Effect of Magnesium on the Hydrophobicity of Sphalerite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Zeta Potential
2.3. Contact Angle
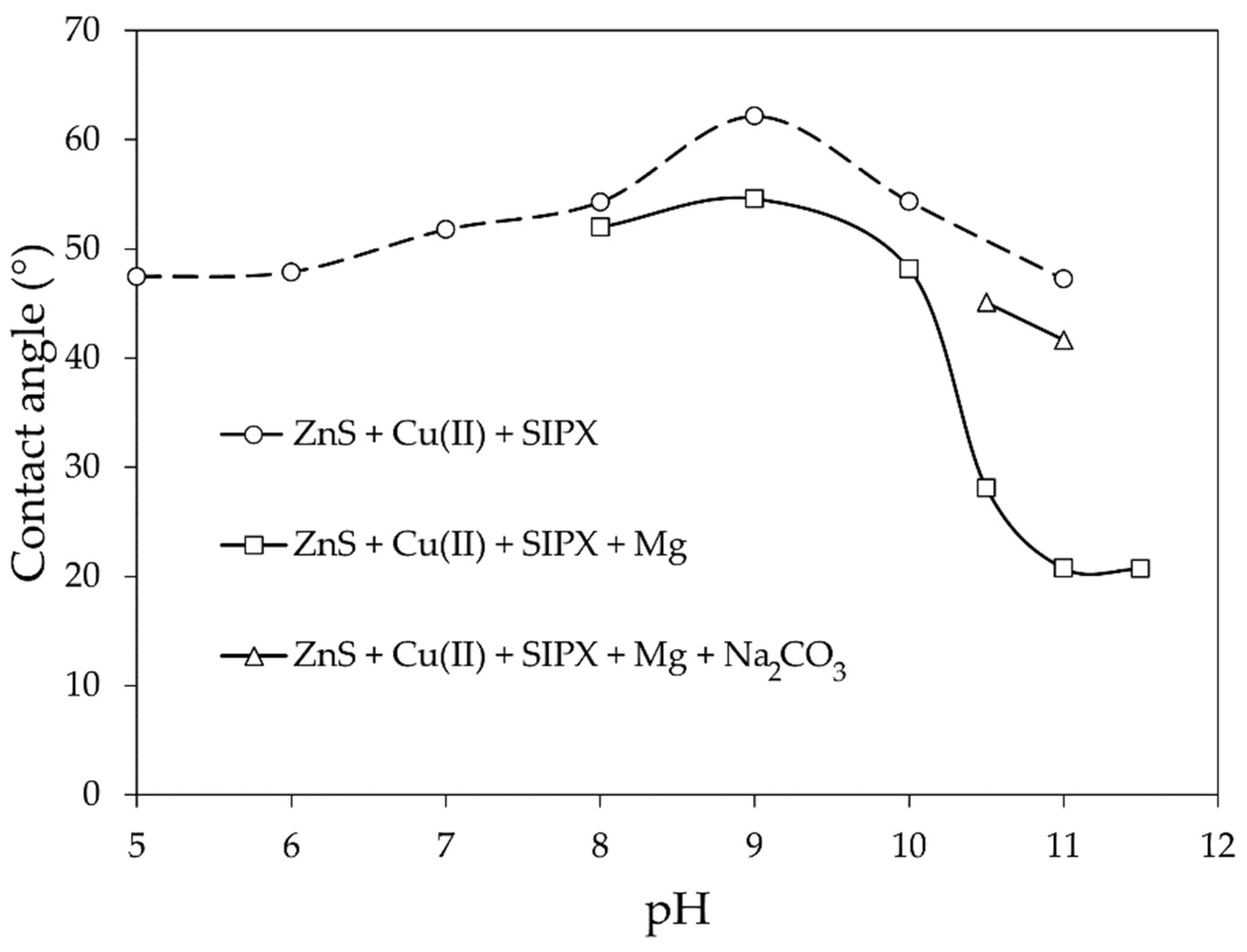
- The sphalerite sample was activated with 50 mg/L of Cu(II) at pH 5.4 for 15 min. The sample was then decanted and further conditioned during 5 min with 10−4 mol/L SIPX at the pH of interest (i.e., from 5 to 11) and the contact angle was measured in the latter solution.
- After copper activation as in previous experiment, the sample was conditioned during 5 min in the presence of both SIPX and magnesium (50 mg/L) at the pH of interest (8 to 11.5). Then, the contact angle was measured.
- After copper activation as in previous cases, the sample was conditioned during 5 min in the presence of SIPX, magnesium, and sodium carbonate (2 g/L). The pH was adjusted at 10.5 and 11 because in previous tests high mineral depression was observed at these values. Then, the contact angle was recorded and measured in a 10−3 mol/L NaNO3 solution of the same pH values (i.e., 10.5 and 11) to avoid the optical interference caused by the precipitates.
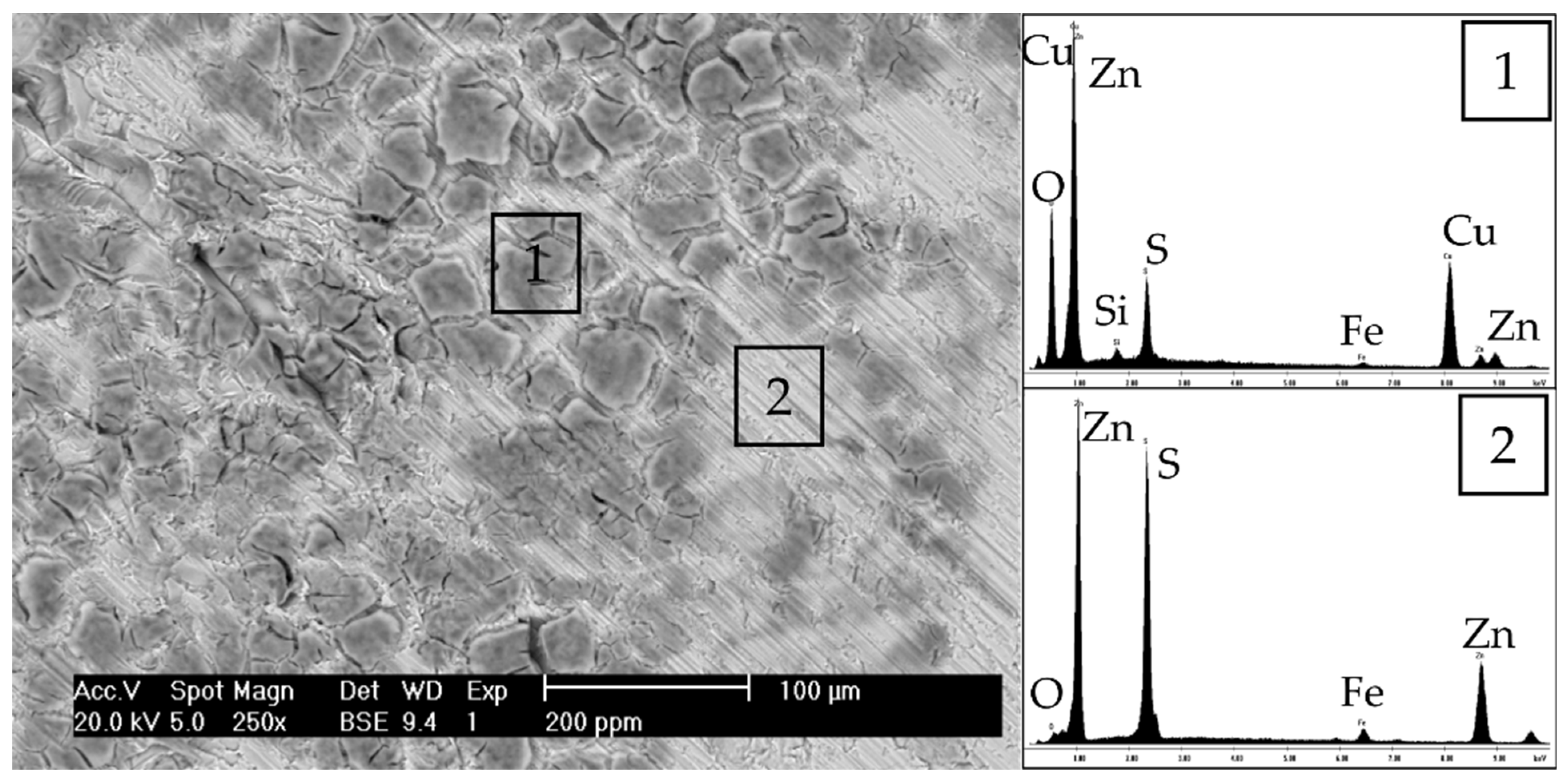
2.4. Thermodynamic Simulation, Surface Analysis, and Characterization
3. Results and Discussion
3.1. Process Water Chemical Charaterization
3.2. Zeta Potential of Sphalerite
3.3. Effect of Magnesium on the Contact Angle of Cu-Activated Sphalerite
4. Conclusions
- Cu-activation of sphalerite enhance its zeta potential up to a concentration of 5 mg/L; at concentrations greater than 5 mg/L, copper hydroxide will adsorb/precipitate onto the sphalerite surface decreasing its zeta potential because Cu(OH)2 is hydrophilic in nature.
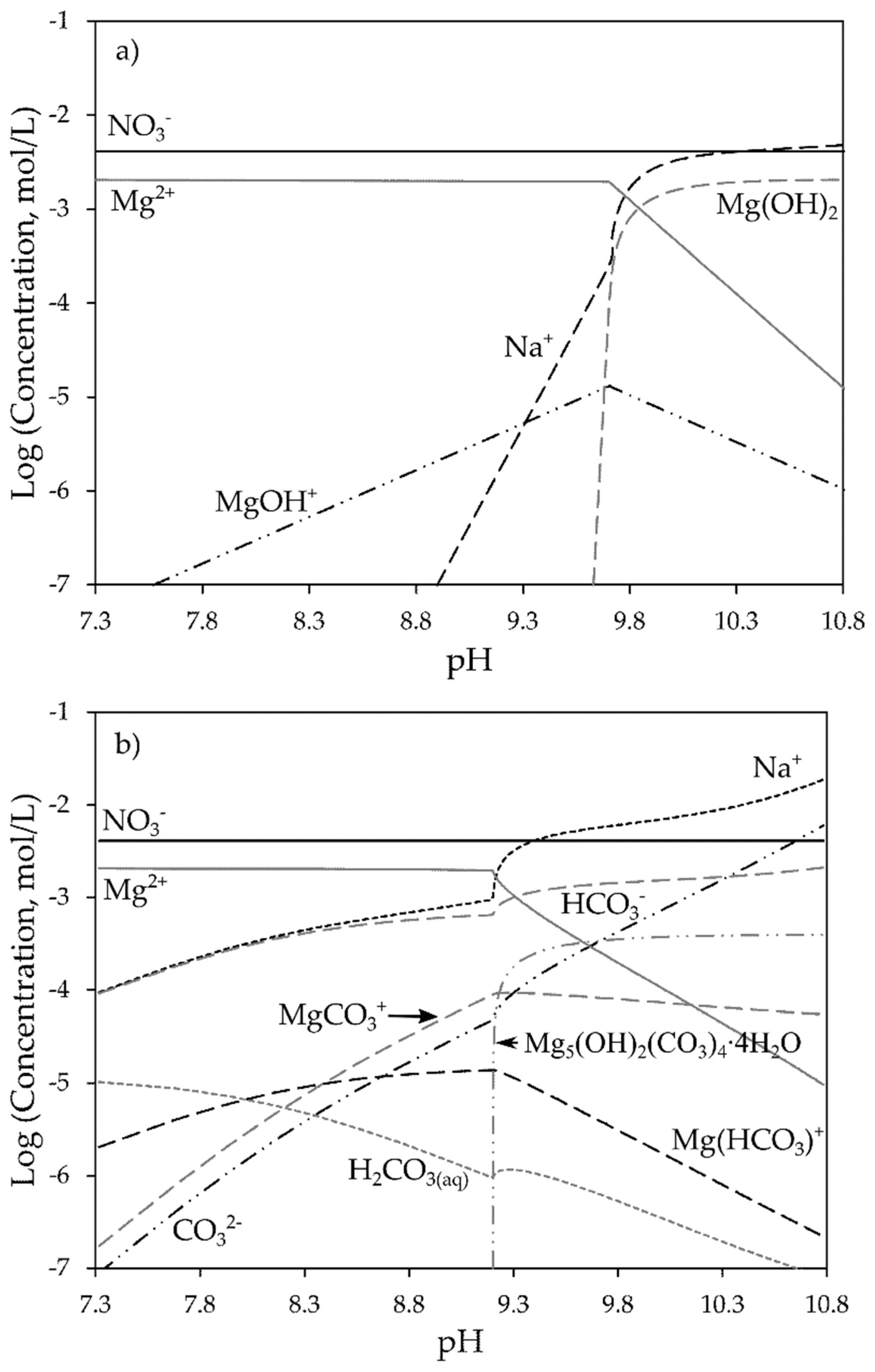
- Magnesium has a detrimental effect on the contact angle of Cu-activated sphalerite conditioned with SIPX beginning at pH of approximately 10 due to the precipitation of magnesium hydroxide onto its surface. The precipitation of this species starts at pH 9.6 and rapidly becomes a predominant species as pH increases. If the process pH is not rigorously controlled to keep it below 10, it will cover the whole sphalerite surface diminishing its recovery.
- The use of sodium carbonate as alkalinizing agent avoids the sodium hydroxide precipitation onto the surface of Cu-activated sphalerite conditioned with SIPX. When sodium carbonate is present, hydromagnesite (Mg5(OH)2(CO3)4∙4H2O) begins to precipitate at pH 9.3 and it will keep precipitating at greater pH values, allowing the recovery of sphalerite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rao, S.R.; Finch, J.A. A review of water reuse in flotation. Miner. Eng. 1989, 2, 65–85. [Google Scholar] [CrossRef]
- Levay, G.; Smart, R.S.C.; Skinner, W.M. The impact of water quality on flotation performance. J. S. Afr. Inst. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Chen, J.; Liu, R.; Sun, W.; Qiu, G. Effect of mineral processing wastewater on flotation of sulfide minerals. T. Nonferr. Metal. Soc. 2009, 19, 454–457. [Google Scholar] [CrossRef]
- October, L.; Corin, K.; Schreithofer, N.; Manono, M.; Wiese, J. Water quality effects on bubble-particle attachment of pyrrhotite. Miner. Eng. 2019, 131, 230–236. [Google Scholar] [CrossRef]
- October, L.L.; Corin, K.C.; Manono, M.S.; Schreithofer, N.; Wiese, J.G. A fundamental study considering specific ion effects on the attachment of sulfide minerals to air bubbles. Miner. Eng. 2020, 151, 106313. [Google Scholar] [CrossRef]
- Coetzer, H.; Du Preez, H.S.; Bredenhann, R. Influence of water resources and metal ions on galena flotation of Rosh Pinah ore. J. S. Afr. Inst. Min. Metall. 2003, 103, 193–207. [Google Scholar]
- Bicak, O.; Ekmekci, Z.; Can, M.; Öztürk, Y. The effect of water chemistry on froth stability and surface chemistry of the flotation of a Cu-Zn sulfide ore. Int. J. Miner. Process. 2012, 102–103, 32–37. [Google Scholar] [CrossRef]
- El-Ammouri, E.; Mirnezami, M.; Lascelles, D.; Finch, J.A. Aggregation index and a methodology to study the role of magnesium in aggregation of sulphide slurries. CIM Bull. 2002, 95, 67–72. [Google Scholar]
- Lascelles, D.; Finch, J.A.; Sui, C. Depressant action of Ca and Mg on flotation of Cu activated sphalerite. Can. Metall. Q. 2003, 42, 133–140. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Uribe-Salas, A. Contact angle study on the activation mechanisms of sphalerite with Cu(II) and Pb(II). Rev. Metal. Madrid 2011, 47, 329–340. [Google Scholar]
- Dávila-Pulido, G.I.; Uribe-Salas, A. Effect of calcium, sulphate and gypsum on copper-activated and non-activated sphalerite surface properties. Miner. Eng. 2014, 55, 147–153. [Google Scholar] [CrossRef]
- Moignard, M.S.; James, R.O.; Healy, T.W. Adsorption of calcium at the zinc sulphide-water interface. Aust. J. Chem. 1977, 30, 733–740. [Google Scholar] [CrossRef]
- Finkelstein, D.P. The activation of sulphide minerals for flotation: A review. Int. J. Miner. Process. 1997, 52, 81–120. [Google Scholar] [CrossRef]
- Sui, C.; Rashchi, F.; Xu, Z.; Kim, J.; Nesset, J.E.; Finch, J.A. Interactions in the sphalerite-Ca-SO4-CO3 systems. Colloid Surf. A 1998, 137, 69–77. [Google Scholar] [CrossRef]
- Fornasiero, D.; Ralston, J. Effect of surface oxide/hydroxide products on the collectorless flotation of copper-activated sphalerite. Int. J. Miner. Process. 2006, 78, 231–237. [Google Scholar] [CrossRef]
- Ikumapayi, F.K. Flotation Chemistry of Complex Sulphide Ores: Recycling of Process Water and Flotation Selectivity. Ph.D. Thesis, Lulea University of Technology, Luleå, Sweden, 2010. [Google Scholar]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interfac. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, M.; Hashemi, M.S.; Finch, J.A. Mechanism of alkaline earth metal ion adsorption on sulphide minerals. In Proceedings of the 48th Conference of Metallurgist of CIM, Sudbury, ON, Canada, 23–26 August 2009; pp. 99–106. [Google Scholar]
- Liu, H.; Yi, J. Polystyrene/magnesium hydroxide nanocomposite particles prepared by surface-initiated in-situ polymerization. Appl. Surf. Sci. 2009, 255, 5714–5720. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I. An Experimental Study of the Effect of Calcium Sulfate in the Flotation Water on the Surface Properties of Sphalerite. Ph.D. Thesis, CINVESTAV, Ramos Arizpe, Mexico, 2014. [Google Scholar]
- Grano, S.R.; Wong, P.L.M.; Skinner, W.; Johnson, N.W.; Ralston, J. Detection and control of calcium sulfate precipitation in the lead circuit of the Hilton concentrator of Mount Isa Mines Limited. In Proceedings of the XIX International Mineral Processing Congress, San Francisco, CA, USA, 22–27 October 1995; pp. 171–179. [Google Scholar]




| Plant | Fe (mg/L) | Pb (mg/L) | Zn (mg/L) | Cu (mg/L) | Ca (mg/L) | Mg (mg/L) | SO4 (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.54 | 2.15 | 112 | 0.35 | 587 | 471 | 4127 |
| 2 | 0.36 | 0.44 | 0.158 | -- | 566 | 124.7 | 1809 |
| 3 | 0.003 | 0.014 | 0.398 | 0.573 | 665 | 11.2 | 1910 |
| 4 | 1.59 | 0.07 | 1.03 | 0.011 | 270 | 37.2 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila-Pulido, G.I.; González-Ibarra, A.A.; Garza-García, M.; Charles, D.A. Effect of Magnesium on the Hydrophobicity of Sphalerite. Minerals 2021, 11, 1359. https://doi.org/10.3390/min11121359
Dávila-Pulido GI, González-Ibarra AA, Garza-García M, Charles DA. Effect of Magnesium on the Hydrophobicity of Sphalerite. Minerals. 2021; 11(12):1359. https://doi.org/10.3390/min11121359
Chicago/Turabian StyleDávila-Pulido, Gloria I., Adrián A. González-Ibarra, Mitzué Garza-García, and Danay A. Charles. 2021. "Effect of Magnesium on the Hydrophobicity of Sphalerite" Minerals 11, no. 12: 1359. https://doi.org/10.3390/min11121359
APA StyleDávila-Pulido, G. I., González-Ibarra, A. A., Garza-García, M., & Charles, D. A. (2021). Effect of Magnesium on the Hydrophobicity of Sphalerite. Minerals, 11(12), 1359. https://doi.org/10.3390/min11121359






