Biomineralization in Polychaete Annelids: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Biomineralization of Serpulids
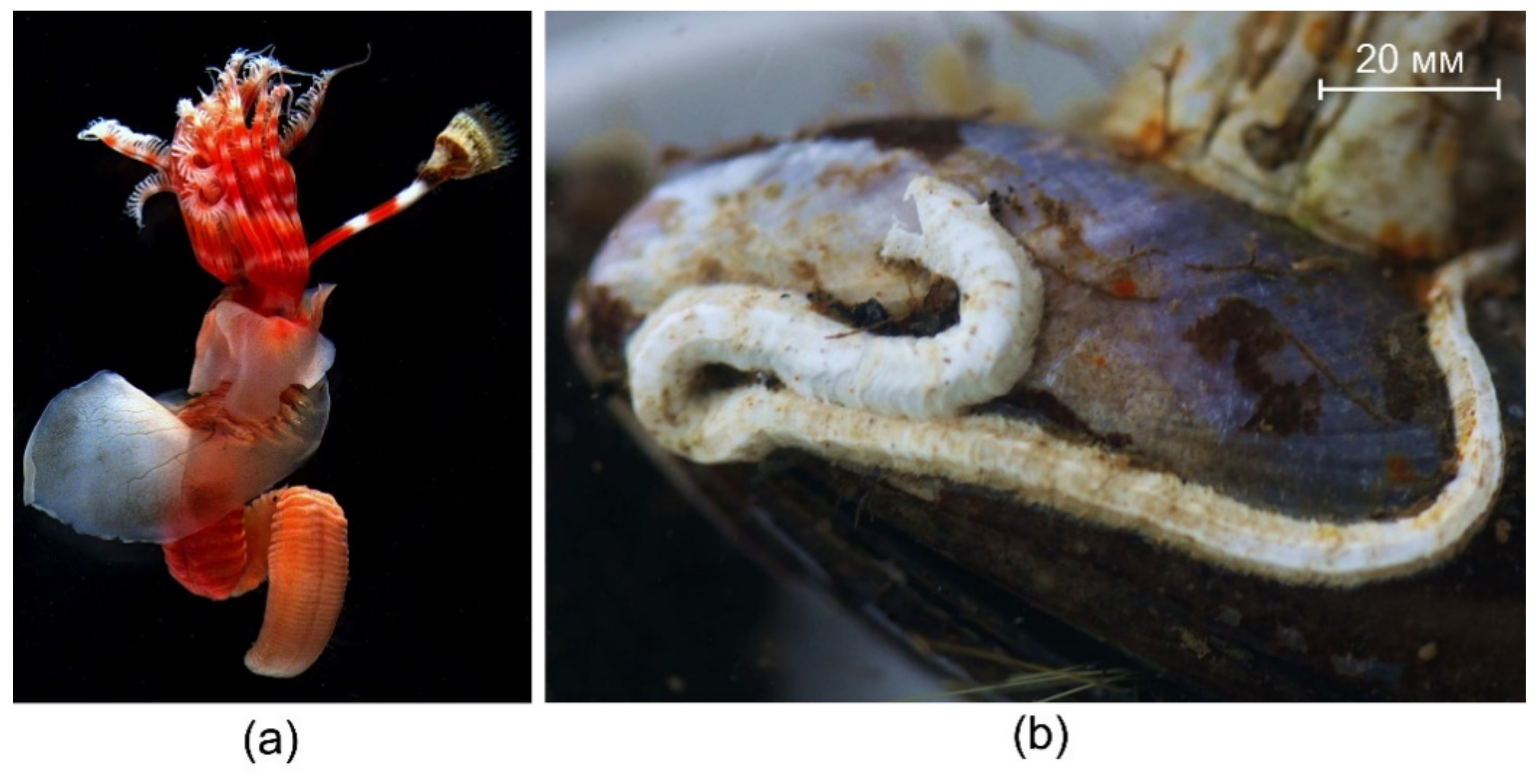
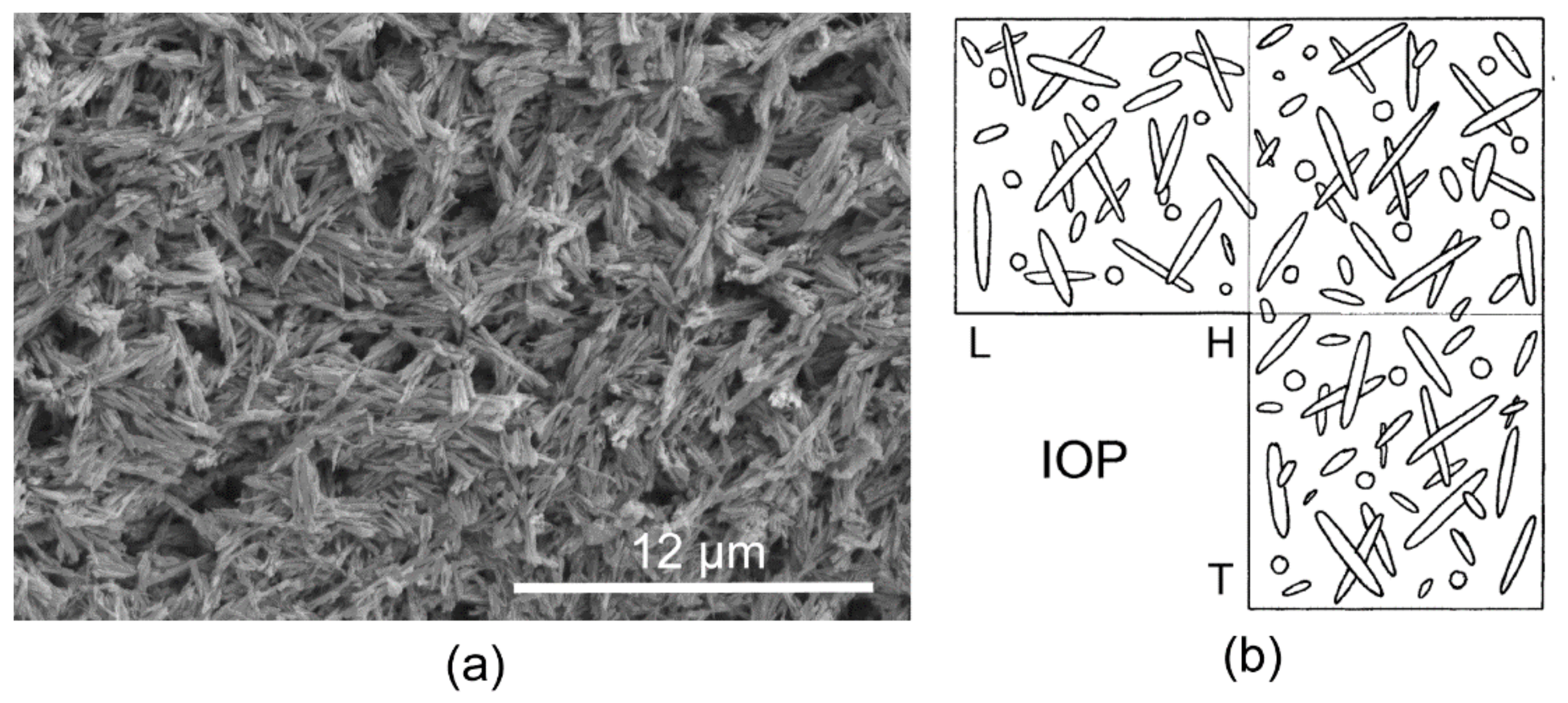
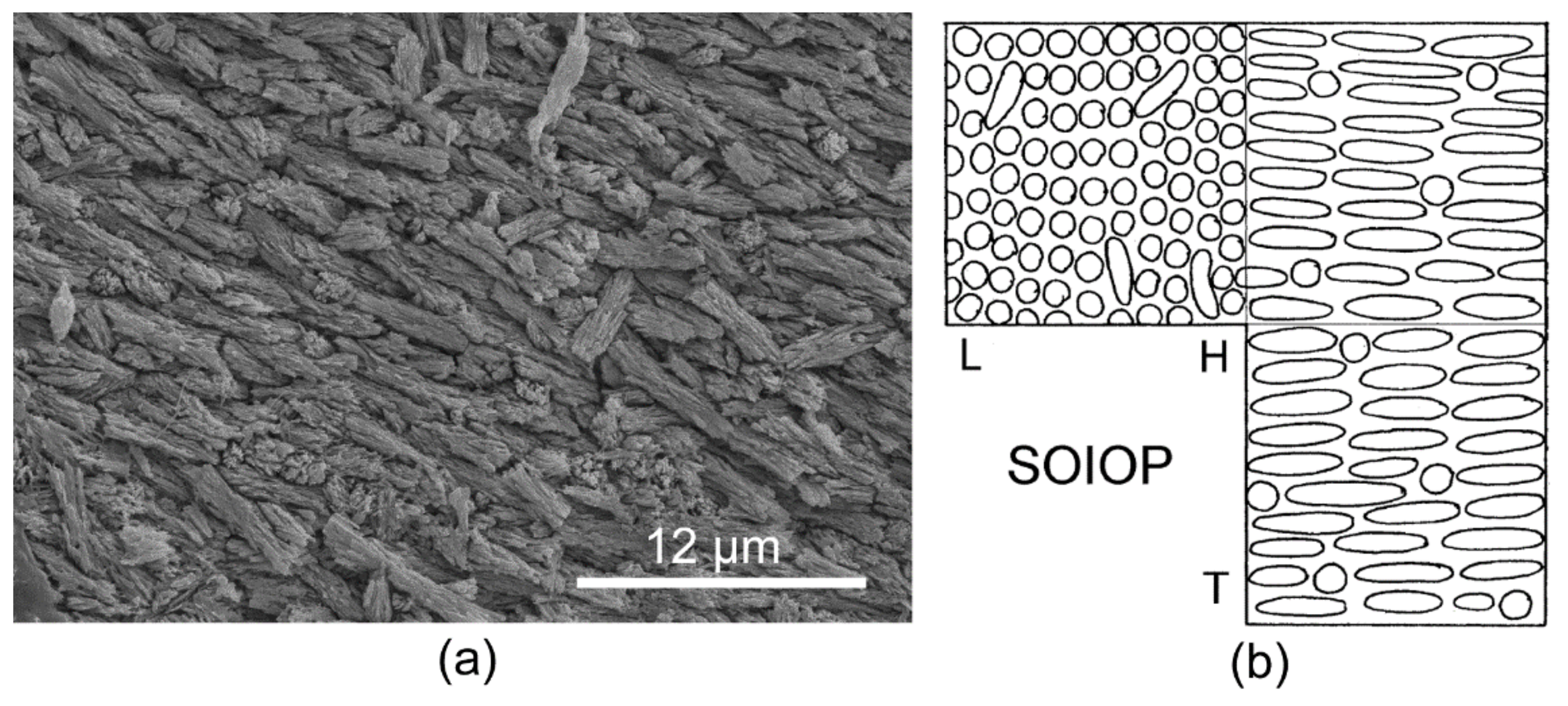
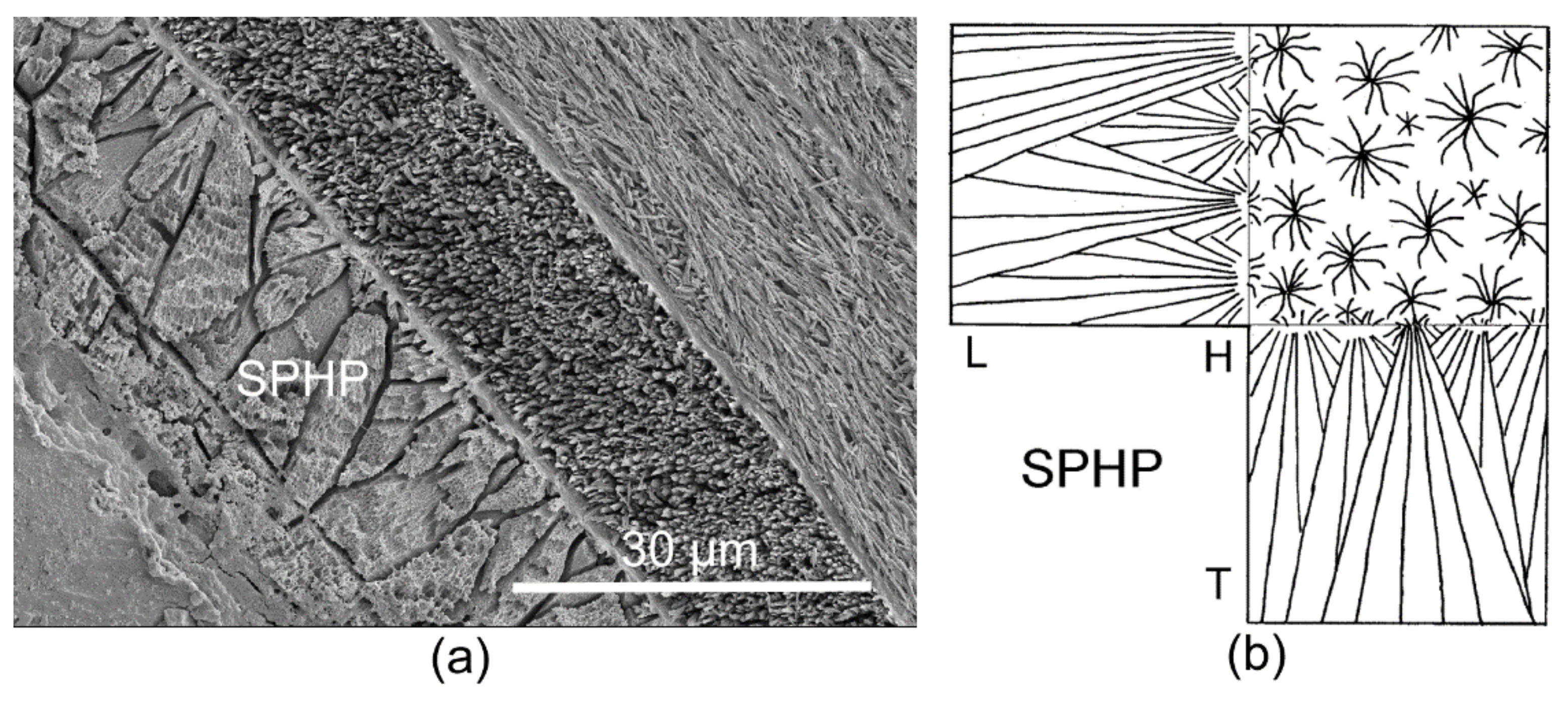
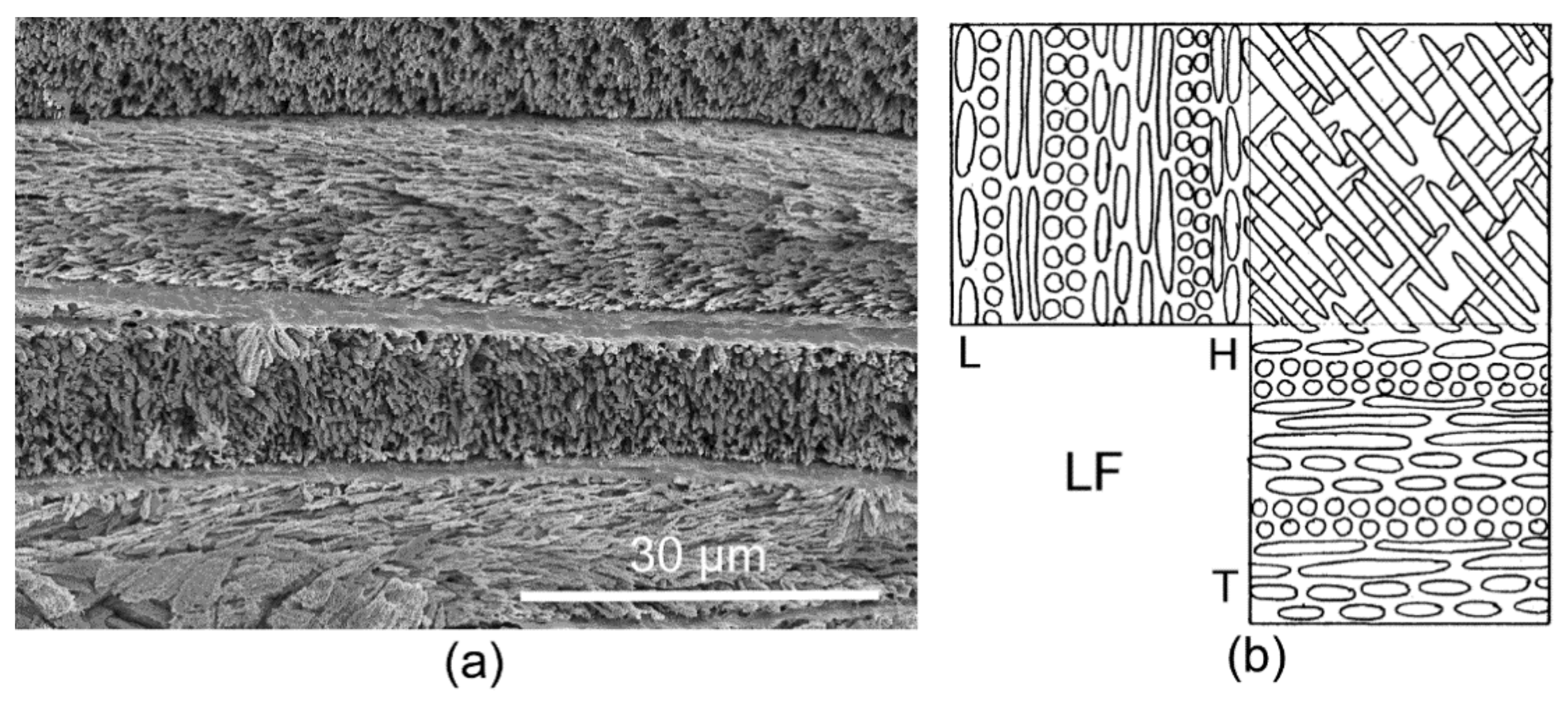
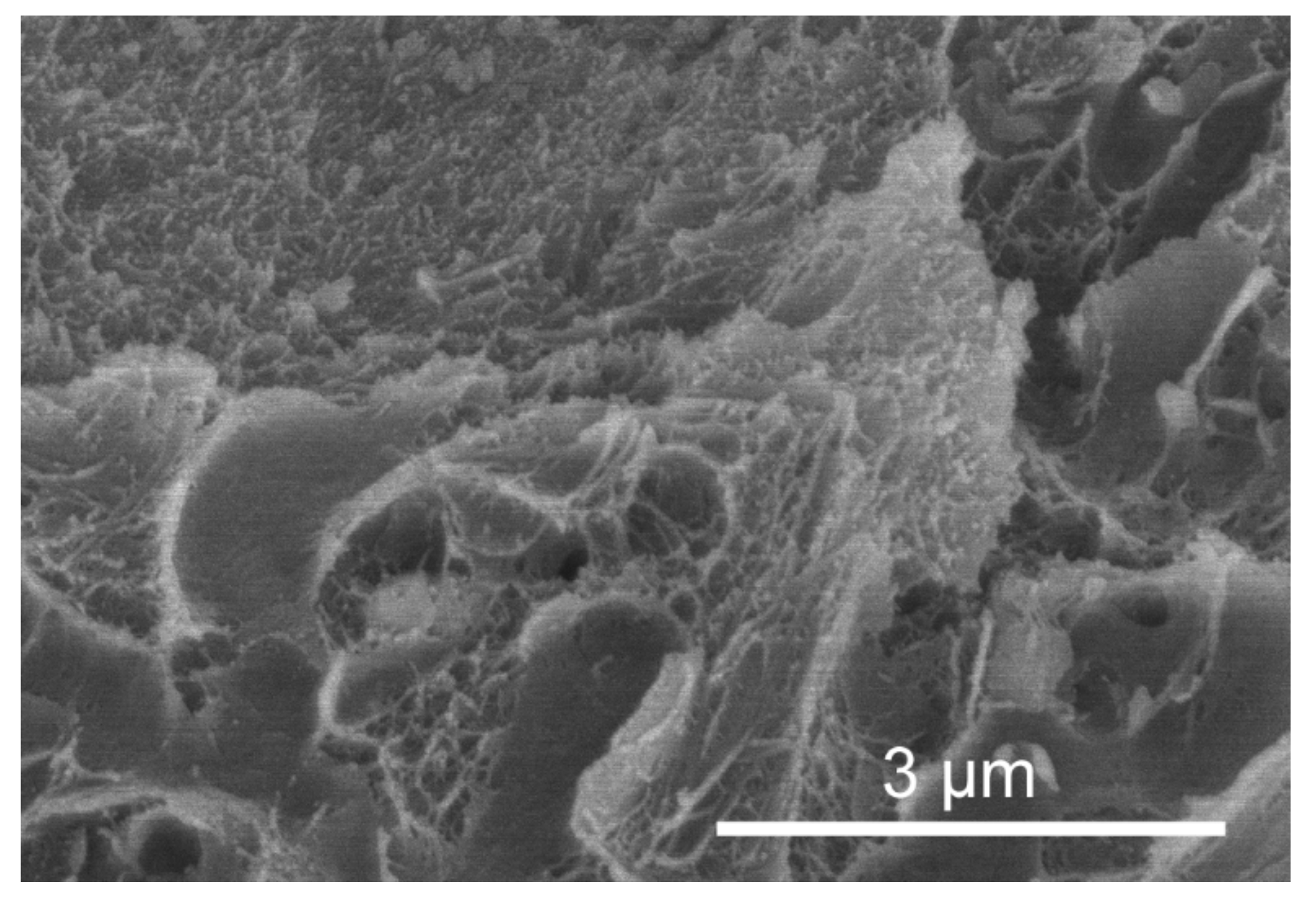
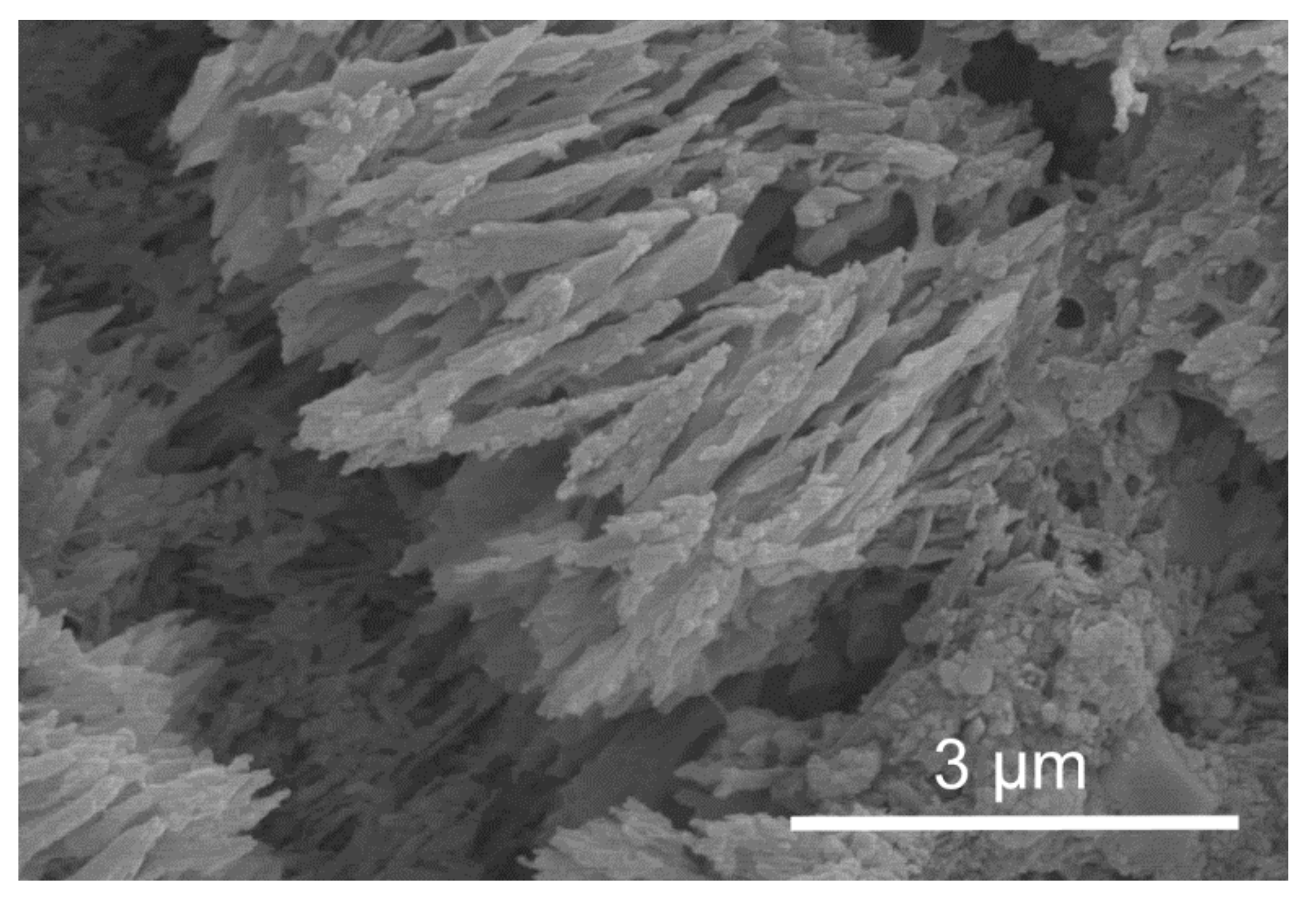
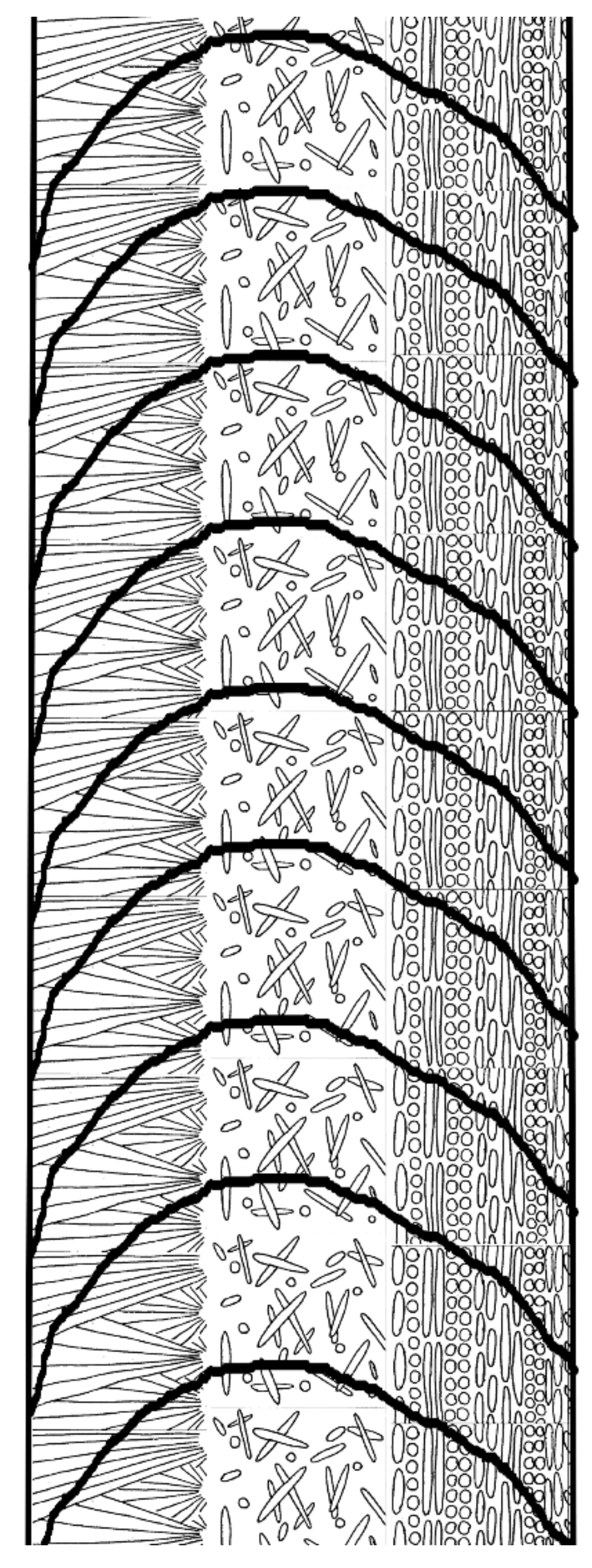
3.1. Skeletal Structures
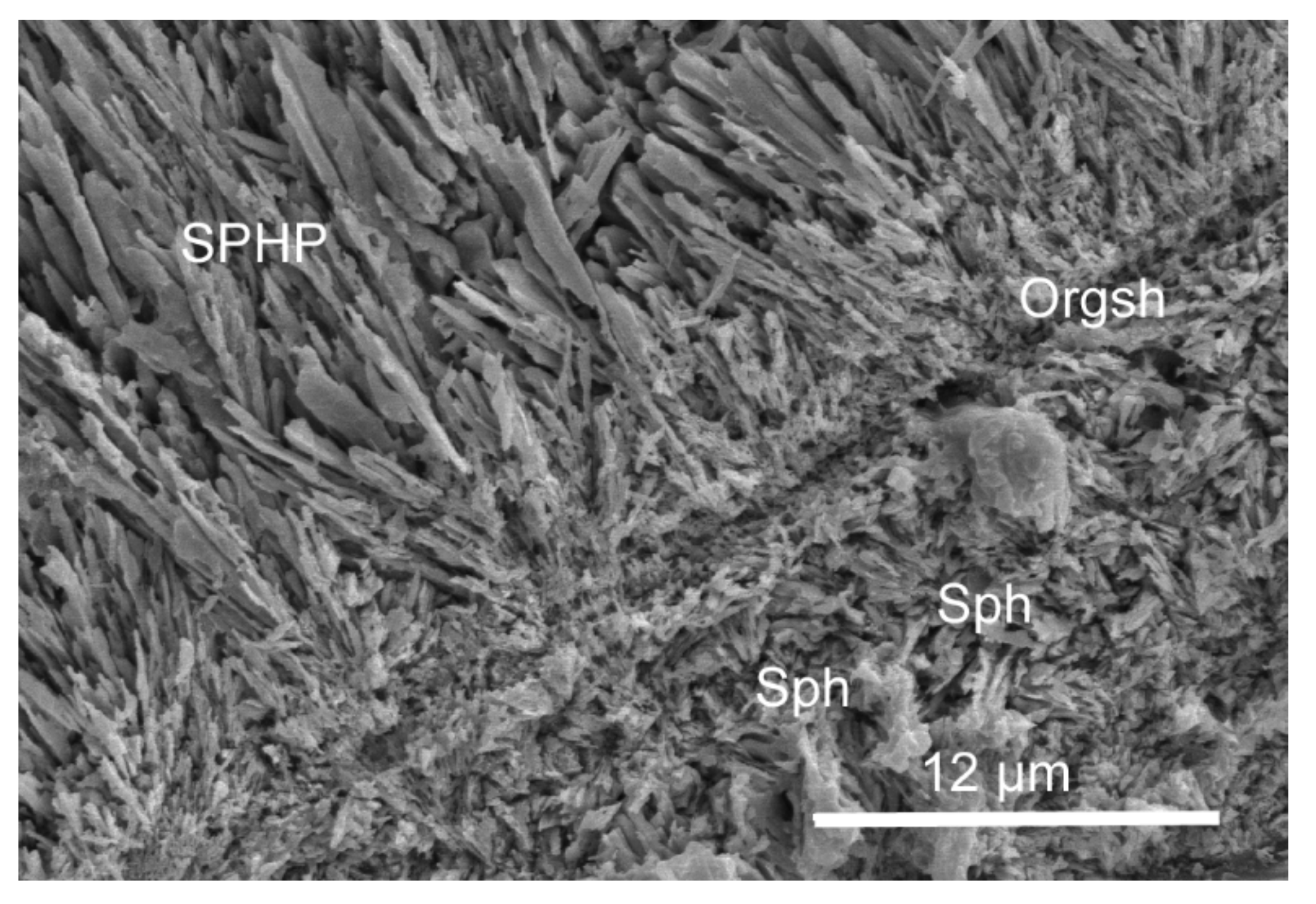
3.2. Organic Sheets in Mineral Structures and the Inner Organic Tube Layer
3.3. Minearal Composition
3.4. Development and Serpulid Biomineralization
3.5. Serpulid Biomineralization and Environment
4. Biomineralization of Sabellids
4.1. Skeletal Structures
4.2. Mineral Composition
5. Biomineralization of Cirratulids
5.1. Skeletal Structures
5.2. Mineral Composition
6. Biomineralization in Non-Tubicolous Polychaetes
7. Discussion
7.1. Serpulid Biomineralization and the Controversy
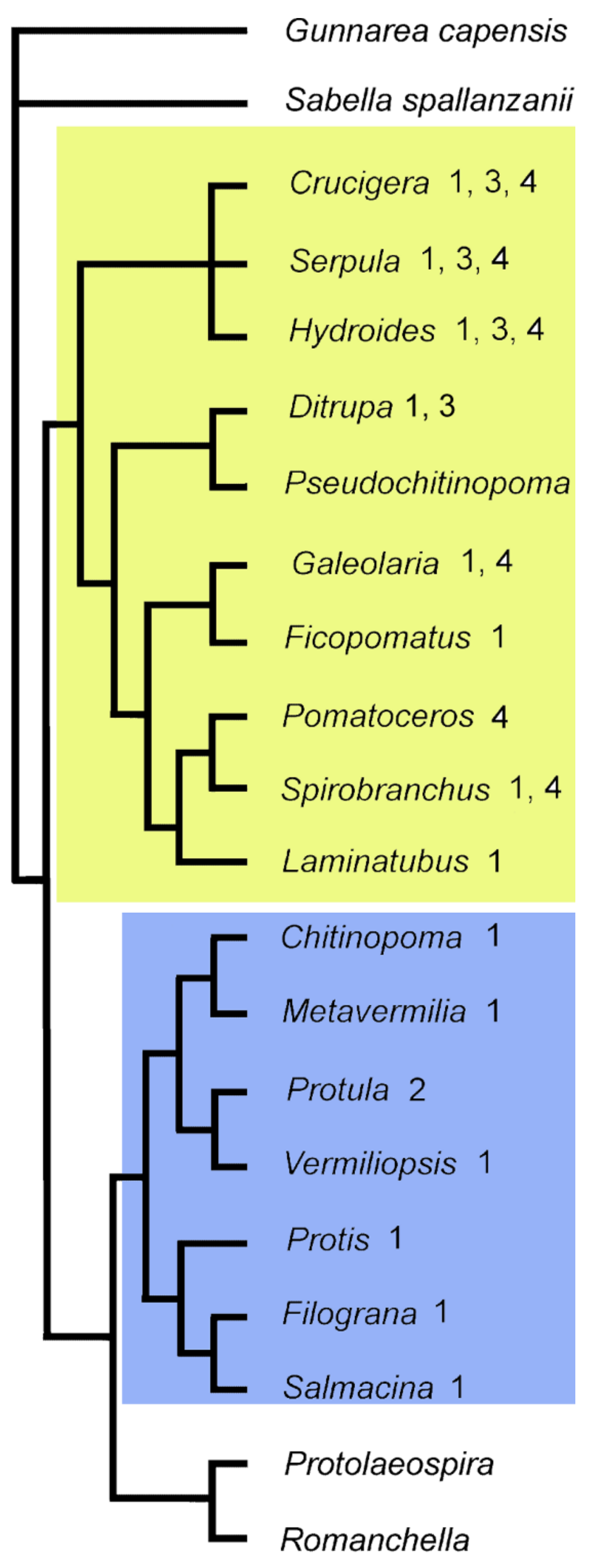
7.2. Evolution of Polychaete Biomineralization
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mastrangelo, P.; Passeri, L. Sedimenti calcareo-argillosi e biolititi a serpulidi nel Mar Piccolo di Taranto. Boll. Soc. Geol. Ital. 1975, 94, 2019–2046. [Google Scholar]
- Medernach, E.; Jordana, A.; Grémare, C.; Nozais, F.; Charles, J.; Amouroux, M. Population dynamics, secondary production and calcification in a Mediterranean population of Ditrupa arietina (Annelida: Polychaeta). Mar. Ecol. Prog. Ser. 2000, 199, 171–184. [Google Scholar] [CrossRef][Green Version]
- Smith, A.M.; Riedi, M.A.; Winter, D.J. Temperate reefs in a changing ocean: Skeletal carbonate mineralogy of serpulids. Mar. Biol. 2013, 160, 2281–2294. [Google Scholar] [CrossRef]
- Videtich, P.E. Stable-isotope compositions of serpulids give insight to calcification processes in marine organisms. Palaios 1986, 1, 189–193. [Google Scholar] [CrossRef]
- Reish, D.J.; Mason, A.Z. Radiocarbon dating and metal analyses of ‘fossil’ and living tubes of Protula (Annelida: Polycaeta). Hydrobiologia 2003, 496, 371–383. [Google Scholar] [CrossRef]
- Carter, J.G.; Bandel, K.; de Burenil, V.; Carlson, S.J.; Castanet, J.; Crenshaw, M.A.; Dalingwater, J.E.; Francillion-Vieillot, H.; Geradie, J.; Meunier, F.J.; et al. Glossary of skeletal biomineralization. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Carter, J.G., Ed.; Wiley: Hoboken, NJ, USA, 1990; pp. 609–671. [Google Scholar]
- Hedley, R.H. Studies on serpulid tube formation. I. The secretion of the calcareous and organic components of the tube by Pomatoceros triqueter. Quart. J. Microsc. Sci. 1956, 97, 411–427. [Google Scholar]
- Hedley, R.H. Studies on serpulid tube formation. II. The calcium-secreting glands in the peristomium of Spirorbis, Hydroides and Serpula. Quart. J. Microsc. Sci. 1956, 97, 421–427. [Google Scholar]
- Hedley, R.H. Tube formation by Pomatoceros triqueter (Polychaeta). J. Mar. Biol. Assoc. UK 1958, 37, 315–322. [Google Scholar] [CrossRef]
- Neff, J.M. Ultrastructural Studies of the Secretion of Calcium Carbonate by the Serpulid Polychaete Worm, Pomatoceras caerulus. Zeit. Zellforsch. 1971, 120, 160–186. [Google Scholar] [CrossRef]
- Neff, J.M. Ultrastructure of calcium phosphate containing cells in the serpulid Pomatoceros caeruleus. Calc. Tiss. Res. 1971, 7, 191–200. [Google Scholar] [CrossRef]
- Ten Hove, H.A.; Smith, R.S. A re-description of Ditrupa gracillima Grube, 1878 (Polychaeta, Serpulidae) from the Indo-Pacific, with a discussion of the genus. Rec. Aust. Mus. 1990, 42, 101–118. [Google Scholar] [CrossRef]
- Vovelle, J.; Grasset, M.; Truchet, M. Sites of biomineralization in the Polychaete Pomatoceros triqueter (Serpulidae) with comments on some other species. Ophelia Suppl. 1991, 5, 661–667. [Google Scholar]
- Nishi, E. On the internal structure of calcified tube walls in Serpulidae and Spirorbidae (Annelida, Polychaeta). Mar. Foul. 1993, 10, 17–20. [Google Scholar] [CrossRef]
- Pillai, T.G.; Ten Hove, H.A. On recent species of Spiraserpula Regenhardt, 1961, a serpulid polychaete genus hitherto known only from Cretaceous and Tertiary fossils. Bull. Nat. Hist. Mus. Lond. 1994, 60, 39–104. [Google Scholar]
- Weedon, M.J. Tube microstructure of Recent and Jurassic serpulid polychaetes and the question of the Palaeozoic ‘spirorbids’. Acta Palaeont. Pol. 1994, 39, 1–15. [Google Scholar]
- Aliani, S.; Bianchi, C.N.; Meloni, C. Scanning electron microscope observations on the tube of the reef-forming serpulid Ficopomatus enigmaticus (Fauvel) (Annelida, Polychaeta). Boll. Zool. 1995, 62, 363–367. [Google Scholar] [CrossRef]
- Sanfilippo, R. Micromorphology, microstructure and functional morphology of the Josephella marenzelleri (Polychaeta Serpulidae) tube. In Autoecology of Selected Fossil Organisms: Achievements and Problems; Bollettino della Società Paleontologica Italiana; Cherchi, A., Ed.; Mucchi: Rome, Italy, 1996; Volume 3, pp. 205–211. [Google Scholar]
- Sanfilippo, R. Tube morphology and structure of the bathyal Mediterranean serpulid Hyalopomatus variorugosus Ben-Eliahu and Fiege, 1996 (Annelida, Polychaeta). Riv. Ital. Paleontol. Stratigr. 1996, 104, 131–138. [Google Scholar]
- Sanfilippo, R.; Mòllica, E. Serpula cavernicola Fassari and Mòllica, 1991 (Annelida Polychaeta): Diagnostic features of the tubes and new Mediterranean records. Mar. Life 2000, 10, 27–32. [Google Scholar]
- Vinn, O.; ten Hove, H.A.; Mutvei, H.; Kirsimäe, K. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zool. J. Linn. Soc. 2008, 154, 633–650. [Google Scholar] [CrossRef]
- Ippolitov, A.P.; Rzhavsky, A.V. On the Microstructure of Tubes of Living Spirorbids (Annelida, Polychaeta). Dokl. Akad. Nauk 2008, 418, 131–133. [Google Scholar]
- Ippolitov, A.P.; Rzhavsky, A.V. Tube morphology, ultrastructures and mineralogy in recent Spirorbinae (Annelida; Polychaeta; Serpulidae). I. General Introduction. Material and methods. Tribe Paralaeospirini. Invert. Zool. 2014, 11, 293–314. [Google Scholar] [CrossRef]
- Ippolitov, A.P.; Rzhavsky, A.V. Tube morphology, ultrastructures and mineralogy in recent Spirorbinae (Annelida: Polychaeta: Serpulidae). II. Tribe Spirorbini. Invert. Zool. 2015, 12, 61–92. [Google Scholar] [CrossRef]
- Tanur, A.E.; Gunari, N.; Sullan, R.M.A.; Kavanagh, C.J.; Walker, G.C. Insights into the composition, morphology, and formation of the calcareous shell of the serpulid Hydroides dianthus. J. Struct. Biol. 2010, 169, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Vinn, O.; ten Hove, H.A. Microstructure and formation of the calcareous operculum in Pyrgopolon ctenactis and Spirobranchus giganteus (Annelida, Serpulidae). Zoomorphology 2011, 130, 181–188. [Google Scholar] [CrossRef]
- Vinn, O. Occurrence, formation and function of organic sheets in the mineral tube structures of Serpulidae (Polychaeta, Annelida). PLoS ONE 2013, 8, e75330. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Vinn, O.; Li, C.; Lu, X.; Kudryavtsev, A.B.; Schopf, J.W.; Shih, K.; Zhang, T.; Thiyagarajan, V. Evidence of compositional and ultrastructural shifts during the development of calcareous tubes in the biofouling tubeworm, Hydroides elegans. J. Struct. Biol. 2015, 189, 230–237. [Google Scholar] [CrossRef]
- Buckman, J.O. The tube of Ditrupa bartonensis (Annelida, Serpulidae), from the Eocene of southern England: Observations on microstructure and its significance. Palaeontol. Electr. 2020, 23, a37. [Google Scholar] [CrossRef]
- Buckman, J.O.; Harries, D.B. Reef forming Serpula vermicularis from Scotland and Ireland: Tube structure, composition and implications. Zool. Anz. 2020, 288, 53–65. [Google Scholar] [CrossRef]
- Vinn, O. Biomineralization of Polychaete Annelids in the Fossil Record. Minerals 2020, 10, 858. [Google Scholar] [CrossRef]
- Vinn, O.; ten Hove, H.A.; Mutvei, H. On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta). Palaeontology 2008, 51, 295–301. [Google Scholar] [CrossRef]
- Vinn, O. The ultrastructure of calcareous cirratulid (Polychaeta, Annelida) tubes. Est. J. Earth Sci. 2009, 58, 153–156. [Google Scholar] [CrossRef]
- Taylor, P.D.; Vinn, O.; Kudryavtsev, A.; Schopf, J.W. Raman spectroscopic study of the mineral composition of cirratulid tubes (Annelida, Polychaeta). J. Struct. Biol. 2010, 171, 402–405. [Google Scholar] [CrossRef]
- Vinn, O.; Kirsimäe, K.; ten Hove, H.A. Tube ultrastructure of Pomatoceros americanus (Polychaeta, Serpulidae): Implications for the tube formation of serpulids. Est. J. Earth Sci. 2009, 58, 148–152. [Google Scholar] [CrossRef]
- Bubel, A. Electron microscope studies on the operculum of Spirorbis spirorbis (L.). In Proceedings of the International Biodegradation Symposium; Sharpley, J.M., Kaplan, A.M., Eds.; Applied Science Publishers: London, UK, 1976; pp. 495–514. [Google Scholar]
- Bubel, A. A fine structural study of the calcareous opercular plate and associated cells in a polychaete annelid. Tiss. Cell 1983, 15, 457–476. [Google Scholar] [CrossRef]
- Bubel, A.; Thorp, C.H.; Fitzsimmons, C.A.L. An histological and electron microscopical study of opercular regeneration in the serpulid, Pileolaria (P.) granulata, with particular reference to the formation of the calcareous opercular plate. In Proceedings of the 4th International Congress on Marine Corrosion and Fouling, Juan-les-Pins, France, 14–18 June 1976; pp. 85–96. [Google Scholar]
- Bubel, A.; Thorp, C.H.; Moore, M.N. An histological, histochemical and ultrastructural study of the operculum of the serpulid Pomatoceros triqueter L. with particular reference to the formation of the calcareous opercular plate during opercular regeneration. In Biodeterioration: The Proceedings of the Fourth International Biodeterioration Symposium; Oxley, T.A., Allsopp, D.A., Becker, G., Eds.; Pitman Publishing: London, UK, 1980; pp. 275–290. [Google Scholar]
- Bubel, A.; Thorp, C.H.; Fenn, R.H.; Livingstone, D. Opercular regeneration in Pomatoceros lamarckii Quatrefages (Polychaeta: Serpulidae). Differentation of the operculum and deposition of the calcareous opercular plate. J. Zool. Lond. Ser. 1985, B1, 49–94. [Google Scholar] [CrossRef]
- Vinn, O.; Mutvei, H.; ten Hove, H.A.; Kirsimäe, K. Unique Mg-calcite skeletal ultrastructure in the tube of the serpulid polychaete Ditrupa. Neues Jahrb. Geol. Paläontol. Abh. 2008, 248, 79–89. [Google Scholar] [CrossRef]
- Vinn, O. On the unique isotropic aragonitic tube microstructure of some serpulids (Polychaeta, Annelida). J. Morph. 2013, 274, 478–482. [Google Scholar] [CrossRef]
- Vinn, O. SEM study of semi-oriented tube microstructures of Serpulidae (Polychaeta, Annelida): Implications for the evolution of complex oriented microstructures. Micr. Res. Tech. 2013, 76, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.K.; Vinn, O.; Taylor, P.D.; Schopf, J.W.; Kudryavtsev, A.; Bailey-Brock, J. Serpulids living deep: Calcareous tubeworms beyond the abyss. Deep Sea Res. 2014, 90, 91–104. [Google Scholar] [CrossRef]
- Vinn, O. The role of an internal organic tube lining in the biomineralization of serpulid tubes. Carnets Géol. 2011, 11, 13–16. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Environmental relations of modification compositions of certain carbonate secreting marine invertebrates. Proc. Natl. Acad. Sci. USA 1954, 40, 39–48. [Google Scholar] [CrossRef]
- Bornhold, B.D.; Milliman, J.D. Generic and environmental control of carbonate mineralogy in serpulid (Polychaete) tubes. J. Geol. 1973, 81, 363–373. [Google Scholar] [CrossRef]
- Simkiss, K.; Wilbur, K.M. Biomineralization: Cell Biology and Mineral Deposition; Academic Press: Cambridge, MA, USA, 1989; p. 337. [Google Scholar]
- Andersson, A.J.; Mackenzie, F.T.; Bates, N.R. Life on the margin: Implication of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 2008, 373, 265–273. [Google Scholar] [CrossRef]
- Ries, J.B. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 2011, 403, 54–64. [Google Scholar] [CrossRef]
- Taylor, P.D.; James, N.P.; Bone, Y.; Kuklinski, P.; Kyser, T.K. Evolving mineralogy of cheilostome bryozoans. Palaios 2009, 24, 440–452. [Google Scholar] [CrossRef]
- Bickert, T. Carbonate compensation depth. In Encyclopedia of Paleoclimatology and Ancient Environments; Gornitz, V., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 136–138. [Google Scholar]
- Chan, V.B.S.; Li, C.; Lane, A.C.; Wang, Y.; Lu, X.; Shih, K.; Zhang, T.; Thiyagarajan, V. CO2-driven ocean acidification alters and weakens integrity of the calcareous tubes produced by the serpulid tubeworm, Hydroides elegans. PLoS ONE 2012, 7, e42718. [Google Scholar] [CrossRef]
- Li, C.Y.; Chan, V.B.S.; He, C.; Meng, Y.; Yao, H.M.; Shih, K.M.; Thiyagarajan, V. Weakening Mechanisms of the Serpulid Tube in a High-CO2 World. Envir. Sci. Tech. 2014, 48, 14158–14167. [Google Scholar] [CrossRef]
- Chan, V.B.S.; Thiyagarajan, V.; Lu, X.W.; Zhang, T.; Shih, K. Temperature Dependent Effects of Elevated CO2 on Shell Composition and Mechanical Properties of Hydroides elegans: Insights from a Multiple Stressor Experiment. PLoS ONE 2013, 8, e78945. [Google Scholar] [CrossRef]
- Li, C.Y.; Meng, Y.; He, C.; Chan, V.B.S.; Yao, H.M.; Thiyagarajan, V. Mechanical robustness of the calcareous tubeworm Hydroides elegans: Warming mitigates the adverse effects of ocean acidification. Biofouling 2016, 32, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Taubner, I.; Böhm, F.; Winde, V.; Böttcher, M.E. Effect of temperature rise and ocean acidification on growth of calcifying tubeworm shells (Spirorbis spirorbis): An in situ benthocosm approach. Bioegeosciences 2018, 15, 1425–1445. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Rosso, A.; Reitano, A.; Insacco, G. First record of sabellid and serpulid polychaetes from the Permian of Sicily. Acta Palaeontol. Pol. 2017, 62, 25–38. [Google Scholar] [CrossRef]
- Jäger, M. Serpulidae und Spirorbidae (Polychaeta sedentaria) aus Campan und Maastricht von Norddeutschland, den Niederlanden, Belgien und angrenzenden Gebieten. Geol. Jahrb. 2004, A157, 121–249. [Google Scholar]
- Perkins, T.H. Calcisabella piloseta, a new genus and species of Sabellinae (Polychaeta: Sabellidae). Bull. Mar. Sci. 1991, 48, 261–267. [Google Scholar]
- Kupriyanova, E.K.; Rouse, G.W. Yet another example of paraphyly in Annelida: Molecular evidence that Sabellidae contains Serpulidae. Molec. Phylogen. Evol. 2008, 46, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Galli Oliver, C.; Reitner, J. Skeletal structure, growth, and paleoecology of the patch reef building polychaete worm Diplochaetetes mexicanus Wilson, 1986 from the Oligocene of Baja California (Mexico). Geobios 1989, 22, 761–775. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Control and design principles in biological mineralization. Ang. Chem. Int. Ed. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Jimi, N.; Fujimoto, S.; Takehara, M.; Imura, S. Black spicules from a new interstitial opheliid polychaete Thoracophelia minuta sp. nov. (Annelida: Opheliidae). Sci. Rep. 2021, 11, 1557. [Google Scholar] [CrossRef]
- Righi, S.; Savioli, M.; Prevedelli, D.; Simonini, R.; Malferrari, D. Unravelling the ultrastructure and mineralogical composition of fireworm stinging bristles. Zoology 2021, 144, 125851. [Google Scholar] [CrossRef]
- Tilic, E.; Pauli, B.; Bartolomaeus, T. Getting to the root of fireworms’ stinging chaetae—Chaetal arrangement and ultrastructure of Eurythoe complanata (Pallas, 1766) (Amphinomida). J. Morphol. 2017, 278, 865–876. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Macdonald, T.; Rouse, G.W. Phylogenetic relationships within Serpulidae (Sabellida, Annelida) inferred from molecular and morphological data. Zool. Scripta 2006, 35, 421–439. [Google Scholar] [CrossRef]
- Taylor, P.D. Seawater chemistry, biomineralization and the fossil record of calcareous organisms. In Proceedings of International Symposium “The Origin and Evolution of Natural Diversity”; Origin and Evolution of Natural Diversity, Sapporo, Japan, 1–5 October 2007; 21st Century COE for Neo-Science of Natural History; Okada, H., Mawatari, S.F., Suzuki, N., Gautam, P., Eds.; Hokkaido University: Sapporo, Japan, 2007; pp. 21–29. [Google Scholar]
- Vinn, O.; Jäger, M.; Kirsimäe, K. Microscopic evidence of serpulid affinities of the problematic fossil tube “Serpula” etalensis from the Lower Jurassic of Germany. Lethaia 2008, 41, 417–421. [Google Scholar] [CrossRef]
- Ries, J.B. The effect of ambient Mg/Ca on Mg fractionation in calcareous marine invertebrates: A record of Phanerozoic Mg/Ca in seawater. Geology 2004, 32, 981–984. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinn, O. Biomineralization in Polychaete Annelids: A Review. Minerals 2021, 11, 1151. https://doi.org/10.3390/min11101151
Vinn O. Biomineralization in Polychaete Annelids: A Review. Minerals. 2021; 11(10):1151. https://doi.org/10.3390/min11101151
Chicago/Turabian StyleVinn, Olev. 2021. "Biomineralization in Polychaete Annelids: A Review" Minerals 11, no. 10: 1151. https://doi.org/10.3390/min11101151
APA StyleVinn, O. (2021). Biomineralization in Polychaete Annelids: A Review. Minerals, 11(10), 1151. https://doi.org/10.3390/min11101151





