Application of Ion Exchange for Recovery of Noble Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Conditioning of the Resins
2.2.2. Batch Experiments
2.2.3. Elution Experiments
2.2.4. Cementation Experiments
2.2.5. Analytical Methods
3. Results and Discussion
3.1. Batch Experiments
3.1.1. Selection of the Ion-Exchange Resins
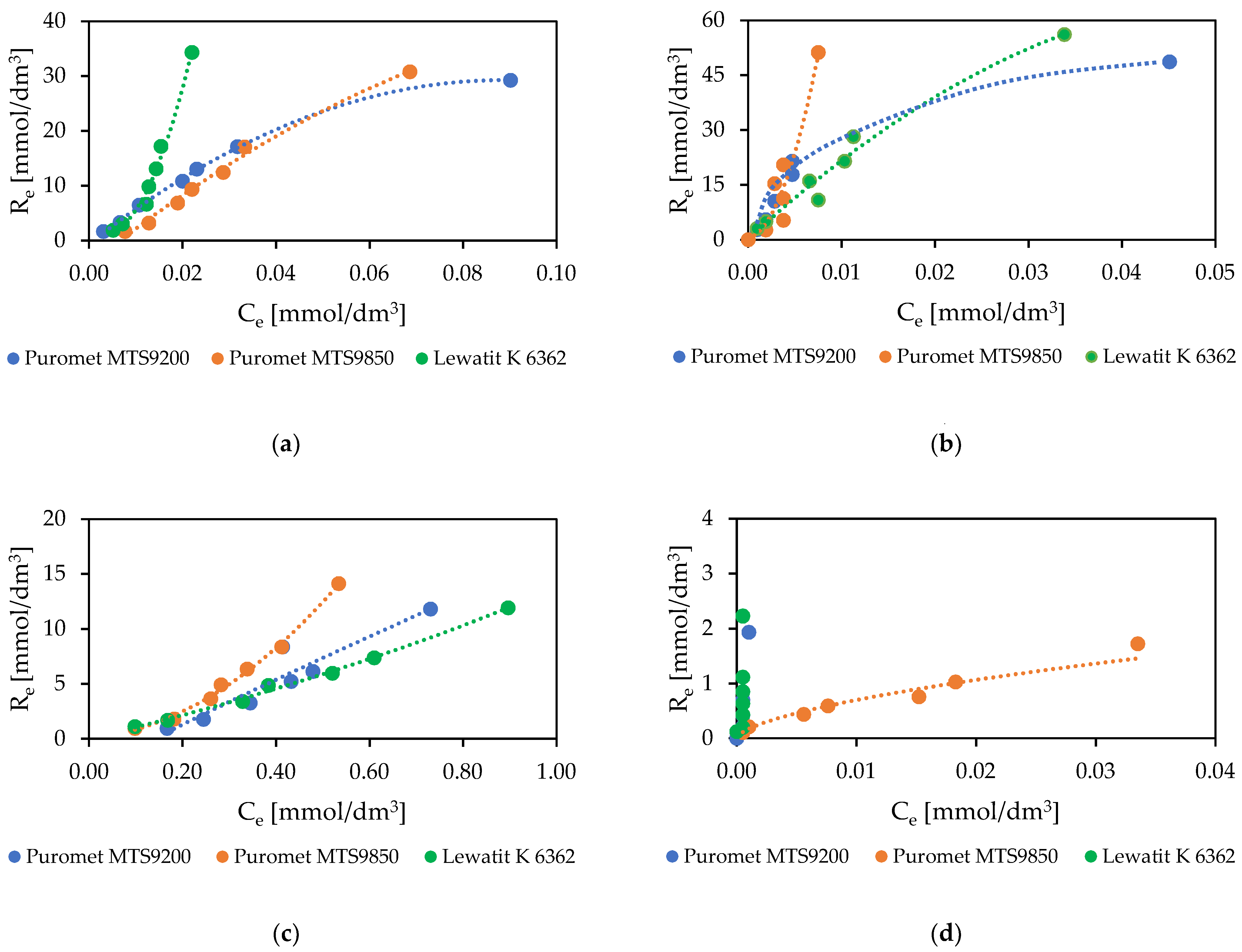
3.1.2. Sorption Isotherms
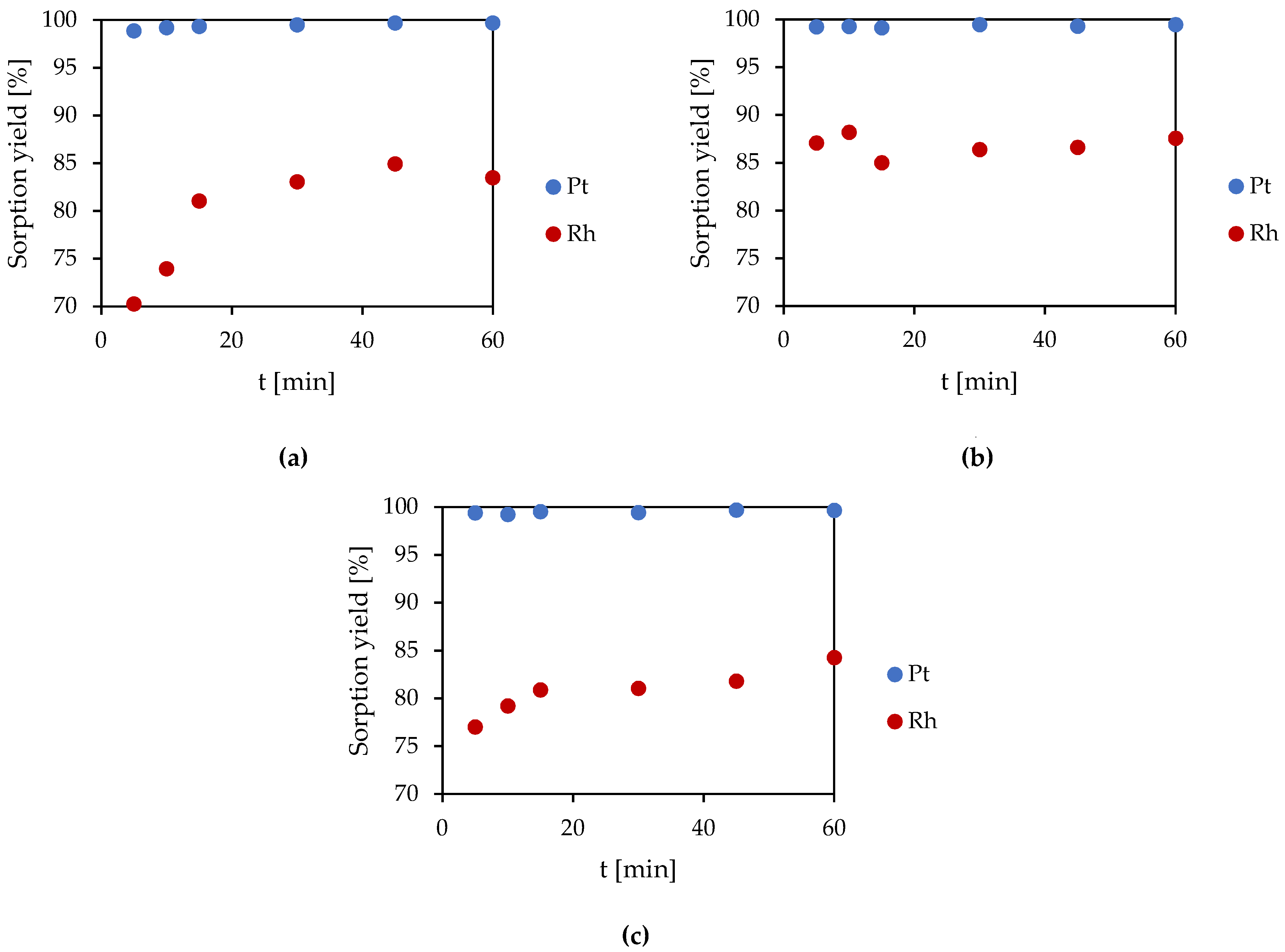
3.1.3. Kinetic Studies
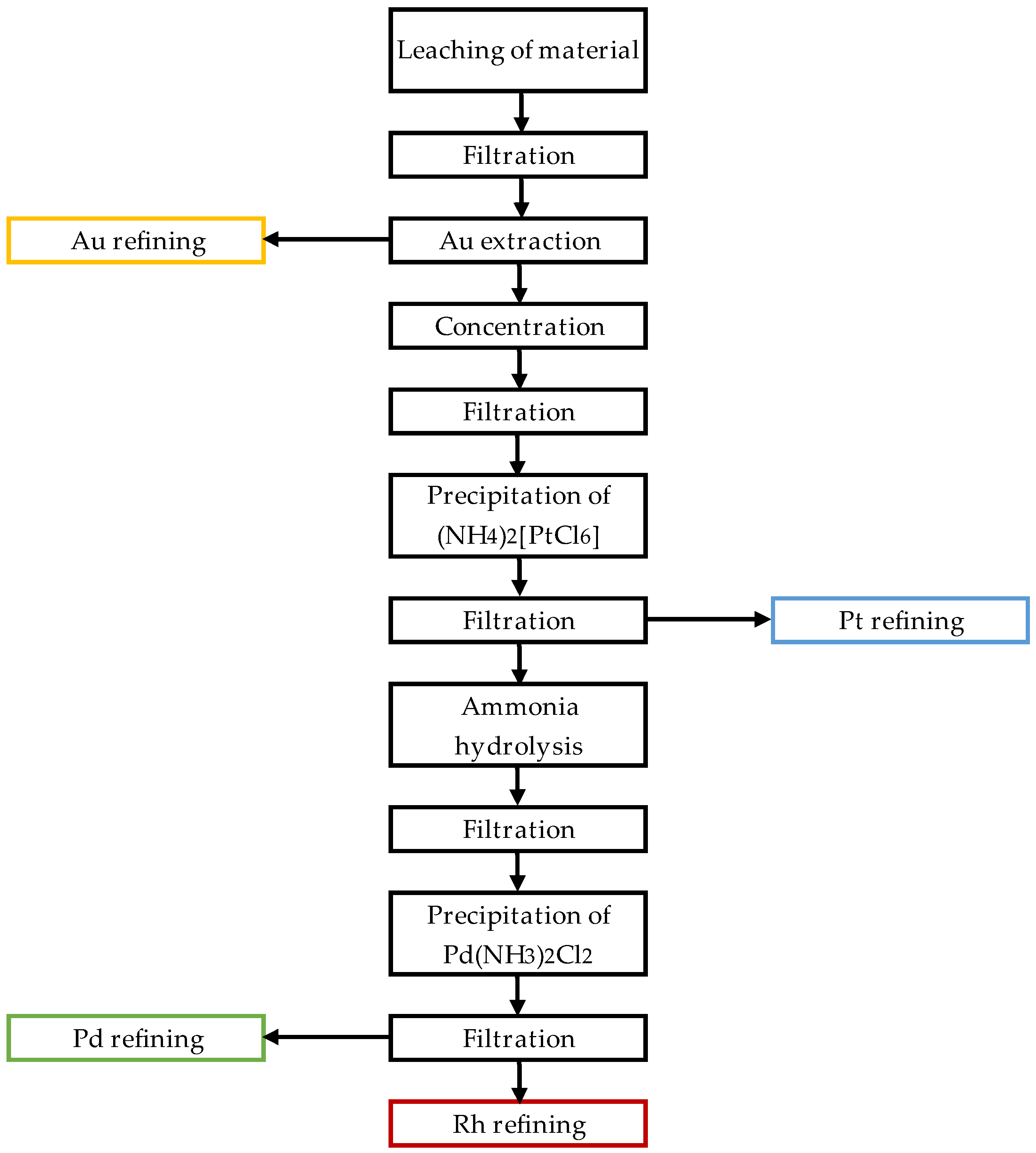
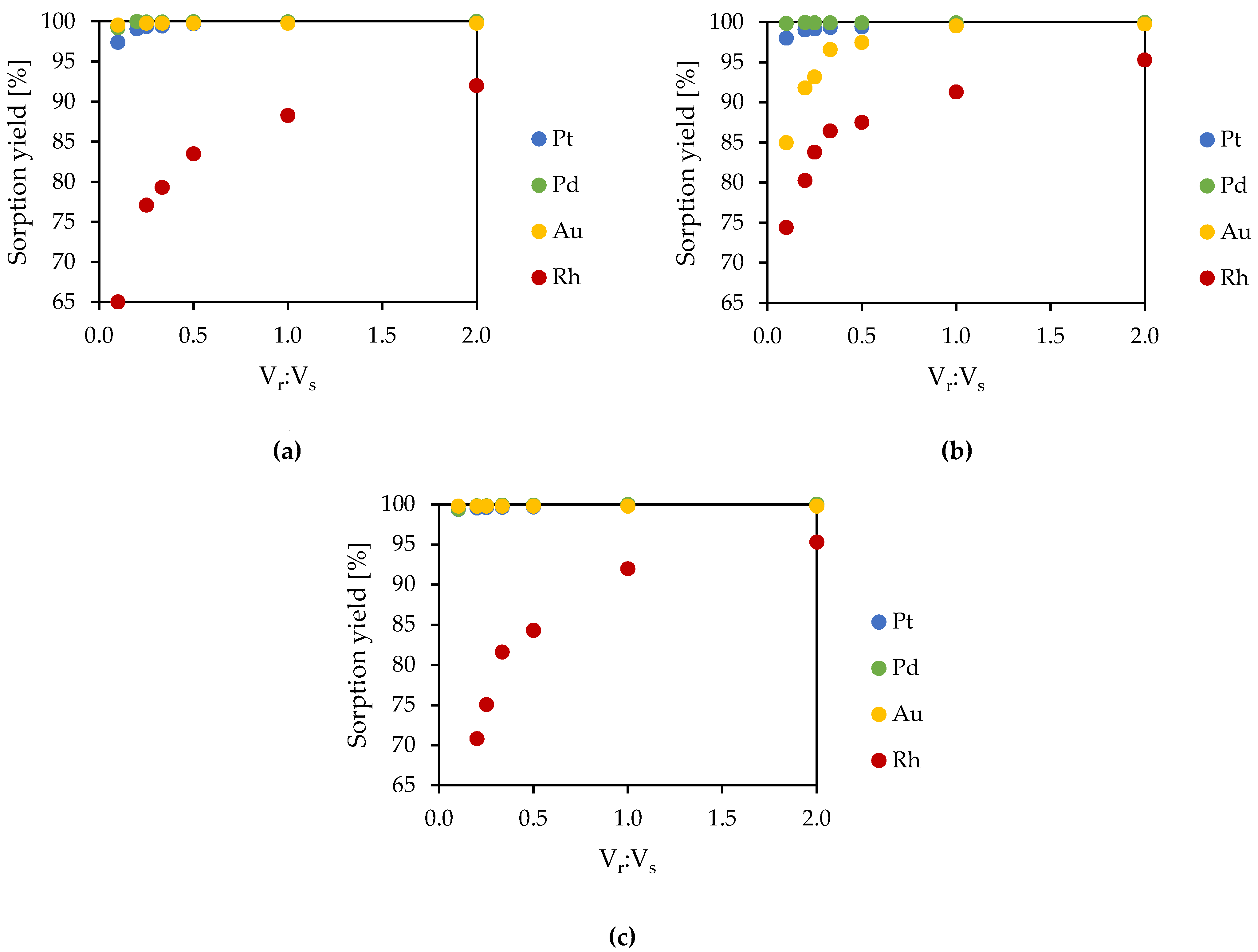
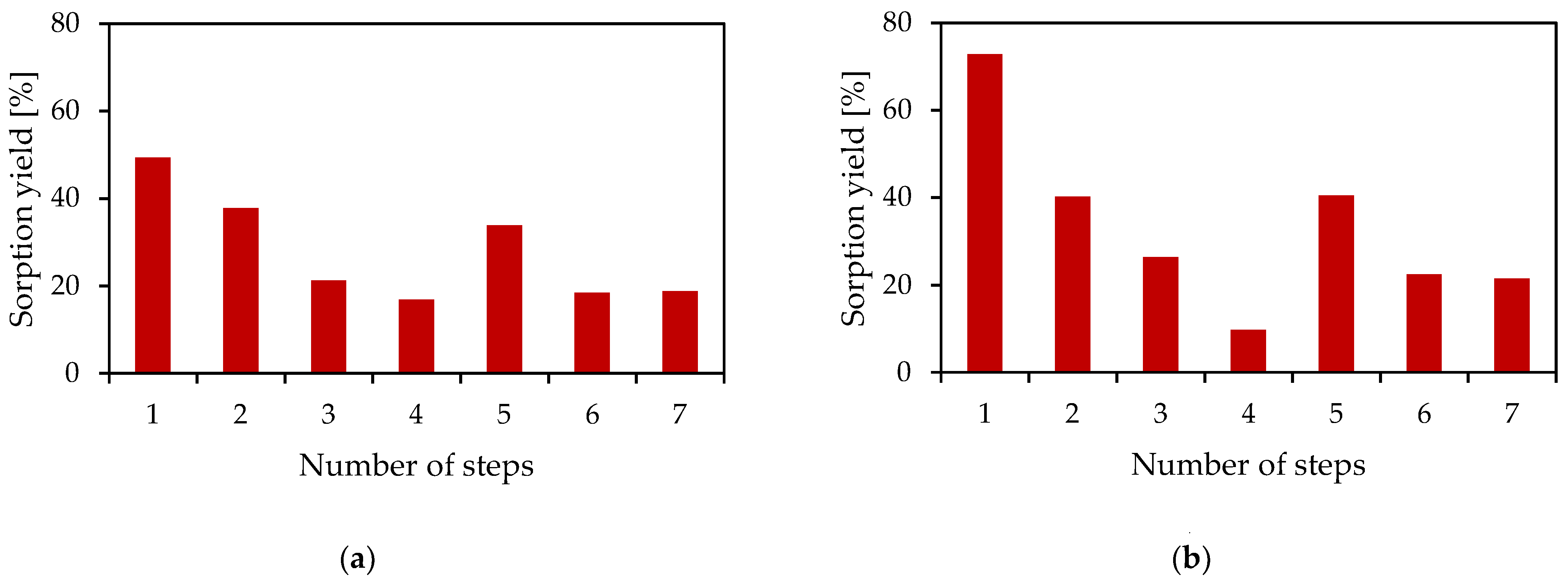
3.1.4. Determination of the Sorption Yield Depending on the Number of Process Steps
3.2. Elution
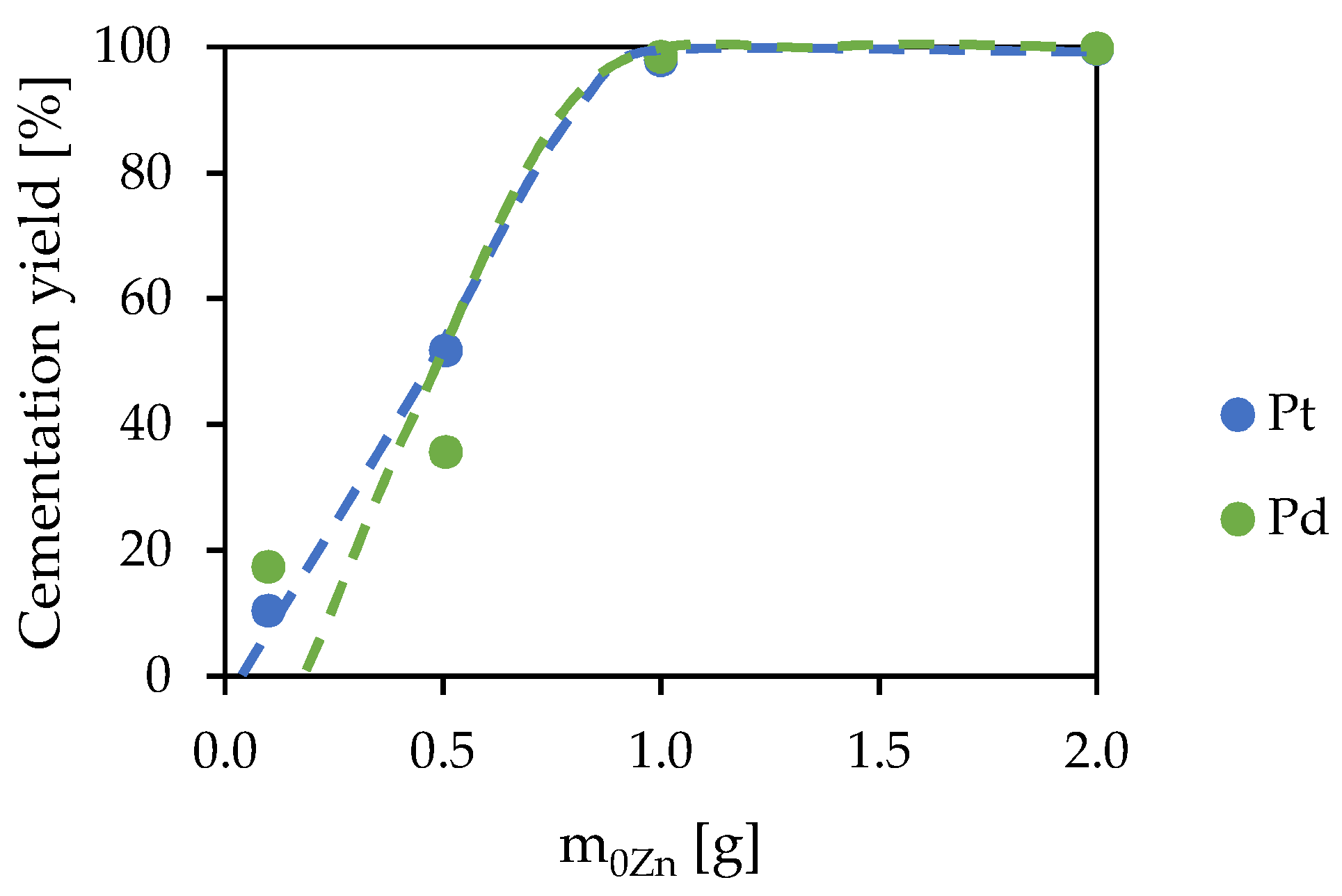
3.3. Cementation
3.4. Comparison with Other Research
4. Conclusions
- The best resins for the sorption of noble metals are Puromet MTS9200, Puromet MTS9850, and Lewatit K 6362. Among the resins, Puromet MTS9200 and Lewatit K 6362 should be used for solutions with a higher concentration of gold (due to the lower gold sorption yields in the isotherm experiments for Puromet MTS9850), and Puromet MTS9850 and Lewatit K 6362 should be used in processes, which need to be conducted using fewer ion exchange steps (due to the lower recovery of noble metals after one step for Puromet MTS9200);
- The volume ratio Vr:Vs = 1:10 and reaction time of 15–30 min could be used as the basic conditions for conducting the experiments using a dynamic method;
- The solution of thiourea in hydrochloric acid could be used as the eluent to recover noble metals;
- From the acidic thiourea eluates, noble metals can be recovered as concentrates using cementation with zinc powder;
- In case of Lewatit K 6362, to obtain a concentrate with a high concentration of noble metals, the resin must be burned and then leached.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey. 2021, Platinum-Group Metals Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/platinum-group-metals-statistics-and-information (accessed on 20 September 2021).
- U.S. Geological Survey. 2021, Gold Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/gold-statistics-and-information (accessed on 20 September 2021).
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Lyon, S.B. Corrosion of noble metals. Shreir’s Corros. 2010, 1, 2205–2223. [Google Scholar]
- De Aberasturi, D.J.; Pinedo, R.; De Larramendi, I.R.; De Larramendi, J.I.R.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Yousif, A.M. Recovery and then individual separation of platinum, palladium, and rhodium from spent car catalytic converters using hydrometallurgical technique followed by successive precipitation methods. J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Pan, T.; Liu, X.; Yuan, L.; Zhang, Y.; Wang, J.; Guo, Z. Adsorption of Pd(II) complexes from chloride solutions obtained by leaching chlorinated spent automotive catalysts on ion exchange resin Diaion WA21J. J. Colloid Interface Sci. 2010, 345, 12–18. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Lee, J.C.; Chagnes, A.; Kim, M.S.; Jeong, J.; Cote, G. Highly selective separation of individual platinum group metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv. 2016, 6, 62717–62728. [Google Scholar] [CrossRef]
- Benke, G.; Anyszkiewicz, K.; Leszczyńska-Sejda, K. Zastosowanie sorpcji w technologii produkcji czystych metali szlachetnych. Przem. Chem. 2003, 82, 808–811. [Google Scholar]
- Kononova, O.N.; Melnikov, A.M.; Duba, E.V. Sorption and Separation of Platinum and Rhodium in Presence of Transition Metals. J. Sib. Fed. Univ. Chem. 2014, 7, 170–184. [Google Scholar]
- Sun, P.P.; Kim, T.Y.; Min, B.J.; Cho, S.Y. Separation of platinum (IV) and rhodium (III) from hydrochloric acid solutions using diaion resins. Mater. Trans. 2015, 56, 1863–1867. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, R.; Mróz, A.; Cejner, M. The enrichment of Pt(IV) ions on Dowex-1 × 8 and Purolite S-920 ion exchangers from aqueous solutions and their determination using slurry sampling and direct solid sampling graphite furnace atomic absorption spectrometry techniques. Anal. Methods. 2016, 8, 5818–5825. [Google Scholar] [CrossRef]
- Hubicki, Z.; Leszczyńska, M.; Łodyga, B.; Łodyga, A. Palladium(II) removal from chloride and chloride-nitrate solutions by chelating ion-exchangers containing N-donor atoms. Miner. Eng. 2006, 19, 1341–1347. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Effect of matrix and structure types of ion exchangers on palladium(II) sorption from acidic medium. Chem. Eng. J. 2010, 160, 660–670. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.L.; Li, D. Recovery of platinum, palladium and rhodium from acidic chloride leach solution using ion exchange resins. Hydrometallurgy 2015, 152, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Kononova, O.N.; Melnikov, A.M.; Borisova, T.V.; Krylov, A.S. Simultaneous ion exchange recovery of platinum and rhodium from chloride solutions. Hydrometallurgy 2011, 105, 341–349. [Google Scholar] [CrossRef]
- Patel, J. Ion exchange resins. XIII-Water-D-Ion Exch. Resins-2 2016, 9, 171–174. [Google Scholar]
- Sahu, P. Ion Exchange Resins: An approach for the Development of Advanced Materials with Industrial, Pharmaceutical and Clinical Applications. Int. J. Adv. Res. Ideas Innov. Technol. 2018, 4, 465–481. [Google Scholar]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Nikoloski, A.N.; Ang, K.L. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Miner. Process. Extr. Metall. Rev. 2014, 35, 369–389. [Google Scholar] [CrossRef]
- Suzuki, T.; Ogata, T.; Tanaka, M.; Kobayashi, T.; Shiwaku, H.; Yaita, T.; Narita, H. Unique anion-exchange properties of 3,3′-diaminobenzidine resulting in high selectivity for rhodium(III) over palladium(II) and platinum(IV) in a concentrated hydrochloric acid solution. Anal. Sci. 2019, 35, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.L.; Lien, H.L. Effective and selective recovery of precious metals by thiourea modified magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 9834–9847. [Google Scholar] [CrossRef] [Green Version]
- Warshawsky, A. Integrated ion exchange and liquid-liquid extraction process for the separation of platinum group metals (PGM). Sep. Purif. Rev. 1982, 11, 95–130. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.G.; Oh, J.K. Cementation behavior of gold and silver onto Zn, Al, and Fe powders from acid thiourea solutions. Can. Metall. Q. 1997, 36, 149–155. [Google Scholar]
- Huang, K.; Chen, J.; Chen, Y.R.; Zhao, J.C.; Li, Q.W.; Yang, Q.X.; Zhang, Y. Enrichment of Platinum Group Metals (PGMs) by two-stage selective pressure leaching cementation from low-grade Pt-Pd sulfide concentrates. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2006, 37, 697–701. [Google Scholar] [CrossRef]
- Perez, J.P.H.; Folens, K.; Leus, K.; Vanhaecke, F.; Van Der Voort, P.; Du Laing, G. Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resour. Conserv. Recycl. 2019, 142, 177–188. [Google Scholar] [CrossRef]
- Chen, J.; Huang, K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 2006, 82, 164–171. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
| Name | Type | Functional Group | Ionic Form | Matrix | Company |
|---|---|---|---|---|---|
| Puromet MTS9200 | weak base | isothiouronium | H+ form | polystyrenic crosslinked with divinylbenzene | Purolite |
| Puromet MTS9850 | polyamine | FB form | polyacrylic crosslinked with divinylbenzene | ||
| Puromet MTS9100 | - | amidoxime | |||
| Lewatit MonoPlus TP 214 | weak base | thiourea | - | polystyrenic | Lanxess |
| Lewatit K 6362 | strong base | quaternary ammonium salt, type I | Cl− form |
| Composition [mg/dm3] | ||||||
|---|---|---|---|---|---|---|
| Pt | Pd | Rh | Au | Mg | Al | P |
| 142 | 182 | 77 | 12 | 0.4 | <5 | <25 |
| K | Ca | Sc | Ti | V | Cr | Mn |
| 98 | 11 | <0.5 | <0.5 | 41 | 3 | <0.5 |
| Fe | Co | Ni | Cu | Zn | Ga | Ge |
| 25 | 2.5 | <2.5 | 69 | 36 | 3 | 0.1 |
| As | Se | Br | Rb | Sr | Y | Zr |
| 16 | <5 | <50 | <0.5 | <0.5 | <0.05 | <0.5 |
| Nb | Mo | Ru | B | Be | Ag | Cd |
| <0.05 | <0.5 | 0.2 | 2 | <0.5 | 3.1 | 3.9 |
| In | Sn | Sb | Te | I | Cs | Ba |
| 2.5 | 5.6 | <0.05 | 0.1 | <50 | <0.5 | <0.5 |
| La | Ce | Pr | Nd | Sm | Eu | Gd |
| <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Tb | Dy | Ho | Er | Tm | Yb | Lu |
| <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Hf | Ta | W | Re | Os | Ir | Li |
| <0.05 | <0.05 | 0.1 | 1.5 | <0.5 | <0.05 | <0.5 |
| Na | Hg | Tl | Pb | Bi | Th | U |
| 20 | <0.5 | <0.05 | 5.7 | 4.2 | <0.05 | <0.05 |
| Concentration [mg/dm3] | |||||
|---|---|---|---|---|---|
| Pt | Pd | Rh | Au | As | Zn |
| 674 | 601 | 215 | 44 | 33 | 159 |
| Cu | K | Na | V | Ca | Fe |
| 241 | <0.5 | 49 | <0.5 | 6 | 4 |
| Puromet MTS9200 | Puromet MTS9850 | Puromet MTS9100 | Lewatit MonoPlus TP 214 | Lewatit K 6362 | ||
|---|---|---|---|---|---|---|
| Sorption yield [%] | Pt | 99.08 | 99.04 | 74.18 | 99.86 | 99.55 |
| Pd | 99.98 | 99.98 | 97.95 | 99.97 | 99.80 | |
| Rh | 80.19 | 80.28 | 43.26 | 74.60 | 70.79 | |
| Au | 92.73 | 91.82 | 96.36 | 99.77 | 99.77 | |
| Cu | 8.71 | 8.30 | 27.88 | 99.17 | 7.68 | |
| Zn | 20.75 | 19.50 | 23.40 | 69.31 | 81.13 |
| Number of Steps | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Metal concentration [mg/dm3] | Puromet MTS9200 | Rh | 109.0 | 67.8 | 53.4 | 44.4 | 29.4 | 24.0 | 19.5 |
| Pt | 36.5 | 2.2 | - | - | - | - | - | ||
| Puromet MTS9850 | Rh | 58.4 | 34.9 | 25.7 | 23.2 | 13.8 | 10.7 | 8.4 | |
| Pt | 14.1 | 4.0 | - | - | - | - | - | ||
| Lewatit K 6362 | Rh | 91.5 | 53.5 | 35.2 | 25.8 | - | - | 9.2 | |
| Pt | 4.9 | 2.4 | - | - | - | - | - | ||
| Metal | 10% HCl | 2 mol/dm3 AT | 2 mol/dm3 AT in 10% HCl | 1 mol/dm3 TU in 2 mol/dm3 HCl | ||
|---|---|---|---|---|---|---|
| Elution yields [%] | Puromet MTS9200 | Pt | 0.15 | 0.15 | 1.84 | 41.28 |
| Pd | 0.17 | 0.34 | 0.69 | 63.11 | ||
| Rh | 1.04 | 0.52 | 4.18 | 7.31 | ||
| Au | 2.35 | 2.35 | 4.71 | 47.08 | ||
| Puromet MTS9850 | Pt | 0.16 | 0.47 | 2.91 | 61.20 | |
| Pd | 5.94 | 1.40 | 2.33 | 81.79 | ||
| Rh | 0.51 | 0.51 | 0.68 | 0.51 | ||
| Au | 2.39 | 2.39 | 3.19 | 74.15 | ||
| Lewatit K 6363 | Pt | 0.54 | 4.86 | 1.69 | 35.66 | |
| Pd | 2.42 | 0.15 | 0.32 | 37.97 | ||
| Rh | 19.88 | 22.08 | 10.12 | 11.93 | ||
| Au | 2.07 | 2.07 | 4.31 | 20.70 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| m0Zn [g] | 0.0996 | 0.5064 | 1.0000 | 2.0000 |
| mfZn [g] | 0.0135 | 0.0302 | 0.1538 | 0.8865 |
| Concentrate composition [%] | Pt 0.444 Pd 0.960 Rh 0.187 Au 0.225 Zn 98.184 | Pt 0.987 Pd 0.879 Rh 0.083 Au 0.101 Zn 97.950 | Pt 0.367 Pd 0.477 Rh 0.016 Au 0.020 Zn 99.120 | Pt 0.065 Pd 0.084 Rh 0.003 Au 0.003 Zn 99.845 |
| pH | 0.00 | 0.12 | 5.82 | 6.00 |
| This Study’s Results | Comparison [13,16,17] | ||
|---|---|---|---|
| Puromet MTS9200 | |||
| Type of solution | Technological solution | Synthetic solution [13] | |
| Sorption studies | Pt 97.0% pH = 0.24, Vr = 10 mL, vs. = 100 mL, t = 1.0 h, T = 20 °C | Pt ~ 91.0% pH = 0.50, m = 0.05 g, V = 50 mL, t = 1.5 h, T = 25 °C | |
| Kinetic studies | Equilibrium time = 5 min pH = 0.24, Vr = 10 mL, vs. = 20 mL, T = 20 °C | Equilibrium time = 10 min pH = 1, m = 0.05 g, V = 50 mL, T = 25 °C | |
| Elution studies | Pt 41.0% CTU = 1 mol/dm3 in 2 mol/dm3 HCl, Vr = 10 mL, vs. = 50 mL, t = 1 h, T = 20 °C | Pt ~ 41.0% CTU = 0.6 mol/dm3, m = 0.05 g, V = 50 mL, t = 1 h, T = 25 °C | |
| Puromet MTS9850 | |||
| Type of solution | Technological solution | Synthetic solution [16] | Process leach solution [16] |
| Sorption studies | Pt 98.0% Pd 99.9% Rh 74.4% Vr = 10 mL, vs. = 100 mL, t = 1 h | Pt 75.6% Pd 52.3% Rh 94.8% | Pt 81.6% Pd 58.8% Rh 87.8% |
| Vr = 5 mL, vs. = 200 mL, t = 24 h | |||
| Kinetic studies | Equilibrium time = 15–30 min Vr = 10 mL, vs. = 20 ml | Equilibrium time = 30 min Vr = 5 mL, vs. = 200 ml | |
| Elution studies | Pt 61.0% Pd 81.0% Rh 0.5% CTU = 1 mol/dm3 in 2 mol/dm3 HCl, Vr = 10 mL, vs. = 50 mL, t = 1 h | Pt 70.0% Pd 93.0% Rh 7.0% CTU = 1 mol/dm3 in 2 mol/dm3 HCl, Vr = 5 mL, vs. = 50 mL, t = 5 h | |
| Puromet MTS9850 | |||
| Type of solution | Technological solution | Synthetic solution [17] | |
| Sorption studies | Pt 98.0% Rh 74.0% CHCl ~ 1 mol/dm3, Vr = 10 mL, Vs = 100 mL, t = 1 h, T = 20 °C | Pt 89.0% Rh 94.0% CHCl = 1 mol/dm3, mr = 0.1–0.2 g, vs. = 10.0–20.0 mL, t = 24 h, T = 20 °C | |
| Kinetic studies | Equilibrium time = 15–30 min Vr = 10 mL, vs. = 20 ml | Equilibrium time = 30 min mr = 0.1 g, vs. = 10 ml | |
| Elution studies | Pt 61.0% Rh 0.5% CTU = 1 mol/dm3 in 2 mol/dm3 HCl, Vr = 10 mL, vs. = 50 mL, t = 1h | Pt 94.0% Rh 94.8% CTU = 1 mol/dm3 in 2 mol/dm3 H2SO4 | Pt 63.1% Rh 97.4% CTU = 1 mol/dm3 in 2 mol/dm3 KOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goc, K.; Kluczka, J.; Benke, G.; Malarz, J.; Pianowska, K.; Leszczyńska-Sejda, K. Application of Ion Exchange for Recovery of Noble Metals. Minerals 2021, 11, 1188. https://doi.org/10.3390/min11111188
Goc K, Kluczka J, Benke G, Malarz J, Pianowska K, Leszczyńska-Sejda K. Application of Ion Exchange for Recovery of Noble Metals. Minerals. 2021; 11(11):1188. https://doi.org/10.3390/min11111188
Chicago/Turabian StyleGoc, Karolina, Joanna Kluczka, Grzegorz Benke, Joanna Malarz, Karolina Pianowska, and Katarzyna Leszczyńska-Sejda. 2021. "Application of Ion Exchange for Recovery of Noble Metals" Minerals 11, no. 11: 1188. https://doi.org/10.3390/min11111188
APA StyleGoc, K., Kluczka, J., Benke, G., Malarz, J., Pianowska, K., & Leszczyńska-Sejda, K. (2021). Application of Ion Exchange for Recovery of Noble Metals. Minerals, 11(11), 1188. https://doi.org/10.3390/min11111188






