X-ray Diffraction Analysis of Clay Particles in Ancient Baekje Black Pottery: Indicator of the Firing Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
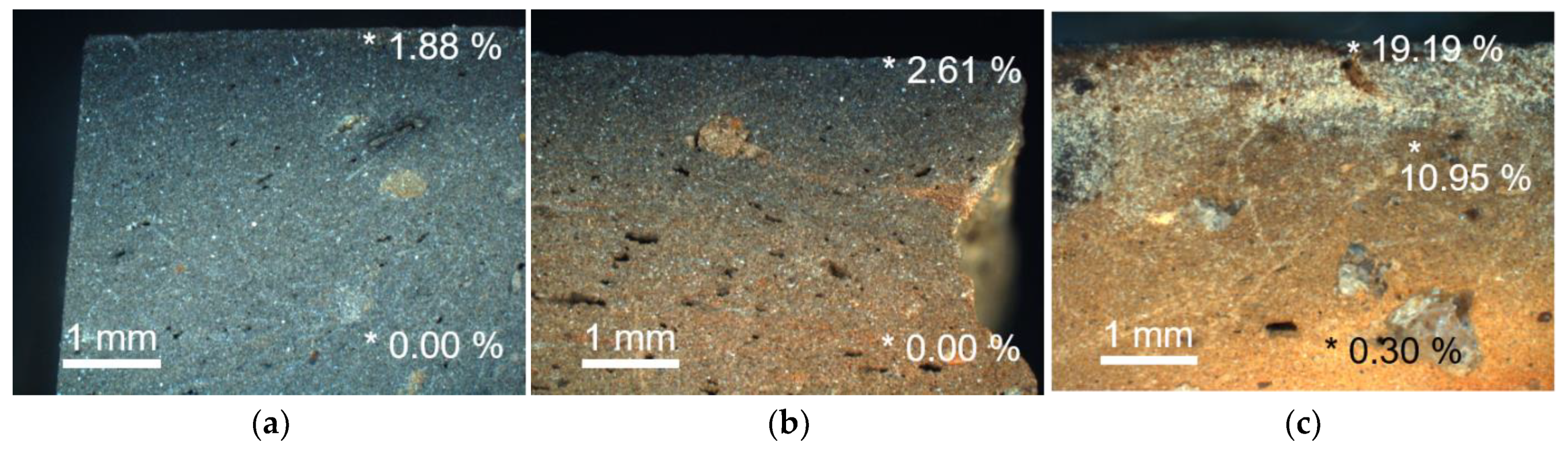
3.1. Blackening Trend and Carbon Content
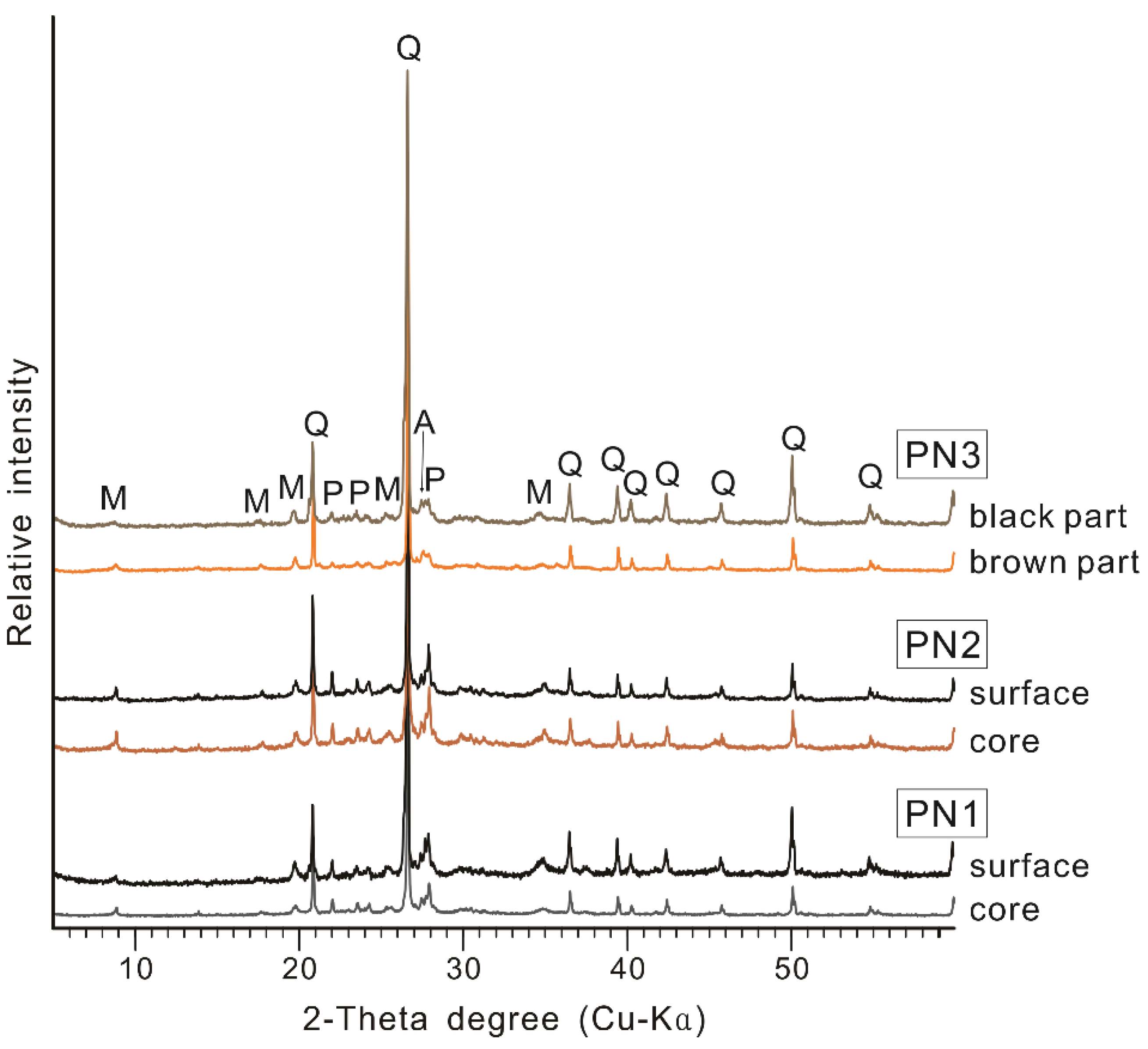
3.2. Major Mineral Composition
3.3. Clay Particle XRD and Firing Parameters
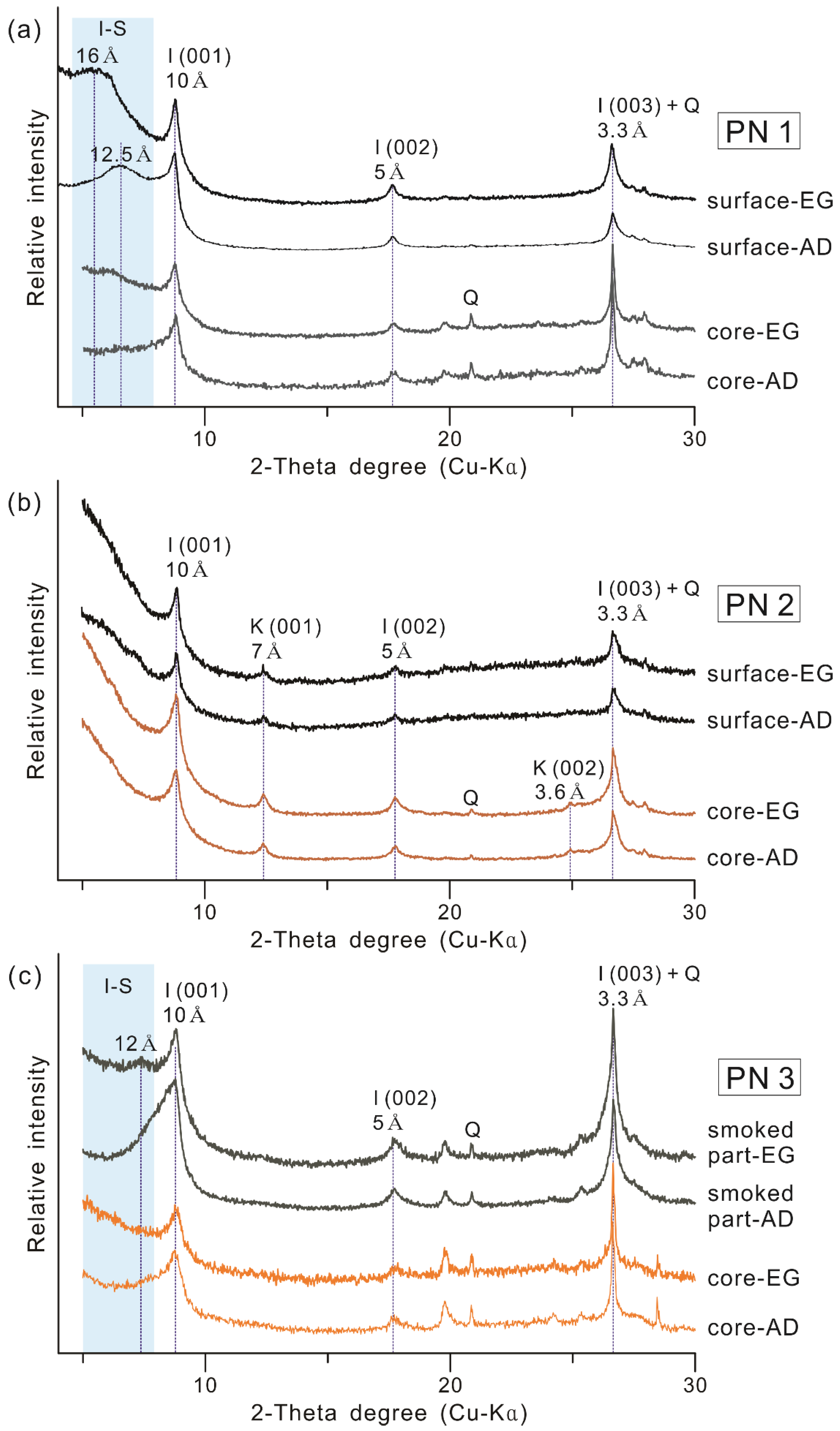
3.3.1. Clay Mineral Composition
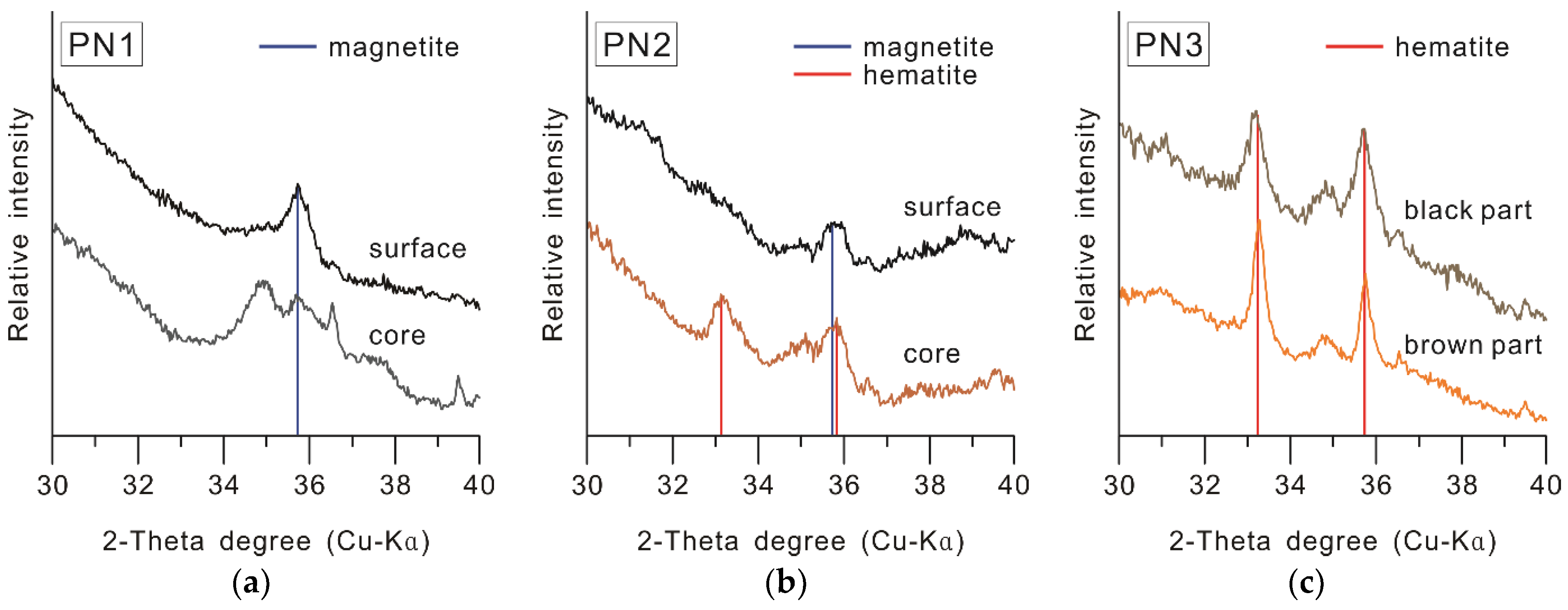
3.3.2. Iron Oxide Composition
3.4. Manufacturing Techniques for Each Type of Black Pottery
- PN1: It is a type of black pottery with a black or grayish black core, in which carbon was not detected. It was estimated to have been produced by reduction firing in the temperature range of 550–900 °C, and it was suggested that the blackening occurred due to the conversion of iron oxides into magnetite in the raw material soil. Furthermore, dark blackening was predicted to have occurred, even in the inner part, through firing in a stronger reduction environment (e.g., higher temperature, reduction gas amount, and reaction duration time) compared to PN2, in which the inner part is brown.
- PN2: It is a type of black pottery in which the core is brown, and a black margin of about 1 mm from the surface has been formed. Since kaolin was detected in both the black surface and the brown core, the result revealed that it was produced by firing at temperatures below 550 °C. Moreover, the study found that it was produced in a reduction firing environment of at least 500 °C, based on the iron oxide composition of the black surface, in which magnetite was detected [76], unlike the brown core, in which hematite was detected. In other words, PN2 was produced by reduction firing in the range of 500–550 °C, and the core seems to have not blackened because it was manufactured at a lower reduction firing condition compared to PN1.
- PN3: The carbon content is distinctly high in the parts where the blackening occurred because of the covering layer of soot. Irrespective of the color in each part, hematite was detected, while kaolin was not. This implies that, in this type, the deposition and penetration of black carbon particles were achieved through firing in a state of contact with vegetable raw material, in an oxidizing environment with temperatures in the range of 550–900 °C.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Branfman, S. Raku: A Practical Approach; Krause Publications: Iola, WI, USA, 2001; p. 176. [Google Scholar]
- Watkins, J.C.; Wandless, P.A. Alternative Kilns and Firing Techniques; Lark crafts: Asheville, NC, USA, 2006; p. 128. [Google Scholar]
- Lazo, E. Naked Raku and Related Bare Clay Techniques; American Ceramic Society: Westerville, OH, USA, 2012; p. 151. [Google Scholar]
- Dassow, S.V. Low-Firing and Burnishing; A & C Black publishers LTD: London, UK, 2013; p. 112. [Google Scholar]
- Trąbska, J.; Wesełucha-Birczyńska, A.; Zięba-Palus, J.; Thagård Runge, M. Black painted pottery, Kildehuse II, Odense County, Denmark. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Hawley, F.M. Prehistoric pottery pigments in the Southwest. Am. Anthropol. 1929, 31, 731–754. [Google Scholar] [CrossRef]
- Stewart, J.D.; Adams, K.R. Evaluating visual criteria for identifying carbon- and iron-based pottery paints from the four corners region using SEM-EDS. Am. Antiq. 1999, 64, 675–696. [Google Scholar] [CrossRef]
- Pendleton, M.; Washburn, D.K.; Ellis, E.A.; Pendleton, B.B. Distinguishing between mineral paint and carbon paint on ancestral Puebloan pottery. Micros. Today 2012, 20, 32–36. [Google Scholar] [CrossRef]
- Pendleton, M.; Washburn, D.K.; Ellis, E.A.; Pendleton, B.B. Comparing the detection of iron-based pottery pigment on a carbon-coated sherd by SEM-EDS and by Micro-XRF-SEM. Yale J. Biol. Med. 2014, 87, 15–20. [Google Scholar]
- Spataro, M.; Cubas, M.; Craig, O.E.; Chapman, J.C.; Boroneant, A.; Bonsall, C. Production and function of Neolithic black-painted pottery from Schela Cladovei (Iron Gates, Romania). Archaeol. Anthropol. Sci. 2019, 11, 6287–6304. [Google Scholar] [CrossRef]
- Rosado, L.; Pevenage, J.V.; Vandenabeele, P.; Candeias, A.; Lopes, M.D.C.; Tavares, D.; Alfenim, R.; Schiavon, N.; Mirao, J. Multi-analytical study of ceramic pigments application in the study of Iron Age decorated pottery from SW Iberia. Measurement 2017, 118, 262–274. [Google Scholar] [CrossRef]
- Gangas, N.H.J.; Kostikas, A.; Simopoulos, A.; Vocotopoulou, J. Mössbauer spectroscopy of ancient Greek pottery. Nature 1971, 229, 485–486. [Google Scholar] [CrossRef]
- Kostikas, A.; Simopoulos, A.; Gangas, N.H.J. Mössbauer study of ancient Greek pottery. J. Phys. 1974, 35, 107–115. [Google Scholar]
- Longworth, G.; Warren, S.E. Mössbauer spectroscopy of Greek ‘Etruscan’ pottery. Nature 1975, 255, 625–627. [Google Scholar] [CrossRef]
- Nagy, S.; Kuzman, E.; Weiszburg, T.; Gyökeres-Tóth, M.; Riedel, M. Oxide transformation during preparation of black pottery in Hungary. J. Radioanal. Nucl. Chem. 2000, 246, 91–96. [Google Scholar] [CrossRef]
- Epossi Ntah, Z.L.; Sobott, R.; Fabbri, B.; Bente, K. Characterization of some archaeological ceramics and clay samples from Zamala—Far-northern part of Cameroon (West Central Africa). Cerâmica 2017, 63, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Noghani, S.; Emami, M. Mineralogical phase transition on sandwich-like structure of clinky pottery from Parthian Period, Iran. Period. Mineral. 2014, 83, 171–185. [Google Scholar]
- Venkatachalapathy, R.; Bakas, T.; Basavaiah, N.; Deenadayalan, K. Mössbauer and mineral magnetic studies on archaeological potteries from Adhichanallur, Tamilnadu, India. Hyperfine Interact. 2008, 186, 89–98. [Google Scholar] [CrossRef]
- Shimada, I.; Häusler, W.; Jakob, M.; Montenegro, J.; Riederer, J.; Wagner, U. Early pottery making in northern coastal Peru. Part IV: Mössbauer study of ceramics from Huaca Sialupe. Hyperfine Interact. 2003, 150, 125–139. [Google Scholar] [CrossRef]
- Bong, W.S.K.; Matsumura, K.; Nakai, I. Firing technologies and raw materials of typical early and middle Bronze Age pottery from Kaman-Kalehöyük; A statistical and chemical analysis. Anat. Archaeol. Stud. 2008, 17, 295–311. [Google Scholar]
- Meirer, F.; Liu, Y.; Pouyet, E.; Fayard, B.; Cotte, M.; Sanchez, C.; Andrews, J.C.; Mehta, A.; Scianu, P. Full-field XANES analysis of Roman ceramics to estimate firing conditions—A novel probe to study hierarchical heterogeneous materials. J. Anal. At. Spectrom. 2013, 28, 1870–1883. [Google Scholar] [CrossRef]
- Tanthanuch, W.; Pattanasiriwisawa, W.; Somphon, W.; Srilomsak, S. Synchrotron Studies of Ban Chiang Ancient Pottery. Suranaree J. Sci. Technol. 2011, 18, 15–28. [Google Scholar]
- Joseph, D. Investigations on Megalithic Pottery Samples by X-ray Emission Spectroscopy (EDXRF and XANES). Asian J. Adv. Basic Sci. 2014, 3, 60–66. [Google Scholar]
- Hormes, J.; Bovenkamp-Langlois, L.; Klysubun, W.; Kizilkaya, O. Calcium X-ray absorption near edge structure (XANES) spectra: A thermometer for the firing temperature of ceramics? Microchem. J. 2020, 154, 104571. [Google Scholar] [CrossRef]
- Sciau, P.; Relaix, S.; Kihn, Y.; Roucau, C. The role of Microstructure, Nanostructure and Composition in the Brilliant Red Slip of Roman Terra Sigillata Pottery from Southern Gaul. MRS Online Proc. Libr. 2005, 852, 7–12. [Google Scholar] [CrossRef]
- Mirguet, C.; Roucau, C.; Sciau, P. Transmission Electron Microscopy a Powerful Means to Investigate the Glazed Coating of Ancient Ceramics. J. Nano Res. 2009, 8, 141–146. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, N.S.; Lee, J.H.; Lee, H.S.; Chae, S.J. A study on the occurrence of Paekche burnished black pottery and their reproduction. Rev. Cult. Herit. Stud. 2001, 34, 4–18. [Google Scholar]
- Kim, S.K.; Han, M.S.; Nam, S.W.; Jang, S. Manufacturing characteristics of black burnished pottery from Pungnaptoseong, Beakje. J. Conserv. Sci. 2017, 33, 417–429. [Google Scholar]
- Yoon, Y.J. Study on the pre-historic relics along the Geumho River. Komunhwa Korea Antiq. 1969, 5, 1–22. [Google Scholar]
- Kim, S.K. A Study on Material Characteristics and Making Techniques for Black Burnished Pottery from Hanseong Period of Baekje in Ancient Korea. Ph.D. Thesis, Kongju National University, Koongju, Korea, 2020. [Google Scholar]
- Han, Y.H. The newly acquired bronze age artifacts of Chinju National Museum (1984–1985). Yeongnam Archaeol. Rev. 1986, 1, 151–167. [Google Scholar]
- Shin, K.S.; Oh, M.M. The production and restoration of Bronze Age polished pottery through experimental archaeology. J. Kor. Field Archaeol. 2010, 8, 35–80. [Google Scholar]
- Nam, S.W. As production technology, the meaning of the black burnished pottery in Baekje Dynasty. Korean Archaeol. 2013, 89, 94–137. [Google Scholar]
- Nam, S.W.; Kim, S.K. Research on the production technology of black-burnished pottery in Baekje period from the perspective of experimental archaeology. Ann. Baekje 2015, 12, 5–34. [Google Scholar]
- Jin, W.S.J. The Developmental Study of Interior Ceramics Which is Using Traditional Smoked Earthenware Techniques. Master’s Thesis, Wonkwang University, Iksan, Korea, 2016. [Google Scholar]
- Moon, D.H.; Lee, M.S.; Nam, S.W.; Cho, H.G. A Study on Magnetic Properties and Role of the Iron Oxides in Ancient Baekje Black Burnished Pottery by Mössbauer Spectroscopy. J. Magn. 2020, 25, 496–502. [Google Scholar] [CrossRef]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Lackschewitz, K.S.; Singer, A.; Botz, R.; Garbe-Schönberg, D.; Stoffers, P. Mineralogy and geochemistry of clay minerals near a hydrothermal site in the Escanaba Trough, Gorda Ridge, northeast Pacific Ocean. In Proceedings of the Ocean Drilling Program, Scientific Results; Zierenberg, R.A., Fouquet, Y., Miller, D.J., Normark, W.R., Eds.; Texas A&M University: College Station, TX, USA, 2000; Volume 169, pp. 1–24. [Google Scholar]
- Bristow, T.F.; Kennedy, M.J.; Derkowski, A.; Droser, M.L.; Jiang, G.; Creaser, R.A. Mineralogical constraints on the paleoenvironments of the Ediacaran Doushantuo Formation. Proc. Natl. Acad. Sci. USA 2009, 106, 13190–13195. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.J.; Cho, H.G. Changes in detrital sediment supply to the central Yellow Sea since the last deglaciation. Ocean Sci. 2020, 16, 1247–1259. [Google Scholar] [CrossRef]
- Moon, D.H. Mineralogical Study on the Raw Materials of Cultural Properties; Interpretation and Rediscovery. Ph.D. Thesis, Gyeongsang National University, Jinju, Korea, 2021. [Google Scholar]
- Cho, H.G.; Kim, S.-O.; Kwak, K.Y.; Choi, H. Clay mineral distribution and provenance in the Heuksan mud belt, Yellow Sea. Geo-Mar. Lett. 2015, 35, 411–419. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, S.-O.; Kim, Y.H. Characterization of Behavior of Colloidal Zero-Valent Iron and Magnetite in Aqueous Environment. J. Miner. Soc. Korea 2015, 28, 95–108. [Google Scholar] [CrossRef]
- Rice, P.M. Pottery Analysis: A Sourcebook, 1st ed.; The University of Chicago Press: Chicago, IL, USA, 1987; p. 559. [Google Scholar]
- Quinn, P.S.; Benzonelli, A. X-ray diffraction and archaeological materials analysis. In The SAS Encyclopedia of Archaeological Sciences, 1st ed.; López Varela, S.L., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Viania, A.; Cultrone, G.; Sotiriadisa, K.; Ševčíka, R.; Šašeka, P. The use of mineralogical indicators for the assessment of firing temperature in fired-clay bodies. Appl. Clay Sci. 2018, 163, 108–118. [Google Scholar] [CrossRef]
- Quinn, P.S. Ceramic Petrography: The Interpretation of Archaeological Pottery and Related Artefacts in Thin Section, 1st ed.; Archaeopress: Oxford, UK, 2013. [Google Scholar]
- Maggetti, M.; Neururer, C.; Ramseyer, D. Temperature evolution inside a pot during experimental surface (bonfire) firing. Appl. Clay Sci. 2011, 53, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Travé Allepuz, E. Colour Transformation and Textural Change in Biotite: Some Remarks for the Interpretation of Firing Technology in Greyware Pottery Thin-Sections. Minerals 2021, 11, 428. [Google Scholar] [CrossRef]
- Middleton, A.P. Technological investigation of the coatings on some ‘hematite-coated’ pottery from southern England. Archaeometry 2007, 29, 250–261. [Google Scholar] [CrossRef]
- De Bonis, A.; Cultrone, G.; Grifa, C.; Langella, A.; Leone, A.P.; Mercurio, M.; Morra, V. Different shades of red: The complexity of mineralogical and physico-chemical factors influencing the colour of ceramics. Ceram. Int. 2017, 43, 8065–8074. [Google Scholar] [CrossRef]
- Heimann, R.; Franklin, U.M. Archaeo-thermometry: The assessment of firing temperatures of ancient ceramics. J. Int. Inst. Conserv.-Can. Group 1980, 4, 23–45. [Google Scholar]
- Van Diepen, A.M.; Popma, J.A. Mössbauer effect and magnetic properties of an amorphous Fe2O3. J. Phys. Colloq. 1976, 37, 755–758. [Google Scholar] [CrossRef]
- Cao, X.; Prozorov, R.; Koltypin, Y.; Kataby, G.; Felner, I.; Gedanken, A. Synthesis of pure amorphous Fe2O3. J. Mater. Sci. 1997, 12, 402–406. [Google Scholar] [CrossRef]
- Schneeweiss, O.; Zbořil, R.; David, B.; Heřmánek, M.; Mashlan, M. Solid-state synthesis of α-Fe and iron carbide nanoparticles by thermal treatment of amorphous Fe2O3. Hyperfine Interact. 2009, 189, 167–173. [Google Scholar] [CrossRef]
- Topsøe, H.; Dumesic, J.; Boudart, M. Mössbauer spectra of stoichiometric and nonstoichiometric Fe3O4 microcrystals. J. Phys. Colloq. 1974, 35, 411–413. [Google Scholar] [CrossRef]
- Koch, C.B.; Jiang, J.Z.; Mørup, S. Mechanical milling of Fe3O4/SiO2: Formation of an amorphous Fe(II)-Si-O-containing phase. Scr. Mater. 1999, 12, 233–236. [Google Scholar]
- Rodriguez, A.F.R.; Coaquira, J.A.H.; Morales, M.A.; Faria, F.S.E.D.V.; Cunha, R.M.; Santos, J.G.; Silveira, L.B.; Candela, D.R.S.; Baggio-Saitovitch, E.M.; Rabelo, D.; et al. Synthesis, characterization and magnetic properties of polymer-Fe3O4 nanocomposite. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013, 100, 101–103. [Google Scholar] [CrossRef]
- Hah, H.Y. Mössbauer Spectroscopy of Iron Oxide Nanoparticles: Materials for Biomedical Applications. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2018. [Google Scholar]
- Zhang, X.W.; Kong, L.W.; Cui, X.L.; Yin, S. Occurrence characteristics of free iron oxides in soil microstructure: Evidence from XRD, SEM and EDS. Bull. Eng. Geol. Environ. 2016, 75, 1493–1503. [Google Scholar] [CrossRef]
- Maggetti, M. 11. Phase analysis and its significance for technology and origin. In Archaeological Ceramics; Olin, J.S., Franklin, A.D., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 121–133. [Google Scholar]
- Wilson, M.A.; Hamilton, A.; Ince, C.; Carter, M.A.; Hall, C. Rehydroxylation (RHX) dating of archaeological pottery. Proc. R. Soc. A 2012, 468, 3476–3493. [Google Scholar] [CrossRef] [Green Version]
- Weiner, S.; Nagorsky, A.; Feldman, Y.; Kossay, A. Archaeological Ceramic Diagenesis: Clay Mineral Recrystallization in Sherds from a Late Byzantine Kiln, Israel. Minerals 2020, 10, 408. [Google Scholar] [CrossRef]
- Bellotto, M.; Gualtieri, A.; Artioli, G.; Clark, S.M. Kinetic study of the kaolinite-mullite reaction sequence. Part I: Kaolinite dehydroxylation. Phys. Chem. Miner. 1995, 22, 207–217. [Google Scholar] [CrossRef]
- Murad, E.; Wagner, U. Pure and impure clays and their firing products. Hyperfine Interact. 1989, 45, 161–177. [Google Scholar] [CrossRef]
- Chevalier, R.; Coey, J.M.D.; Bouchez, R. A study of iron in fired clay: Mössbauer effect and magnetic measurement. J. Phys. Colloq. 1976, 37, 861–865. [Google Scholar] [CrossRef]
- Maniatis, Y.; Tite, M.S. Technological examination of Neolithic-Bronze age pottery from central and southeast Europe and from the near East. J. Archaeol. Sci. 1981, 8, 59–76. [Google Scholar] [CrossRef]
- Mackenzie, K.J.D.; Cardile, C.M. A 57Fe Mössbauer study of black coring phenomena in clay-based ceramic materials. J. Mater. Sci. 1990, 25, 2937–2942. [Google Scholar] [CrossRef]
- Maritan, L.; Nodari, L.; Mazzoli, C.; Milano, A.; Russo, U. Influence of firing conditions on ceramic products: Experimental study on clay rich in organic matter. Applied Clay Sci. 2006, 31, 1–15. [Google Scholar] [CrossRef]
- Dawson, D.; Kent, O. Experiments in reduction firing. Bull. Exp. Firing Grp. 1987, 5, 34–41. [Google Scholar]
- Dawson, D.; Kent, O. Reduction fired low-temperature ceramics. Postmediev. Archaeol. 1999, 33, 164–178. [Google Scholar] [CrossRef]
- Thér, R.; Gregor, M. Experimental reconstruction of the pottery firing process of Late Bronze Age pottery from north-eastern Bohemia. In Archaeological Ceramics: A Review of Current Research; Scarcella, S., Ed.; Archaeopress: Oxford, UK, 2011; pp. 128–142. [Google Scholar]
- Kingery, W.D. Attic Pottery Gloss Technology. Archaeomaterials 1991, 5, 47–54. [Google Scholar]
- Zhushchikhovskaya, I.S.; Nikitin, G. Ceramic firing structures in Prehisoric and ancient societies of the Russian far East. Asian Perspect. 2015, 53, 121–149. [Google Scholar] [CrossRef] [Green Version]
- Heimann, R.B.; Maggetti, M. The struggle between thermodynamics and kinetics: Phase evolution of ancient and historial ceramics. In EMU Notes in Mineralogy: The Contribution of Mineralogy to Cultural Heritage; Artioli, G., Oberti, R., Eds.; Mineralogical Society: London, UK, 2019; Volume 20, pp. 233–281. [Google Scholar]
- Yu, J.; Han, Y.; Li, Y.; Gao, P.; Li, W. Mechanism and kinetics of the reduction of hematite to magnetite with CO-CO2 in a micro-fluidized bed. Minerals 2017, 7, 209. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.-H.; Kim, S.-J.; Nam, S.-W.; Cho, H.-G. X-ray Diffraction Analysis of Clay Particles in Ancient Baekje Black Pottery: Indicator of the Firing Parameters. Minerals 2021, 11, 1239. https://doi.org/10.3390/min11111239
Moon D-H, Kim S-J, Nam S-W, Cho H-G. X-ray Diffraction Analysis of Clay Particles in Ancient Baekje Black Pottery: Indicator of the Firing Parameters. Minerals. 2021; 11(11):1239. https://doi.org/10.3390/min11111239
Chicago/Turabian StyleMoon, Dong-Hyeok, So-Jin Kim, Sang-Won Nam, and Hyen-Goo Cho. 2021. "X-ray Diffraction Analysis of Clay Particles in Ancient Baekje Black Pottery: Indicator of the Firing Parameters" Minerals 11, no. 11: 1239. https://doi.org/10.3390/min11111239
APA StyleMoon, D.-H., Kim, S.-J., Nam, S.-W., & Cho, H.-G. (2021). X-ray Diffraction Analysis of Clay Particles in Ancient Baekje Black Pottery: Indicator of the Firing Parameters. Minerals, 11(11), 1239. https://doi.org/10.3390/min11111239




