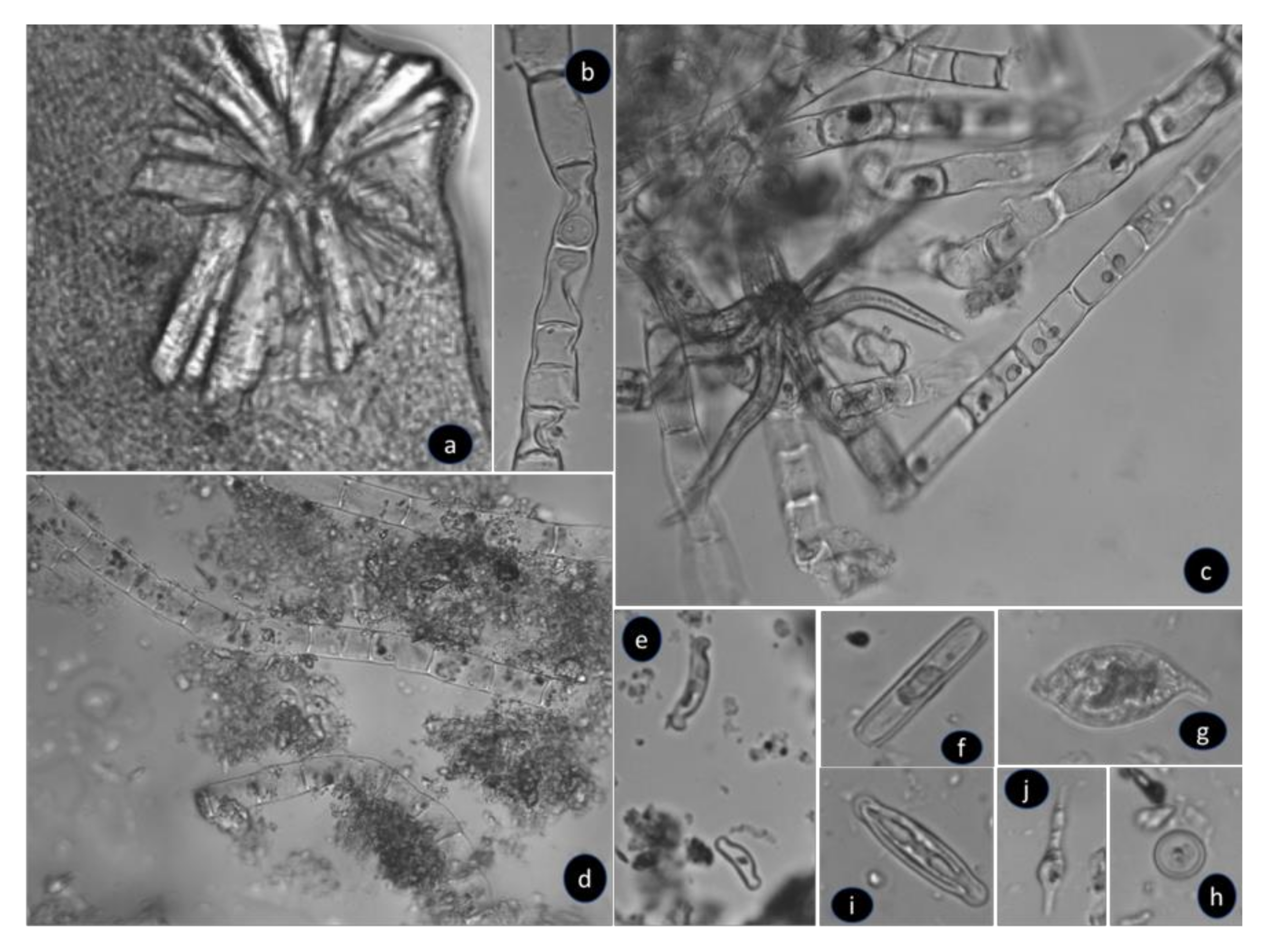
3.3.1. Field Evidence
3.3.2. Identification by Optical Microscopy
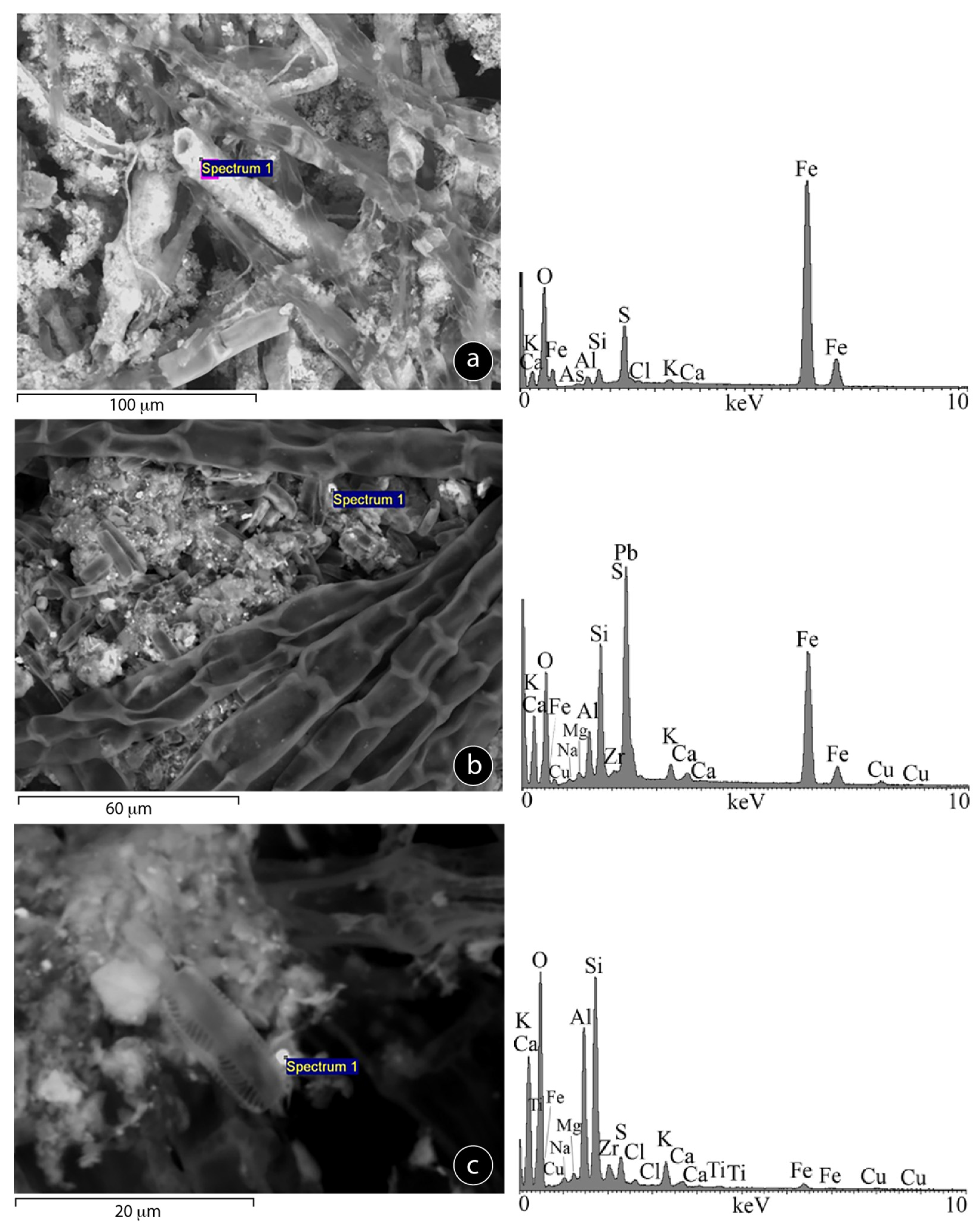
3.3.3. Mineral-Algae Interactions (SEM Study)
3.3.4. Spatial Distribution of Algae Taxa
3.3.5. Relationships between Hydrochemistry, Sample Location and Algae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomes, P.; Valente, T. Physical and chemical conditions for colonization by Euglena mutabilis: Case studies in two acid mine drainage sites. In Proceedings of the IMWA: Mine Water: Technological and Ecological Challenges, Perm, Russia, 15–19 July 2019; Wolkersdorfer, C., Khayrulina, E., Polyakova, S., Bogush, A., Eds.; International Mine Water Association: Wendelstein, Germany, 2019; pp. 419–424. [Google Scholar]
- Gross, W. Ecophysiology of algae living in highly acidic environments. Hydrobiology 2000, 433, 31–37. [Google Scholar] [CrossRef]
- Gomes, P.; Valente, T.; Geraldo, D.; Ribeiro, C. Photosynthetic pigments in acid mine drainage: Seasonal patterns and associations with stressful abiotic characteristics. Chemosphere 2020, 239, 124774. [Google Scholar] [CrossRef]
- Valente, T.; Rivera, M.J.; Almeida, S.F.P.; Delgado, C.; Gomes, P.; Grande, A.; de la Torre, M.L.; Santisteban, M. Characterization of water reservoirs affected by acid mine drainage: Geochemical, mineralogical, and biological (diatoms) properties of the water. Environ. Sci. Pollut. Res. 2015, 23, 6002–6011. [Google Scholar] [CrossRef]
- Lessmann, D.; Fyson, A.; Nixdorf, B. Phytoplankton of the extremely acidic mining lakes of Lusatia (Germany) with pH ≤ 3. Hydrobiology 2000, 433, 123–128. [Google Scholar] [CrossRef]
- Valente, T.; Gomes, C.L. The role of two acidophilic algae as ecological indicators of acid mine drainage sites. J. Iber Geol. 2007, 33, 283–294. [Google Scholar]
- Luís, A.T.; Durães, N.; de Almeida, S.F.P.; da Silva, E.F. Integrating geochemical (surface waters, stream sediments) and biological (diatoms) approaches to assess AMD environmental impact in a pyritic mining area: Aljustrel (Alentejo, Portugal). J. Environ. Sci. 2016, 42, 215–226. [Google Scholar] [CrossRef]
- Valente, T. Modelos de Caracterização de Impacte Ambiental para Escombreiras Reactivas-Equilíbrio e Evolução de Resíduos de Actividade Extractiva. Ph.D. Thesis, University of Minho, Braga, Portugal, 2004; 301p. [Google Scholar]
- Levings, C.D.; Varela, D.E.; Mehlenbacher, N.M.; Barry, K.L.; Piercey, G.E.; Guo, M.; Harrison, P.J. Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at Britannia Beach, Howe Sound, British Columbia. Mar. Pollut. Bull. 2005, 50, 1585–1594. [Google Scholar] [CrossRef]
- Sabater, S.; Buchaca, T.; Cambra, J.; Catalan, J.; Guasch, H.; Ivorra, N.; Munõz, I.; Navarro, E.; Real, M.; Romaní, A. Structure and function of benthic algal communities in an extremely acid river. J. Phycol. 2003, 39, 481–489. [Google Scholar] [CrossRef]
- Amils, R.; Gonzalez-Toril, E.; Aguilera, A.; Rodríguez, N.; Fernandez-Remolar, D.; Gomez, F.; García-Moyano, A.; Malki, M.; Oggerin, M.; Sanchez-Andrea, I.; et al. From Río Tinto to mars. The terrestrial and extraterrestrial ecology of acidophiles. Adv. Appl Microbiol. 2011, 77, 41–70. [Google Scholar] [CrossRef]
- Wolowski, K.; Turnau, K.; Henriques, F.S. The algal fora of an extremely acidic, metal-rich drainage pond of São Domingos pyrite mine (Portugal). Cryptogam. Algol. 2008, 29, 313–324. [Google Scholar]
- Tavares, M.T.; Abreu, M.M.; Vairinho, M.M.; Sousa, A.J.; Quental, L. Comportamento geoquímico de alguns elementos vestigiais na envolvente das Minas de S.; Domingos, Alentejo: Áreas da Tapada e do Telheiro. Rev. Ciências Agrárias 2009, 32, 182–194. [Google Scholar]
- Quental, L.; Sousa, A.J.; Marsh, S.; Abreu, M.M. Identification of materials related to acid mine drainage using multi-source spectra at S. Domingos Mine, southeast Portugal. Int J. Remote Sens. 2013, 34, 1928–1948. [Google Scholar] [CrossRef]
- Gomes, P.; Valente, T.; Grande, J.A.; Cordeiro, M. Occurrence of sulphate efflorescences in São Domingos mine. Comun. Geol. 2017, 104, 83–89. [Google Scholar]
- Abreu, M.; Tavares, M.T.; Batista, M.J. Potential Use of Erica andevalensis and Erica australis in Phytoremediation of Sulphide Mine Environments: São Domingos, Portugal. J. Geochem. Explor. 2008, 96, 210–222. [Google Scholar] [CrossRef]
- Gonçalves, J.A.; Henriques, R. UAV photogrammetry for topographic monitoring of coastal areas. ISPRS J. Photogramm. Remote Sens. 2015, 104, 101–111. [Google Scholar] [CrossRef]
- Arge, L.; Chase, J.S.; Halpin, P.N.; Toma, L.; Vitter, J.S.; Urban, D.; Wickremesinghe, R. Flow computation on massive grids. Proc. ACM Work Adv. Geogr. Inf. Syst. 2001, 82–87. [Google Scholar] [CrossRef]
- Arge, L.; Chase, J.S.; Halpin, P.N.; Toma, L.; Vitter, J.S.; Urban, D.; Wickremesinghe, R. Flow computation on massive grid terrains. GeoInformatica Int. J. Adv. Comput. Sci. Geogr. Inf. Syst. 2003, 7, 283–313. [Google Scholar]
- AWWA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- ASTM Committee on Standards. Standard Practice for Cleaning Laboratory Glassware, Plasticware and Equipment Used in Microbiological Analysis; American Society for Testing and Materials: West Conshohocken, PN, USA, 1992; Volume 5. [Google Scholar]
- Benzécri, J.P. L’Analyse des Correspondences. Cah. Anal. Données 1977, 2, 125–142. [Google Scholar]
- Pereira, H.G.; Brito, M.G.; Albuquerque, T.; Ribeiro, J. Geostatistical Estimation of a Summary Recovery Index for Marble Quarries, Geostatistics Troia’92; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; Volume 2. [Google Scholar]
- Sousa, P.; Sousa, J. ANDAD, Version 7.12 Copyright; CVRM/IST: Lisboa, Portugal, 2000. [Google Scholar]
- Robbins, E.I.; Rodgers, T.M.; Alpers, C.N.; Nordstrom, D.K. Ecogeochemistry of the subsurface food web at pH 0–2.5 in Iron Mountain, California, U.S.A. Hydrobiology 2000, 433, 15–23. [Google Scholar] [CrossRef]
- Robbins, E.I.; Anderson, J.E.; Podwysocki, M.H.; Nord, G.L. Seasonal variations in spectral reflectance of microbial flocculates, precipitates, and oil-like films associated with neutral and acidic mine drainage. In Environmental Monitoring and Biodiagnostics of Hazardous Contaminants; Healy, M., Wise, D.L., Moo-Young, M., Eds.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar] [CrossRef]
- Anderson, J.E.; Robbin, E.I. Spectral reflectance and detection of iron-oxide precipitates associated with acidic mine drainage. Photogramm. Eng. Remote Sens. 1998, 64, 1201–1208. [Google Scholar]
- Soyol-Erdene, T.O.; Valente, T.; Grande, J.A.; de la Torre, M.L. Mineralogical controls on mobility of rare earth elements in acid mine drainage environments. Chemosphere 2018, 205, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M. Caracterização ambiental do complexo mineiro de São Domingos–Cartografia de Infra-Estruturas e Impacte Sobre o Meio Hídrico. Master’s Thesis, University of Minho, Braga, Portugal, 2017; 84p. [Google Scholar]
- Smucker, N.J.; Vis, M.L. Acid mine drainage affects the development and function of epilithic biofilms in streams. J. N. Am. Benthol. Soc. 2011, 30, 728–738. [Google Scholar] [CrossRef]
- Valente, T.; Gomes, P.; Pamplona, J.; de la Torre, M.L. Natural stabilization of mine waste-dumps—Evolution of the vegetation cover in distinctive geochemical and mineralogical environments. J. Geochem. Explor. 2012, 123, 152–161. [Google Scholar] [CrossRef]
- Gomes, P.; Valente, T.; Sequeira Braga, M.A.; Grande, J.A.; de la Torre, M.L. Enrichment of trace elements in the clay size fraction of mining soils. Environ. Sci. Pollut. Res. 2016, 23, 6039–6045. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Antunes, I.M.; Sequeira Braga, M.A.; Neiva, A.; Santos, A.; Moreno, F. Mobility control of uranium and other potentially toxic elements in mine waters by ochre-precipitates. In Proceedings of the IMWA: Mine Water: Technological and Ecological Challenges, Perm, Russia, 15–19 July 2019; Wolkersdorfer, C., Khayrulina, E., Polyakova, S., Bogush, A., Eds.; International Mine Water Association: Wendelstein, Germany, 2019; pp. 458–462. [Google Scholar]
- Peng, X.; Chen, S.; Xu, H. Formation of biogenic sheath-like Fe oxyhydroxides in a near-neutral pH hot spring: Implications for the origin of microfossils in high-temperature, Fe-rich environments. J. Geophys. Res. Biogeosci. 2013, 118, 1397–1413. [Google Scholar] [CrossRef]
- Ishihara, H.; Hashimoto, H.; Taketa, E.; Suzuki, T.; Mandai, K.; Kunoh, H.; Takada, J. Silicon-Rich, Iron Oxide Microtubular Sheath Produced by an Iron-Oxidizing Bacterium, Leptothrix sp. Strain OUMS1, in Culture. Minerals 2014, 4, 565–577. [Google Scholar] [CrossRef]
- Prygiel, J.; Coste, M. Guide Méthodologique pour la Mise en Oeuvre de l’Indice Biologique Diatomées NF T 90–354; Agence de l’eau Artois Picardie: Douai, France, 2000; p. 340. [Google Scholar]
- Freitas, A.P.P.; Schneider, I.A.H.; Schwartzbold, A. Biosorption of heavy metals by algal communities in water streams affected by the acid mine drainage in the coal-mining region of Santa Catarina state, Brazil. Miner. Eng. 2011, 24, 1215–1218. [Google Scholar] [CrossRef]
- Kumar, R.N.; McCullough, C.D.; Lund, M.A.; Larranaga, S.A. Assessment of factors limiting algal growth in acidic pit lakes-a case study from Western Australia, Australia. Environ. Sci. Pollut. Res. 2015, 23, 5915–5924. [Google Scholar] [CrossRef]
- Wolowski, K. Phylum Euglenophyta. In Freshwater Algal Flora of the British Isles; John, D.M., Whitton, B.A., Brook, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 144–179. [Google Scholar]



| Samples | pH | EC | T °C | SO4−2 | Al | As | Ca | Cr | Cu | Fe | K | Mg | Mn | Ni | Co | Zn | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P3 | Mean | 2.7 | 2877.2 | 23.3 | 1608.3 | 148.858 | 0.075 | 93.409 | 0.071 | 12.721 | 71.260 | 3.684 | 71.819 | 7.564 | 0.197 | 0.669 | 7.109 | 0.028 | 0.030 |
| Min | 2.3 | 228.0 | 16.2 | 661.5 | 67.872 | 0.026 | 44.817 | 0.030 | 5.750 | 19.677 | 1.281 | 32.784 | 3.039 | 0.100 | 0.319 | 3.265 | 0.014 | 0.005 | |
| Max | 3.5 | 4378.0 | 29.6 | 2760.5 | 251.000 | 0.160 | 163.000 | 0.100 | 22.200 | 170.000 | 8.380 | 169.000 | 14.700 | 0.483 | 1.300 | 10.855 | 0.041 | 0.051 | |
| P4 | Mean | 2.7 | 2654.8 | 23.3 | 1728.2 | 135.585 | 0.081 | 59.266 | 0.063 | 1.660 | 95.830 | 4.071 | 117.758 | 22.614 | 0.340 | 1.025 | 63.778 | 0.085 | 0.017 |
| Min | 2.3 | 310.0 | 14.5 | 552.3 | 42.306 | 0.015 | 19.784 | 0.020 | 0.589 | 29.084 | 2.333 | 39.060 | 6.996 | 0.056 | 0.342 | 20.550 | 0.050 | 0.011 | |
| Max | 3 | 4230.0 | 31.3 | 2600.4 | 199.073 | 0.240 | 105.000 | 0.100 | 3.210 | 160.007 | 6.190 | 189.000 | 34.265 | 0.738 | 1.555 | 96.076 | 0.118 | 0.030 | |
| P5 | Mean | 2.5 | 4034.7 | 23.2 | 3155.3 | 288.885 | 1.506 | 101.635 | 0.121 | 32.429 | 253.137 | 1.393 | 105.460 | 15.584 | 0.309 | 1.277 | 62.080 | 0.373 | 0.102 |
| Min | 2.1 | 251.0 | 13.7 | 865.7 | 4.830 | 0.061 | 14.842 | 0.040 | 0.381 | 3.020 | 0.600 | 8.440 | 0.594 | 0.050 | 0.334 | 0.977 | 0.033 | 0.020 | |
| Max | 2.8 | 6943.0 | 30.0 | 6564.9 | 675.000 | 6.260 | 205.000 | 0.285 | 83.900 | 801.000 | 2.262 | 252.000 | 37.200 | 1.040 | 3.020 | 199.000 | 1.300 | 0.286 | |
| P6 | Mean | 3 | 1734.8 | 23.2 | 1001.9 | 83.493 | 0.849 | 50.865 | 0.062 | 9.427 | 70.664 | 4.869 | 47.338 | 5.065 | 0.129 | 0.389 | 19.837 | 0.099 | 0.289 |
| Min | 2.6 | 117.3 | 15.6 | 68.2 | 1.757 | 0.003 | 9.639 | 0.001 | 0.378 | 0.995 | 1.966 | 8.374 | 0.165 | 0.033 | 0.077 | 0.595 | 0.002 | 0.031 | |
| Max | 4 | 4320.0 | 31.9 | 2955.1 | 354.446 | 9.498 | 86.900 | 0.119 | 48.057 | 459.577 | 8.400 | 108.104 | 11.346 | 0.360 | 1.479 | 155.883 | 0.819 | 0.626 | |
| P8 | Mean | 2.8 | 3392.7 | 22.2 | 2498.0 | 211.161 | 0.119 | 117.307 | 0.103 | 16.886 | 220.407 | 4.785 | 81.232 | 10.737 | 0.207 | 0.895 | 31.697 | 0.080 | 0.307 |
| Min | 2.2 | 181.9 | 14.7 | 58.5 | 0.075 | 0.060 | 10.480 | 0.050 | 0.166 | 1.201 | 0.421 | 8.554 | 0.162 | 0.100 | 0.100 | 0.687 | 0.004 | 0.011 | |
| Max | 4.7 | 7320.0 | 35.1 | 5731.9 | 497.000 | 0.299 | 256.000 | 0.171 | 38.000 | 499.000 | 29.142 | 170.389 | 24.152 | 0.499 | 1.913 | 82.000 | 0.194 | 1.181 | |
| P9 | Mean | 2.9 | 1728.7 | 22.4 | 836.4 | 67.196 | 0.108 | 47.504 | 0.062 | 7.090 | 66.351 | 5.825 | 33.874 | 3.699 | 0.099 | 0.336 | 10.848 | 0.044 | 0.407 |
| Min | 2.4 | 204.0 | 16.8 | 110.4 | 4.809 | 0.021 | 13.724 | 0.020 | 0.590 | 4.676 | 2.483 | 9.781 | 0.666 | 0.048 | 0.069 | 1.544 | 0.011 | 0.152 | |
| Max | 3.5 | 3186.0 | 30.2 | 1641.5 | 131.366 | 0.279 | 86.456 | 0.100 | 13.343 | 166.920 | 29.143 | 63.881 | 7.355 | 0.137 | 0.652 | 23.491 | 0.080 | 0.765 | |
| P10 | Mean | 2.9 | 3199.5 | 20.3 | 2055.2 | 103.202 | 0.113 | 195.022 | 0.060 | 6.803 | 67.240 | 4.123 | 181.316 | 12.684 | 0.176 | 0.563 | 15.995 | 0.072 | 0.135 |
| Min | 2.4 | 185.7 | 8.0 | 151.3 | 9.741 | 0.030 | 16.088 | 0.020 | 1.144 | 1.779 | 0.100 | 11.363 | 0.613 | 0.062 | 0.061 | 1.944 | 0.019 | 0.005 | |
| Max | 3.7 | 8598.0 | 26.2 | 6753.2 | 310.774 | 0.320 | 658.000 | 0.100 | 15.791 | 303.574 | 11.000 | 651.026 | 42.800 | 0.638 | 1.770 | 45.852 | 0.204 | 0.477 | |
| P11 | Mean | 4 | 740.8 | 20.5 | 300.6 | 26.502 | 0.067 | 52.233 | 0.053 | 2.321 | 14.231 | 5.790 | 33.537 | 2.799 | 0.071 | 0.147 | 4.103 | 0.019 | 0.129 |
| Min | 2.9 | 168.7 | 12.6 | 58.4 | 0.100 | 0.007 | 15.833 | 0.003 | 0.019 | 0.330 | 1.330 | 10.500 | 0.140 | 0.005 | 0.003 | 0.042 | 0.002 | 0.001 | |
| Max | 6.3 | 1527.0 | 28.4 | 790.0 | 98.570 | 0.149 | 130.137 | 0.100 | 11.023 | 78.501 | 29.145 | 73.218 | 9.803 | 0.109 | 0.434 | 12.616 | 0.054 | 0.393 |
| Genus | P3 | P4 | P5 | P6 | P8 | P9 | P10 | P11 |
|---|---|---|---|---|---|---|---|---|
| Mougeotia sp. | +++ | + | ++ | + | ++ | + | ++ | |
| Ulothrix sp. | ++ | + | ||||||
| Spirogyra sp. | + | + | + | + | + | |||
| Klebsormidium sp. | + | + | ||||||
| Euglena mutabilis | + | + | ||||||
| Pinnularia sp. | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Eunotia exigua | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Navicula sp. | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Oedogonium | ++ | |||||||
| Chlamydomonas sp. | +++ | |||||||
| Characium sp. | + | |||||||
| Dinobryon sp. | +++ | |||||||
| Chlorella sp. | + | |||||||
| Cyclotella sp. | + | |||||||
| Bacteria | +++ | +++ | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, P.; Valente, T.; Albuquerque, T.; Henriques, R.; Flor-Arnau, N.; Pamplona, J.; Macías, F. Algae in Acid Mine Drainage and Relationships with Pollutants in a Degraded Mining Ecosystem. Minerals 2021, 11, 110. https://doi.org/10.3390/min11020110
Gomes P, Valente T, Albuquerque T, Henriques R, Flor-Arnau N, Pamplona J, Macías F. Algae in Acid Mine Drainage and Relationships with Pollutants in a Degraded Mining Ecosystem. Minerals. 2021; 11(2):110. https://doi.org/10.3390/min11020110
Chicago/Turabian StyleGomes, Patrícia, Teresa Valente, Teresa Albuquerque, Renato Henriques, Núria Flor-Arnau, Jorge Pamplona, and Felipe Macías. 2021. "Algae in Acid Mine Drainage and Relationships with Pollutants in a Degraded Mining Ecosystem" Minerals 11, no. 2: 110. https://doi.org/10.3390/min11020110
APA StyleGomes, P., Valente, T., Albuquerque, T., Henriques, R., Flor-Arnau, N., Pamplona, J., & Macías, F. (2021). Algae in Acid Mine Drainage and Relationships with Pollutants in a Degraded Mining Ecosystem. Minerals, 11(2), 110. https://doi.org/10.3390/min11020110







