Abstract
There has been little research on the metal isotopic composition of adakitic rock. The main objective of our investigation was to obtain more knowledge on the iron isotopic composition of adakitic rocks and provide new evidence for the genesis of Shangcheng pluton from an iron isotope perspective. The Dabie orogen is divided into eastern and western areas by the Shangcheng-Macheng fault, and the Shangcheng pluton is located in the western Dabie orogen area. The iron isotopic composition of these rocks ranges from 0.08‰ to 0.20‰ (2SD, n = 3). The δ56Fe values of two rocks from the SGD (Sigudun) unit are relatively low (0.11 ± 0.03‰ and 0.08 ± 0.04‰), while the δ56Fe values of the other samples are basically consistent (0.18–0.2‰). Evidence from elemental geochemical characteristics and petrogenesis defines the Shangcheng pluton as adakitic rocks. Our investigation on the elemental and isotopic compositions hints that the enrichment of heavy iron isotopes cannot be explained by weathering/alteration and fluid exsolution. Fractional crystallization of magnetite may account for the enrichment of light iron isotopes in two rocks from the SGD unit, while the fractional iron isotope trend in the other five samples can be explained by Δ56Fecrystal-melt = ~0.035‰. Two investigated rocks from SGD units may have been derived from the partial melting of amphibolite, while the other five samples may have been derived from the partial melting of eclogite containing 10–15% garnet.
1. Introduction
Recent theoretical developments have revealed that the fractionation of iron isotopes occurs during magmatic processes. Partial melting, fractional crystallization, and fluid exsolution can all be responsible for the enrichment of heavy iron isotopes [1,2,3,4,5,6,7,8,9,10,11,12,13]. Nevertheless, factors besides magmatic processes can also cause significant fractionation of iron isotopes. Due to the different mineral compositions of the source rock, the δ56Fe value of melt generated by the molten lower crust in the eastern Dabie orogen ranges from 0.120‰ to 0.196‰, according to simulations and observations, contributing to the heterogeneity of δ56Fe after crystallization [14]. Redox conditions also account for the fractionation of iron isotopes. Li et al. [15] reported that the δ56Fe content of the Archaean BIF deposit is 1.5–2.6‰, due to a low degree of Fe (II) oxidation along with very low oxidation rates, resulting in the enrichment of heavy iron isotopes. On the contrary, Fe3+ released from primary minerals will form secondary minerals under an oxidative environment, preventing a loss of Fe and a change in the iron isotopic composition [16,17].
A popular explanation of adakites is that they originated from partial melting of subducted slab, which endows them with a unique geochemical composition compared with general granitic rocks (Al2O3 ≥ 15%, Sr > 300 ppm, Y < 20 ppm, unobvious Eu anomaly, etc.) [18]. It should be noted that there are genetic differences between adakite and adakitic rocks. The genesis of adakitic rocks generally involves four methods: (1) partial melting of the lower crust; (2) partial melting of the delaminated crust; (3) differentiation of mafic magma under high pressure; and (4) mixed magma [19]. The above four types of adakitic rocks have been confirmed or reported [20,21,22,23,24,25].
Previous studies on adakitic rocks have been mainly based on the geochemical characteristics of major and trace elements. However, few studies have been conducted on its metal isotopes. In this study, the iron isotopic composition and elemental content of seven adakitic rocks from the Shangcheng pluton were measured. Considering the complexity of iron isotopic fractionation, the effects of weathering/alteration and fluid exsolution on isotopic fractionation are first discussed by assessing the elemental and iron isotopic features. Then, the contributions to isotopic enrichment of fractional crystallization and the mineral composition of the source rock are evaluated by modelling, and evidence on the elemental and isotopic connection is revealed. The main objective of our investigation is to obtain more knowledge on the iron isotopic composition of adakitic rocks and gain new evidence on the genesis of the Shangcheng pluton from an iron isotope perspective.
2. Geological Setting
The Dabie orogen is located at the Central China Orogenic, which is in the eastern part of the Qinling-Tongbai orogen (see Figure 1) [26]. It formed when the South China block subducted beneath the North China block during the Triassic and resulted in volcanic activity during the Cretaceous [27,28,29,30,31,32,33,34,35]. A large volume of intrusive intermediate-acid magma provides us a good chance to research the iron isotope composition of adakitic rocks. The Shangcheng-Macheng fault divides the Dabie orogen into eastern and western areas (Figure 1), and the Shangcheng pluton is located in the western area of the Dabie orogen.
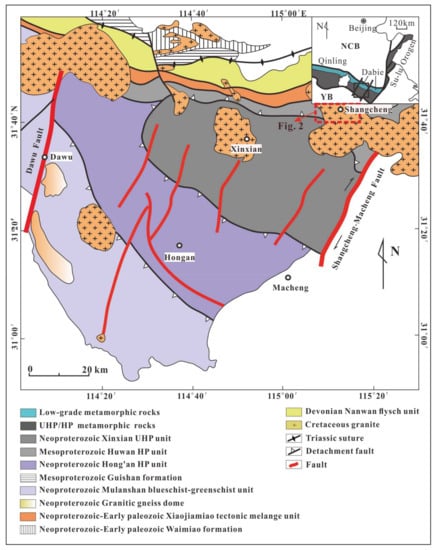
Figure 1.
Geological map of the western Dabie orogen (modified after [36,37]).
The Shangcheng pluton contains the Guishan Formation, Nanwan flysch Unit, Yangxiaozhuang Formation, and Xiaojiamiao Formation, and it is covered by late Cretaceous Jingangtai volcanic rocks [27]. Previous studies have divided the Shangcheng pluton into Sigudun (128–137 Ma), Shiguwa (130–139 Ma), and Loufangwan (133–141 Ma) intrusive units, according to their textures, mineral compositions, and contact relationships [38,39,40,41] (Figure 2). We named these the SGD unit (Sigudun), SGW unit (Shiguwa), and LFW unit (Loufangwan).
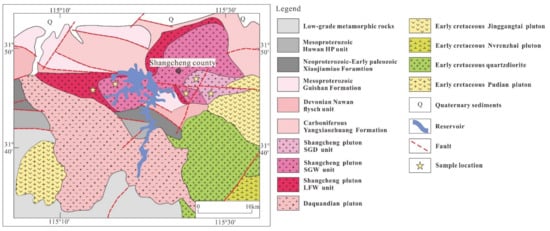
Figure 2.
Simplified geological map of the Shangcheng pluton (modified after [38]).
A porphyritic texture can be observed in SGD unit samples, and the phenocryst is dominated by potassium feldspar, which accounts for about 20% of the total content. The matrix is dominated by plagioclase (35%) and quartz (25%). Biotite (5–10%) and amphibole (5%) can be seen under single polarizer (3a), as can accessory minerals, such as apatite and magnetite (Figure 3a–c). A porphyritic-like structure can be observed in samples from the SGW unit, and potassium feldspar dominates the phenocrysts (20%). The matrix includes plagioclase (30%), quartz (30%), biotite (5%), and hornblende (Figure 3d–f). Some plagioclase has a ring structure (Figure 3e). Large potassic feldspar crystals (Figure 3h,i) can be seen locally in samples from the LFW unit. Quartz, plagioclase, and biotite are encapsulated in the potassic feldspar phenocrysts (Figure 3g,h). The matrix is dominated by quartz (30%) and plagioclase (30%), with small amounts of biotite (5%), apatite, and magnetite.
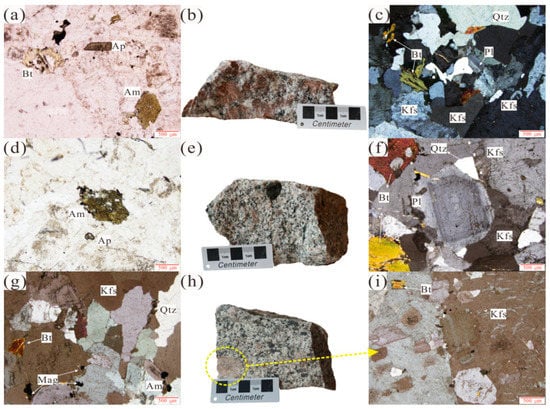
Figure 3.
Representative field photographs and photomicrographs (single and crossed polars) of the Shangcheng pluton. (a,d) are samples of the SGD unit and SGW unit viewed under a single polar microscope. They include apatite, hornblende, biotite, magnetite, and other mineral particles; (b,e,h) are specimen photographs of the SGD unit, SGW unit, and LFW unit, respectively. A xenolith with a diameter of about 1 cm can be seen in the SGW unit; (c) shows the main rock-forming minerals, which are potassium feldspar, plagioclase, and quartz under crossed polar; (f) is a sample from the SGW unit, showing the ring structure of plagioclase; (g) is from the LFW unit and shows rock-forming minerals, such as quartz and potassium feldspar; (i) is a microscopic photo of giant potassium feldspar particles, which are wrapped in biotite, plagioclase, and quatrtz. Kfs—potassium feldspar; Pl—plagioclase; Qtz—quartz; Am—amphibole; Bt—biotite; Mag-magnetite; Ap—apatite.
3. Analytical Method
Twenty-three samples were collected from the Shangcheng pluton, and seven samples were selected for this study by excluding the altered samples (SGD unit: SGD-2-1, SGD-2-3, SGD-3-1; SGW unit: SGW-1-2; LFW unit: LFW-1-1, LFW-1-2, LFW-2-1).
Major element analyses of whole rock were conducted on XRF (Primus II, Rigaku, Japan) at the Wuhan Samplesolution Analytical Technology Co., Ltd., Wuhan, China, and the analysis error was less than 1% (except P2O5). Trace element analyses of whole rock were conducted with an Agilent 7700e ICP-MS at the Wuhan SampleSolution Analytical Technology Co., Ltd., Wuhan, China. For the specific process followed, see [42].
Iron isotope analyses of samples were conducted at the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, Wuhan, China. Detailed descriptions of sample digestion, chemical evaporation, and other chemical procedures are given in [43]. Iron isotopic measurements were performed on a Nu Plasma 1700 MC-ICP-MS using the sample-standard bracketing (SSB) method. Dissolved IRMM-014 Fe isotope standard with 5 μg g−1 Fe was used as the bracketing standard, and the internal precision was better than 15 × 10−6 (relative standard error) for the 56Fe/54Fe ratio. Iron isotopic compositions were reported using the standard per mil (‰) notation of δ56Fe for the 56Fe/54Fe ratio and δ57Fe for the 57Fe/54Fe ratio, where:
δ56Fe = [(56Fe/54Fesample)/(56Fe/54FeIRMM-014) − 1] × 1000
δ57Fe = [(57Fe/54Fesample)/(57Fe/54FeIRMM-014) − 1] × 1000
4. Results
4.1. Elemental Composition
The major and trace element compositions for these rocks are summarized in Table 1 and Table 2. Samples from the Shangcheng pluton were generally found to be metaluminous, but one (LFW-1-2) was weakly peraluminous.

Table 1.
Major element compositions of of Shangcheng adakitic rocks.

Table 2.
Trace element compositions of Shangcheng adakitic rocks.
The samples from the Shangcheng pluton had high concentrations of SiO2 (67.29~71.89%), Al2O3 (14.45~15.54%), and Na2O (4.07~4.73%), along with low concentrations of Fe2O3T (1.73~3.65%) and P2O5(0.10~0.20%). The A/CNK (Al2O3/ (CaO + Na2O + K2O) mole ratio) of the samples ranged from 0.92 to1.02. The Mg# values ((Mg/Mg + Fe) mole ratio) ranged from 36.34 to 46.33.
The total REE content of the Shangcheng pluton ranged from 138.09 to 218.5 ppm, exhibiting LREE enrichment and unobvious Eu anomalies (δEu = 0.89–1.02) on chondrite-normalized REE patterns (Figure 4a). On the primitive mantle-normalized trace element spider diagram (Figure 4b), it was observed that the samples were depleted of high-field-strength elements, such as P, Ti, Nb and Ta, while they were enriched in large ion lithophile elements, such as Pb, K, Ba.
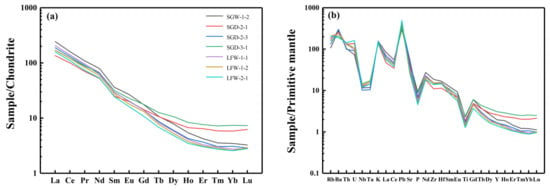
Figure 4.
Chondrite-normalized REE patterns (a) and primitive mantle-normalized trace element spider diagram (b). Chondrite and mantle-normalized values were taken from [44,45].
4.2. Iron Isotopic Composition
All seven samples had δ56Fe concentrations ranging from 0.08‰ to 0.20‰ (2SD, n = 3) (Table 3). The δ56Fe values of one sample (SGD-2-1 and SGD-3-1) were relatively low (0.11 ± 0.03‰ and 0.08 ± 0.04‰), while the δ56Fe values of the other samples were in accordance (0.18–0.2‰). On the whole, the δ56Fe content of the samples was in accordance with the trend of iron isotopic fractionation in granite.

Table 3.
Iron isotope compositions of representative samples.
5. Discussion
5.1. Petrogenesis of Shangcheng Pluton
After combining the mineralogy and elemental composition characteristics (Figure 5a,b), we propose that the Shangcheng pluton mainly includes two kinds of rocks: biotite monzonite and quartz monzonite.
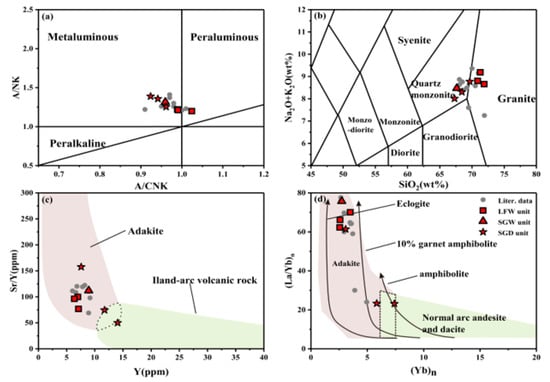
Figure 5.
(a) A/CNK vs. ANK, (b) SiO2 vs. Na2O + K2O [46,47], (c) Y vs. Sr/Y [18], (d) (Yb)n vs. (La/Yb)n [48]; the curves in Figure 5d indicate the degree of melting.
A previous study [38] showed that the rock type of the Shangcheng pluton is high-K calc-alkaline I type granite, which originated from low-degree partial melting of eclogite or amphibolite in the presence of garnet. In this study, the Y-Sr/Y diagram (Figure 5c) shows that SGD-2-1 and SGD-3-1 may have a conjoint background. In the (Yb)n-(La/Yb)n diagram (Figure 5d), two points are also present at the junction. Those two anomalies will be discussed in a later chapter.
The Sr/Y-(La/Yb)n (Figure 6a) and MgO-SiO2 diagrams (Figure 6b) indicate that the investigated rocks used in this study may have originated from both thickened and delaminated lower crust through low-degree partial melting. All samples in this study are basically consistent with the compositional definition of adakite [18], with SiO2 ≥ 56 (wt.%), Al2O3 ≥ 15 (wt.%), MgO ≤ 3 (wt.%), Sr > 400 ppm, Sr/Y > 20, and no obvious Eu anomalies. Previous studies [38,39,40,41] have reached a consistent conclusion on the genesis of the Shangcheng pluton, which is that it was derived from the partial melting of mafic rocks in the lower crust. The Shangcheng pluton can be considered adakitic instead of adakite rocks, based on the genetic differences between them.
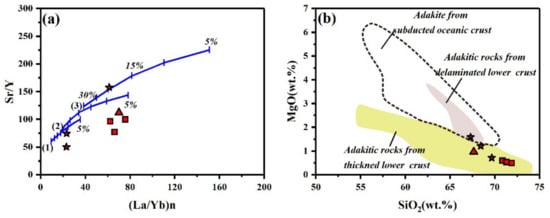
Figure 6.
(a) Sr/Y vs. (La/Yb)n [21], (b) SiO2 vs. MgO [49]. Curves 1, 2, and 3 in Figure 6a show batch partial melting with residual phases of 30% garnet + 69% clinopyroxene + 1% rutile (1), 15% garnet + 84.5% clinopyroxene + 0.5% rutile, (2), and 5% garnet + 94.9% clinopyroxene + 0.1% rutile (3). Segments on curves indicate 5% increments in the melt fraction.
5.2. Geological Information on the Iron Isotopic Composition
5.2.1. Weathering and Alteration
A slight alteration was presented in potassic feldspar, plagioclase, amphibole, and biotite, according to mineralogy observations (Figure 3c,e,f). This was found to be strongly connected to weathering and environmental alterations occurring after the magmatic process. Fe3+ is enriched in heavy iron isotopes due to its stronger bond strength, while Fe2+ is enriched in light iron isotopes as a result of its larger ionic radius and weaker bond strength [50,51,52]. Fe2+ is released from primary minerals under the influence of weathering and migrates with light iron isotopes. On the contrary, under strong oxidative conditions, the release of Fe3+ from primary minerals leads to the formation of secondary minerals, preventing the loss of Fe and fractionation of the iron isotope composition [17].
CIA (Chemical index of alteration, CIA = Al2O3/(Al2O3 + Na2O + K2O + CaO), as a mole ratio, [53]) has been utilized to indicate the influence of the weathering degree on samples [54,55,56]. The δ56Fe of the samples did not significantly change with increased weathering (Figure 7a,b). Ba and U, as fluid mobile elements, are found preferentially in fluid under weathering conditions, resulting in a decrease in Ba/Th and an increase in Nb/U. The evolving trend exhibited in the Ba/Th vs. δ56Fe and Nb/U vs. δ56Fe diagrams shows no connection between two indices (Figure 7c,d). The above results illustrate negligible effects of weathering and alteration on the δ56Fe content of samples.
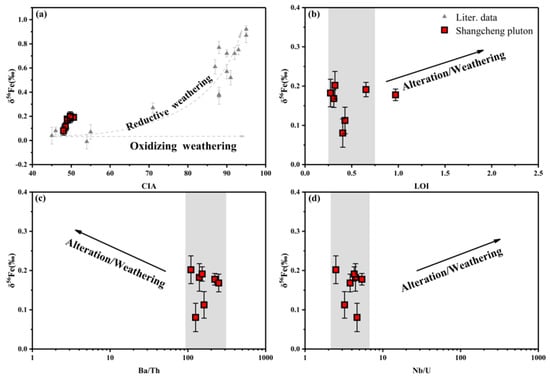
Figure 7.
(a) CIA vs. δ56Fe, (b) LOI vs. δ56Fe, (c) Ba/Th vs. δ56Fe, (d) Nb/U vs. δ56Fe; data were taken from [17].
5.2.2. Fluid Exsolution
The solubility of water decreases with magmatic upwelling and a decrease in pressure, resulting in the exsolution of Fe2+-enriched fluids. The fluid carries away light iron isotopes and contributes to the enrichment of heavy iron isotopes in the residual magma [3,11,12]. The Zr/Hf of igneous rocks generally ranges from 26 to 46, but Zr is more likely to be enriched in F-bearing fluids than Hf [13], and same applies to Nb and Ta. Therefore, the exsolution of fluid causes Zr/Hf < 26 and Nb/Ta < 5 [13,57]. Both Rb and La and Th and U are incompatible pairs that are not significantly affected by magmatic processes. However, fluid exsolution will reduce the Rb/La value and increase the Th/U value due to the higher fluid mobility of Rb and U compared with that of La and Th [14,58].
A correlation was not observed between δ56Fe and the four indices in this study (Figure 8a–d). On the Th/U vs. δ56Fe diagram (Figure 8c), the Th/U of the samples remains within the average Th/U value of the lower crust (~5.9 [14]) nearby. In addition, Du et al. [13] proposed that the control of iron isotope fractionation by fluid exudation is weak (<0.07‰). Therefore, fluid exsolution may not be the mechanism controlling the iron isotopic composition of the Shangcheng pluton.
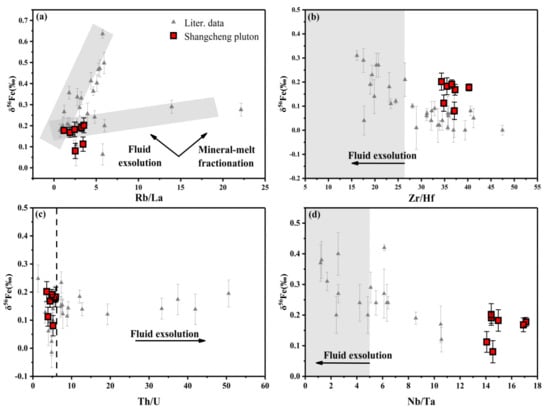
Figure 8.
(a) Rb/La vs. δ56Fe, (b) Zr/Hf vs. δ56Fe, (c) Th/U vs. δ56Fe, (d) Nb/Ta vs. δ56Fe. Data were taken from [12,13,14,56,58].
5.2.3. Fractional Crystallization and Mineralogy of the Source Rock
He et al. [14] reported the iron isotopic compositions of non-adakitic rock, low-Mg adakitic rock, and high-Mg Adak from the east Dabie orogen in Costa Rica/Panama, Central America and simulated the variation range of δ56Fe melt-residual during the partial melting of low-Mg adakite. The (Dy/Yb)n vs. δ56Fe diagram (Figure 9b) shows most samples plotted on the gray area, which may indicate the partial melting of eclogite containing 10~15% garnet, as the (Dy/Yb)n of melt = 1.9~2.3, δ56Fe melt-residual = 0.067~0.089‰, and δ56Fe melt = 0.138~0.155‰, according to previous simulation results [14]. This is similar to what can be observed on the (Yb)n vs. (La/Yb)n diagram, which displays an origin from melted amphibolite containing 10% garnet (Figure 9a or Figure 5d). It is notable that SGD-2-1 and SGD-3-1 may stem from the partial melting of eclogite containing 5% garnet or amphibolite, as shown in the (Dy/Yb)n vs. δ56Fe (Figure 9b) and (Yb)n vs. (La/Yb)n diagrams (Figure 9a or Figure 5d). The differences between the two samples from SGD and the other five samples suggest that the mineralogy of source rocks has had an effect on the fractionation of iron isotopes in the Shangcheng pluton.
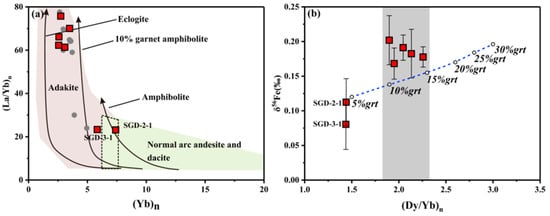
Figure 9.
(a) (Yb)n vs. (La/Yb)n [48], (b) (Dy/Yb)n vs. δ56Fe. The percentage denotes the content of garnet within the assumed source rock, and the blue dotted line is based on the simulative results from [14].
In terms of the other five samples, the elemental results indicate that they may have been generated from the partial melting of amphibolite containing 10% garnet, while the iron isotope results suggest the partial melting from eclogite containing 10~15% garnet as the origin. We conclude that the latter origin is more likely. Firstly, previous studies have demonstrated that there were two periods of major magmatic processes in the Dabie orogen during the Mesozoic. Rock produced in the first period are aged 145–130 Ma and have the geochemical characteristics of adakitic rocks [33,59,60], while rock produced in the second period is aged 130~110 Ma and has the characteristics of ordinary granite [61,62,63]. The thinning of the lower crust is considered to have occurred 130 Ma ago [21,64]. According to the reported data [38,39,40,41], the age of SGD unit ranges from 128 Ma to 137 Ma, while the ages of the SGW unit (130–139 Ma) and the LFW unit (133–141 Ma) are both older than 130 Ma. Secondly, from the perspective of (Dy/Yb)n, amphibole favours Dy over Yb. Thus, the (Dy/Yb)n should be lower if it was melted from amphibolite [21]. This may suggest that SGD-2-1 and SGD-3-1 were derived from the partial melting of amphibolite when the lower crust thinned with the removal of the eclogite source, while the other five samples may have been derived from the partial melting of eclogite containing 10~15% garnet, as the lower crust is still thickened with the existence of eclogite.
The δ56Fe of the samples increased with an increasing Fe3+/∑Fe and decreasing Mg# (Figure 10a,b), suggesting that the crystallization fractionation may have an effect on the iron isotopic composition of the sample. In the SiO2 vs. δ56Fe diagram (Figure 10c), the crystallization trend is similar to what is displayed by reported data. δ56Fecrystal-melt can be simulated using the Rayleigh fractionation equation.
where δ56Femelt(0) represents the δ56Fe value of the initial melt, and fFe represents the Fe fraction in the remaining melt (Figure 10d). Δ56Fecrystal-mel = ~0.035‰ may account for the fractional crystallization of most samples, apart from SGD-2-1 and SGD-3-1.
δ56Femelt = δ56Femelt(0) − Δ56Fecrystal-melt × ln(fFe)
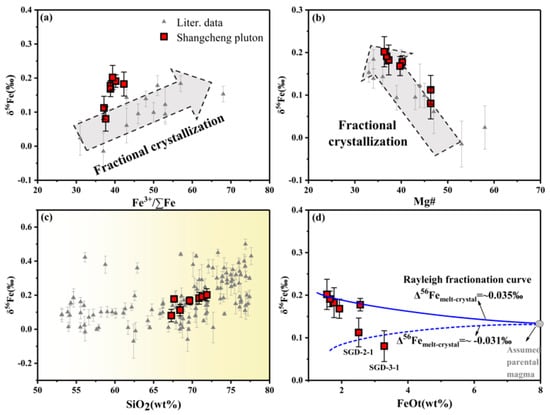
Figure 10.
(a) Fe3+/∑Fe vs. δ56Fe, (b) Mg# vs. δ56Fe, (c) SiO2 vs. δ56Fe, (d) FeOt vs. δ56Fe. The data were taken from [7,12,14,65]. The blue line stands for the modelled fractionation trend of samples.
Considering the DNb/ DTa = ~1 in magnetite (DNb = 2.2, DTa = ~2.5; [66]), the fractional crystallization of magnetite may not lead to the variation of Nb/Ta (Figure 11a), but the concentration of Fe3+ or Fe2O3T in the residual melt decreases as fractional crystallization proceeds, resulting in enrichment with heavy iron isotopes (Figure 11b). Thus, the fractional trend of iron isotopes in SGD-2-1 and SGD-3-1 may demonstrate the fractional crystallization of magnetite.
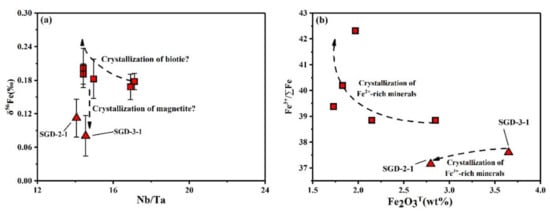
Figure 11.
(a) Nb/Ta vs. δ56Fe, (b) Fe2O3T vs. δ56Fe.
6. Conclusions
Evidence from the elemental geochemical characteristics and petrogenesis suggests that the Shangcheng pluton contains adakitic rock. Our investigation on the elemental and isotopic compositions hints that iron isotope enrichment cannot be explained by weathering/alteration and fluid exsolution. The fractional crystallization of magnetite accounts for enrichment with light iron isotopes in two rocks from the SGD unit due to the variation trends of Nb/Ta and Fe2O3T, while the fractional trend of iron isotopes in the other five samples can be explained by Δ56Fecrystal-melt = ~0.035‰. Considering that one SGD unit sample was found to have evolved similarly to the SGW and LFW unit samples, it can be concluded that the other two SGD unit rocks are more likely to have been derived from the partial melting of amphibolite, while the other five samples may have been derived from the partial melting of eclogite containing 10–15% garnet.
Author Contributions
Conceptualization, C.D.; methodology, C.D.; software, C.D.; validation, C.D. and W.L.; formal analysis, C.D.; sample collection, C.D. and C.H.; resources, C.D.; data curation, M.L.; writing—original draft preparation, C.D.; writing—review and editing, C.D. and W.L.; visualization, C.D.; supervision, W.L.; project administration, C.D. and W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant no. 41972169) and the priority academic program development of Jiangsu Higher Education on Institutions (2018–2021).
Data Availability Statement
The data presented in this study are openly available.
Acknowledgments
We appreciate Zhaochu Hu for his help in the measurement of iron isotopes. We also thank our team members who contributed to this study.
Conflicts of Interest
This study involved no conflicts of interest exist, and all authors have approved the manuscript for publication. We also declare that the work described is original research that has not been published previously and is not under consideration of publication elsewhere, in whole or part.
References
- Williams, H.M.; Peslier, A.H.; McCammon, C.; Halliday, A.N.; Levasseur, S.; Teutsch, N.; Burg, J.P. Systematic Iron Isotope Variations in Mantle Rocks and Minerals: The Effects of Partial Melting and Oxygen Fugacity. Earth Planet. Sci. Lett. 2005, 235, 435–452. [Google Scholar] [CrossRef]
- Weyer, S.; Ionov, D.A. Partial Melting and Melt Percolation in the Mantle: The Message from Fe Isotopes. Earth Planet. Sci. Lett. 2007, 259, 119–133. [Google Scholar] [CrossRef]
- Telus, M.; Dauphas, N.; Moynier, F.; Tissot, F.; Teng, F.Z.; Nabelek, P.; Craddock, P.R.; Groat, L.A. Iron, Zinc, Magnesium and Uranium Isotopic Fractionation During Continental Crust Differentiation: The Tale from Migmatites, Granitoids, and Pegmatites. Geochim. Cosmochim. Acta 2012, 97, 247–265. [Google Scholar] [CrossRef]
- Xu, L.J.; He, Y.; Wang, S.J.; Wu, H.; Li, S. Iron Isotop-E Fractionation During Crustal Anatexis: Constraint-S from Migmatites from the Dabie Orogen, Central China. Lithos 2017, 284, 171–179. [Google Scholar] [CrossRef]
- Schuessler, J.A.; Schoenberg, R.; Sigmarsson, O. Iron and Lithium Isotope Systematics of the Hekla Volcano, Iceland: Evidence for Fe Isotope Fractionation During Magma Differentiation. Chem. Geol. 2009, 258, 78–91. [Google Scholar] [CrossRef]
- Teng, F.Z.; Dauphas, N.; Helz, R.T.; Huang, S. Diffusion-driven Magnesium and Iron Isotope Fractionat-ion in Hawaiian Olivine. Earth Planet. Sci. Lett. 2011, 308, 317–324. [Google Scholar] [CrossRef]
- Sossi, P.A.; Foden, J.D.; Halverson, G.P. Redox-Controlled Iron Isotope Fractionation During Magmatic Differentiation: An Example from the Red Hill Intrusion, S. Tasmania. Contrib. Mineral. Petrol. 2012, 164, 757–772. [Google Scholar] [CrossRef]
- Collinet, M.; Charlier, B.; Namur, O.; Oeser, M.; Médard, E.; Weyer, S. Crystallization History of Enriched Shergottites from Feand Mg Isotope Fractionation in Olivine Megacrysts. Geochim. Cosmochim. Acta 2017, 207, 277–297. [Google Scholar] [CrossRef]
- Du, D.H.; Wang, X.L.; Yang, T.; Chen, X.; Li, J.Y.; Li, W. Origin of Heavy Fe Isotope Compositions in High-Siica Igneous Rocks: A Rhyolite Perspective. Geochim. Cosmochim. Acta 2017, 218, 58–72. [Google Scholar] [CrossRef]
- Chen, Y.H.; Niu, Y.L.; Duan, M.; Gong, H.M.; Guo, P.Y. Fractional Crystallization Causes the Iron Isotope Contrast Between Mid-Ocean Ridge Basalts and Abyssal Peridotites. Commun. Earth Environ. 2021, 2, 65. [Google Scholar] [CrossRef]
- Poitrasson, F.; Freydier, R. Heavy Iron Isotope Composition of Granites Determined by High Resolution MC-ICP-MS. Chem. Geol. 2005, 222, 132–147. [Google Scholar] [CrossRef]
- Heimann, A.; Beard, L.B.; Johnson, C.M. The Role of Volatile Exsolution and Sub-solidus Fluid/Rock Interactions in Producing High 56Fe/54Fe Ratios in Siliceous Igneous Rocks. Geochim. Cosmochim. Acta 2008, 72, 4379–4396. [Google Scholar] [CrossRef]
- Du, D.H.; Li, W.Q.; Wang, X.L.; Shu, X.J.; Yang, T.; Sun, T. Fe Isotopic Fractionation During the Magmatic-Hydrothermal Stage of Granitic Magmatism. Lithos 2019, 350, 105265. [Google Scholar] [CrossRef]
- He, Y.S.; Wu, H.J.; Ke, S.; Liu, S.A.; Wang, Q. Iron Isotopic Compositions of Adakitic and Non-Adakitic Graniti-C Magmas: Magma Compositional Control and Subtle Residual Garnet Effect. Geochim. CosmochImica Acta 2017, 203, 89–103. [Google Scholar] [CrossRef]
- Li, W.Q.; Czaja, A.D.; Van Kranendonk, M.J.; Beard, B.L.; Roden, E.E.; Johnson, C.M. An anoxic, Fe(II)-Rich, U-Poor ocean 3.46 billion years ago. Geochim. Cosmochim. Acta 2013, 120, 65–79. [Google Scholar] [CrossRef]
- Poitrasson, F.; Viers, J.; Martin, F.; Braun, J.J. Limited Iron Isotope Variations in Recent Lateritic Soils from Nsimi, Cameroon: Implications for the Global Fe Geochemical cycle. Chem. Geol. 2008, 253, 54–63. [Google Scholar] [CrossRef]
- Liu, S.A.; Teng, F.Z.; Li, S.G.; Wei, G.J.; Ma, J.L. Copper and Iron Isotope Fractionation during Weathering and Pedogenesis: Insights from Saprolite Profiles. Geochim. Cosmochim. Acta 2014, 146, 59–75. [Google Scholar] [CrossRef]
- Defant, M.J.; Drummond, M.S. Derivation of Some Modern Arc Magmas by Melting of Young Subducted Lithosphere. Nature 1990, 347, 662–665. [Google Scholar] [CrossRef]
- Xu, J.F.; Wu, J.Q.; Wang, Q.; Chen, J.L.; Cao, K. Research Advances of Adakites and Adakitic Rocks in China. Bull. Mineral. Geochem. 2014, 33, 6–13. [Google Scholar]
- Jiang, J.S.; Hu, P.; Xiang, W.S.; Wang, J.X.; Lei, Y.J.; Zhao, K.; Zeng, G.P.; Wu, F.F.; Xiang, P. Geochronology, geochemistry and its implication for regional tectonic evolution of adakite-like rock in the Burearea, western Ethiopia. Acta Geol. Sin. 2021, 95, 1260–1267. [Google Scholar]
- He, Y.S.; Li, S.G.; Hoefs, J.; Huang, F.; Liu, S.A.; Hou, Z.H. Post-Collisional Granitoids from the Dabie Orogen: New Evidence for Partial Melting of a Thickened Continental Crust. Geochim. Cosmochim. Acta 2011, 75, 3815–3838. [Google Scholar] [CrossRef]
- Chen, B.; Jahn, B.M.; Suzuki, K. Petrological and Nd-Sr-Os Isotopic Constraints on The Origin of High-Mg Adakitic Rocks from The North China Craton: Tectonic Implications. Geology 2013, 41, 91–94. [Google Scholar] [CrossRef]
- Gao, S.; Rudnick, R.; Yuan, H.L.; Liu, X.M.; Xu, W.L.; Ling, W.L.; Ayers, J.; Wang, X.C.; Wang, Q.H. Recycling Lower Continental Crust in the North China Craton. Nature 2004, 432, 892–897. [Google Scholar]
- Guo, F.; Nakamuru, E.; Fan, W.; Kobayoshi, K.; Li, C.W. Generation of Palaeocene Adakitic Andesites by Magma Mixing; Yanji Area, NE China. J. Petrol. 2007, 48, 661–692. [Google Scholar] [CrossRef]
- Chung, S.L.; Liu, D.; Ji, J.Q.; Chu, M.F.; Lee, H.Y.; Wen, D.J.; Lo, C.H.; Lee, T.Y.; Qian, Q.; Zhang, Q. Adakites from Continental Collision Zones: Melting of Thickened Lower Crust Beneath Southern Tibet. Geology 2003, 31, 1021–1024. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Yang, Y.; Jiang, W.J.; Yuan, L. Diverse Partial Melting during Continental Rifting, Subduction-Exhumation and Mountain-Root Collapse in the Dabie Orogen, Central China. Earth Sci. 2019, 44, 4195–4202. [Google Scholar]
- Zhao, Z.F.; Dai, F.Q.; Chen, Q. Continental Slab-Mantle Interaction: Geochemical Evidence from Post-Collisional Andesitic Rocks in the Dabie Orogen. Earth Sci. 2019, 44, 4119–4127. [Google Scholar]
- Zheng, Y.F.; Wang, Z.R.; Li, S.G. Oxygen Isotope Equilibrium Ultrahigh-Pressure Metamorphic Minerals and Its Constraints on Sm-Nd and Rb-Sr Chronometers. Geol. Soc. Lond. Spec. Publ. 2003, 220, 93–117. [Google Scholar] [CrossRef]
- Li, S.G.; Jagoutz, E.; Lo, C.H.; Chen, Y.Z.; Li, Q.L.; Xiao, Y.L. Sm/Nd, Rb/Sr, and 40Ar/39Ar Isotopic Systematics of the Ult-Rahigh-Pressure Metamorphic Rocks in the Dabie-Sulu Orogenic Belt, Central China: A Retrospective View. Int. Geol. Rev. 1999, 41, 1114–1124. [Google Scholar]
- Zhao, Z.F.; Zheng, Y.F.; Wei, C.S.; Wu, Y.B. Post-collisional Granitoids from the Dabie Orogen in China: Zircon U–Pb Age, Element and O Isotope Evidence for Recycling of Subducted Continental Crust. Lithos 2007, 93, 248–272. [Google Scholar] [CrossRef]
- Zheng, Y.F. Research Progress on UHP Metamorphism and Continental Collision: A Case Study of Dabie Sulu Orogenic Belt. Chin. Sci. Bull. 2008, 53, 2129–2152. [Google Scholar]
- Suo, S.T.; Zhong, Z.Q.; Zhou, H.W.; You, Z.D.; Zhang, L. Two Fresh Types of Eclogites in the Dabie-Sulu UHP Metamorphic Belt, China: Implications for the Deep Subduction and Earliest Stages of Exhumation of the Continental Crust. J. Earth Sci. 2012, 23, 775–785. [Google Scholar] [CrossRef]
- Xu, H.; Ma, C.; Zhang, J.; Ye, K. Early Cretaceous Low-Mg adakitic granites from the Dabie orogen, eastern China: Petrogenesis and implications for destruction of the over-thickened lower continental crust. Gondwana Res. 2013, 23, 190–207. [Google Scholar] [CrossRef]
- Liu, S.X.; Xu, H. Geochemistry, Zircon U-Pb Age and Hf Isotope of the Huilanshan Granitoids in the North Dabie Terrane: Implications for SynCollapse Magmatism of Orogen. J. Earth Sci. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Niu, P.P.; Jiang, S.Y. Petrogenesis of the Late Mesozoic Qijinfeng Granite Complex in the Tongbai Orogen: Geochronological, Geochemical and Sr-Nd-Pb-Hf Isotope Evidence. Lithos 2020, 356, 105290. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Fu, B.; Gong, B.; Li, L. Stable iso-Tope geochemistry of ultrahigh pressure metamorphi-C rocks from the Dabie–Sulu orogen in China: Implications for geodynamics and fluid regime. Earth-Sci. Rev. 2003, 62, 105–161. [Google Scholar] [CrossRef]
- Liu, X.C.; Jahn, B.M.; Liu, D.Y.; Dong, S.W.; Li, S.Z. SHRIMP U-Pb Zircon Dating of a Metagabbro and Eclogites from Western Dabieshan (Hong’an Block), China, and Its Tectonic Implications. Tectonophysics 2004, 394, 171–192. [Google Scholar]
- Gao, X.Y.; Zhao, T.P.; Shi, X.B.; Zhang, Z.H.; Bao, Z.W. Geochemisty and petrogenesis of the early cretaceous Shangcheng and Daquandian granites in the north Dabie mountains. Geochimica 2013, 42, 307–339. [Google Scholar]
- Liu, W.B.; Liu, Z.H.; Zhang, S.J. Geological and geochemical features of Shangcheng granite body and its genetic implication, Henan. Geol. Miner. Resour. South China 2004, 4, 17–23. [Google Scholar]
- Huang, D.F.; Lu, X.X.; Luo, Z.H.; Song, Y.W.; Lv, G.Y. Zircon SHRIMP U-Pb Age, Geochemical Characteristics and Geological Implications of Shangcheng Pluton in the Northern Margin of Dabie Mountain. Earth Sci. 2019, 44, 3829–3844. [Google Scholar]
- Yang, C.Y. Geochemical Feature and Petrogensis of Shangcheng Pluton and Xinxian Pluton, Dabie Orogenic Belt, China. Master’s Thesis, University of Science and Technology, Hefei, China, 2020. [Google Scholar]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, F.; Xu, J.; Gao, C.G.; Chen, H.H. In Situ Analysis of Major and Trace Elements of Anhydrous Minerals by LA-ICP-MS without Applying an Internal Standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Lei, Y.T.; Li, M.; Wang, Z.C.; Zhu, Y.T.; Hu, Z.C.; Liu, Y.S.; Chai, X.N. High resolution iron isotopic measurement using large geometry Multi-Collector Inductively Coupled Plasma Mass Spectrometer. At. Spectrosc. 2021, in press. [Google Scholar]
- Sun, S.S.; McDonough, W.F. Chemical and Isotopic Systematics of Oceanic Basalts: Implications for Mantle Composition and Processes. In Magmatism in the Oceanic Basalts; Saunder, A.D., Norry, M.J., Eds.; Geological Society London Special Publications: London, UK, 1989; pp. 313–345. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W. A Guide to the Chemical Classification of the Common Volcanic Rocks. Can. J. Earth Sci. 1971, 8, 523–548. [Google Scholar] [CrossRef]
- Middlemost, E. Naming materials in the magma/igneous rock system. Earth Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Qin, J.F.; Lai, S.C.; Diwu, C.R.; Ju, Y.J.; Li, Y.F. Magma mixing origin for the post-collisional adakitic monzogranite of the Triassic Yangba pluton, Northwestern margin of the South China block: Geochemistry, Sr–Nd isotopic, zircon U–Pb dating and Hf isotopic evidences. Contrib. Mineral. Petrol. 2010, 159, 389–409. [Google Scholar] [CrossRef]
- Xue, H.M. Geochronology, geochemistry and stratospheric interactions of Late Mesozoic granitoids near the boundary between Anhui and Zhejiang provinces in the eastern segment of the Jiangnan orogenic belt. Acta Petrol. Sin. 2021, 37, 433–461. [Google Scholar]
- Wu, H.J.; He, Y.S.; Bao, L.; Zhu, C.W.; Li, S.G. Mineral Composition Control on Inter-mineral Iron Isotopic Fractionation in Granitoids. Geochim. Et Cosmochim. Acta 2017, 198, 208–217. [Google Scholar] [CrossRef]
- Dauphas, N.; Roskosz, M.; Alp, E.; Neuville, D.R.; Hu, M.Y.; Sio, C.K.; Tissot, F.L.H.; Zhao, J.; Tissandier, L.; Médard, E.; et al. Magma redox and structural controls on iron isotope variations in Earth’s mantle and crust. Earth Planet. Sci. Lett. 2014, 398, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Schauble, E.A. Applying Stable Isotope Fractionation Theory to New Systems. Geochemistry of Non-traditional Stable Isotopes. Geochem. Non Tradit. Stable Isot. 2004, 55, 65–111. [Google Scholar]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic Climates and Plate Motions Inferred from Major Element Chemistry of Lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Li, C.; Yang, S. Is Chemical Index of Alteration (CIA) a Reliable Proxy for Chemical Weathering in Global Drainage Basins? Am. J. Sci. 2010, 310, 111–127. [Google Scholar] [CrossRef]
- Goldberg, K.; Humayun., M. The Applicability of the Chemical Index of Alteration as a Paleoclimatic Indicator: An Example from The Permian of The ParanÁ Basin, Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 175–183. [Google Scholar] [CrossRef]
- Li, R.Y. Evolution of the Archean Continental Crust and Link to the Great Oxidation Event traced by Mg, Fe isotopes. Ph.D. Thesis, China University of Geoscience Beijing, Beijing, China, 2020. [Google Scholar]
- Bea, A.F.; Montero, P.; Ortega, M. A LA-ICP-MS Evaluation of Zr Reservoirs in Common Crustal Rocks: Implications for Zr and Hf Geochemistry, and Zircon-Forming Processes. Can. Mineral. 2006, 44, 693–714. [Google Scholar] [CrossRef]
- Xia, Y.; Li, S.Q.; Huang, F. Iron and Zinc Isotope Fractionation During Magmatism in the Continental Crust: Evidence from Bimodal Volcanic Rocks from Hailar Basin, NE China. Geochim. Cosmochim. Acta 2017, 213, 35–46. [Google Scholar]
- Wang, Q.; Wyman, D.A.; Xu, J.F.; Jian, P.; Zhao, Z.H.; Li, C.F.; Xu, W.; Ma, J.L.; He, B. Early Cretaceous adakitic granites in the Northern Dabie Complex, central China: Implications for partial melting and delamination of thickened lower crust. Geochim. Cosmochim. Acta 2007, 71, 2609–2636. [Google Scholar] [CrossRef]
- Huang, F.; Li, S.; Feng, D.; He, Y.S.; Chen, F.K. High-Mg adakitic rocks in the Dabie orogen, central China: Implications for foundering mechanism of lower continental crust. Chem. Geol. 2008, 255, 1–13. [Google Scholar] [CrossRef]
- Jahn, B.M.; Wu, Y.B.; Lo, C.H.; Tsai, C.H. Crust–mantle interaction induced by deep subduction of the continental crust: Geochemical and Sr–Nd isotopic evidence from post-Collisional mafic–Ultramafic intrusions of the northern Dabie complex, central China. Chem. Geol. 1999, 157, 119–146. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fan, W.M.; Peng, T.P.; Zhang, H.F.; Guo, F. Nature of the Mesozoic lithospheric mantle and tectonic decoupling beneath the Dabie Orogen, Central China: Evidence from 40Ar/ 39Ar geochronology, elemental and Sr–Nd–Pb isotopic compositions of early Cretaceous mafic igneous rocks. Chem. Geol. 2005, 220, 165–189. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Zheng, Y.F.; Wei, C.S.; Wu, Y.B.; Chen, F.K.; Jahn, B.M. Zircon U–Pb age, element and C–O isotope geochemistry of post-Collisional mafic-ultramafic rocks from the Dabie orogen in east-Central China. Lithos 2005, 83, 1–28. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Z.W.; Li, H.C.; Yang, X.N.; Yang, J.Q.; Wang, H.; Wang, S.H. Petrogenesis and Origin of the Xinxian Granitic Batholith in Henan Province and Its Implication for the Tectonic Evolution of the Western Dabie Area. Acta Geol. Sin. 2013, 87, 1510–1524. [Google Scholar]
- Foden, J.; Sossi, P.A.; Wawryk, C.M. Fe isotopes and the contrasting petrogenesis of A-, I-and S-type granite. Lithos 2015, 212–215, 32–44. [Google Scholar]
- Nash, W.P.; Crecraft, H.R. Partition Coefficients for Trace Elements in Silicic Magmas. Geochim. Cosmochim. Acta 1985, 49, 2309–2322. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).