Influence of Microbubble on Fine Wolframite Flotation
Abstract
:1. Introduction
2. Materials and Methods
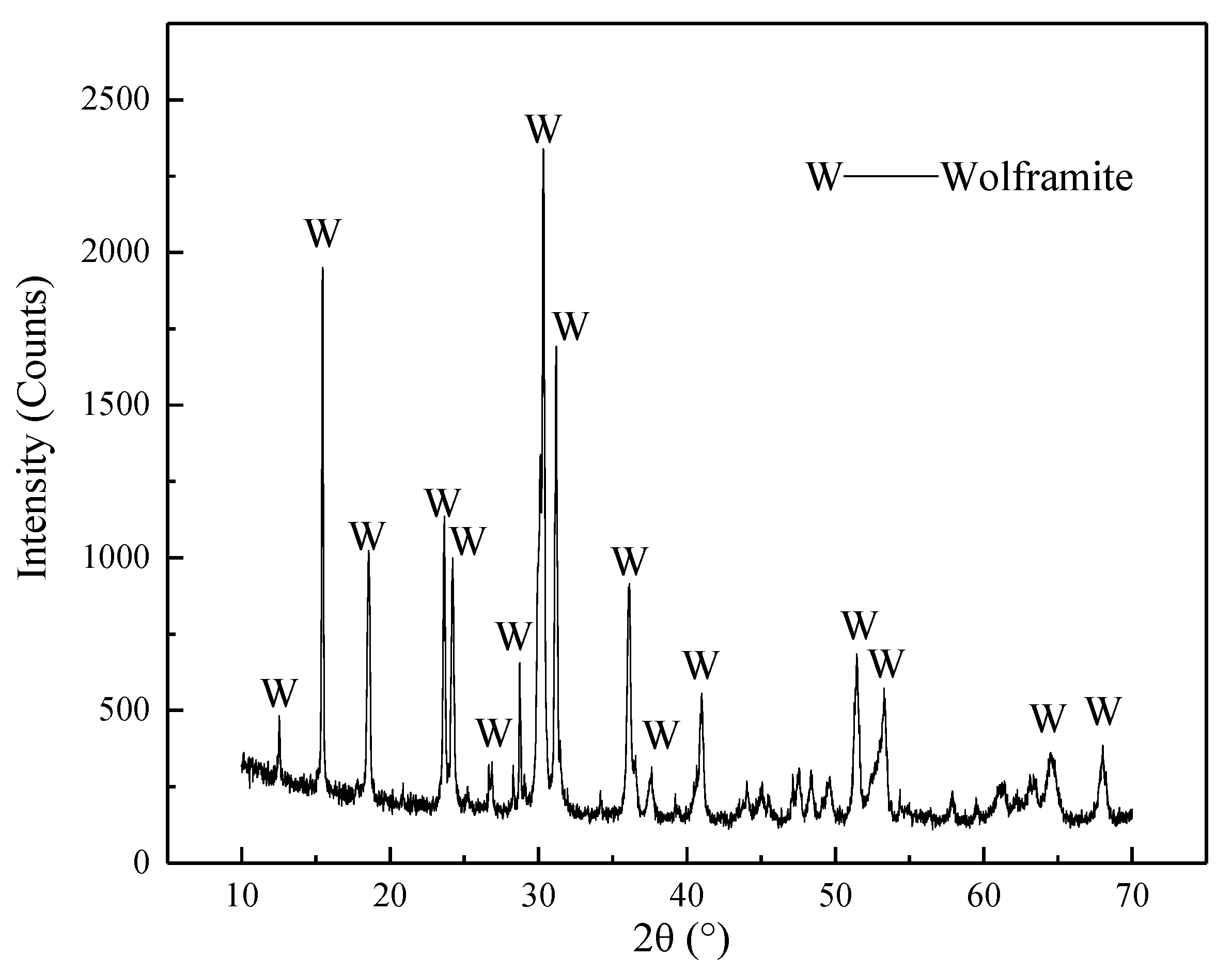
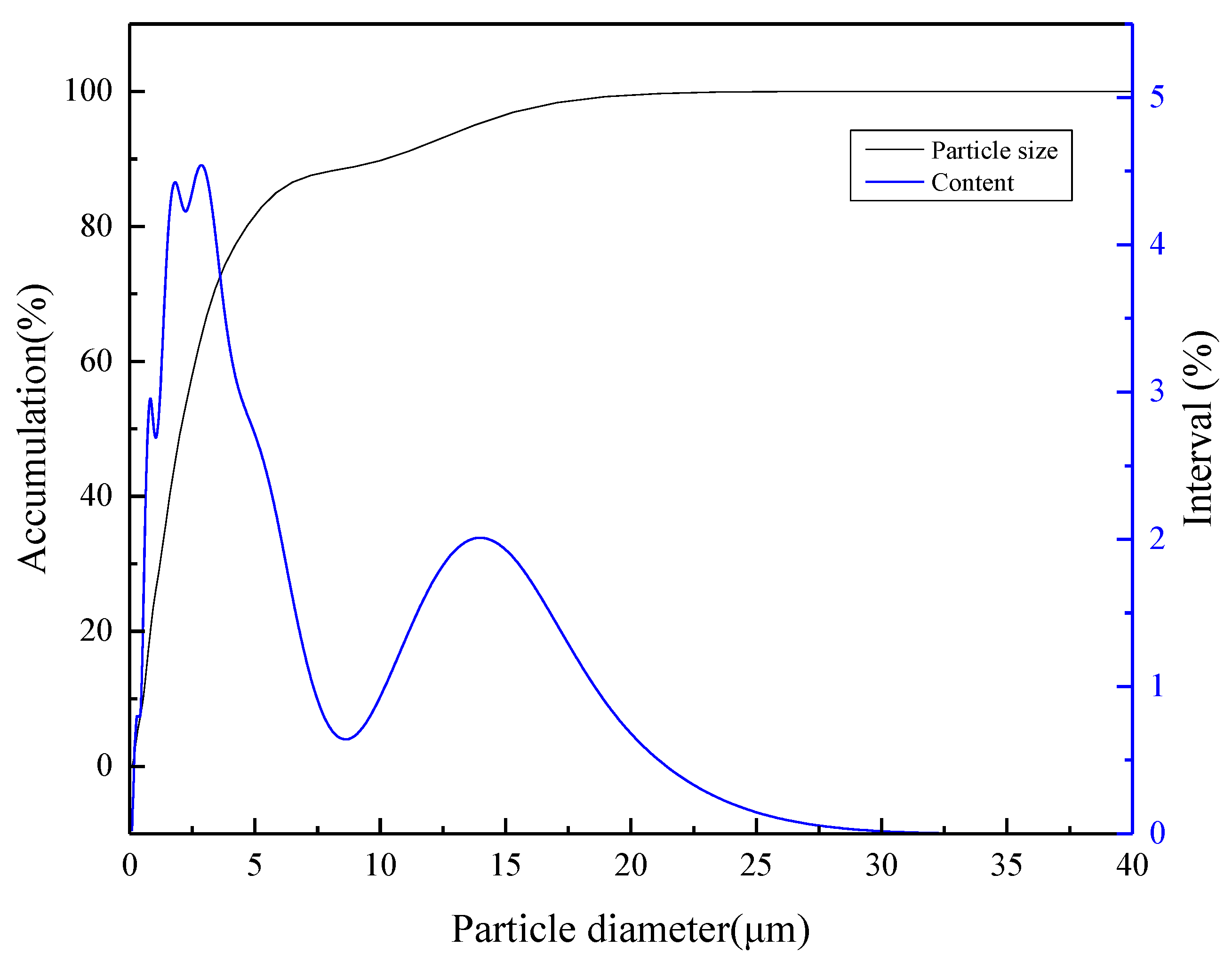
2.1. Materials
2.2. Single Mineral Flotation
2.3. Zeta Potential Analysis
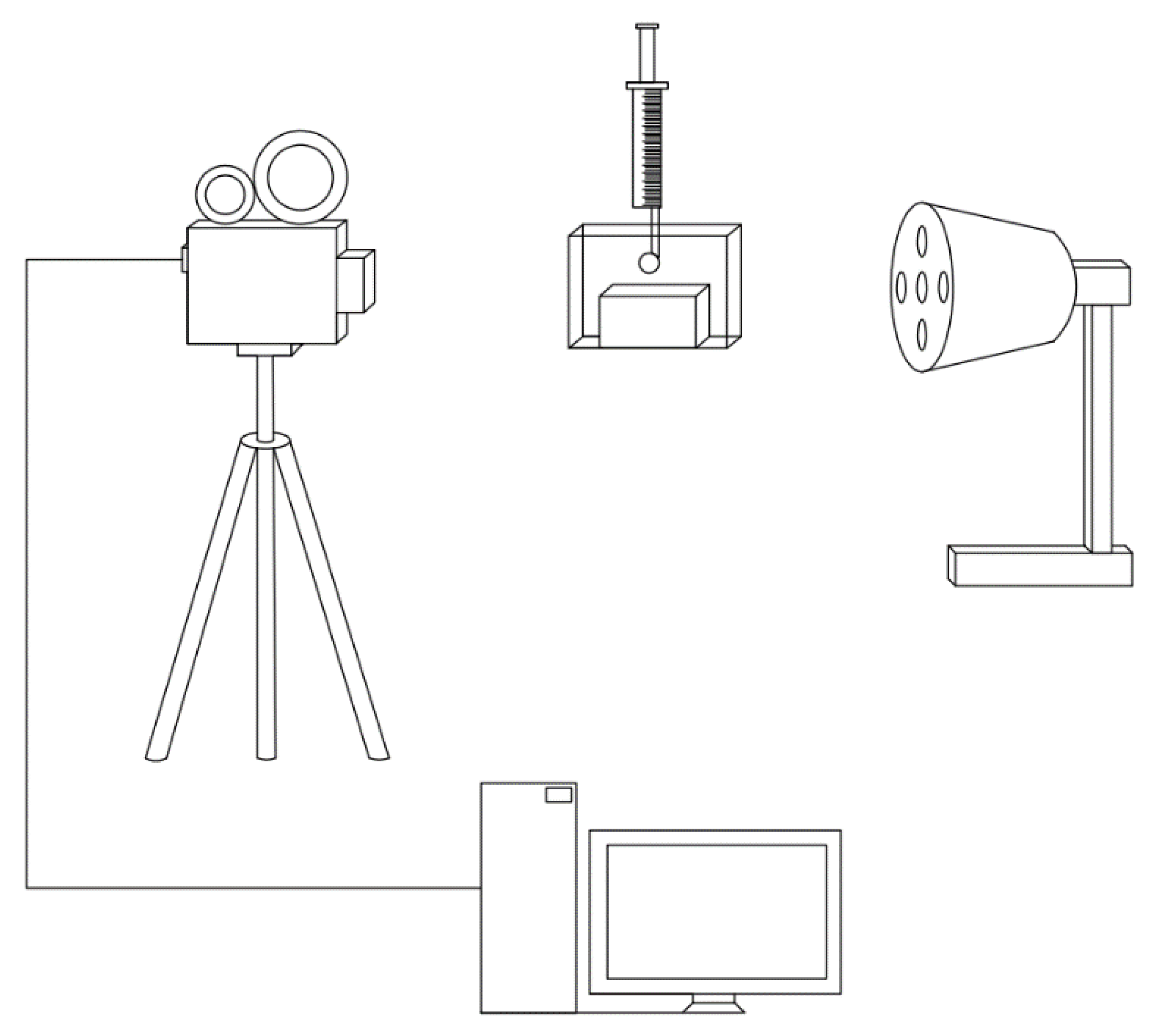
2.4. High-Speed Camera Analysis
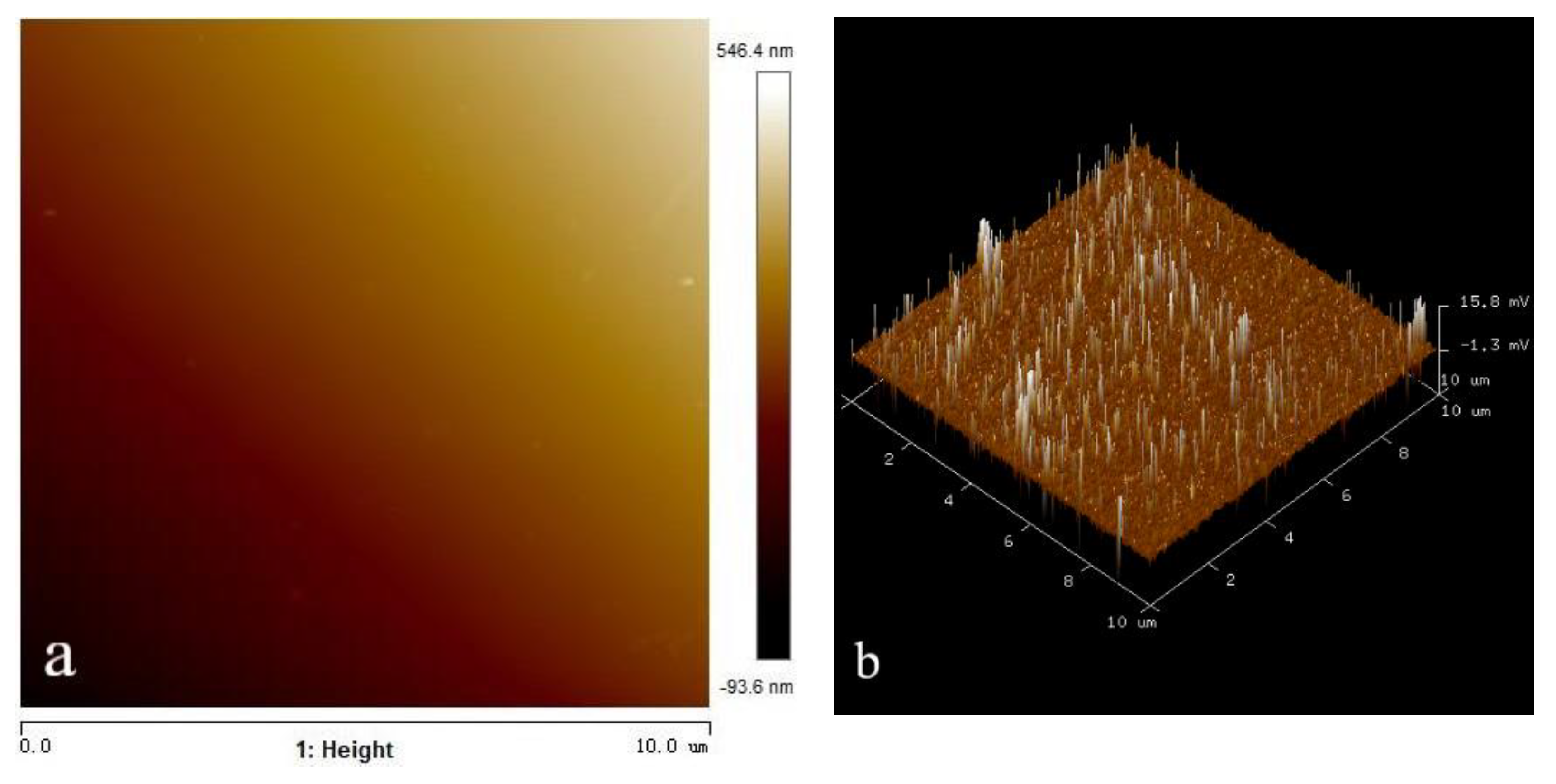
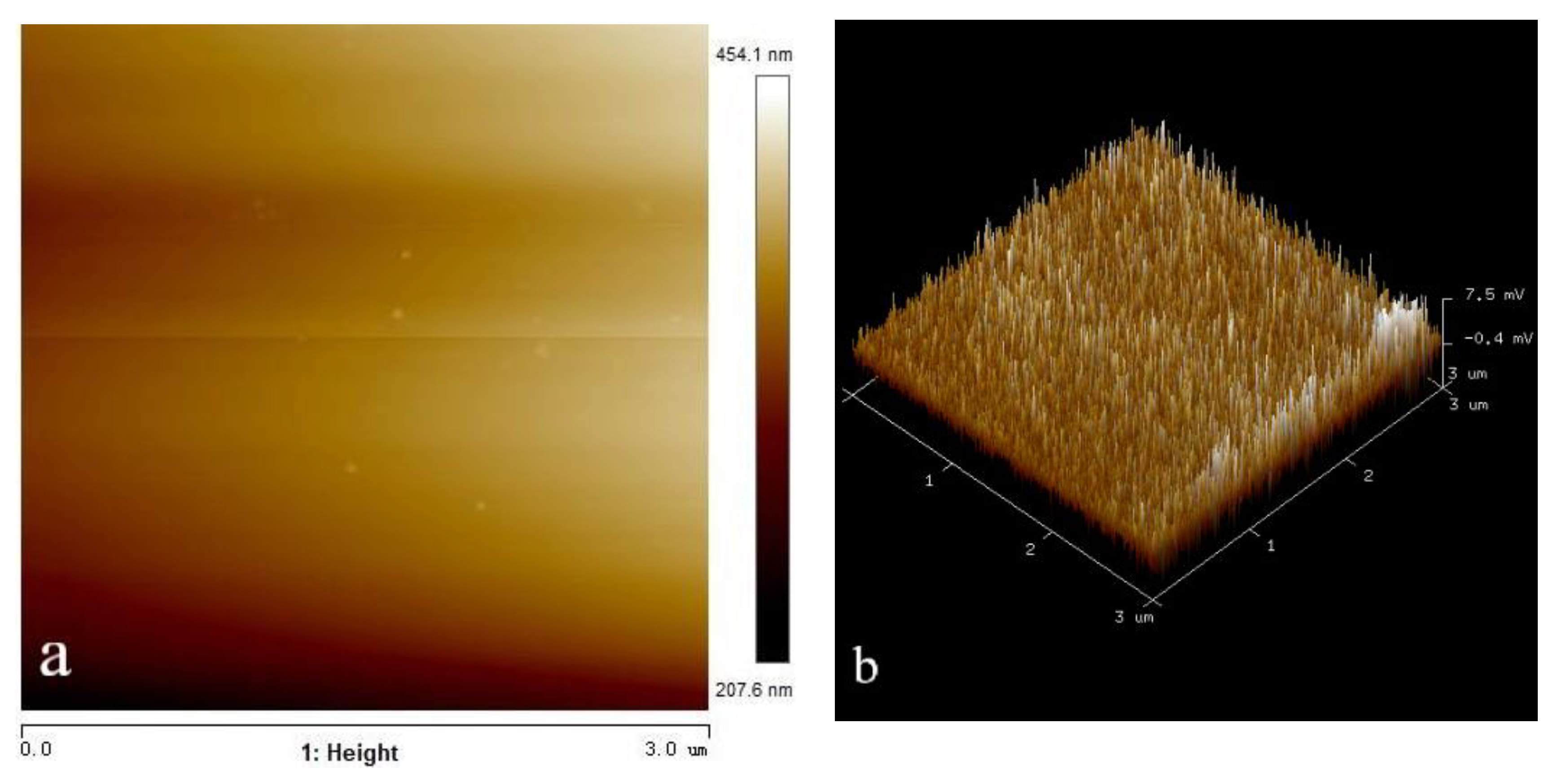
2.5. AFM Analysis
3. Results and Discussion
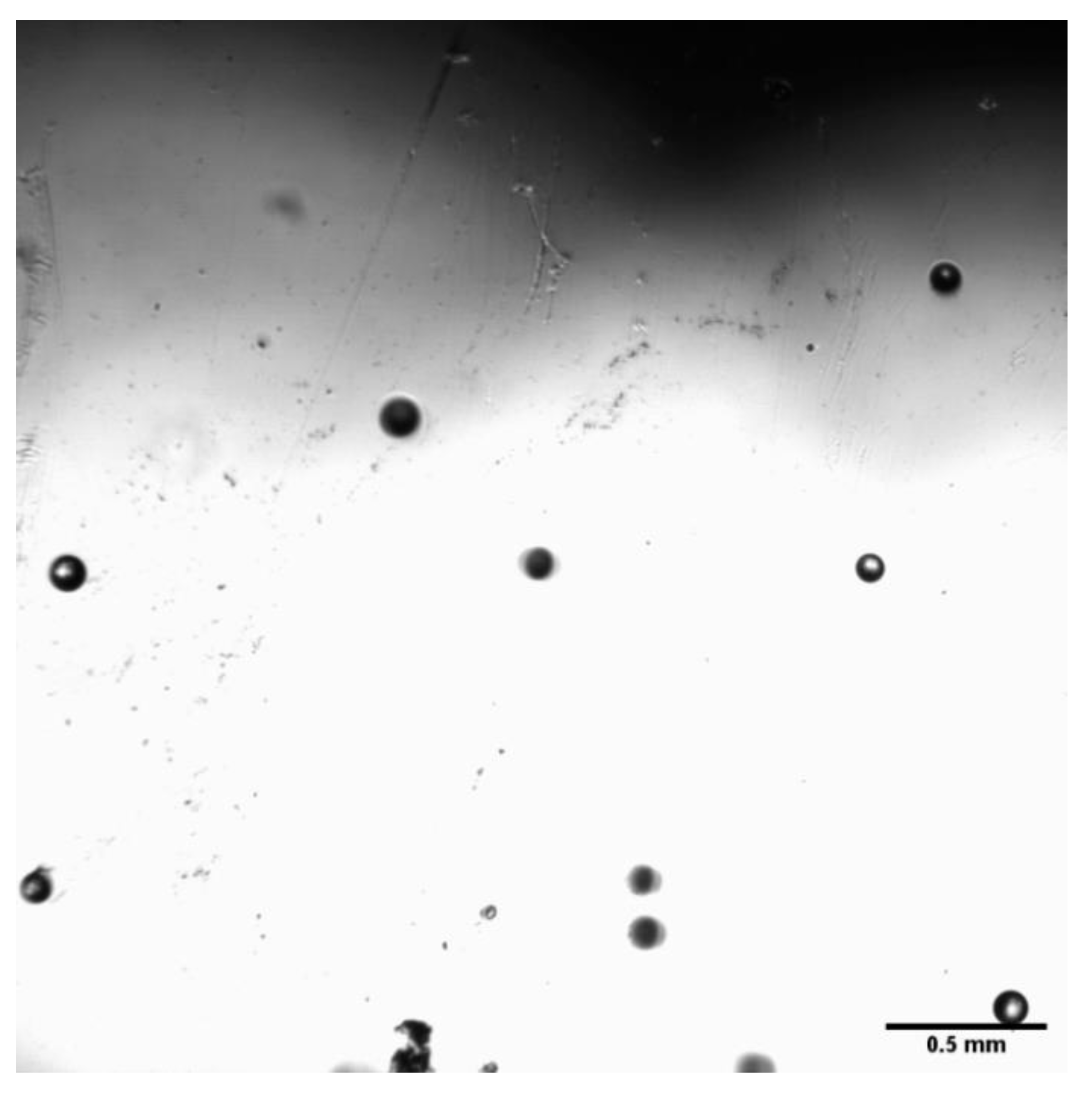
3.1. Bubble Size Distribution
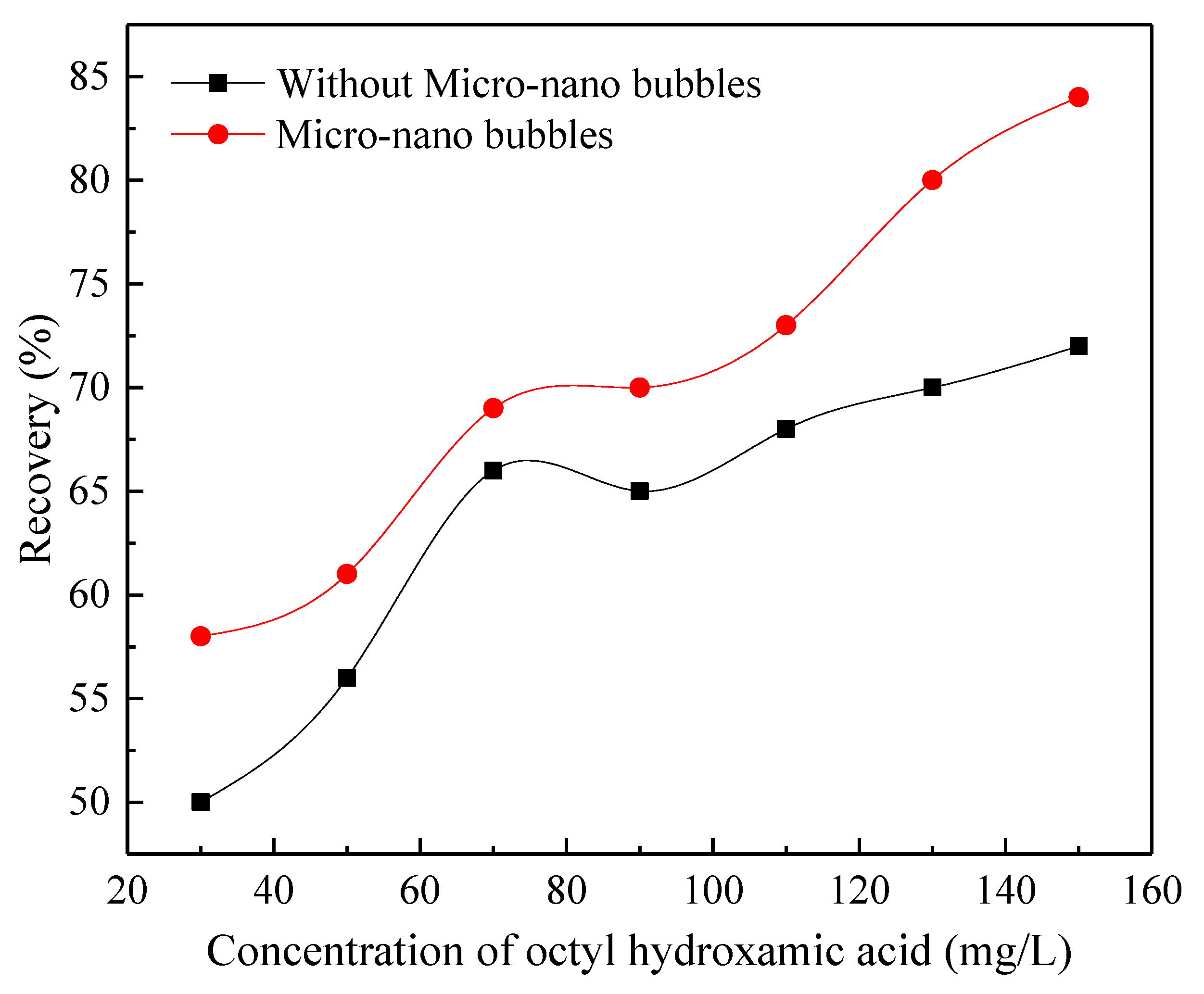
3.2. Effect of Micro-Nanobubbles on the Flotation of Fine Wolframite
3.3. Effect of Microbubble on Zeta Potential
3.4. Interaction between Wolframite and Bubble
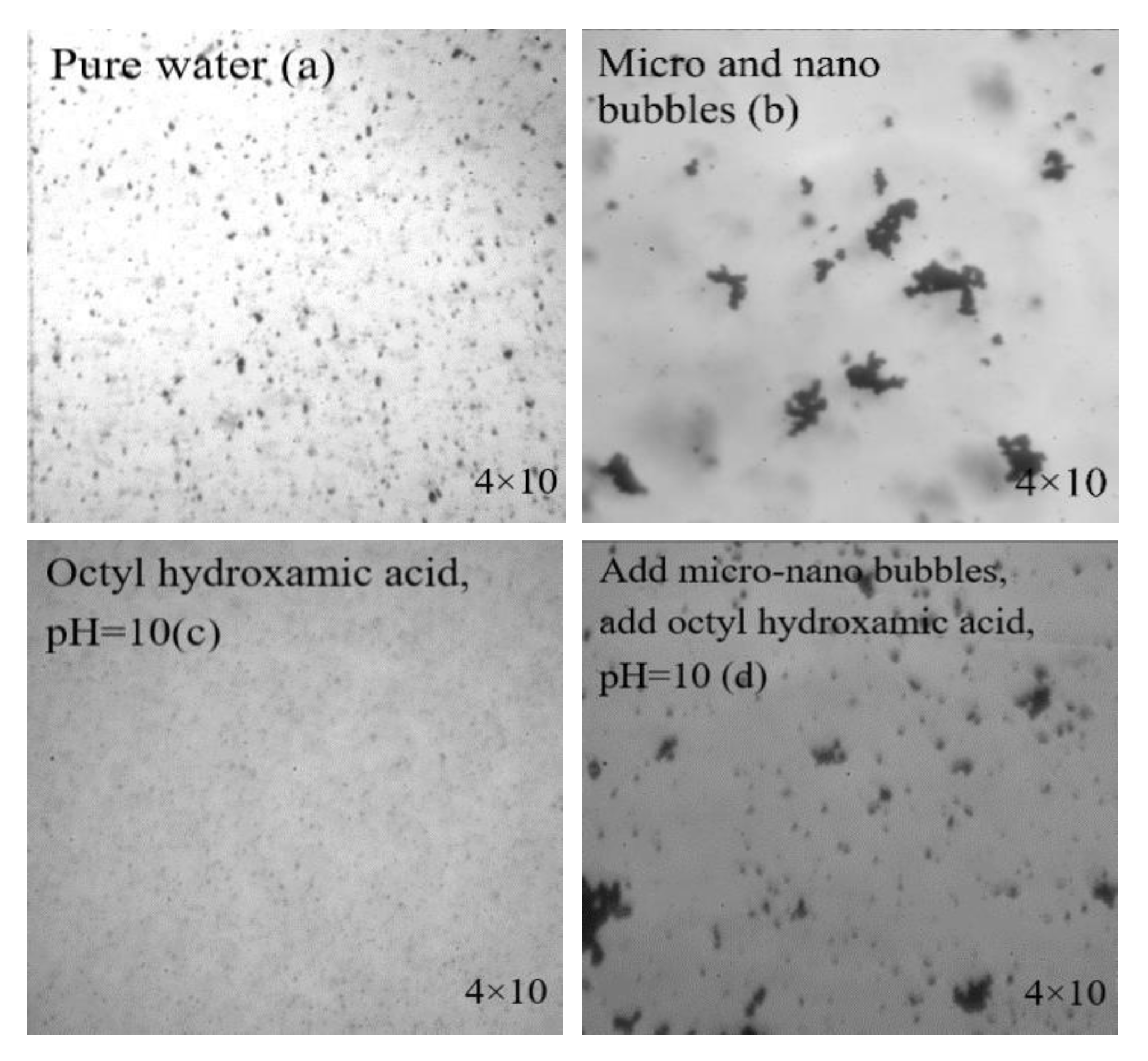
3.4.1. Agglomeration of Wolframite in Slurry under Different Conditions
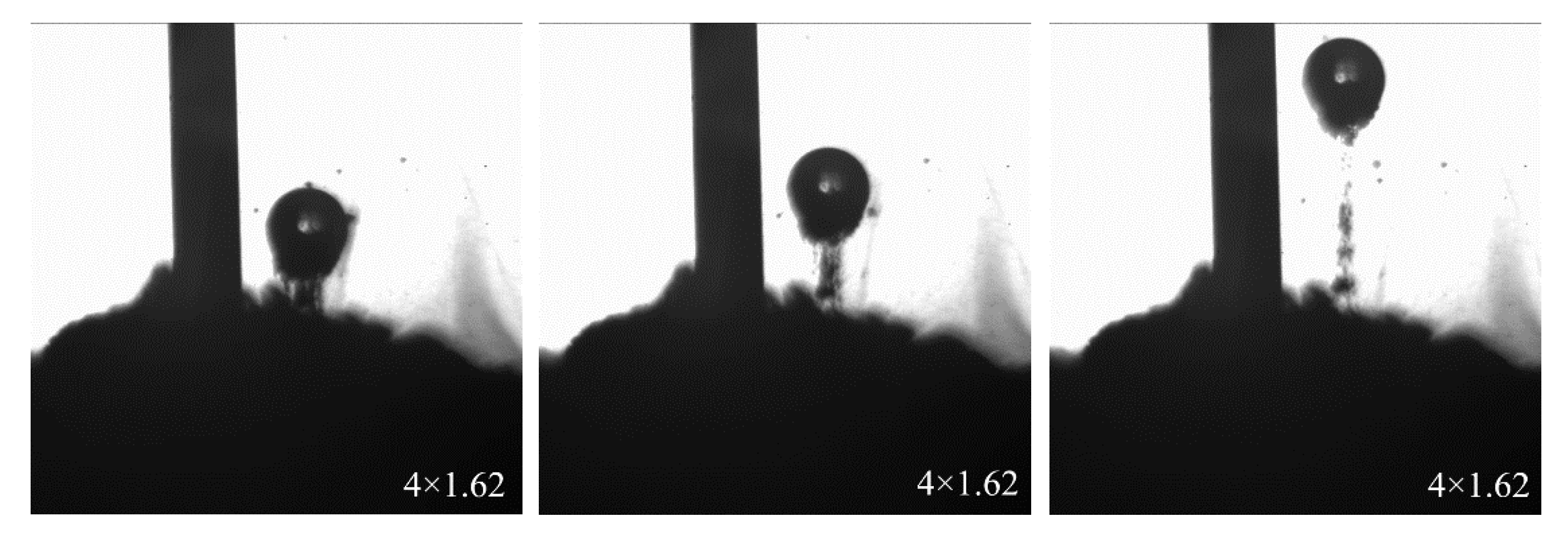
3.4.2. Collision between Bubble and Mineral Particle
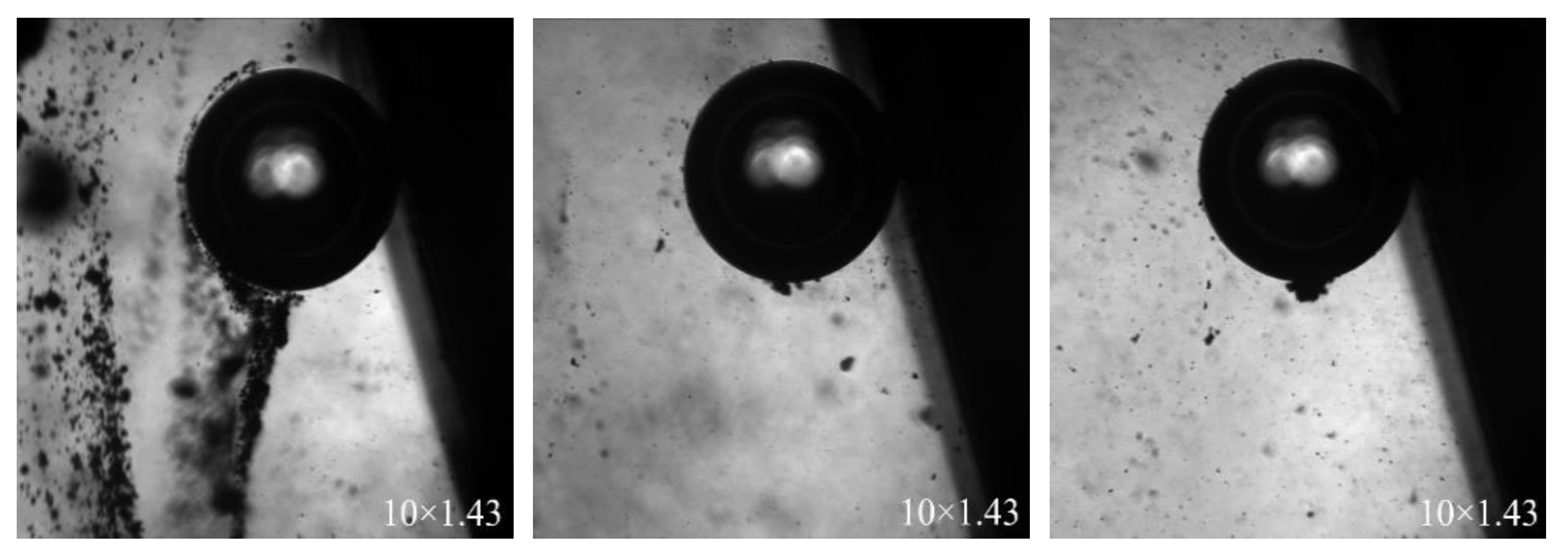
3.4.3. Adhesion between Mineral Particles and Bubbles
3.5. Effect of Microbubble on the Adsorption of OHA on the Wolframite Surface
4. Conclusions
- The introduction of micro-nanobubbles can significantly improve the recovery of fine wolframite flotation.
- The fine-grain wolframite in the slurry had obvious agglomeration after adding microbubbles. The addition of microbubbles changed the agglomerating way of fine mineral particles, making it easier for fine wolframite particles to agglomerate together.
- The AFM analysis results showed that the introduction of micro-nanobubbles on the surface of the flake wolframite reduced the adsorption of octyl hydroxamic acid, which resulted in the competitive adsorption of microbubbles and collectors.
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Y. The Characteristics and Research Advances of Mineral Processing Technologies of Chinese Tungsten Resources. China Tungeten. Ind. 2016, 31, 35–39. [Google Scholar] [CrossRef]
- Ai, G.; Li, X. Study situation and prospects of concentration of micro-fine wolframite. Min. Process. Equip. 2011, 39, 89–96. [Google Scholar]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of Fine Particles: A Review. Min. Proc. Ext. Met. Rev. 2021, 42, 473–483. [Google Scholar] [CrossRef]
- Zawala, J.; Karaguzel, C.; Wiertel, A.; Sahbaz, O.; Malysa, K. Kinetics of the bubble attachment and quartz flotation in mixed solutions of cationic and non-ionic surface-active substances. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 118–126. [Google Scholar] [CrossRef]
- Ai, G.; Yang, X.; Li, X. Flotation characteristics and flotation kinetics of fine wolframite. Powder Technol. 2017, 305, 377–381. [Google Scholar] [CrossRef]
- Brabcová, Z.; Karapantsios, T.; Kostoglou, M.; Basařová, P.; Matis, K. Bubble–particle collision interaction in flotation systems. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 95–103. [Google Scholar] [CrossRef]
- Testa, F.G.; Safari, M.; Deglon, D.; Leal Filho, L.D.S. Influence of agitation intensity on flotation rate of apatite particles. REM Int. Eng. J. 2017, 70, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xia, Y.; Zhang, Y.; Ding, S.; Rong, G.; Cao, Y.; Xing, Y.; Gui, X. Mechanism of shale oil as an effective collector for oxidized coal flotation: From bubble–particle attachment and detachment point of view. Fuel 2019, 255, 115885. [Google Scholar] [CrossRef]
- Frederick Bloom, T.J.H. On the structure of collision and detachment frequencies in $otation models. Chem. Eng. Sci. 2002, 57, 2467–2473. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hassas, B.V.; Kouachi, S.; Brabcova, Z.; Çelik, M.S. Effect of bubble size and velocity on collision efficiency in chalcopyrite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 498, 258–267. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Filippov, L.O.; Kravchenko, O.V. Combined microflotation of glass beads. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124810. [Google Scholar] [CrossRef]
- Fan, M.; Daniel, T.; Rick, H.; Luo, Z. Nanobubble generation and its application in froth flotation (part I): Nanobubble generation and its effects on properties of microbubble and millimeter scale bubble solutions. Min. Sci. Technol. 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Rh, Y. The role of hydrodynamic and surface forces in bubble–particle interaction. Int. J. Miner. Process. 2000, 58, 129–143. [Google Scholar] [CrossRef]
- Safari, M.; Deglon, D. An attachment-detachment kinetic model for the effect of energy input on flotation. Miner. Eng. 2018, 117, 8–13. [Google Scholar] [CrossRef]
- Safari, M.; Deglon, D. Evaluation of an Attachment–Detachment Kinetic Model for Flotation. Minerals 2020, 10, 978. [Google Scholar] [CrossRef]
- Leila, Z.; Janet, A.W.E. Thermodynamics of Surface Nanobubbles. Langmuir 2016, 32, 11309–11320. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippova, I.; Filippov, L.; Picarra, A.; Rulyov, N.; Fornasiero, D. Flotation of fine particles in the presence of combined microbubbles and conventional bubbles. Miner. Eng. 2020, 155, 106439. [Google Scholar] [CrossRef]
- Bu, X.; Zhang, T.; Chen, Y.; Xie, G.; Peng, Y. Comparative study of conventional cell and cyclonic microbubble flotation column for upgrading a difficult-to-float Chinese coking coal using statistical evaluation. Int. J. Coal Prep. Util. 2020, 40, 359–375. [Google Scholar] [CrossRef]
- Santana, R.; Ribeiro, J.; Santos, M.; Reis, A.; Ataíde, C.; Barrozo, M. Flotation of fine apatitic ore using microbubbles. Sep. Purif. Technol. 2012, 98, 402–409. [Google Scholar] [CrossRef]
- Ahmadi, R.; Khodadadi, D.A.; Abdollahy, M.; Fan, M. Nano-microbubble flotation of fine and ultrafine chalcopyrite particles. Int. J. Min. Sci. Technol. 2014, 24, 559–566. [Google Scholar] [CrossRef]
- Rajeev, P.; Kumarmajumder, S.; Ghosh, P. Flotation of fine particles from binary mixture by ionic microbubbles. ChemXpres 2015, 2, 133–137. [Google Scholar]
- Chen, B.; Bao, S.; Zhang, Y.; Zheng, R. Ultrasound-assisted synthesis of N235-impregnated resins for vanadium (V) adsorption. R Soc. Open Sci. 2018, 5, 171746. [Google Scholar] [CrossRef] [Green Version]
- Xu, J. Mechanism of Micro-bubble Air Flotation in Water Treatment Process. and Flotation Experiment; Kunming University of Science and Technology: Kunming, China, 2013. [Google Scholar]
- Liu, H.; Yang, C.; Wang, Y.; Gui, W. Influence analysis and parameter optimization of bubble diameter in Micro-bubbles flotation process. Miner. Eng. Res. 2009, 24, 58–61. [Google Scholar]
- Hu, H.; Li, M.; Li, L.; Tao, X. Improving bubble-particle attachment during the flotation of low rank coal by surface modification. Int. J. Min. Sci. Technol. 2020, 30, 217–223. [Google Scholar] [CrossRef]
- Zhang, S. Study on Collision and Attachment Behavior Between Particle and Bubble in Coal Slime Flotation; China University of Mining & Technology: Beijing, China, 2015. [Google Scholar]
- Yang, J. Visualized Study on the Interaction between Single Bubbles and Curved Solid Surface in Flotation Separation Process; Chang'an University: Xi’an, China, 2014. [Google Scholar]
- Ma, L. Study on Minerals Particles Containing Calcium and Bubble Interaction; Cental South University: Changsha, China, 2011. [Google Scholar]
- Nazari, S.; Shafaei, S.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Fan, M. Effects of nanobubble and hydrodynamic parameters on coarse quartz flotation. Int. J. Min. Sci. Technol. 2019, 29, 289–295. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Ren, L.; Zhang, Y.; Bao, S. Influence of Microbubble on Fine Wolframite Flotation. Minerals 2021, 11, 1079. https://doi.org/10.3390/min11101079
Wei P, Ren L, Zhang Y, Bao S. Influence of Microbubble on Fine Wolframite Flotation. Minerals. 2021; 11(10):1079. https://doi.org/10.3390/min11101079
Chicago/Turabian StyleWei, Penggang, Liuyi Ren, Yimin Zhang, and Shenxu Bao. 2021. "Influence of Microbubble on Fine Wolframite Flotation" Minerals 11, no. 10: 1079. https://doi.org/10.3390/min11101079
APA StyleWei, P., Ren, L., Zhang, Y., & Bao, S. (2021). Influence of Microbubble on Fine Wolframite Flotation. Minerals, 11(10), 1079. https://doi.org/10.3390/min11101079







