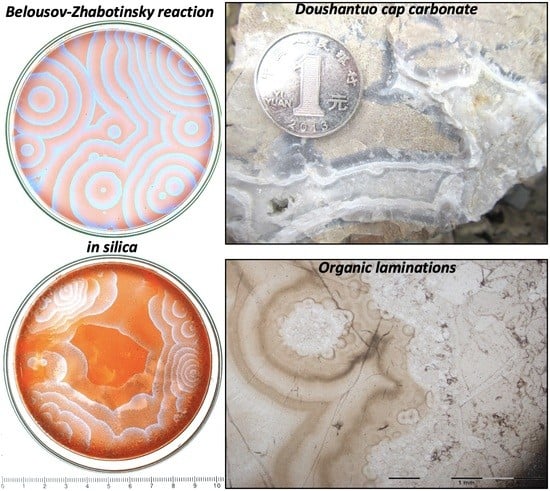
Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids
Abstract
:1. Introduction
2. Geological Context of Studied Material
3. Experimental and Analytical Methods
3.1. Chemically Oscillating Reactions and the Classical BZ Reaction
- 6 mL of Solution A: 15 g NaBrO3 [1M] + H2S4O [0.33 M] premixed solution in H2O
- 1 mL of Solution B: Malonic acid (C3H4O4) [1 M] diluted in H2O
- 0.5 mL of Solution C: NaBr [1 M] diluted in water
- 1 mL of phenanthroline ferrous sulfate solution: Ferroin (C36H24FeN62+) [25 mM]–1 mL
- 1 drop of a non-ionic surfactant: Triton X-100 ((C14H22O(C2H4O)n)
3.2. Chemically Oscillating Reaction Experiments with Other Carboxylic Acids
3.3. Chemically Oscillating Reaction Experiments with Humic Acid
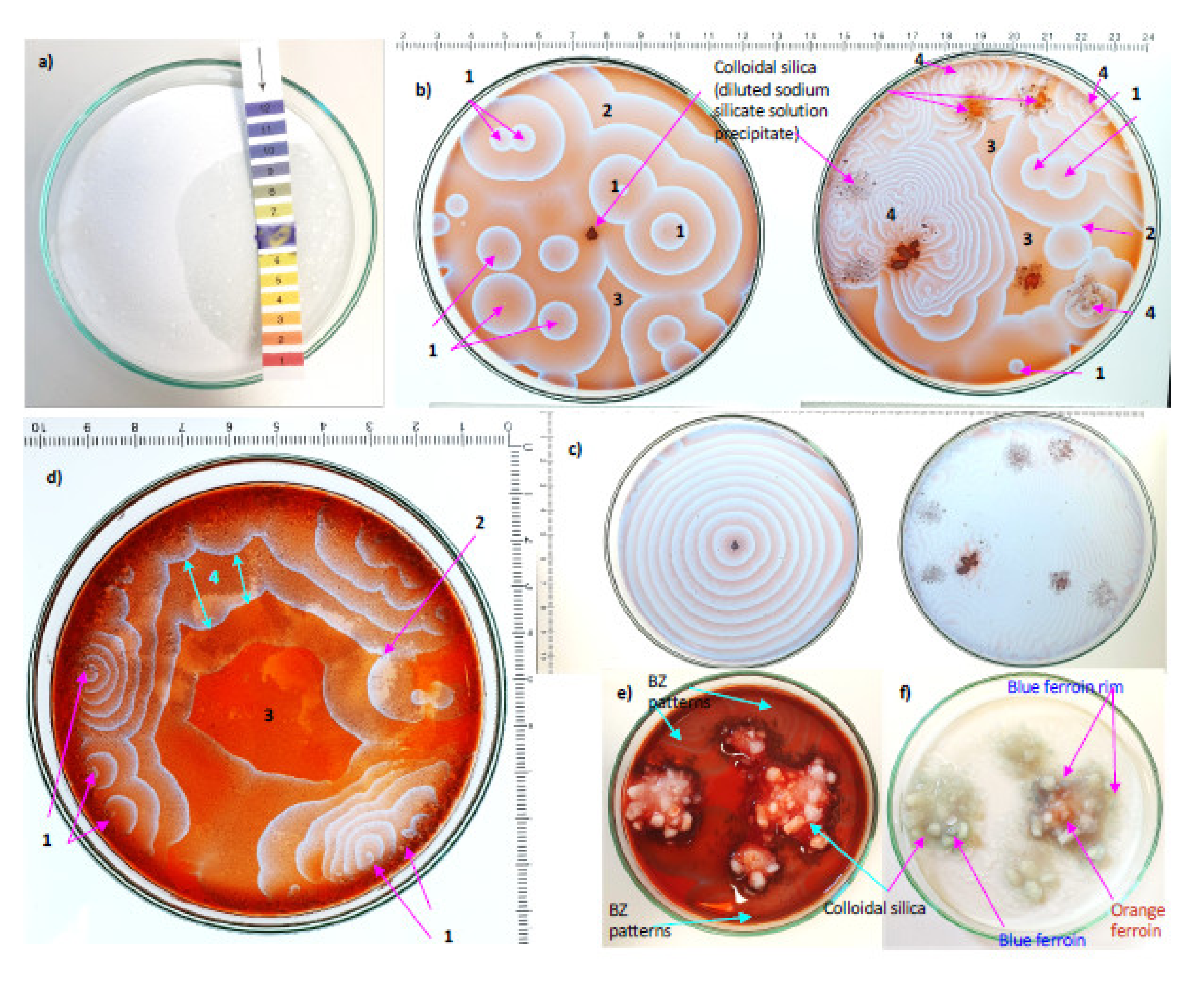
3.4. Chemically Oscillating Reactions in Colloidal Silica
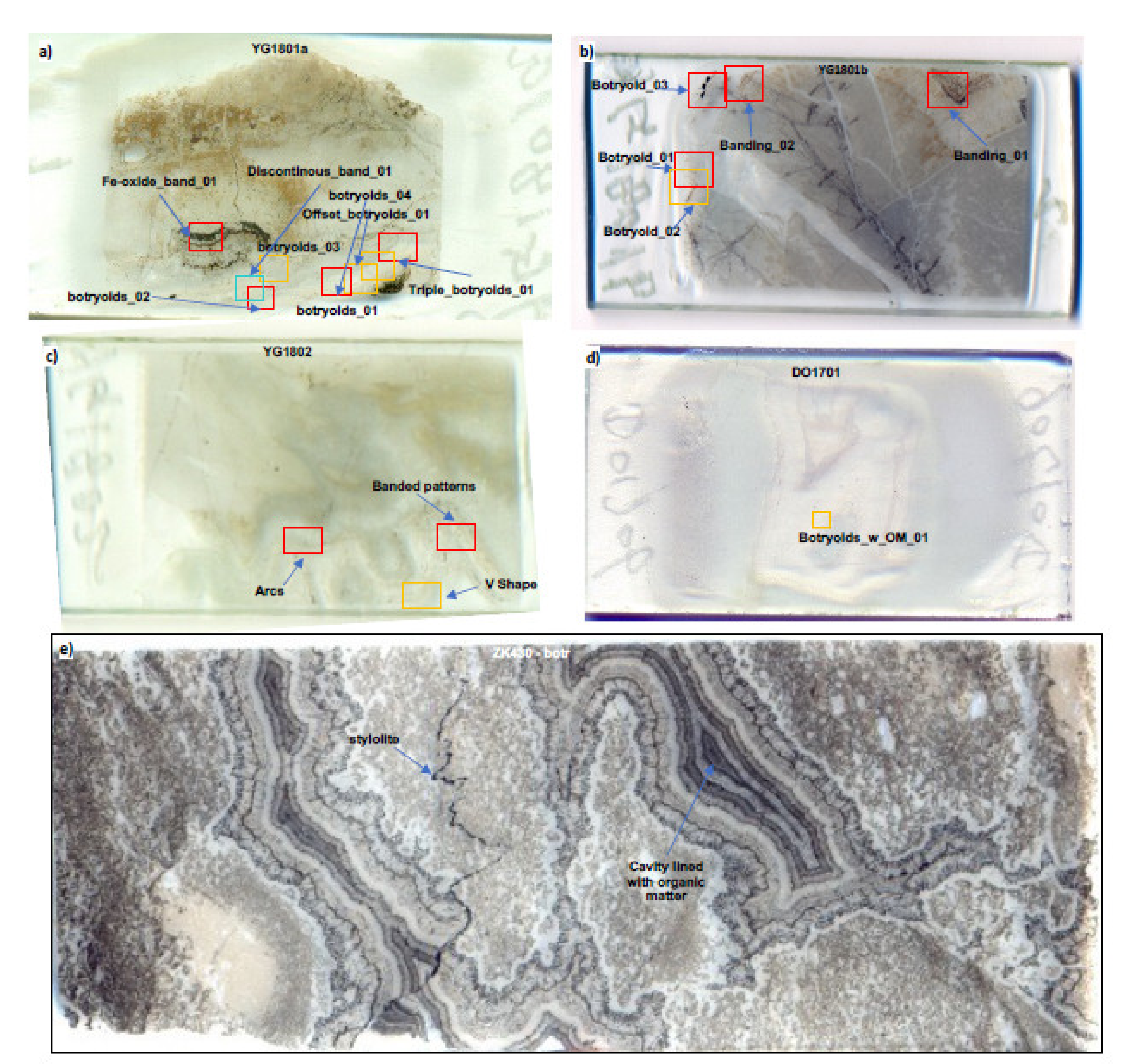
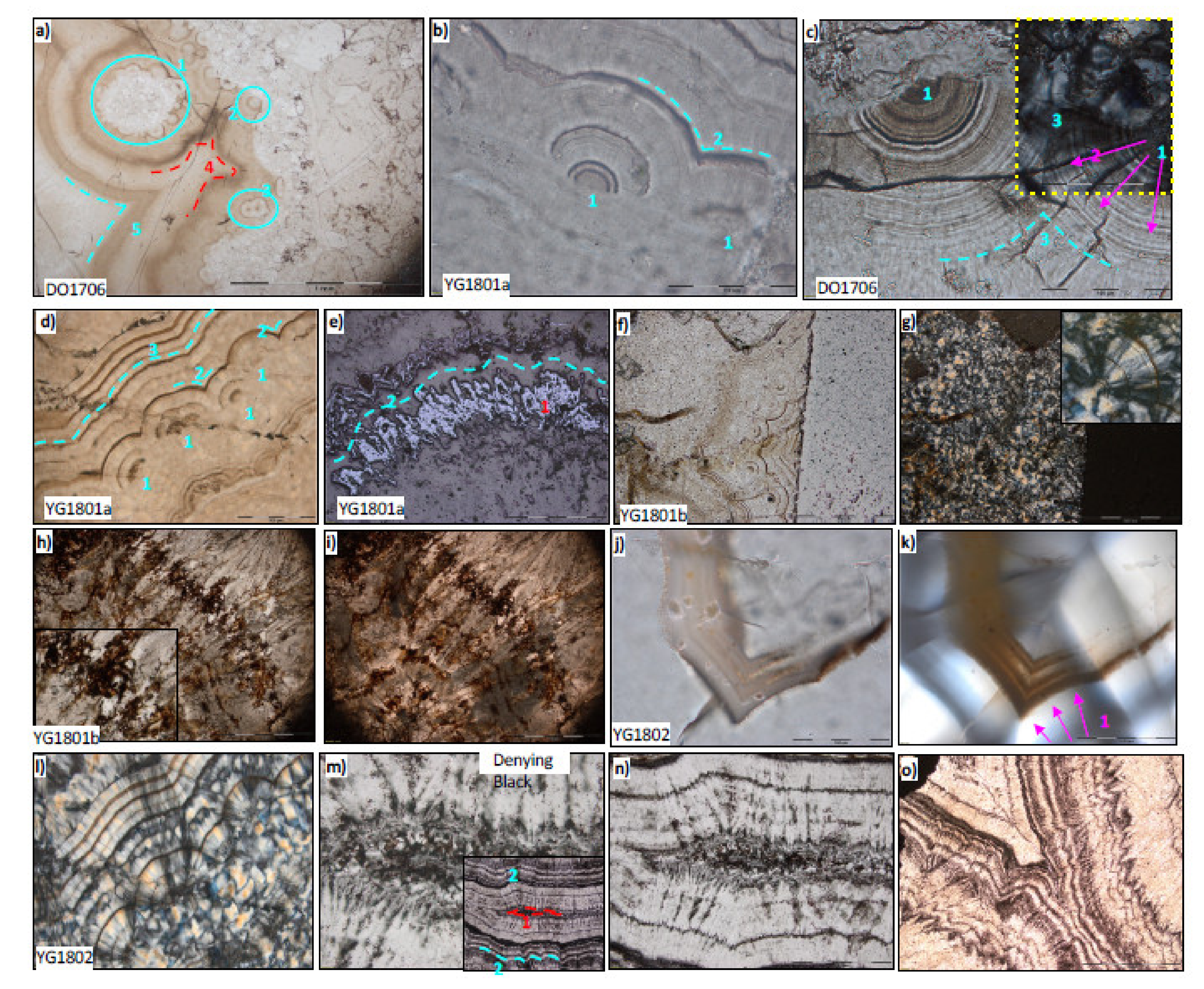
3.5. Polarising Microscopy of Petrographic Thin Sections of Quartz Botryoids
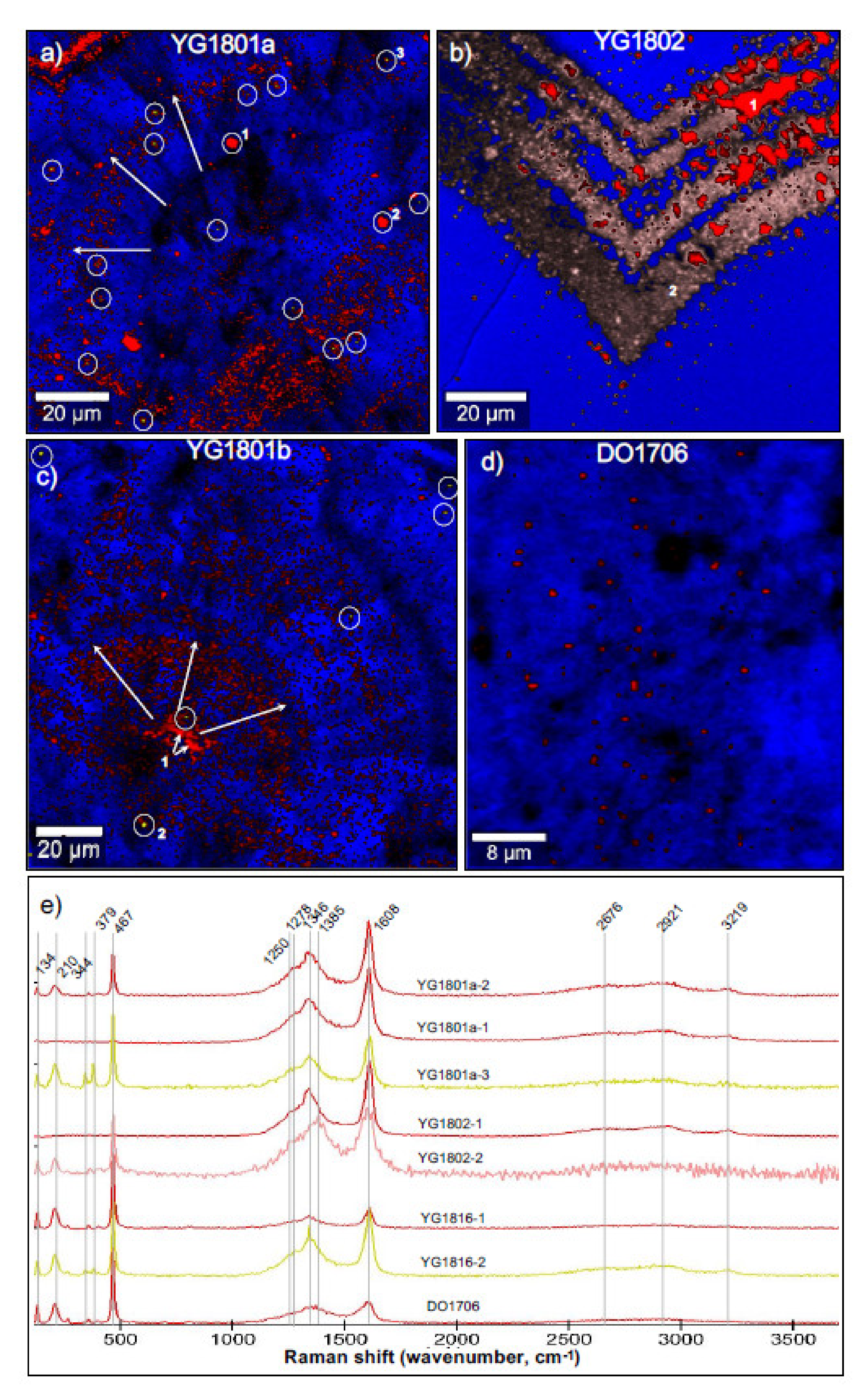
3.6. Micro-Raman Spectroscopic Imaging
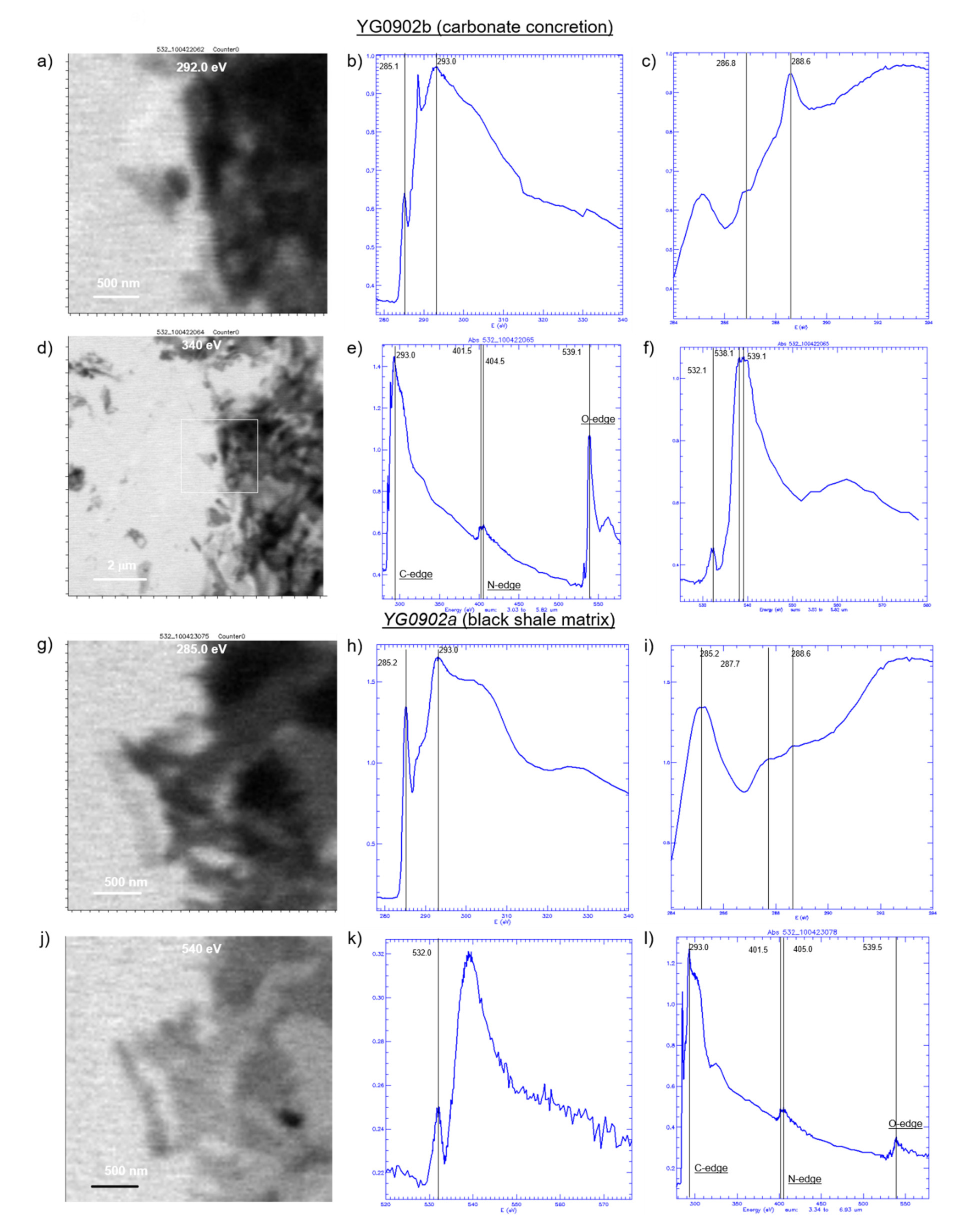
3.7. Scanning Transmission X-ray Microspectroscopy (STXM) of Acid-Insoluble Organic Matter
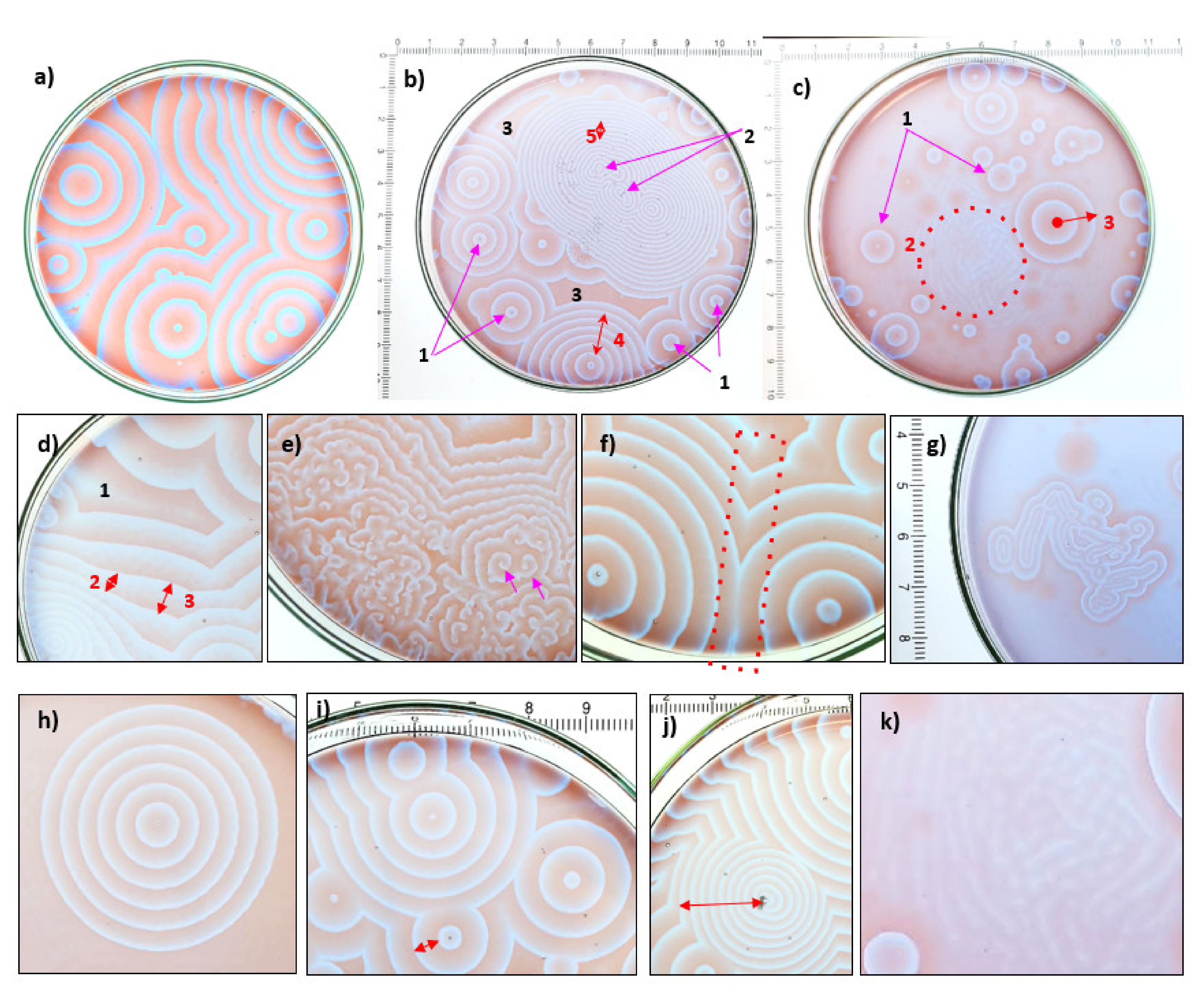
4. Observations of Circularly Concentric Fractal Patterns
4.1. Results from New Chemically Oscillating Experiments
4.2. Results from Polarising Microscopy of Quartz Botryoids from the Post-Snowball Carbonate
4.3. Raman Imaging of Quartz Botryoids from Doushantuo Cap Carbonate
4.4. Molecular Functional Groups in Organic Matter from Niutitang Concretions in Black Shale
5. Discussion
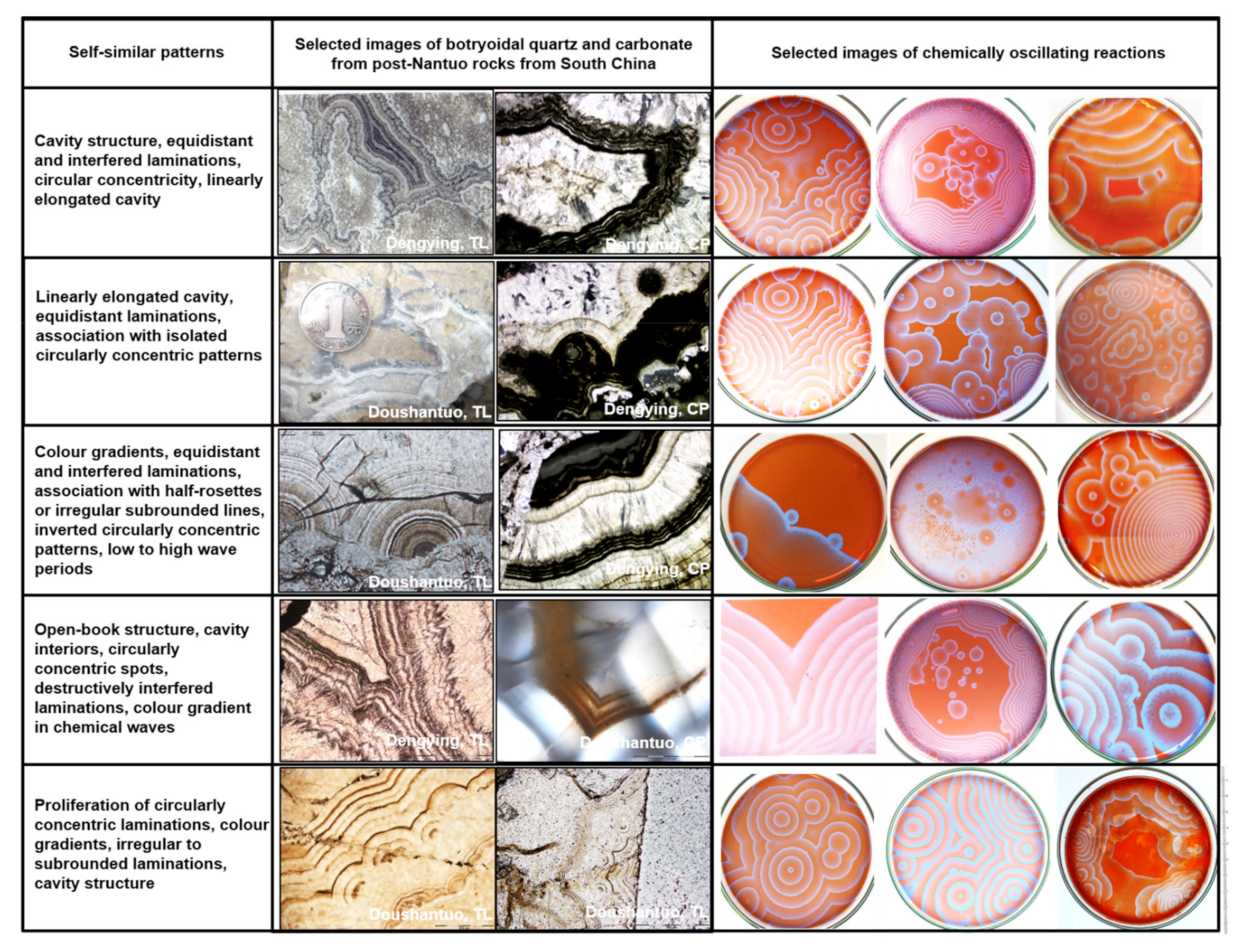
5.1. Self-Similar, Fractal Patterns in Chemically Oscillating Reactions and in Botryoidal Quartz and Carbonate
5.1.1. Comparisons of Geometry, Compositions, Dimension Size
5.1.2. An Imperfect Comparison
5.1.3. Effects of Colloidal Silica vs. Humic Acid
5.1.4. Comparison with Mineralised Organic Decomposition during Early Diagenesis in Nature
5.1.5. Organic Geochemistry of Autochthonous Botryoidal Quartz
5.2. Implications for the Post-Snowball Earth Conditions
5.3. Broad Significance of Botryoid Mineral Formation: From Mineralogy to Exobiology
6. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Götze, J.; Zenz, J. Agate-fascination between legend and science. In Agates III; Bode Verlag: Salzhemmendorf, Germany, 2011; pp. 19–133. [Google Scholar]
- Heaney, P.J.; Davis, A.M. Observation and origin of self-organized textures in agates. Science 1995, 269, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, geochemistry and genesis of agate—A review. Minerals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Papineau, D.; De Gregorio, B.; Fearn, S.; Kilcoyne, D.; McMahon, G.; Purohit, R.; Fogel, M. Nanoscale petrographic and geochemical insights on the origin of the Palaeoproterozoic stromatolitic phosphorites from Aravalli Supergroup, India. Geobiology 2016, 14, 3–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papineau, D.; She, Z.; Dodd, M.S. Chemically-oscillating reactions during the diagenetic oxidation of organic matter and in the formation of gran- ules in late Palaeoproterozoic chert from Lake Superior. Chem. Geol. 2017, 470, 33–54. [Google Scholar] [CrossRef] [Green Version]
- Dodd, M.S.; Papineau, D.; She, Z.; Fogel, M.L.; Nederbragt, S.; Pirajno, F. Organic remains in late Palaeoproterozoic granular iron formations and im-plications for the origin of granules. Precambrian Res. 2018, 310, 133–152. [Google Scholar] [CrossRef] [Green Version]
- Papineau, D. Chemically oscillating reactions in the formation of botryoidal malachite. Am. Mineral. 2020, 105, 447–454. [Google Scholar] [CrossRef]
- Gabriel, N.W.; Papineau, D.; She, Z.; Leider, A.; Fogel, M.L. Organic diagenesis in stromatolitic dolomite and chert from the late Palaeoproterozoic McLeary Formation. Precambrian Res. 2021, 354, 106052. [Google Scholar] [CrossRef]
- Zaikin, A.; Zhabotinsky, A. Concentration wave propagation in two- dimensional liquid-phase self-oscillating system. Nature 1970, 225, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H. Global distribution of the Proterozoic sphaeromorph acritarch Valeria lophostriata (Jankauskas). Wei ti gu Sheng wu xue bao. Acta Micropalaeontol. Sin. 1999, 16, 215–224. [Google Scholar]
- Som, S.M.; Buick, R.; Hagadorn, J.W.; Blake, T.S.; Perreault, J.M.; Harnmeijer, J.P.; Catling, D. Earth’s air pressure 2.7 billion years ago constrained to less than half of modern levels. Nat. Geosci. 2016, 9, 448–451. [Google Scholar] [CrossRef]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef]
- Jiang, G.; Kennedy, M.; Christie-Blick, N. Stable isotopic evidence for methane seeps in Neoproterozoic postglacial cap carbonates. Nat. Cell Biol. 2003, 426, 822–826. [Google Scholar] [CrossRef]
- Condon, D.; Zhu, M.; Bowring, S.; Wang, W.; Yang, A.; Jin, Y. U-Pb Ages from the Neoproterozoic Doushantuo Formation. China Sci. 2005, 308, 95–98. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Jiang, S.-Y.; Ling, H.-F.; Yang, J.-H. Pb–Pb dating of black shales from the Lower Cambrian and Neoproterozoic strata, South China. Geochemistry 2009, 69, 183–189. [Google Scholar] [CrossRef]
- Barfod, G.H.; Albarède, F.; Knoll, A.H.; Xiao, S.; Télouk, P.; Frei, R.; Baker, J. New Lu–Hf and Pb–Pb age constraints on the earliest animal fossils. Earth Planet. Sci. Lett. 2002, 201, 203–212. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.-H.; Xiao, S.; Lan, Z.; Ouyang, Q.; Guan, C.; Chen, Z. A new SIMS zircon U–Pb date from the Ediacaran Doushantuo Formation: Age constraint on the Weng’an biota. Geol. Mag. 2017, 154, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- McFadden, K.A.; Xiao, S.; Zhou, C.; Kowalewski, M. Quantitative evaluation of the biostratigraphic distribution of acanthomorphic acritarchs in the Ediacaran Doushantuo Formation in the Yangtze Gorges area, South China. Precambrian Res. 2009, 173, 170–190. [Google Scholar] [CrossRef]
- She, Z.-B.; Strother, P.; Papineau, D. Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites II: Microbial diversity and C isotopes. Precambrian Res. 2014, 251, 62–79. [Google Scholar] [CrossRef]
- Li, C.; Love, G.D.; Lyons, T.W.; Fike, D.A.; Sessions, A.L.; Chu, X. A Stratified Redox Model for the Ediacaran Ocean. Science 2010, 328, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Lu, M.; Zhang, J.; Zhao, F.; Li, G.; Aihua, Y.; Zhao, X.; Zhao, M. Carbon isotope chemostratigraphy and sedimentary facies evolution of the Ediacaran Doushantuo Formation in western Hubei, South China. Precambrian Res. 2013, 225, 7–28. [Google Scholar] [CrossRef]
- Cui, H.; Xiao, S.; Cai, Y.; Peek, S.; Plummer, R.E.; Kaufman, A.J. Sedimentology and chemostratigraphy of the terminal Ediacaran Dengying Formation at the Gaojiashan section, South China. Geol. Mag. 2019, 156, 1924–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Wang, P.; Li, T.; Zhao, K.; Yan, H.; Li, J.; Zhou, L. Nitrogen geo- chemistry and abnormal mercury enrichment of shales from the lowermost Cambrian Niutitang Formation in South China: Implications for the marine redox conditions and hydrothermal activity. Glob. Planet. Chang. 2021, 199, 103449. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Fogel, M.; Yabuta, H.; Cody, G.D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 2007, 71, 4380–4403. [Google Scholar] [CrossRef]
- Kilcoyne, A.; Tyliszczak, T.; Steele, W.F.; Fakra, S.; Hitchcock, P.; Franck, K.; Anderson, E.; Harteneck, B.; Rightor, E.G.; Mitchell, G.E.; et al. Interferometer-controlled scanning transmission X-ray microscopes at the Advanced Light Source. J. Synchrotron Radiat. 2003, 10, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Papineau, D.; De Gregorio, B.T.; Sagar, J.; Thorogate, R.; Wang, J.; Nittler, L.; Kilcoyne, D.A.; Marbach, H.; Drost, M.; Thornton, G. Fossil biomass preserved as graphitic carbon in a late Paleoproterozoic banded iron for-mation metamorphosed at more than 550 °C. J. Geol. Soc. 2019, 176, 651–668. [Google Scholar] [CrossRef]
- Lahfid, A.; Beyssac, O.; Deville, E.; Negro, F.; Chopin, C.; Goffé, B. Evolution of the Raman spectrum of carbonaceous material in low-grade metasediments of the Glarus Alps (Switzerland). Terra Nova 2010, 22, 354–360. [Google Scholar] [CrossRef]
- Kouketsu, Y.; Mizukami, T.; Mori, H.; Endo, S.; Aoya, M.; Hara, H.; Nakamura, D.; Wallis, S. A new approach to develop the Raman carbonaceous material geothermometer for low-grade metamorphism using peak width. Isl. Arc. 2014, 23, 33–50. [Google Scholar] [CrossRef]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Menguy, N.; Guyot, F.; Brown, G.E., Jr.; Goffé, B. Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth Planet. Sci. Lett. 2007, 262, 257–272. [Google Scholar] [CrossRef]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Brown, G., Jr.; Stamm, L.G.; Duringer, P. Ultrastructural and chemical study of modern and fossil sporoderms by Scanning Transmission X-ray Microscopy (STXM). Rev. Palaeobot. Palynol. 2009, 156, 248–261. [Google Scholar] [CrossRef]
- Bernard, S.; Beyssac, O.; Benzerara, K.; Findling, N.; Tzvetkov, G.; Brown, G., Jr. XANES, Raman and XRD study of anthracene-based cokes and saccharose-based chars submitted to high-temperature pyrolysis. Carbon 2010, 48, 2506–2516. [Google Scholar] [CrossRef]
- Bernard, S.; Horsfield, B.; Schulz, H.-M.; Wirth, R.; Schreiber, A.; Sherwood, N. Geochemical evolution of organic-rich shales with increasing maturity: A STXM and TEM study of the Posidonia Shale (Lower Toarcian, northern Germany). Mar. Pet. Geol. 2012, 31, 70–89. [Google Scholar] [CrossRef]
- Boyce, C.K.; Cody, G.D.; Feser, M.; Jacobsen, C.; Knoll, A.H.; Wirick, S. Organic chemical differentiation within fossil plant cell walls detected with X-ray spectromicroscopy. Geology 2002, 30, 1039–1042. [Google Scholar] [CrossRef]
- Myneni, S.C.B. Soft X-ray Spectroscopy and Spectromicroscopy Studies of Organic Molecules in the Environment. Rev. Mineral. Geochem. 2002, 49, 485–579. [Google Scholar] [CrossRef]
- Shard, A.G.; Whittle, J.D.; Beck, A.J.; Brookes, P.N.; Bullett, N.A.; Talib, R.A.; Mistry, A.; Barton, D.; McArthur, S.L. A NEXAFS examination of un- saturation in plasma polymers of allylamine and propylamine. J. Phys. Chem. B 2004, 108, 12472–12480. [Google Scholar] [CrossRef]
- Nuevo, M.; Milam, S.; Sandford, S.; De Gregorio, B.; Cody, G.; Kilcoyne, A. XANES analysis of organic residues produced from the UV irradiation of astrophysical ice analogs. Adv. Space Res. 2011, 48, 1126–1135. [Google Scholar] [CrossRef]
- Alleon, J.; Bernard, S.; Le Guillou, C.; Marin-Carbonne, J.; Pont, S.; Beyssac, O.; McKeegan, K.D.; Robert, F. Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. Nat. Commun. 2016, 7, 11977. [Google Scholar] [CrossRef] [Green Version]
- Cody, G.; Botto, R.; Ade, H.; Wirick, S. The application of soft X-ray microscopy to the in-situ analysis of sporinite in coal. Int. J. Coal Geol. 1996, 32, 69–86. [Google Scholar] [CrossRef]
- Cody, G.D.; Gupta, N.S.; Briggs, D.E.; Kilcoyne, A.; Summons, R.E.; Kenig, F.; Plotnick, R.E.; Scott, A. Molecular signature of chitin-protein complex in Paleozoic arthropods. Geology 2011, 39, 255–258. [Google Scholar] [CrossRef] [Green Version]
- Berner, R.A. Calcium carbonate concretions formed by the decomposition of organic matter. Science 1968, 159, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hardisty, D.S.; Luo, G.; Huang, J.; Algeo, T.J.; Cheng, M.; Shi, W.; An, Z.; Tong, J.; Xie, S.; et al. Uncovering the spatial heterogeneity of Ediacaran carbon cycling. Geobiology 2017, 15, 211–224. [Google Scholar] [CrossRef]
- Papineau, D.; Mojzsis, S.J.; Schmitt, A.K. Multiple sulfur isotopes from Paleoproterozoic Huronian interglacial sediments and the rise of atmospheric oxygen. Earth Planet. Sci. Lett. 2007, 255, 188–212. [Google Scholar] [CrossRef]
- Hardisty, D.S.; Lu, Z.; Planavsky, N.J.; Bekker, A.; Philippot, P.; Zhou, X.; Lyons, T.W. An iodine record of Paleoproterozoic surface ocean oxygenation. Geology 2014, 42, 619–622. [Google Scholar] [CrossRef]
- Boyd, S.R. Nitrogen in future biosphere studies. Chem. Geol. 2001, 176, 1–30. [Google Scholar] [CrossRef]
- Sheik, C.S.; Cleaves, H.J.; Johnson-Finn, K.N.; Giovannelli, D.; Kieft, T.L.; Papineau, D.; Schrenk, M.O.; Tumiati, S. Abiotic and biotic processes that drive carboxylation and decarboxylation reactions. Am. Mineral. 2020, 105, 609–615. [Google Scholar] [CrossRef]
- Ferralis, N.; Matys, E.D.; Knoll, A.H.; Hallmann, C.; Summons, R.E. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon 2016, 108, 440–449. [Google Scholar] [CrossRef] [Green Version]
- She, Z.-B.; Strother, P.; McMahon, G.; Nittler, L.R.; Wang, J.; Zhang, J.; Sang, L.; Ma, C.; Papineau, D. Terminal Proterozoic cyanobacterial blooms and phosphogenesis documented by the Doushantuo granular phosphorites I: In situ micro-analysis of textures and composition. Precambrian Res. 2013, 235, 20–35. [Google Scholar] [CrossRef]
- She, Z.-B.; Zhang, Y.-T.; Liu, W.; Song, J.; Zhang, Y.; Li, C.; Strother, P.; Papineau, D. New observations of Ambient Inclusion Trails (AITs) and pyrite framboids in the Ediacaran Doushantuo Formation, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 461, 374–388. [Google Scholar] [CrossRef]
- Alleon, J.; Bernard, S.; Le Guillou, C.; Beyssac, O.; Sugitani, K.; Robert, F. Chemical nature of the 3.4 Ga Strelley Pool microfossils. Geochem. Perspect. Lett. 2018, 37–42. [Google Scholar] [CrossRef]
- Xiao, S.; Schiffbauer, J.D.; McFadden, K.A.; Hunter, J. Petrographic and SIMS pyrite sulfur isotope analyses of Ediacaran chert nodules: Implications for microbial processes in pyrite rim formation, silicification, and exceptional fossil preservation. Earth Planet. Sci. Lett. 2010, 297, 481–495. [Google Scholar] [CrossRef]
- Cui, H.; Kaufman, A.J.; Xiao, S.; Zhou, C.; Liu, X.-M. Was the Ediacaran Shuram Excursion a globally synchronized early diagenetic event? Insights from methane-derived authigenic carbonates in the uppermost Doushantuo Formation, South China. Chem. Geol. 2017, 450, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Hardisty, D.S.; Lu, Z.; Bekker, A.; Diamond, C.W.; Gill, B.; Jiang, G.; Kah, L.C.; Knoll, A.H.; Loyd, S.J.; Osburn, M.R.; et al. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate. Earth Planet. Sci. Lett. 2017, 463, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, X.; Shi, X.; Jiang, G.; Tang, D.; Wang, L.; An, Z. Iodine content of the carbonates from the Doushantuo Formation and shallow ocean redox change on the Ediacaran Yangtze Platform, South China. Precambrian Res. 2019, 322, 160–169. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Barge, L.M.; Cardoso, S.S.S.; Cartwright, J.H.E.; Cooper, G.J.T.; Cronin, L.; De Wit, A.; Doloboff, I.J.; Escribano, B.; Goldstein, R.; Haudin, F.; et al. From Chemical Gardens to Chemobrionics. Chem. Rev. 2015, 115, 8652–8703. [Google Scholar] [CrossRef] [Green Version]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Minerol. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Chan, M.A.; Hinman, N.W.; Potter-McIntyre, S.L.; Schubert, K.E.; Gillams, R.J.; Awramik, S.M.; Boston, P.J.; Bower, D.M.; Des Marais, D.J.; Farmer, J.D.; et al. De-ciphering biosignatures in planetary contexts. Astrobiology 2019, 19, 1075–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, A.; Benning, L.G.; Wirth, R.; Siljeström, S.; Fries, M.D.; Hauri, E.; Conrad, P.G.; Rogers, K.; Eigenbrode, J.; Schreiber, A.; et al. Organic synthesis on Mars by electrochemical reduction of CO. Sci. Adv. 2018, 4, eaat5118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Guo, Z.; Du, M.; Czaja, A.; Papineau, D.; Chen, S.; Xu, H.; Li, J.; Ta, K.; Bai, S.; et al. Past endolithic life in metamorphic ocean crust. Geochem. Perspect. Lett. 2020, 14, 14–19. [Google Scholar] [CrossRef]
- Hazen, R.M.; Bekker, A.; Bish, D.L.; Bleeker, W.; Downs, R.T.; Farquhar, J.; Ferry, J.M.; Grew, E.S.; Knoll, A.H.; Papineau, D.; et al. Needs and opportunities in mineral evolution research. Am. Minerol. 2011, 96, 953–963. [Google Scholar] [CrossRef]
- Squyres, S.W.; Arvidson, R.E.; Ruff, S.; Gellert, R.; Morris, R.V.; Ming, D.W.; Crumpler, L.; Farmer, J.D.; Marais, D.J.D.; Yen, A.; et al. Detection of Silica-Rich Deposits on Mars. Science 2008, 320, 1063–1067. [Google Scholar] [CrossRef]


| Features | Botryoidal Quartz in Doushantuo Cap Carbonate | Classical BZ Reaction | BZ with Malonic and Succinic | BZ with Malonic and Alpha-Ketoglutaric | BZ with Humic Acid | BZ with Humic Acid and all Three Carboxylic Acids | BZ with Colloidal Sillica |
|---|---|---|---|---|---|---|---|
| Parallel banding/destructively interfered laminations | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Circularly concentric oxidation spot | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Oxidation spiral | ? | Yes | Yes | Yes | Yes | Yes | Yes |
| Cavity structure | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Irregular/subrounded lines | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Open book structure | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Colour gradient in chemical waves | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| fingerprint/ripple pattern | No | Yes | Yes | Yes | No | No | No |
| Spectral Parameters | Sample | Dengying Carbonate Botryoid | YG1801a | YG1802 | YG18016 |
|---|---|---|---|---|---|
| G-peak | Peak position | 1601 | 1585 | 1608 | 1580 |
| Peak FWHM | 36 | 45 | 32 | 60 | |
| Peak area | 14,800 | 1500 | 28,500 | 435 | |
| D1-peak | Peak position | 1343 | 1347 | 1337 | 1350 |
| Peak FWHM | 110 | 112 | 100 | 120 | |
| Peak area | 27,500 | 6500 | 57,000 | 2050 | |
| D2-peak | Peak position | 1620 | 1610 | 1620 | 1610 |
| Peak FWHM | 20 | 25 | 11 | 29 | |
| Peak area | 2500 | 2260 | 2100 | 920 | |
| D3-peak | Peak position | 1510 | 1510 | 1510 | 1510 |
| Peak FWHM | 150 | 130 | 140 | 130 | |
| Peak area | 8000 | 1200 | 11,000 | 280 | |
| D4-peak | Peak position | 1245 | 1249 | 1245 | 1250 |
| Peak FWHM | 100 | 109 | 80 | 115 | |
| Peak area | 9000 | 1990 | 10,000 | 764 | |
| Correlation coefficients | R2 | 0.9729 | 0.9835 | 0.9852 | 0.9798 |
| R | 0.9896 | 0.9917 | 0.9926 | 0.9899 | |
| Peak metamorphic temperature * | Model 1 (°C) | 268.5 | 319.3 | 301.4 | 320.9 |
| Model 2 (°C) | 260.6 | 320.4 | 297.9 | 322.5 | |
| Model 3 (°C) | 241.6 | 237.3 | 263.1 | 220.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papineau, D.; Yin, J.; Devine, K.G.; Liu, D.; She, Z. Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids. Minerals 2021, 11, 1060. https://doi.org/10.3390/min11101060
Papineau D, Yin J, Devine KG, Liu D, She Z. Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids. Minerals. 2021; 11(10):1060. https://doi.org/10.3390/min11101060
Chicago/Turabian StylePapineau, Dominic, Jiayu Yin, Kevin G. Devine, Deng Liu, and Zhenbing She. 2021. "Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids" Minerals 11, no. 10: 1060. https://doi.org/10.3390/min11101060
APA StylePapineau, D., Yin, J., Devine, K. G., Liu, D., & She, Z. (2021). Chemically Oscillating Reactions during the Diagenetic Formation of Ediacaran Siliceous and Carbonate Botryoids. Minerals, 11(10), 1060. https://doi.org/10.3390/min11101060