LIBS as a Spectral Sensor for Monitoring Metallic Molten Phase in Metallurgical Applications—A Review
Abstract
:1. Introduction
1.1. Need for Process Monitoring and Sensing
1.2. Measurement Technologies for Melting Processes
- High-temperature conditions: Measurement components and equipment are affected by ambient temperatures near the molten process. Specifically, optical-based devices suffer from alignment losses when exposed to temperature changes. In this context, high strength (“heavy duty”) and isolated devices are needed. Nevertheless, ambient temperatures, corrosive atmospheres, and dust produce accelerated deterioration and failure of these devices.
- Distance: Equipment and molten sample need to be adequately separated to ensure equipment safety. Nevertheless, there is a limited number of techniques that can ensure precise measurements without endangering the equipment. Relatively long distances produce sensitivity loss and influence detection limits. On the other hand, the proximity of the sensors increases the detrimental effect of the heat. Therefore, the housing of the equipment must be specifically designed to ensure the safety of the optical elements.
- Spectral background: The spectral background in molten conditions follows Planck’s law, describing the black body emission of light. This issue requires special attention in data treatment for avoiding spectral interferences of the continuous emission of molten samples. Additionally, the fluctuation caused due to the temperature, must be considered for better sensitivity and detection limits.
- Slag phase interference: Estimation of chemical composition in molten phases implies the spectral characterization of the interaction surface. The surface chemical composition is different from that of the complete sample. Thus, spectral techniques are limited to the acquisition of chemical composition in the lighter phase, located in the upper part of a molten bath, e.g., slag measurement. This phase impedes the analysis of the phases located below the slag. Overcoming this drawback is possible by using immersion and non-immersion probes, depending on whether the investigation is carried out on the surface or inside the molten material. Nevertheless, immersion probes suffer from rapid deterioration, and short-term maintenance is needed. In this sense, it will require major effort to expand the limited capacity of optical sensors to obtain the so-called deep profile of the sample.
- Data processing: Chemical reactions in the molten phase are rapid. High-frequency sampling is, therefore, required for following all chemical changes. This implies the need for fast data acquisition and processing.
1.3. Why do We Need a Review?
2. Laser-Induced Breakdown Spectroscopy (LIBS)
- i.
- Multi-elemental analysis can be performed from metals to non-metals.
- ii.
- They require minimum or no-sample preparation.
- iii.
- The sample can be measured regardless of whether it is solid, liquid, or gaseous.
- iv.
- They can be applied to any material irrespective of its conducting or non-conducting properties.
- v.
- Fast measurement capability.
- vi.
- Single-shot analysis ability.
- vii.
- Since they are laser-based techniques, visual line of sight is sufficient, enabling the employment of LIBS in harsh environments, where human intervention or reach is difficult.
2.1. Optimization of LIBS
- Detector choice: It is mandatory to use detectors (detectors such as the linear array gated CCD (charge-coupled device), ICCD (intensified CCD) that produce time-resolved plasma measurements. Temporal parameters of detectors such as delay time, integration time, and accumulation of multiple laser shots should ensure that emission lines result distinguishable from background noise, i.e., a reasonable signal-to-noise ratio is obtained.
- Spectral region: At higher temperatures, thermal radiation from molten samples begins to interfere with the visible regions of LIBS. Apparently, wavelengths over 500 nm contribute to the background signal [26]. The efficient collection and transmission of the radiation emanating from the plasma generated on the melt surface is an important aspect to consider for achieving high sensitivity [27]. A solution to this problem is to increase the light amplification at shorter wavelengths in the UV regions [28].
- Line selection: The selection of specific spectral lines is an important factor since interference from adjacent, closely overlapping spectral lines of other elements can hinder calibration results. Thus, the use of high-resolution spectrometers is desirable [28]. The involvement of self-absorption lines also makes the calibration nonlinear. Therefore, the upper limit of linearity before self-absorption should be carefully quantified to be within the required concentration range, which improves limits of detection (LOD) [29]. For these reasons, the selection of strong spectral intensity lines for calibration sometimes leads to saturation effects and non-linear spectral behavior.
- Averaging/accumulation of spectra: Changes in molten surface composition and plasma variations induce intensity fluctuations of LIBS signals. These fluctuations are usually addressed by averaging or accumulating LIBS signals, which enhances the signal-to-noise ratio along with improvement in repeatability and reliability. However, there are some instances where averaging may not be suitable. One of these cases is the analysis of liquid steel with slag under stirring, where the spectral differentiation of both phases is important. Gruber et al. suggested acquiring multiple single-shot spectra and excluding the slag-related spectra by monitoring Ca [30]. Therefore, the selection of spectra needs to be addressed on a case-by-case basis.
- Surrounding atmosphere and matrix effect: When stoichiometric ablation and detection is disturbed by physical and chemical properties of the sample, the resulting spectrum is considered to be under influence of matrix effect. The composition of plasma is dependent not only on the stoichiometry of the sample but as well as thermal, optical properties of the sample, surface of the sample and laser parameters. The atmosphere surrounding the molten sample can influence the chemical composition of the surface. In the case of molten aluminum alloy, oxidation can occur in the presence of air. Consequently, the selection of argon is recommended for avoiding oxidation and increasing the emission line-to-background ratio [31]. The argon purging also reduces the involvement of ambient atmosphere emission lines in the LIBS plasma avoiding matrix effects related to it. Moreover, this selection ensures the repeatability of measurements with better figures of merit [29]. In some reports, argon was also used for displacing slag-related components on the surface.
- Spectral normalization: Spectral normalization can reduce the uncertainties related to plasma fluctuations. One of the normalization methods is the so-called ratio method, in which the ratio between intensities of the analytical spectral lines (IEl) and the internal standard reference lines (IRef) is calculated, which compensates for the changes in plasma conditions, such as plasma temperature [32]. The internal standard reference lines are matrix-related lines that are considered to be constant throughout the measurement process. However, one should be cautious in selecting proper reference lines. If the upper energy levels of spectral and reference lines of elements are close, then the line intensity ratio (IEl/IRef) becomes temperature independent. The use of expanded spectral region detectors, such as echelle spectrograph, is advantageous in such scenarios because more lines may be available for the selection of reference lines. This method has been adopted by most of the researchers in the field of LIBS of metals in the molten phase [28,30,31,33].
- Laser Fluence: Depending on the type of measurement and analysis, the laser fluence must be optimized taking into account the different phases of the metals. At higher temperatures, the interaction of the laser with the sample produces higher ablation rates and leads to higher intensities that could saturate the detector. Hence, the use of optimized laser energy for solid slag at high temperatures is necessary to obtain relevant spectra for analysis [34].
- Optical arrangements: The laser focusing on the sample surface is crucial in a LIBS setup. If the system comprises an open-path arrangement, the use of longer focal length lenses is advantageous for focusing. By increasing the Rayleigh length, the influence of fluctuations of the focal distance is reduced [35]. Sometimes, aerosol condensation spreads in the surroundings of the molten sample, which causes pre-breakdown of plasmas; thus, a careful focus on the surface sample is highly desired [36].
- Calibration: The liquid metal concentration must be verified with experiments established in the solid phase, before and after melting. Ideally, all measurements should be carried out under the same conditions. However, in practice, system parameters may occasionally vary, resulting in different plasma conditions and rendering calibration curves invalid.
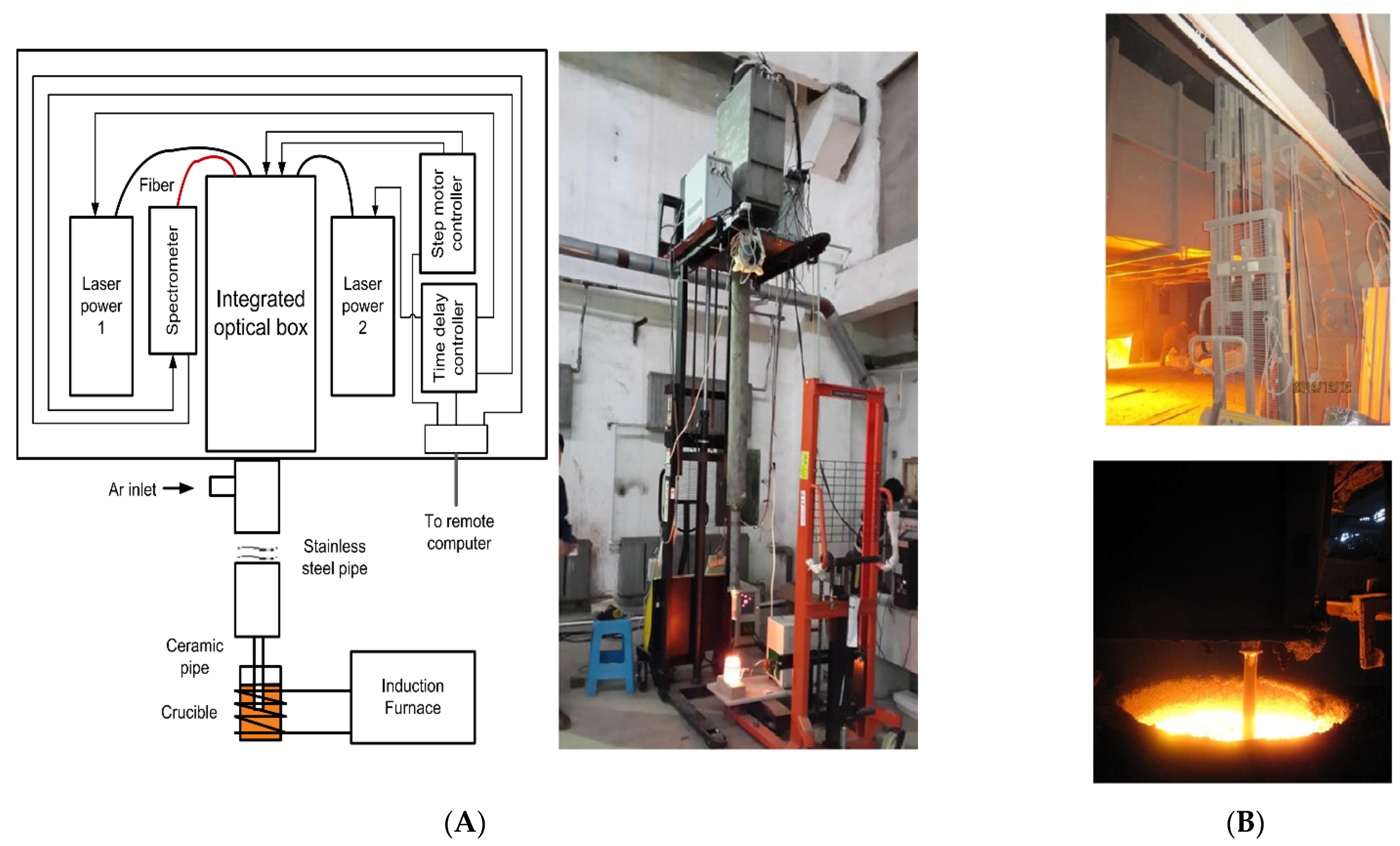
2.2. Developments in LIBS Sensors for Monitoring Molten Phase
2.2.1. Steel and Iron-Based Melt
2.2.2. Aluminum
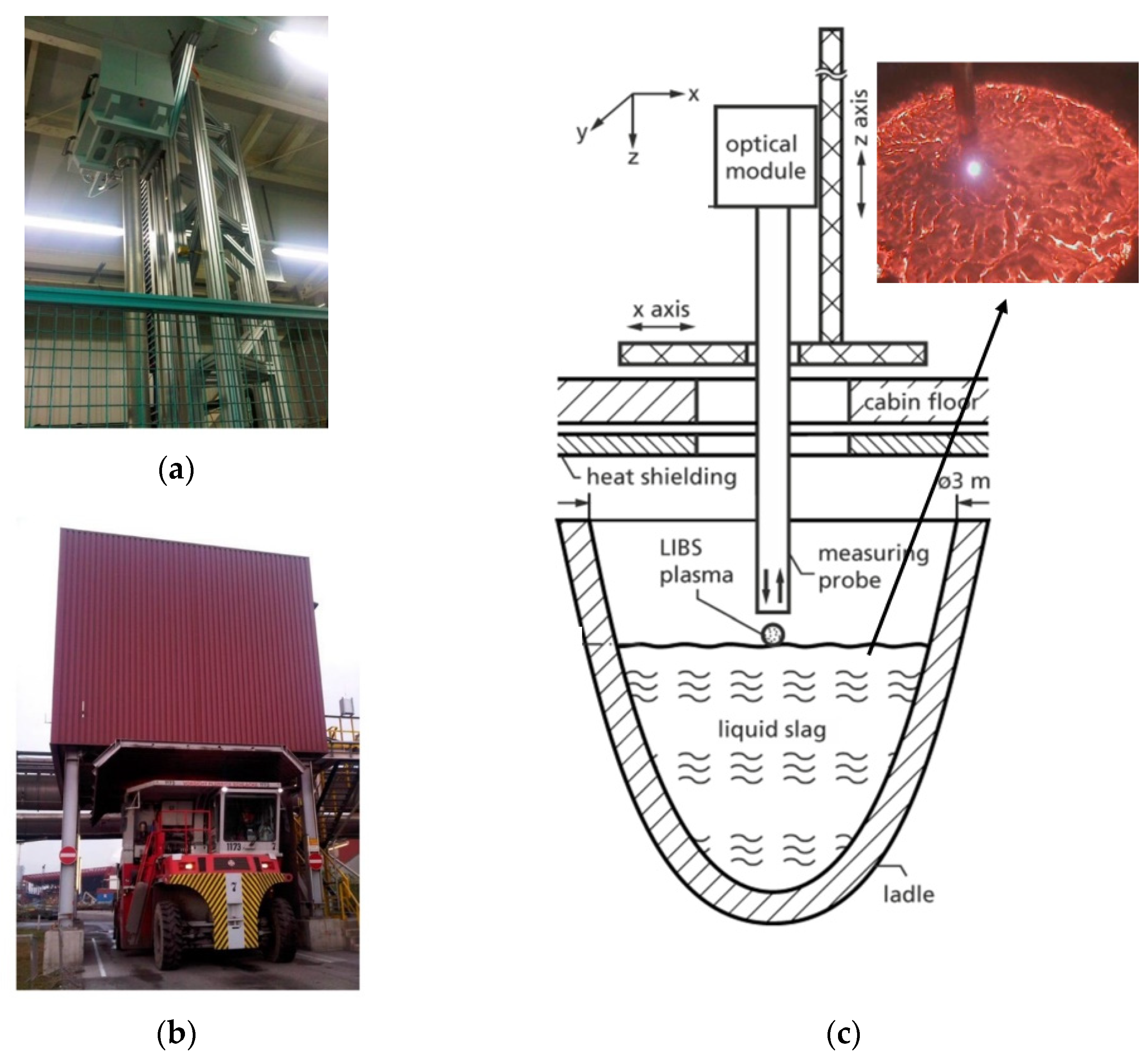
2.2.3. Slags
2.2.4. Other Metals and Minerals
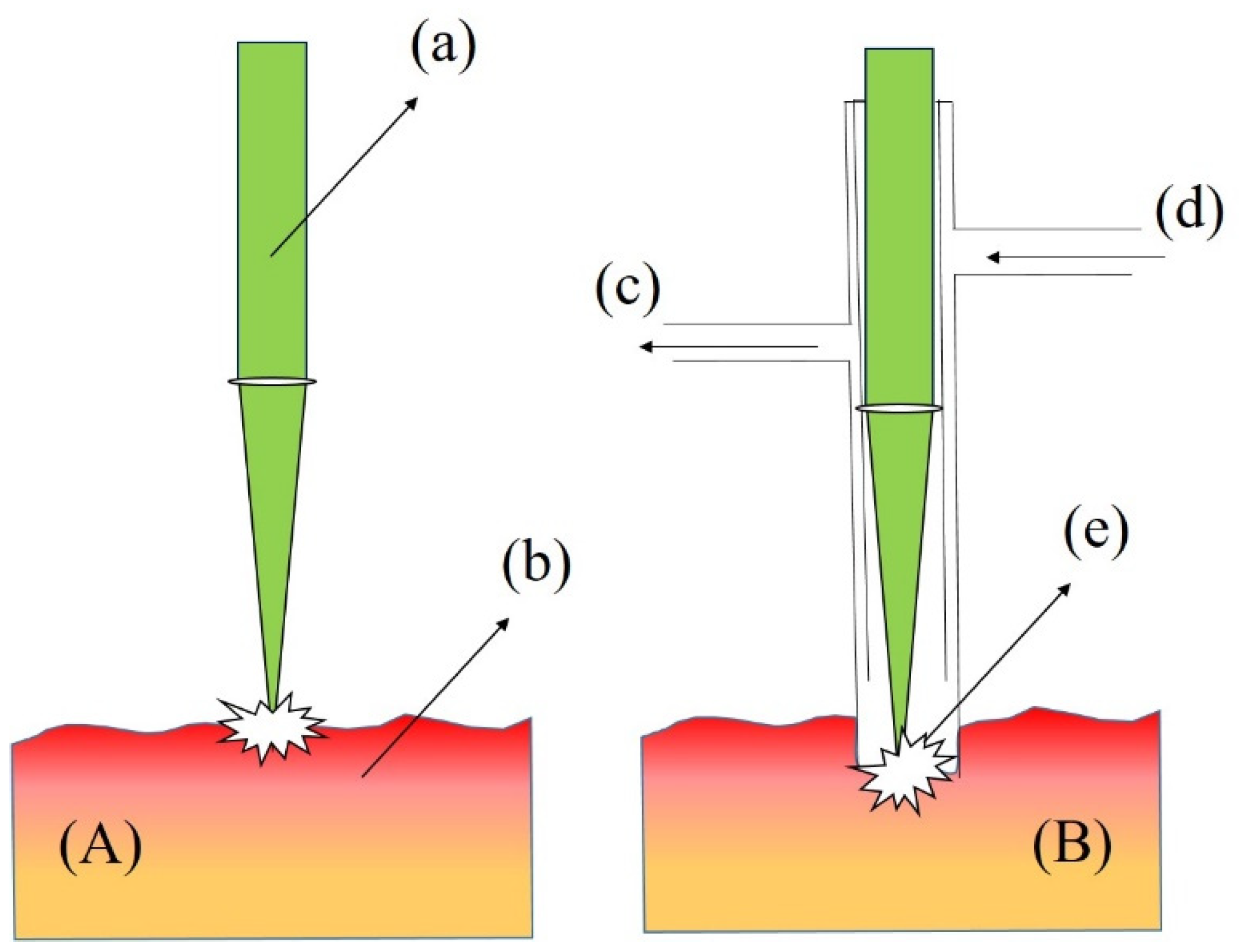
3. Probes and Systems for Molten Metal Monitoring Utilizing LIBS
- The inner faces of the probe walls should not absorb the laser or plasma light while delivering and returning signals to the probe. Losses should be minimized when sending signals to the spectrometer. In addition, the lens optics inside the probe should be protected from heat.
- Immersive probes must be submerged into molten material vertically, which increases the pressure. This effect should be compensated by the probe.
- Molten metals, especially aluminum, absorb oxygen from the ambient and form oxide layers on their surface. Hence, it is important to purge with the noble gases along the probe to obtain clean spectroscopic measurements.
- Temperatures involved in pyrometallurgical processes of metals are very high. Steel is treated at 1500 °C [26], aluminum at 660 °C [52], copper at 1250 °C [76,85] and silicon at 1410 °C [73]. These metals undergo different pyrometallurgical processes at different temperatures. The developed probe should withstand such temperatures and extremely harsh environments.
- Autofocusing is a crucial factor to compensate the effect of the fluctuating surface or the turbulence inside the melt. Technologies such as ultrasonic laser ranging can be utilized for measuring the distance and compensate any deviation from a target value.
- Probe orientation influences the position of the laser focus and pressure values inside the probe. If the argon purge is utilized in the wrong orientation, the pressure will change and molten material will be likely to enter inside the probe, causing damage. The spectral information obtained by the interrogation of laser pulses on the surface of molten metal pool sometimes is not representative of the melt. Materials such as silicon and aluminum often undergo nitridation or oxidation due to the development of slag surfaces. Because of the accumulation of several impurities, slag does not possess a similar chemical composition as the metal [87]. The use of immersive probes for inspecting the composition of the molten sample is an alternative and has been mostly adopted during the past three decades.
4. Non-Conventional Calibration Model Schemes for Molten Metal Analysis
5. Recommendations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Themelis, N.J. Pyrometallurgy near the end of the 20th century. JOM 1994, 46, 51–57. [Google Scholar] [CrossRef]
- Martinez-Alier, J. Mining conflicts, environmental justice, and valuation. J. Hazard. Mater. 2001, 86, 153–170. [Google Scholar] [CrossRef]
- Arnórsson, Ú.K. Probe for Portable Laser Induced Breakdown Spectroscopy of Molten Aluminum and Cryolite. Bachelor’s Thesis, Reykjavík University, Reykjavík, Iceland, 2014. Available online: http://hdl.handle.net/1946/20479 (accessed on 18 June 2021).
- Vásquez, A.; Pérez, F.; Roa, M.; Sanhueza, I.; Rojas, H.; Parra, V.; Balladares, E.; Parra, R.; Torres, S. A Radiometric Technique for Monitoring the Desulfurization Process of Blister Copper. Sensors 2021, 21, 842. [Google Scholar] [CrossRef]
- Reyes, G.; Diaz, W.; Toro, C.; Balladares, E.; Torres, S.; Parra, R.; Vásquez, A. Copper Oxide Spectral Emission Detection in Chalcopyrite and Copper Concentrate Combustion. Processes 2021, 9, 188. [Google Scholar] [CrossRef]
- Arias, L.; Balladares, E.; Parra, R.; Sbarbaro, D.; Torres, S. Sensors and Process Control in Copper Smelters: A Review of Current Systems and Some Opportunities. Minerals 2021, 11, 1. [Google Scholar] [CrossRef]
- Yañez, J.; Torres, S.; Sbarbaro, D.; Parra, R.; Saavedra, C. Analytical instrumentation for copper pyrometallurgy: Challenges and opportunities. IFAC-PapersOnLine 2018, 51, 251–256. [Google Scholar] [CrossRef]
- Luarte, D.; Myakalwar, A.K.; Velásquez, M.; Álvarez, J.; Sandoval, C.; Fuentes, R.; Yañez, J.; Sbarbaro, D. Combining prior knowledge with input selection algorithms for quantitative analysis using neural networks in laser induced breakdown spectroscopy. Anal. Methods 2021, 13, 1181–1190. [Google Scholar] [CrossRef]
- Fergus, J.W. Sensors for monitoring the quality of molten aluminum during casting. J. Mater. Eng. Perform. 2005, 14, 267–275. [Google Scholar] [CrossRef]
- Dong, F.-Z.; Chen, X.-L.; Wang, Q.; Sun, L.-X.; Yu, H.-B.; Liang, Y.-X.; Wang, J.-G.; Ni, Z.-B.; Du, Z.-H.; Ma, Y.-W. Recent progress on the application of LIBS for metallurgical online analysis in China. Front. Phys. 2012, 7, 679–689. [Google Scholar] [CrossRef]
- Rai, A.K.; Yueh, F.Y.; Singh, J.P.; Rai, D.K. Chapter 11-Laser-Induced Breakdown Spectroscopy of Solid and Molten Material. In Laser-Induced Breakdown Spectroscopy; Singh, J.P., Thakur, S.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 255–285. [Google Scholar]
- Senk, D.; Wiens, O.; Mavrommatis, K.; Sancho, L.; Rivas, O.; Androutsopoulos, N.; Galvao, C.; Laserna, J.; Papamantellos, D.; Palangas, C. In-situ, quick sensing system for measurements of critical components in steelmaking slags (Inquisss). EUR 2007, 22818, 1–157. [Google Scholar]
- Noll, R.; Bette, H.; Brysch, A.; Kraushaar, M.; Mönch, I.; Peter, L.; Sturm, V. Laser-induced breakdown spectrometry—applications for production control and quality assurance in the steel industry. Spectrochim. Acta B 2001, 56, 637–649. [Google Scholar] [CrossRef]
- Bengtson, A. Laser Induced Breakdown Spectroscopy compared with conventional plasma optical emission techniques for the analysis of metals–A review of applications and analytical performance. Spectrochim. Acta B 2017, 134, 123–132. [Google Scholar] [CrossRef]
- Wang, Z.; Deguchi, Y.; Shiou, F.; Yan, J.; Liu, J. Application of laser-induced breakdown spectroscopy to real-time elemental monitoring of iron and steel making processes. ISIJ Int. 2016, 56, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Hudson, S.W.; Craparo, J.; De Saro, R.; Apelian, D. Applications of laser-induced breakdown spectroscopy (LIBS) in molten metal processing. Metall. Mater. Trans. B 2017, 48, 2731–2742. [Google Scholar] [CrossRef]
- Lee, Y.I.; Song, K.; Sneddon, J. Laser-Induced Breakdown Spectrometry; Nova Science Publishers: Hapag, NY, USA, 2000. [Google Scholar]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Singh, J.P.; Thakur, S.N. Laser-Induced Breakdown Spectroscopy; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Miziolek, A.W.; Palleschi, V.; Schechter, I. Laser Induced Breakdown Spectroscopy; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Noll, R. Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Musazzi, S.; Perini, U. Laser-Induced Breakdown Spectroscopy: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Álvarez, J.; Velásquez, M.; Myakalwar, A.K.; Sandoval, C.; Fuentes, R.; Castillo, R.; Sbarbaro, D.; Yáñez, J. Determination of copper-based mineral species by laser induced breakdown spectroscopy and chemometric methods. J. Anal. At. Spectrom. 2019, 34, 2459–2468. [Google Scholar] [CrossRef]
- Anubham, S.; Junjuri, R.; Myakalwar, A.; Gundawar, M. An Approach to Reduce the Sample Consumption for LIBS based Identification of Explosive Materials. Def. Sci. J. 2017, 67, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Sreedhar, S.; Kumar, M.A.; Kumar, G.M.; Kiran, P.P.; Tewari, S. Laser-induced breakdown spectroscopy of RDX and HMX with nanosecond, picosecond, and femtosecond pulses. In Proceedings of the Proceedings SPIE 7665, Chemical, Biological, Radiological, Nuclear and Explosives (CBRNE), Orlando, FL, USA, 5 May 2010; p. 76650T. [Google Scholar]
- Monfort, G.; Bellavia, L.; Tonteling, M.; Ojeda, C.; Ansseau, O. On-line measurement of the hot metal temperature and composition in the blast furnace runners by LIBS. J. Appl. Laser Spectrosc. 2014, 1, 6. [Google Scholar]
- Peter, L.; Sturm, V.; Noll, R. Liquid steel analysis with laser-induced breakdown spectrometry in the vacuum ultraviolet. Appl. Opt. 2003, 42, 6199–6204. [Google Scholar] [CrossRef] [Green Version]
- Gruber, J.; Heitz, J.; Strasser, H.; Bäuerle, D.; Ramaseder, N. Rapid in-situ analysis of liquid steel by laser-induced breakdown spectroscopy. Spectrochim. Acta B 2001, 56, 685–693. [Google Scholar] [CrossRef]
- Paksy, L.; Német, B.; Lengyel, A.; Kozma, L.; Czekkel, J. Production control of metal alloys by laser spectroscopy of the molten metals. Part I. Preliminary investigations. Spectrochim. Acta B 1996, 51, 279–290. [Google Scholar] [CrossRef]
- Gruber, J.; Heitz, J.; Arnold, N.; Bäuerle, D.; Ramaseder, N.; Meyer, W.; Hochörtler, J.; Koch, F. In situ analysis of metal melts in metallurgic vacuum devices by laser-induced breakdown spectroscopy. Appl. Spectrosc. 2004, 58, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hubmer, G.; Kitzberger, R.; Mörwald, K. Application of LIBS to the in-line process control of liquid high-alloy steel under pressure. Anal. Bioanal. Chem. 2006, 385, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, C.J.; Carlhoff, C.; Hahn, U.; Jogwich, M. Applications of laser-induced emission spectral analysis for industrial process and quality control. J. Anal. At. Spectrom. 1992, 7, 1029–1035. [Google Scholar] [CrossRef]
- Guezenoc, J.; Gallet-Budynek, A.; Bousquet, B. Critical review and advices on spectral-based normalization methods for LIBS quantitative analysis. Spectrochim. Acta B 2019, 160, 105688. [Google Scholar] [CrossRef]
- Petersson, J.; Gilbert-Gatty, M.; Bengtson, A. Rapid chemical analysis of steel slag by laser-induced breakdown spectroscopy for near-the-line applications. J. Anal. At. Spectrom. 2020, 35, 1848–1858. [Google Scholar] [CrossRef]
- François, E.; Gazeli, O.; Couris, S.; Angelopoulos, G.N.; Blanpain, B.; Malfliet, A. Laser-induced breakdown spectroscopy analysis of the free surface of liquid secondary copper slag. Spectrochim. Acta B 2020, 170, 105921. [Google Scholar] [CrossRef]
- Panne, U.; Neuhauser, R.; Haisch, C.; Fink, H.; Niessner, R. Remote analysis of a mineral melt by laser-induced plasma spectroscopy. Appl. Spectrosc. 2002, 56, 375–380. [Google Scholar] [CrossRef]
- Kondo, H. Comparison between the characteristics of the plasmas generated by laser on solid and molten steels. Spectrochim. Acta B 2012, 73, 20–25. [Google Scholar] [CrossRef]
- Palanco, S.; Conesa, S.; Laserna, J.J. Analytical control of liquid steel in an induction melting furnace using a remote laser induced plasma spectrometer. J. Anal. At. Spectrom. 2004, 19, 462–467. [Google Scholar] [CrossRef]
- Sneddon, J.; Thiem, T.L.; Lee, Y.I. Lasers in Analytical Atomic Spectroscopy; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Gudmundsson, S.H.; Matthiasson, J.; Björnsson, B.M.; Gudmundsson, H.; Leosson, K. Quantitative in-situ analysis of impurity elements in primary aluminum processing using laser-induced breakdown spectroscopy. Spectrochim. Acta B 2019, 158, 105646. [Google Scholar] [CrossRef]
- Sun, L.; Yu, H.; Cong, Z.; Xin, Y.; Li, Y.; Qi, L. In situ analysis of steel melt by double-pulse laser-induced breakdown spectroscopy with a Cassegrain telescope. Spectrochim. Acta B 2015, 112, 40–48. [Google Scholar] [CrossRef]
- Runge, E.F.; Bonfiglio, S.; Bryan, F.R. Spectrochemical analysis of molten metal using a pulsed laser source. Spectrochim. Acta 1966, 22, 1678–1680. [Google Scholar] [CrossRef]
- Ozaki, T.; Takahashi, T.; Iwai, Y.; Gunji, K.; Sudo, E. Giant pulse laser direct spectrochemical analysis of C, Si, and Mn in liquid iron. Trans. Iron Steel Inst. Jpn. 1984, 24, 463–470. [Google Scholar] [CrossRef]
- Carlhoff, C. Laserinduzierte Emissionsspektroskopie für die Direktanalyse von flüssigem Stahl im Konverter. Laser Optoelectron. 1991, 23, 50–52. [Google Scholar]
- Aragon, C.; Aguilera, J.; Campos, J. Determination of carbon content in molten steel using laser-induced breakdown spectroscopy. Appl. Spectrosc. 1993, 47, 606–608. [Google Scholar] [CrossRef]
- Palanco, S.; Cabalin, L.; Romero, D.; Laserna, J. Infrared laser ablation and atomic emission spectrometry of stainless steel at high temperatures. J. Anal. At. Spectrom. 1999, 14, 1883–1887. [Google Scholar] [CrossRef]
- Ramaseder, N.; Gruber, J.; Heitz, J.; Baeuerle, D.; Meyer, W.; Hochoertler, J. VAI-CON® Chem—A New Continuous Chemical Analysis System of Liquid Steel in Metallurgical Vessels. Metall. Ital. 2004, 60, 2. [Google Scholar]
- Sun, L.X.; Xin, Y.; Cong, Z.B.; Li, Y.; Qi, L.F. Online compositional analysis of molten steel by laser-induced breakdown spectroscopy. In Proceedings of the Advanced Materials Research; Trans Tech Publications: Bach, Switzerland, 2013; Volume 694, pp. 1260–1266. [Google Scholar]
- Xiaomei, L.; Penghui, C.; Gehua, C.; Jingjun, L.; Ruixiang, L.; Hao, Y. Effect of Melting Iron-Based Alloy Temperature on Carbon Content Observed in Laser-Induced Breakdown Spectroscopy. Plasma Sci. Technol. 2015, 17, 933. [Google Scholar]
- Qiang, Z.; Congyuan, P.; Teng, F.; Xiaokang, D.; Shengbo, W.; Qiuping, W. Composition and Temperature Monitoring of Molten Metal by a Combined LIBS-IR Thermometry System. J. Appl. Spectrosc. 2018, 85, 817–822. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Zhong, Q.; Xiao, H.; Nie, S.; Lian, F.; Sun, S.; Fan, Z. Quantitative Analysis of Vacuum Induction Melting by Laser-induced Breakdown Spectroscopy. J. Vis. Exp. 2019, 148, e57903. [Google Scholar] [CrossRef]
- Rai, A.K.; Yueh, F.Y.; Singh, J.P.; Zhang, H. High temperature fiber optic laser-induced breakdown spectroscopy sensor for analysis of molten alloy constituents. Rev. Sci. Instrum. 2002, 73, 3589–3599. [Google Scholar] [CrossRef]
- Rai, A.K.; Yueh, F.-Y.; Singh, J.P. Laser-induced breakdown spectroscopy of molten aluminum alloy. Appl. Opt. 2003, 42, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- De Saro, R.; Weisberg, A. Apparatus and Method for In Situ, Real Time Measurements of Properties of Liquids. U.S. Patent No. 6,784,429, 31 August 2004. [Google Scholar]
- De Saro, R. In-Situ, Real-Time Measurement of Melt Constituents in the Aluminum, Glass, and Steel Industries (No. DOE/CH/10974); Energy Research Company: Plainfield, NJ, USA, 2006. Available online: https://www.osti.gov/servlets/purl/882367 (accessed on 18 June 2021).
- Herbert, J.; Fernandez, J.; De Saro, R.; Craparo, J. The Industrial Application of Molten Metal Analysis (LIBS); Springer: Cham, Switzerland, 2019; pp. 945–952. [Google Scholar]
- Gudmundsson, S.H.; Matthiasson, J.; Leosson, K. Quantification of trace elements in molten aluminum with randomized impurity concentrations using laser-induced breakdown spectroscopy. OSA Continuum 2020, 3, 2544–2552. [Google Scholar] [CrossRef]
- Gudmundsson, S.H.; Matthiasson, J.; Leosson, K. Accurate Real-Time Elemental (LIBS) Analysis of Molten Aluminum and Aluminum Alloys. In Proceedings of the Light Metals; Springer: Cham, Switzerland, 2020; pp. 860–864. [Google Scholar]
- Sun, L.; Yu, H.; Cong, Z.; Lu, H.; Cao, B.; Zeng, P.; Dong, W.; Li, Y. Applications of laser-induced breakdown spectroscopy in the aluminum electrolysis industry. Spectrochim. Acta B 2018, 142, 29–36. [Google Scholar] [CrossRef]
- Lu, H.; Hu, X.; Ma, L.; Li, M.; Cao, B. Measurement of the molecular ratio of Aluminum electrolytes using laser-induced breakdown spectroscopy. Spectrochim. Acta B 2020, 164, 105753. [Google Scholar] [CrossRef]
- Hudson, S.W.; Apelian, D. Clean Aluminum Processing: New Avenues for Measurement and Analysis. In Light Metals 2014; Grandfield, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1025–1029. [Google Scholar]
- Hudson, S.; Craparo, J.; De Saro, R.; Apelian, D. Laser-induced breakdown spectroscopy: A new tool for real time melt cognition. Metall. Ital. 2016, 6, 5–8. [Google Scholar]
- Hudson, S.W.; Apelian, D. Inclusion Detection in Molten Aluminum: Current Art and New Avenues for In Situ Analysis. Int. J. Met. 2016, 10, 289–305. [Google Scholar] [CrossRef]
- Hudson, S.; Craparo, J.; De Saro, R.; Apelian, D. TiB 2 Particle Detection in Liquid Aluminum via Laser Induced Breakdown Spectroscopy. In Light Metals 2016; Springer: Berlin/Heidelberg, Germany, 2016; pp. 809–813. [Google Scholar]
- Kraushaar, M.; Noll, R.; Schmitz, H.-U. Slag analysis with laser-induced breakdown spectrometry. Appl. Spectrosc. 2003, 57, 1282–1287. [Google Scholar] [CrossRef]
- Sturm, V.; Schmitz, H.-U.; Reuter, T.; Fleige, R.; Noll, R. Fast vacuum slag analysis in a steel works by laser-induced breakdown spectroscopy. Spectrochim. Acta B 2008, 63, 1167–1170. [Google Scholar] [CrossRef]
- Sturm, V.; Fleige, R.; de Kanter, M.; Leitner, R.; Pilz, K.; Fischer, D.; Hubmer, G.; Noll, R. Laser-Induced Breakdown Spectroscopy for 24/7 Automatic Liquid Slag Analysis at a Steel Works. Anal. Chem. 2014, 86, 9687–9692. [Google Scholar] [CrossRef]
- López-Moreno, C.; Palanco, S.; Laserna, J.J. Quantitative analysis of samples at high temperature with remote laser-induced breakdown spectrometry using a room-temperature calibration plot. Spectrochim. Acta B 2005, 60, 1034–1039. [Google Scholar] [CrossRef]
- Czekkel, J.; Paksy, L.; Lengyel, A.; Bánhidi, O. Quality control of production of alloys in liquid state by laser spectroscopy. IFAC Proc. Vol. 2000, 33, 407–412. [Google Scholar] [CrossRef]
- Baril, E.; St-Onge, L.; Sabsabi, M.; Lucas, J.; Gagné, M. On-line chemical analysis of CGL baths using Laser-Induced Breakdown Spectroscopy. In Proceedings of the 96th Galvanizers Association Meeting, Charleston, SC, USA, 3–6 October 2004. [Google Scholar]
- Giorgi, M.L.; Guirado, S.; Ruiz, J.; Cabalin, L.M.; Vestin, F.; Laserna, J.; Jacques-Beyssen, S.; Simonnet, M.; Sager, M.; Mougeolle, J.M.; et al. Situ Analysis of Hot Dip Galvanizing Baths (Zincana); European Comission: Luxembourg, 2013; Available online: https://op.europa.eu/s/sm9w (accessed on 15 April 2013).
- Vaculovič, T.; Zvěřina, Z.; Otruba, V.; Kanický, V. Laser-induced breakdown spectroscopy of molten metals: Influence of sample temperature. Z. Nat. A 2011, 66, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Darwiche, S.; Benrabbah, R.; Benmansour, M.; Morvan, D. Impurity detection in solid and molten silicon by laser induced breakdown spectroscopy. Spectrochim. Acta B 2012, 74, 115–118. [Google Scholar] [CrossRef]
- Dutouquet, C.; Gallou, G.; Le Bihan, O.; Sirven, J.B.; Dermigny, A.; Torralba, B.; Frejafon, E. Monitoring of heavy metal particle emission in the exhaust duct of a foundry using LIBS. Talanta 2014, 127, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Sun, L.-X.; Yang, Z.-J.; Zeng, P.; Cong, Z.-B.; Qi, L.-F. In situ analysis of magnesium alloy using a standoff and double-pulse laser-induced breakdown spectroscopy system. Front. Phys. 2016, 11, 115207. [Google Scholar] [CrossRef]
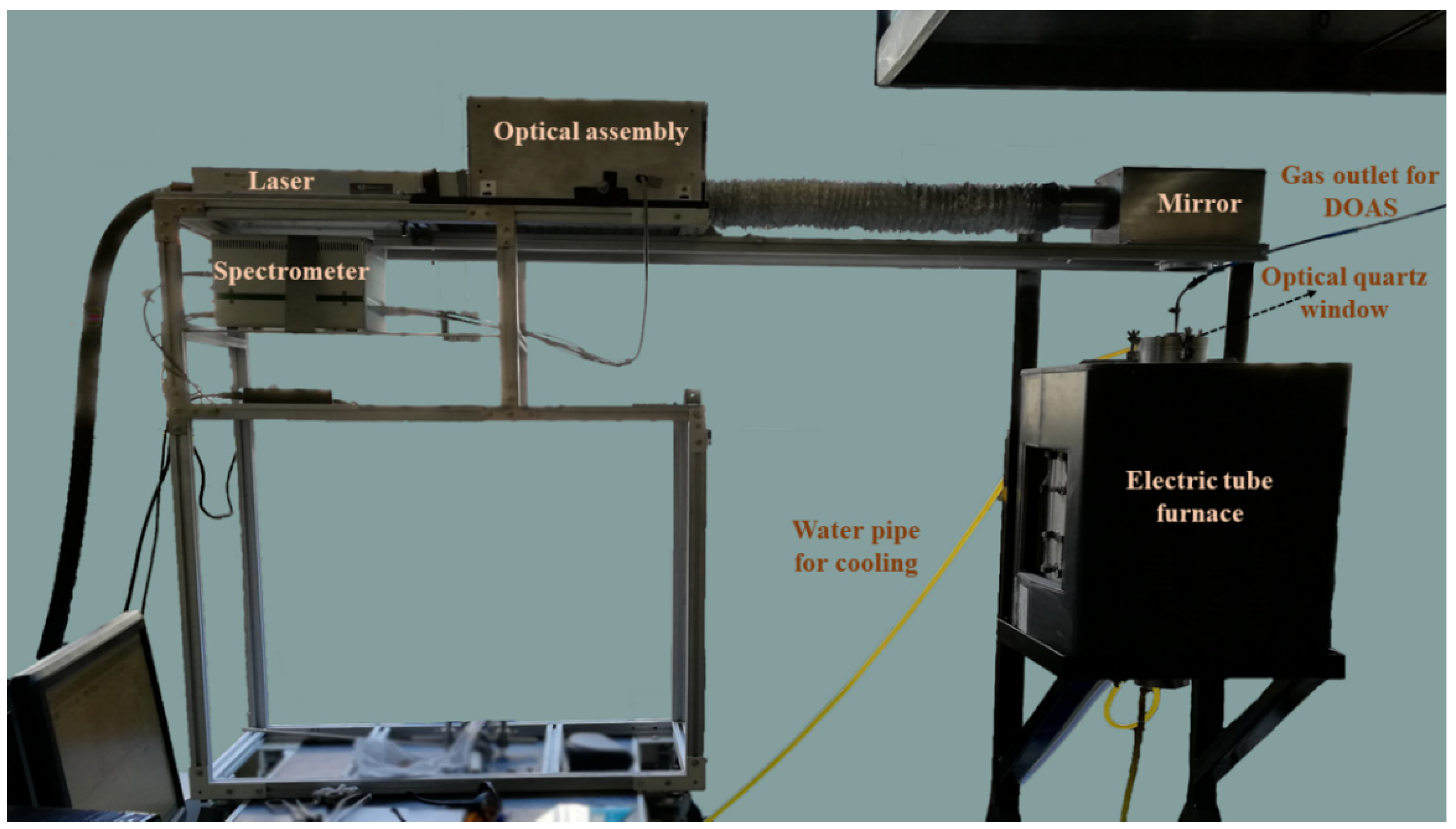
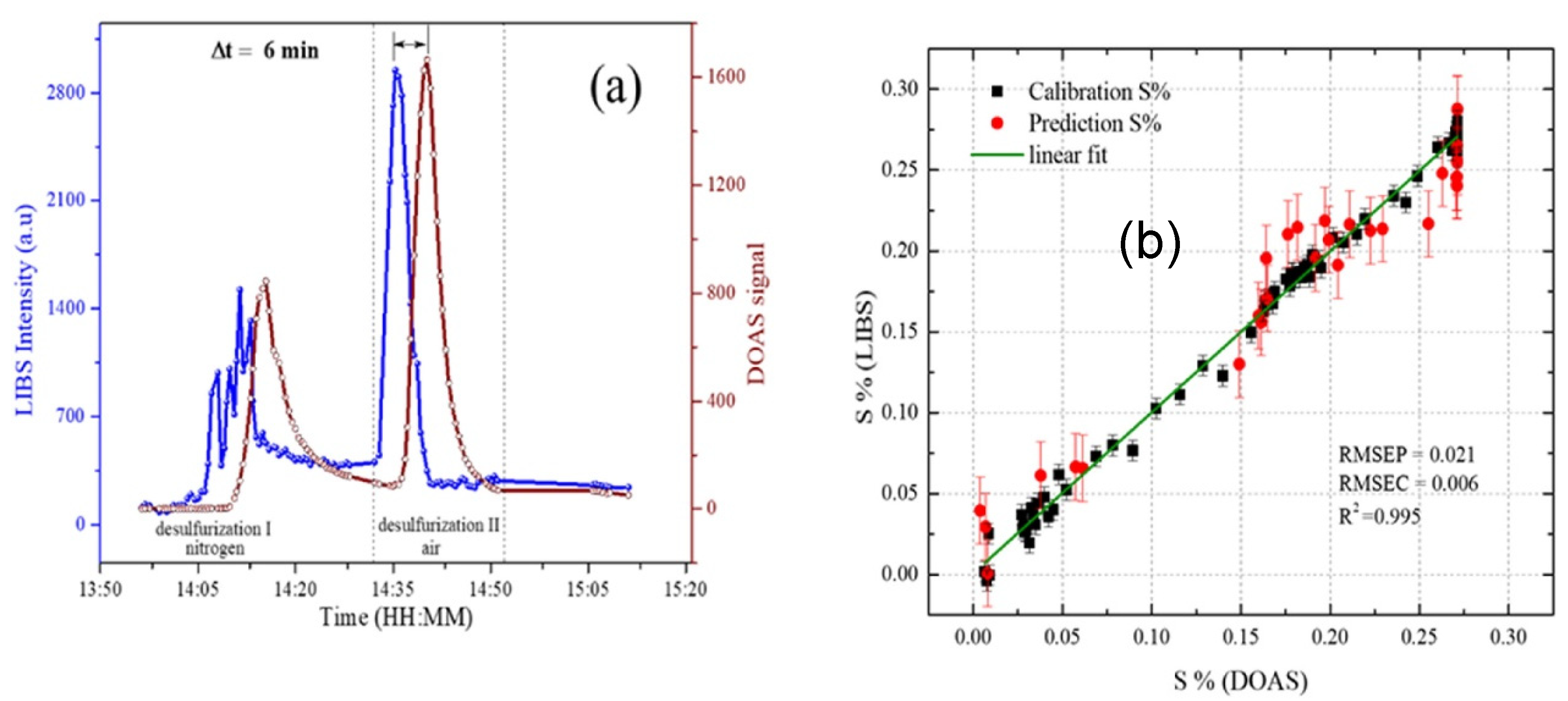
- Myakalwar, A.K.; Sandoval, C.; Sepúlveda, B.; Fuentes, R.; Parra, R.; Balladares, E.; Vásquez, A.; Sbarbaro, D.; Yáñez, J. Laser induced breakdown spectroscopy for monitoring the molten phase desulfurization process of blister copper. Anal. Chim. Acta 2021, 1178, 338805. [Google Scholar] [CrossRef]
- Cremers, D.; Archuleta, F.; Dilworth, H. Rapid Analysis of Steels Using Laser-Based Techniques; Los Alamos National Lab.: Washington, DC, USA; Armco, Inc.: Middletown, OH, USA, 1985. [Google Scholar]
- Carlhoff, C.; Lorenzen, C.-J.; Nick, K.-P. Method and Apparatus for Optically Coupling an Element Analysis System and a Laser to Liquid Metal in a Melting Vessel. U.S. Patent No. 4,995,723, 26 February 1991. [Google Scholar]
- Carlhoff, C.; Lorenzen, C.-J.; Nick, K.-P. Spectrometer for the Simultaneous Measurement of Intensity in Various Spectral Regions. U.S. Patent No. 4,993,834, 19 February 1991. [Google Scholar]
- Kim, Y.W. Transient Spectroscopic Method and Apparatus for in-Process Analysis of Molten Metal. U.S. Patent No. 4,986,658A, 22 January 1991. [Google Scholar]
- Cates, L.E. Pressurized Port for Viewing and Measuring Properties of a Molten Metal Bath. U.S. Patent No. 5,830,407, 3 November 1998. [Google Scholar]
- Cates, L.E.; Reich, P. Submergible Probe for Viewing and Analyzing Properties of a Molten Metal Bath. U.S. Patent No. 6,071,466, 6 June 2000. [Google Scholar]
- Sabsabi, M.; Héon, R.; Lucas, J.M. Method and Apparatus for In-Process Liquid Analysis by Laser Induced Plasma Spectroscopy. U.S. Patent No. 6,700,660, 2 March 2004. [Google Scholar]
- Zhang, H.; Rai, A.K.; Singh, J.P.; Yueh, F.-Y. Fiber Optic Laser-Induced Breakdown Spectroscopy Sensor for Molten Material Analysis. U.S. Patent No. 6,762,835, 13 July 2004. [Google Scholar]
- Lucas, J.M.; Sabsabi, M.; Héon, R. Method and Apparatus for Molten Material Analysis by Laser Induced Breakdown Spectroscopy. U.S. Patent No. 6,909,505B2, 21 June 2005. [Google Scholar]
- Gruber, J.; Dallinger, M. Immersion Probe for Lips Apparatuses. U.S. Patent No. 2,009,026,234,5A1, 22 October 2009. [Google Scholar]
- Benmansour, M.; Benrabbah, R.; Garandet, J.-P.; Morvan, D. Device for Analysing an Oxidisable Molten Metal Using a Libs Technique. U.S. Patent No. 9,933,368B2, 3 April 2018. [Google Scholar]
- Hudson, S.W.; Apelian, D.; DeSaro, R.; Craparo, J.C. Molten Metal Inclusion Testing. U.S. Patent No. 2,019,012,881,1A1, 2 May 2019. [Google Scholar]
- Matiaske, A.-M.; Gornushkin, I.B.; Panne, U. Double-pulse laser-induced breakdown spectroscopy for analysis of molten glass. Anal. Bioanal. Chem. 2012, 402, 2597–2606. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Lu, H.; Xu, J.; Li, H. An LIBS quantitative analysis method for alloy steel at high temperature based on transfer learning. J. Anal. At. Spectrom. 2018, 33, 1184–1195. [Google Scholar] [CrossRef]
- Chang, F.; Lu, H.; Sun, H.; Yang, J. Assessment of the performance of quantitative feature-based transfer learning LIBS analysis of chromium in high temperature alloy steel samples. J. Anal. At. Spectrom. 2020, 35, 2639–2648. [Google Scholar] [CrossRef]

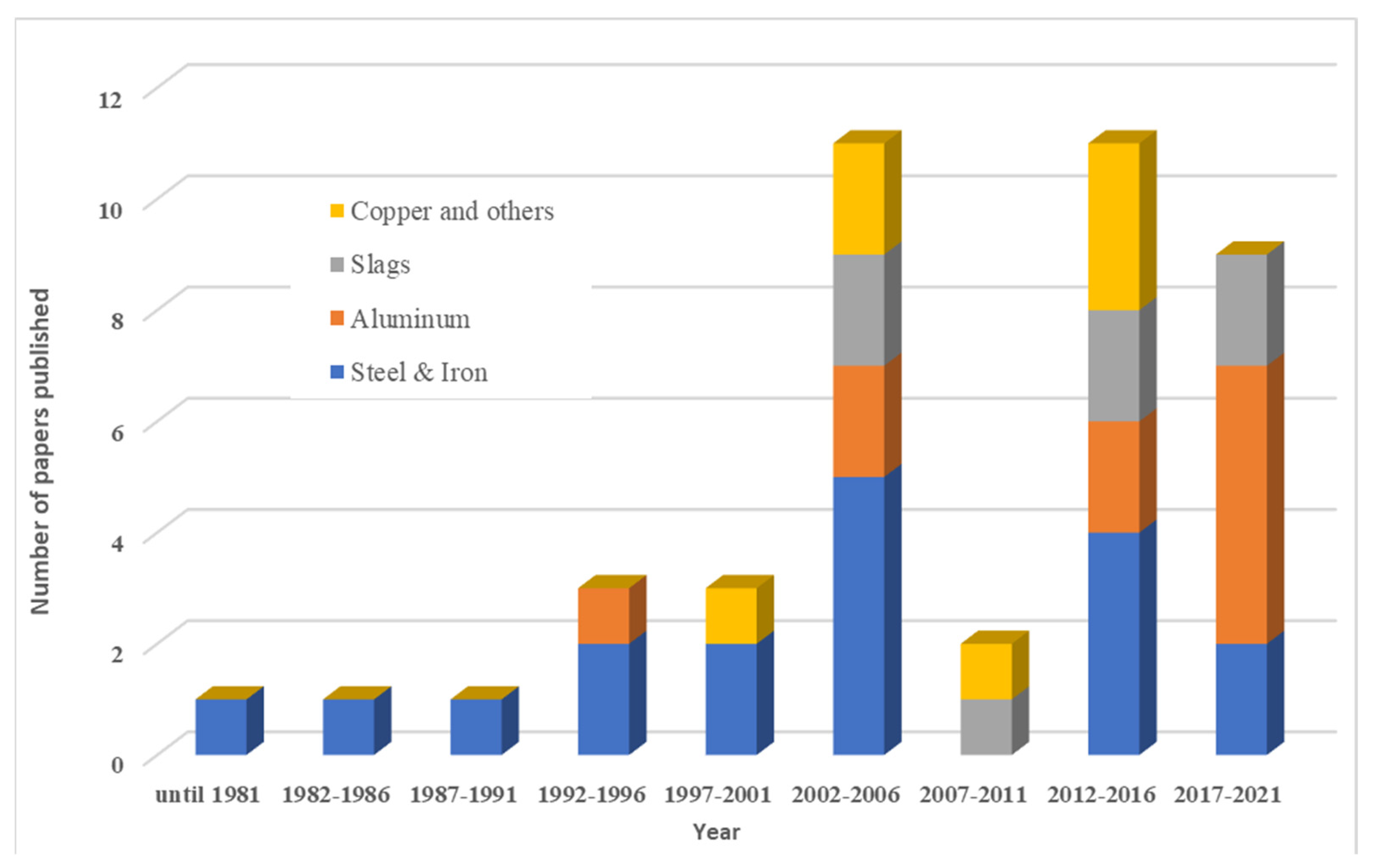
| Categories | Count of Papers |
|---|---|
| Steel and Iron Based melt | 18 |
| Aluminum | 12 |
| Slags | 5 |
| Copper and other metals and minerals | 7 |
| Title | Inventors/Researchers | Company (Patent N°) | System/Probe Details | Notes |
|---|---|---|---|---|
| Method and apparatus for optically coupling an element analysis system and a laser to liquid metal in a melting vessel | Carlhoff et al. [78,79] | Fried. Krupp GmbH (US 4995723, US 4993834) | A technique for investigating elements of hot liquid metal by means of providing a stationary tube at a sidewall of the vessel comprising the molten metal. Laser pulses are passed through the tube and focused on the surface of the melt to create plasma. The light emitted from plasma is directed through an assembly of optical waveguides and lenses to reach the spectrometer. | This method only provides details on the composition of the sample at a fixed position, due to the position of the tube of the vessel being fixed at all times. Maintaining counter pressure is an essential failing that causes the flow of molten metal in the reverse direction. However, this method is advantageous, since it provides access to slag-free liquid of the metal bath. |
| Transient spectroscopic method apparatus for in-process analysis molten metal | Kim et al. [80] | Lehigh University (US4986658) | The system consists of a laser, spectrometer, optics and other supported electronics placed close to the furnace, which can analyze the constituents of molten metal. | This system is complicated and expensive, and in the event of any accident, it would be exposed to temperatures higher than those it can withstand. Such accidents could involve the falling of components, cooling system failures, among others. |
| 1. Pressurized port for viewing and measuring properties of a molten metal bath -(1998) 2. Submergible probe for viewing and analyzing properties of a molten metal bath -(2000) | Cates et al. [81,82] | Kvaerner U.S. Inc. (US5830407), Voest Alpine Industries, Inc., (US6071466) | An immobile concentric tube was inserted into the bottom of a vessel containing molten metal. A pressurized transparent gas was allowed through the center of the pipe, which had an opening. The flow of gas was maintained in such a way that molten melt did not enter the opening. Optical response detectors such as spectrometers were able to assess molten metal through a glass assembly. | Due to the fixed position in the bottom of the metallurgical vessel, the system can determine the chemistry at a fixed location only. |
| Method and apparatus for in-process liquid analysis by laser-induced plasma spectroscopy | Sabsabi et al. [83] | Noranda Inc., Toronto; National Research Council of Canada (USOO67OO660B2) | The apparatus comprises a measurement cell, a pump for maintaining a steady flow of liquid in the cell, a high-power laser, a spectrometer, and a detector assembly. To prevent aerosols and droplets from interfering in sampling, gas is blown above the liquid ensuring that waves and bubbles are removed. | A small discrepancy in the flow of gas and liquid stream changes the position of the liquid surface and alters the laser pulse focus. |
| Fiber-optic laser-induced breakdown spectroscopy sensor for molten material analysis | Singh et al. [84] | Mississippi state university (US6762835) | The invention of a fiber optic LIBS probe can determine a molten material composition online and in real-time. The probe consists of fiber optics and lens arrangements for delivering the laser to the sample and collecting light emitted from the plasma. | The probe can interrogate surface analysis of liquid metal only. |
| Apparatus and method for in situ, real-time measurements of properties of liquids | De Saro et al. [54] | Energy Research Company (US6784429 B2) | The apparatus can measure several properties of liquid metal or any material contained in a vessel on site. The system comprises hollow pipes to supply inert and pressurized gases that cool the system down and protect the assembly while providing an opening for measurement. | In the immersive mode, it is necessary to supply inert gas to stop the melt from entering the probe assembly. |
| Method and apparatus for molten material analysis by laser-induced breakdown spectroscopy | Lucas et al. [85] | National Research Council of Canada (US6909505 B2) | The invention aids LIBS acquisition in the bubbles formed by applying a suitably pressurized gas in the molten metal. | The measurement of the bubbles creates highly fluctuating spectral data where repeatability is a major concern. However, averaging over a summation of signals could be a solution. |
| Immersion probe for LIPS apparatuses | Gruber et al. [86] | GREENBLUM and BERNSTEIN, P.L.C. (US20090262345 A1) | The immersion probe has two lateral openings and one bottom opening. One lateral opening is a rectangular slit used to measure the composition of molten material flowing through it, in the form of the jet at half of the height of the probe tube. The bottom opening maintains pressure and empties the material that flows inside. The last opening is used for inflow of material and measurements at pressurized conditions. | The components are assembled with many individual parts likely to be regularly replaced. Being an opening-dependent device, only molten liquid measurements are possible. If any solid particles were present in the melt they could stop the flow through the openings. |
| Device for analysing an oxidisable molten metal using a libs technique | Benmansour et al. [87] | COMMISSARIAT A L ‘ ENERGIE ATOMIQUE ET AUX ENERGIES (US9933368 B2) | The device is particularly intended for highly oxidizable molten metals such as silicon, zirconium, manganese, aluminum, or titanium. This device comprises hollow tubes for LIBS analysis, inert gas flow, and mechanical stirring paddles. The use of mechanical stirring paddles and inert gas provides a clear surface of the molten pool, limiting oxidization, nitridation, and slag formation. | This device is limited to surface analysis. Mechanical parts may destabilize the position of the molten surface when focusing the laser |
| Molten metal inclusion testing | Hudson et al. [88] | Worcester Polytechnic Institute (US 20190128811A1) | The apparatus consists of spectroscopic arrangements with a probe inserted in the molten metal. The probe has four provisions where two optical fibers deliver laser light and carry plasma emission signals to the spectrometer. Moreover, two gas-purging inlets and outlets are provided. | This apparatus is beneficial for monitoring the cleanliness of metal before casting. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myakalwar, A.K.; Sandoval, C.; Velásquez, M.; Sbarbaro, D.; Sepúlveda, B.; Yáñez, J. LIBS as a Spectral Sensor for Monitoring Metallic Molten Phase in Metallurgical Applications—A Review. Minerals 2021, 11, 1073. https://doi.org/10.3390/min11101073
Myakalwar AK, Sandoval C, Velásquez M, Sbarbaro D, Sepúlveda B, Yáñez J. LIBS as a Spectral Sensor for Monitoring Metallic Molten Phase in Metallurgical Applications—A Review. Minerals. 2021; 11(10):1073. https://doi.org/10.3390/min11101073
Chicago/Turabian StyleMyakalwar, Ashwin Kumar, Claudio Sandoval, Marizú Velásquez, Daniel Sbarbaro, Benjamín Sepúlveda, and Jorge Yáñez. 2021. "LIBS as a Spectral Sensor for Monitoring Metallic Molten Phase in Metallurgical Applications—A Review" Minerals 11, no. 10: 1073. https://doi.org/10.3390/min11101073
APA StyleMyakalwar, A. K., Sandoval, C., Velásquez, M., Sbarbaro, D., Sepúlveda, B., & Yáñez, J. (2021). LIBS as a Spectral Sensor for Monitoring Metallic Molten Phase in Metallurgical Applications—A Review. Minerals, 11(10), 1073. https://doi.org/10.3390/min11101073







