Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Pillaring Agent under Different Temperatures
2.2. Evaluation of the Intercalation Time
2.3. Diluted Pillaring Solutions and Re-Pillaring Procedures
2.4. Characterization Techniques
3. Results and Discussion
3.1. Influence of Temperature in the Synthesis of the Pillaring Agent
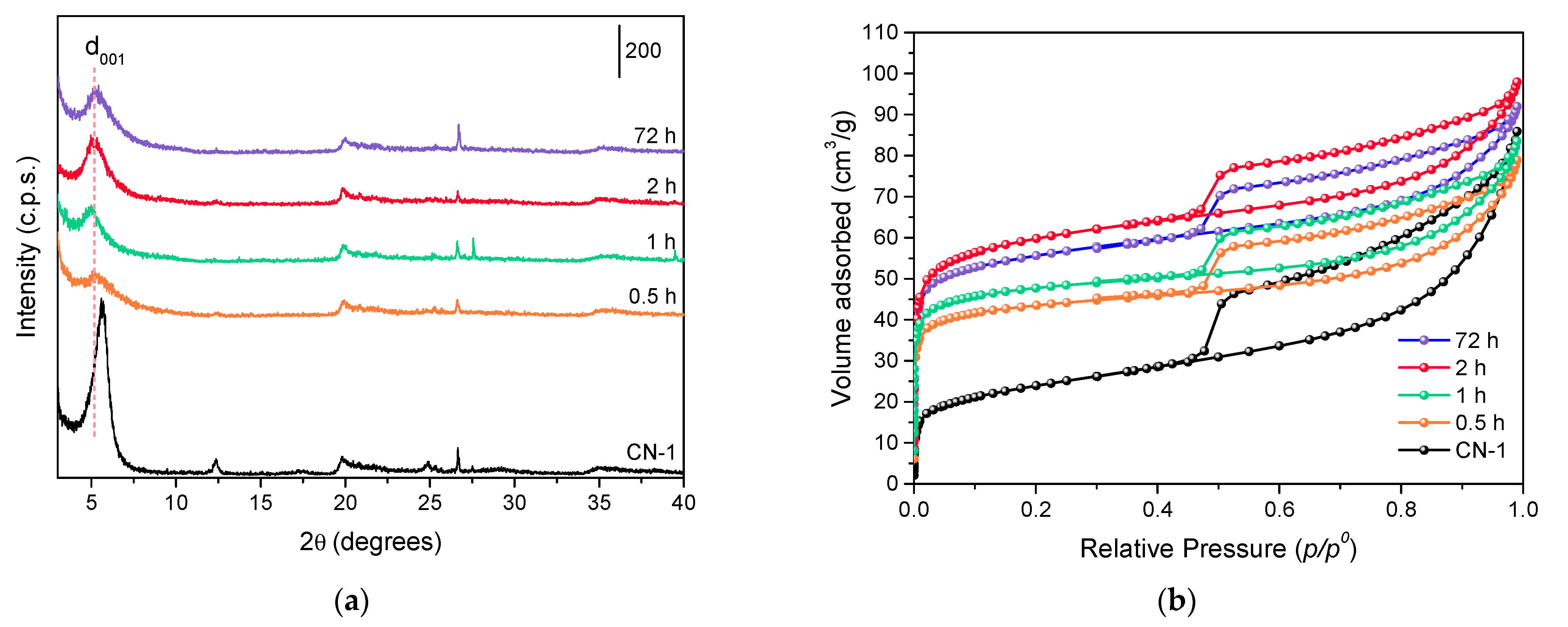
3.2. Influence of the Intercalation Time
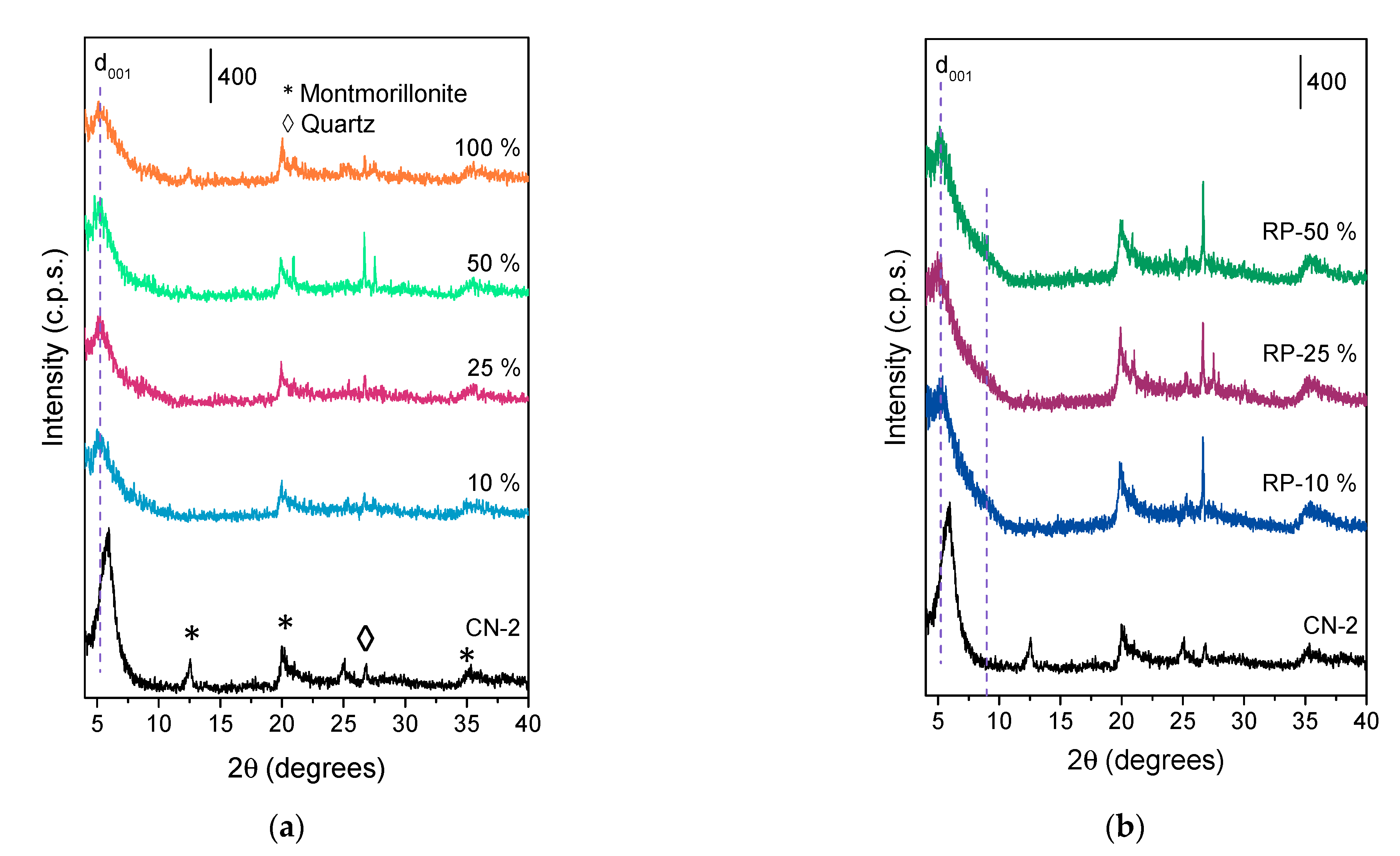
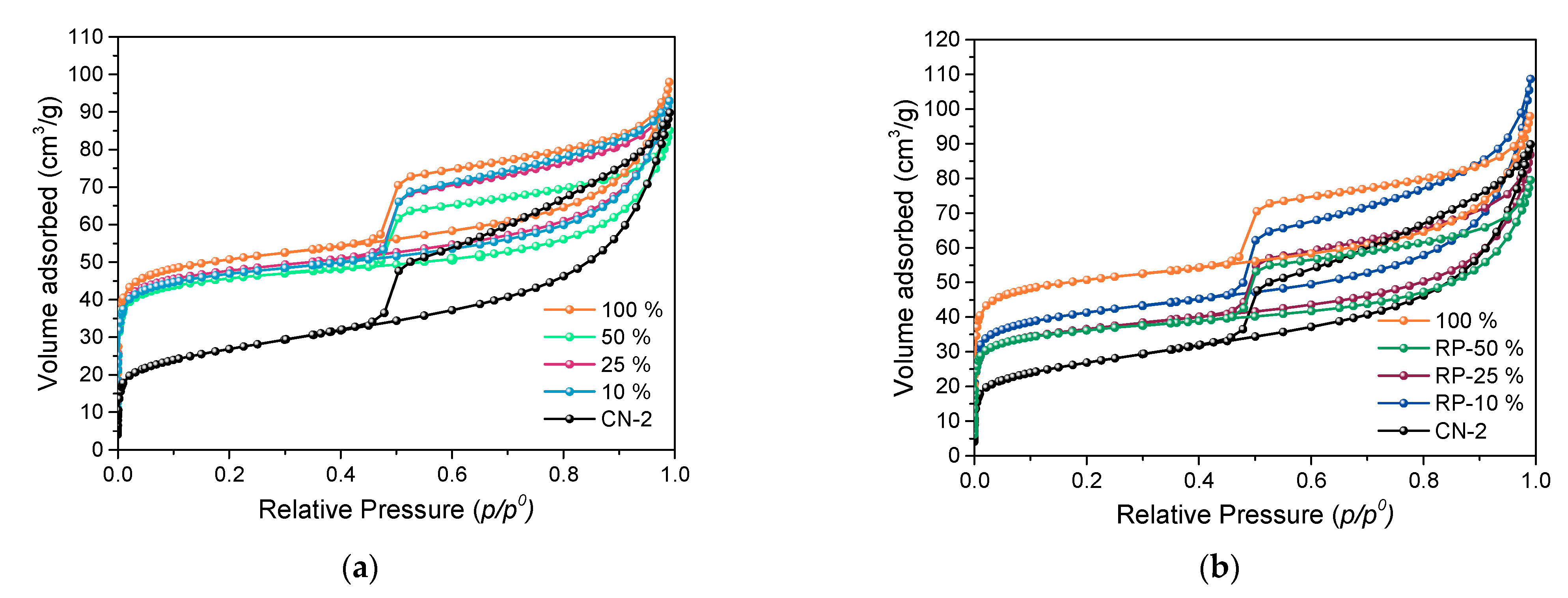
3.3. Control of Pillar Insertion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente, M.A.; Gil, A.; Bergaya, F. Chapter 10.5-Pillared Clays and Clay Minerals. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 523–557. [Google Scholar]
- Pergher, S.B.C.; Corma, A.; Fornes, V. Materiales laminares pilareados preparacion y propriedades. Quim. Nova 1999, 22, 693–709. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Removal of Ciprofloxacin from Aqueous Solutions Using Pillared Clays. Materials 2017, 10, 1345. [Google Scholar] [CrossRef] [Green Version]
- Cardona, Y.; Vicente, M.A.; Korili, S.A.; Gil, A. Progress and perspectives for the use of pillared clays as adsorbents for organic compounds in aqueous solution. Rev. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wu, R.; Dai, H. Alkali metal-promoted aluminum-pillared montmorillonites: High-performance CO2 adsorbents. J. Solid State Chem. 2020, 291, 121585. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M.; Vicente, M.A. Recent Advances in the Synthesis and Catalytic Applications of Pillared Clays. Catal. Rev.-Sci. Eng. 2000, 42, 145–212. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Kloprogge, J.T.; Frost, R.L.; Lu, G.Q.; Zhu, H.Y. Porous Clays and Pillared Clays-Based Catalysts. Part 2: A Review. J. Porous Mater. 2001, 8, 273–293. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Synthesis of Smectites and Porous Pillared Clay Catalysts A Review. J. Porous Mater. 1998, 5, 5–41. [Google Scholar] [CrossRef]
- Cardona, Y.; Korili, S.A.; Gil, A. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13-and Al30-PILC montmorillonites: A review. Appl. Clay Sci. 2021, 203, 105996. [Google Scholar] [CrossRef]
- Catrinescu, C.; Arsene, D.; Apopei, P.; Teodosiu, C. Degradation of 4-chlorophenol from wastewater through heterogeneous Fenton and photo-Fenton process, catalyzed by Al–Fe PILC. Appl. Clay Sci. 2012, 58, 96–101. [Google Scholar] [CrossRef]
- Vallejo, C.A.; Galeano, L.A.; Trujillano, R.; Vicente, M.Á.; Gil, A. Preparation of Al/Fe-PILC clay catalysts from concentrated precursors: Enhanced hydrolysis of pillaring metals and intercalation. RSC Adv. 2020, 10, 40450–40460. [Google Scholar] [CrossRef]
- Bertella, F.; Pergher, S.B.C. Pillaring of bentonite clay with Al and Co. Microporous Mesoporous Mater. 2015, 201, 116–123. [Google Scholar] [CrossRef]
- Mojović, Z.; Banković, P.; Milutinović-Nikolić, A.; Dostanić, J.; Jović-Jovičić, N.; Jovanović, D. Al,Cu-pillared clays as catalysts in environmental protection. Chem. Eng. J. 2009, 154, 149–155. [Google Scholar] [CrossRef]
- Sun Kou, M.R.; Mendioroz, S.; Muñoz, V. Evaluation of the acidity of pillared montmorillonites by pyridine adsorption. Clays Clay Miner. 2000, 48, 528–536. [Google Scholar]
- Mnasri, S.; Frini-Srasra, N. Evolution of Brönsted and Lewis acidity of single and mixed pillared bentonite. Infrared Phys. Technol. 2013, 58, 15–20. [Google Scholar] [CrossRef]
- Moreno, S.; Gutierrez, E.; Alvarez, A.; Papayannakos, N.G.; Poncelet, G. Al-pillared clays: From lab syntheses to pilot scale production characterisation and catalytic properties. Appl. Catal. A Gen. 1997, 165, 103–114. [Google Scholar] [CrossRef]
- Sánchez, A.; Montes, M. Influence of the preparation parameters (particle size and aluminium concentration) on the textural properties of Al-pillared clays for a scale-up process. Microporous Mesoporous Mater. 1998, 21, 117–125. [Google Scholar] [CrossRef]
- Salerno, P.; Mendioroz, S. Preparation of Al-pillared montmorillonite from concentrated dispersions. Appl. Clay Sci. 2002, 22, 115–123. [Google Scholar] [CrossRef]
- Aouad, A.; Pineau, A.; Tchoubar, D.; Bergaya, F. Al-pillared Montmorillonite Obtained in Concentrated Media. Effect of the Anions (nitrate, Sulfate and Chloride) Associated With the Al Species. Clays Clay Miner. 2006, 54, 626–637. [Google Scholar] [CrossRef]
- Olaya, A.; Moreno, S.; Molina, R. Synthesis of pillared clays with aluminum by means of concentrated suspensions and microwave radiation. Catal. Commun. 2009, 10, 697–701. [Google Scholar] [CrossRef]
- Bertella, F.; Pergher, S.B.C. Scale Up Pillaring: A Study of the Parameters That Influence the Process. Materials 2017, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaiah, M.V.; Petit, S.; Brendlé, J.; Patrier, P. Rapid synthesis of aluminium polycations by microwave assisted hydrolysis of aluminium via decomposition of urea and preparation of Al-pillared montmorillonite. Appl. Clay Sci. 2010, 48, 138–145. [Google Scholar] [CrossRef]
- Bertella, F.; Pergher, S.B.C. Reuse of Pillaring Agent in Sequential Bentonite Pillaring Processes. Materials 2017, 10, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Wen, K.; Zhang, P.; Wang, Y.; Ma, L.; Xi, Y.; Zhu, R.; Liu, H.; He, H. Keggin-Al 30 pillared montmorillonite. Microporous Mesoporous Mater. 2017, 242, 256–263. [Google Scholar] [CrossRef]
- Wen, K.; Wei, J.; He, H.; Zhu, J.; Xi, Y. Keggin-Al30: An intercalant for Keggin-Al30 pillared montmorillonite. Appl. Clay Sci. 2019, 180, 105203. [Google Scholar] [CrossRef]
- Lahav, N.; Shani, U.; Shabtai, J. Cross-Linked Smectites. I. Synthesis and Properties of Hydroxy-Aluminum-Montmorillonite. Clays Clay Miner. 1978, 26, 107–115. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, B.V.; Krishna, K.N.; Jai Prakash, B.S. Pillaring of smectites using an aluminium oligomer: A study of pillar density and thermal stability. Appl. Clay Sci. 1996, 10, 439–450. [Google Scholar] [CrossRef]
- Gomes, C.F. Argilas: O Que São e Para Que Servem; Fundação Galouste Gulbenian: Lisboa, Portugal, 1988. [Google Scholar]
- Roca Jalil, M.E.; Vieira, R.S.; Azevedo, D.; Baschini, M.; Sapag, K. Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl. Clay Sci. 2013, 71, 55–63. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kloprogge, J.T.; Booy, E.; Jansen, J.B.H.; Geus, J.W. The Effect of Thermal Treatment on the Properties of Hydroxy-Al and Hydroxy-Ga Pillared Montmorillonite and Beidellite. Clay Miner. 1994, 29, 153–167. [Google Scholar] [CrossRef]
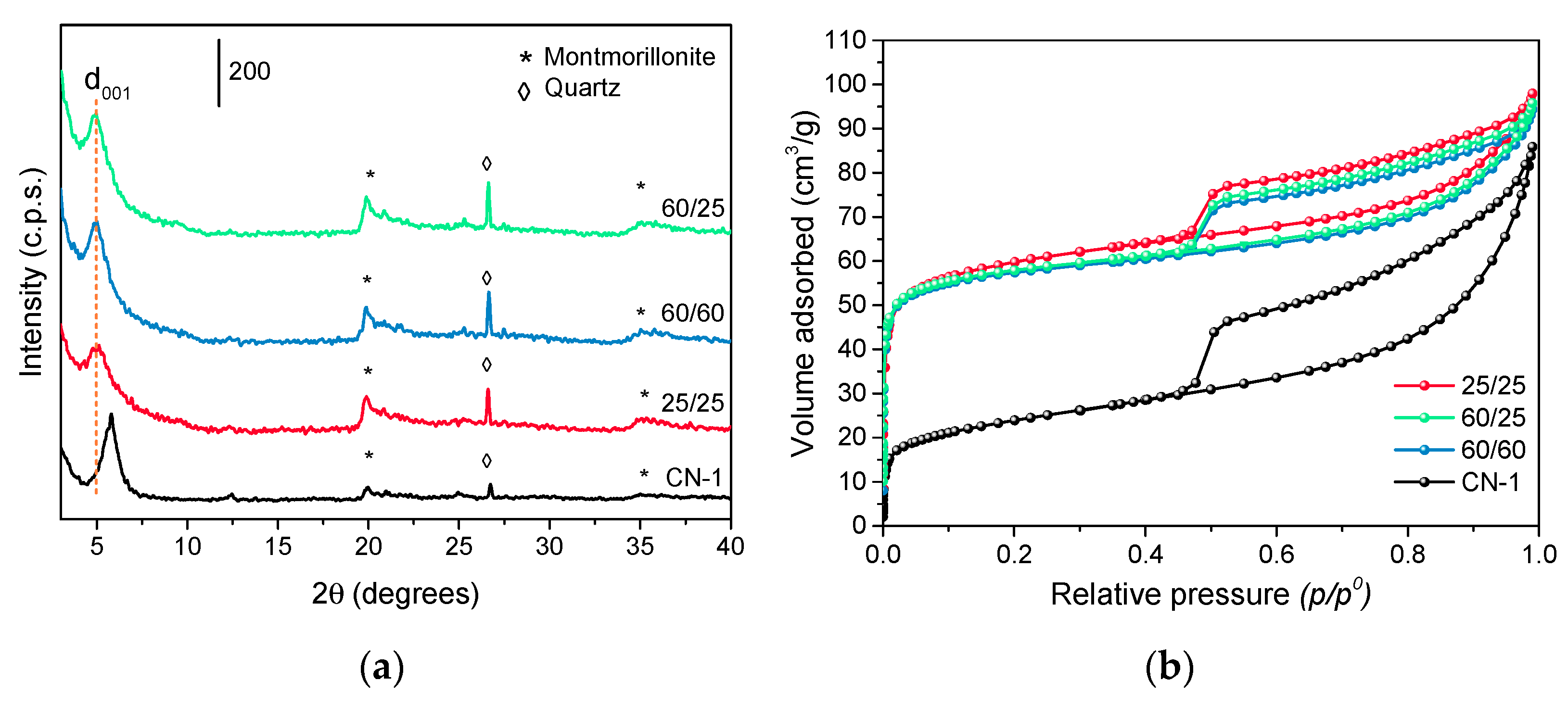

| Sample | d001 1 (nm) | SBET 2 (m2/g) | Sext 3 (m2/g) | Vµ 4 (cm3/g) | TPV 5 (cm3/g) | Si/Al 6 |
|---|---|---|---|---|---|---|
| CN-1 | 1.57 | 90 | 42 | 0.03 | 0.13 | 2.48 |
| 25/25 | 1.75 | 226 | 95 | 0.07 | 0.15 | 1.77 |
| 60/60 | 1.71 | 224 | 79 | 0.07 | 0.15 | 1.77 |
| 60/25 | 1.75 | 224 | 79 | 0.07 | 0.15 | 1.77 |
| Sample | d001 1 (nm) | SBET 2 (m2/g) | Sext 3 (m2/g) | Vµ 4 (cm3/g) | TPV 5 (cm3/g) | Si/Al 6 |
|---|---|---|---|---|---|---|
| CN-1 | 1.57 | 90 | 42 | 0.03 | 0.13 | 2.48 |
| 0.5 h | 1.64 | 168 | 46 | 0.06 | 0.12 | 1.76 |
| 1 h | 1.80 | 213 | 65 | 0.07 | 0.13 | 1.74 |
| 2 h | 1.75 | 226 | 95 | 0.07 | 0.15 | 1.77 |
| 72 h | 1.72 | 184 | 52 | 0.06 | 0.14 | 1.74 |
| Sample | d001 1 (nm) | SBET 2 (m2/g) | Sext 3 (m2/g) | Sµ 4 (m2/g) | Vµ 5 (cm3/g) | TPV 6 (cm3/g) | Si/Al 7 | AlVI/AlIV 9 |
|---|---|---|---|---|---|---|---|---|
| CN-2 | 1.48 | 95 | 49 | 46 | 0.03 | 0.13 | 1.95 | 5.75 |
| 10% | 1.70 | 179 | 35 | 144 | 0.06 | 0.14 | n.d. 8 | 6.39 |
| 25% | 1.68 | 180 | 67 | 113 | 0.06 | 0.14 | 1.62 | 6.79 |
| 50% | 1.70 | 175 | 51 | 124 | 0.06 | 0.13 | 1.65 | 7.74 |
| 100% | 1.71 | 195 | 45 | 150 | 0.07 | 0.15 | 1.54 | 8.66 |
| RP-10% | 1.66 | 154 | 64 | 90 | 0.05 | 0.17 | 1.66 | n.d. 8 |
| RP-25% | 1.68 | 137 | 60 | 77 | 0.04 | 0.13 | 1.52 | n.d. 8 |
| RP-50% | 1.68 | 137 | 59 | 78 | 0.04 | 0.12 | 1.57 | n.d. 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parodia, A.; Prasniski, J.A.; Bertella, F.; Pergher, S.B.C. Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays. Minerals 2021, 11, 1211. https://doi.org/10.3390/min11111211
Parodia A, Prasniski JA, Bertella F, Pergher SBC. Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays. Minerals. 2021; 11(11):1211. https://doi.org/10.3390/min11111211
Chicago/Turabian StyleParodia, Anderson, Janaina A. Prasniski, Francine Bertella, and Sibele B. C. Pergher. 2021. "Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays" Minerals 11, no. 11: 1211. https://doi.org/10.3390/min11111211
APA StyleParodia, A., Prasniski, J. A., Bertella, F., & Pergher, S. B. C. (2021). Keggin-Al13 Polycations: Influence of Synthesis and Intercalation Parameters on the Structural Properties of Al-Pillared Clays. Minerals, 11(11), 1211. https://doi.org/10.3390/min11111211








