Biodepression of Copper-Activated Pyrite with Acidithiobacillus ferrooxidans in Flotation with Fresh and Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Preparation
2.2. Microorganisms Preparation
2.3. Seawater Preparation
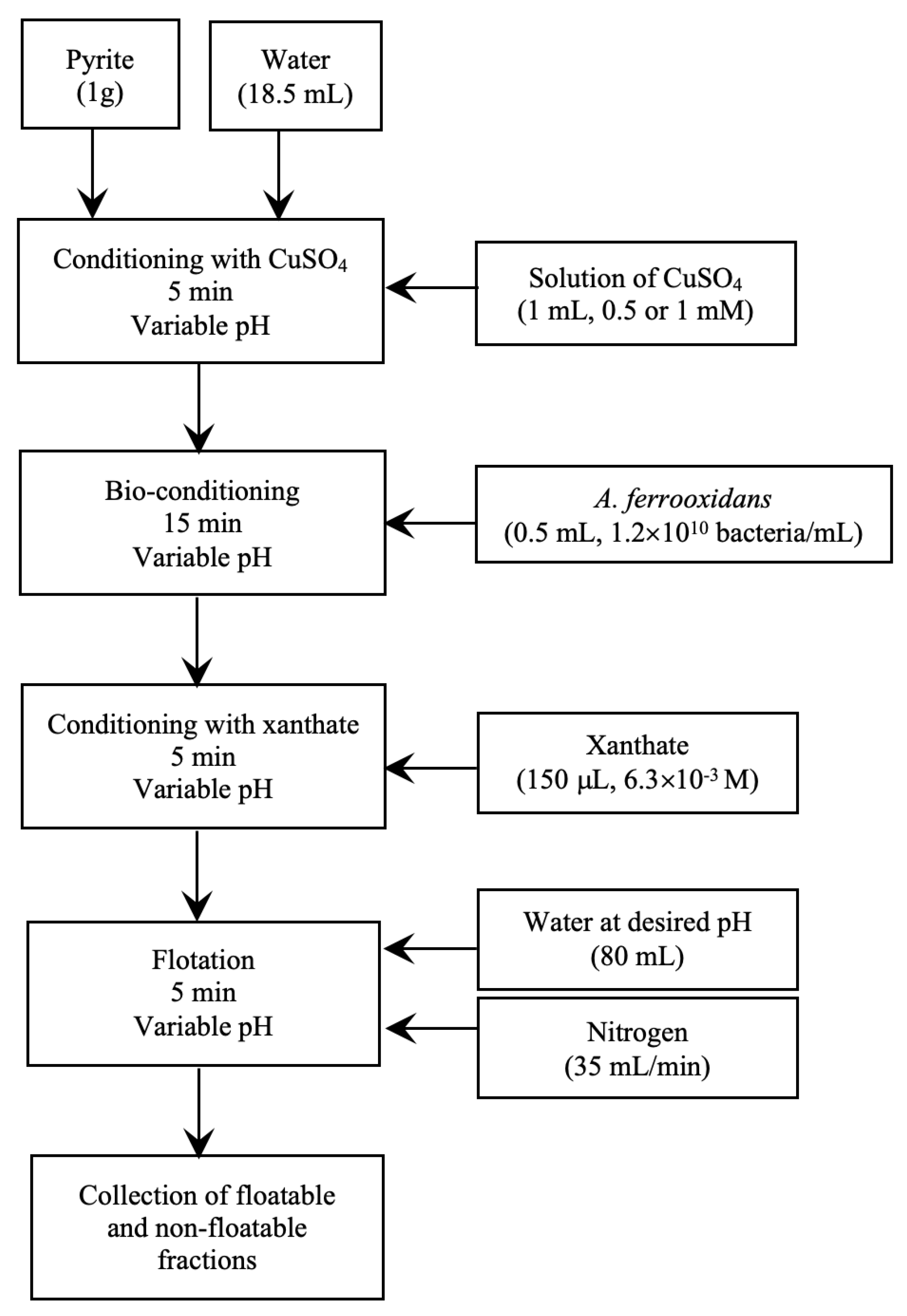
2.4. Flotation and Bioflotation of Cu-Activated Pyrite
2.5. X-ray Photoelectron Spectroscopy
3. Results and Discussion
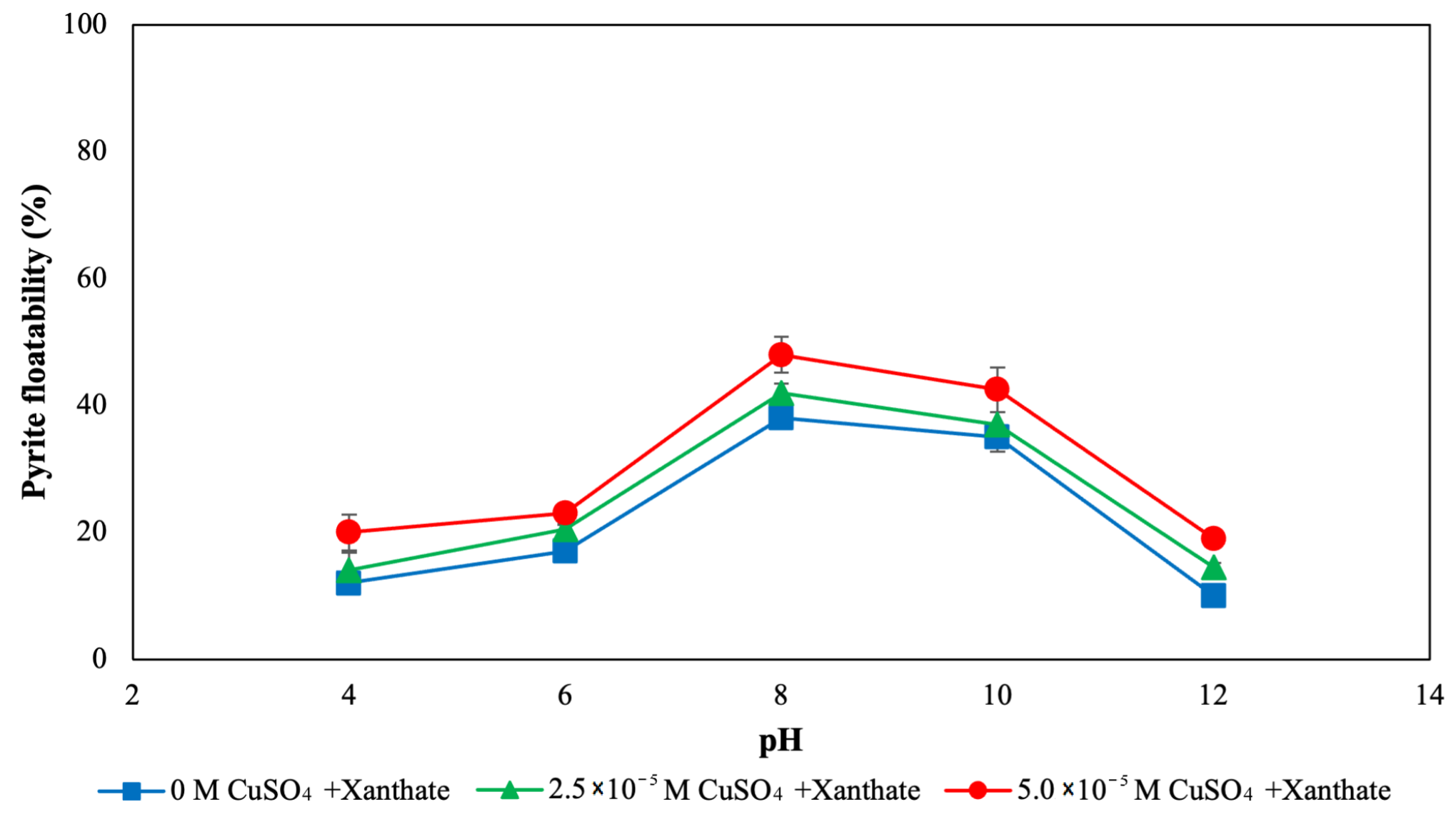
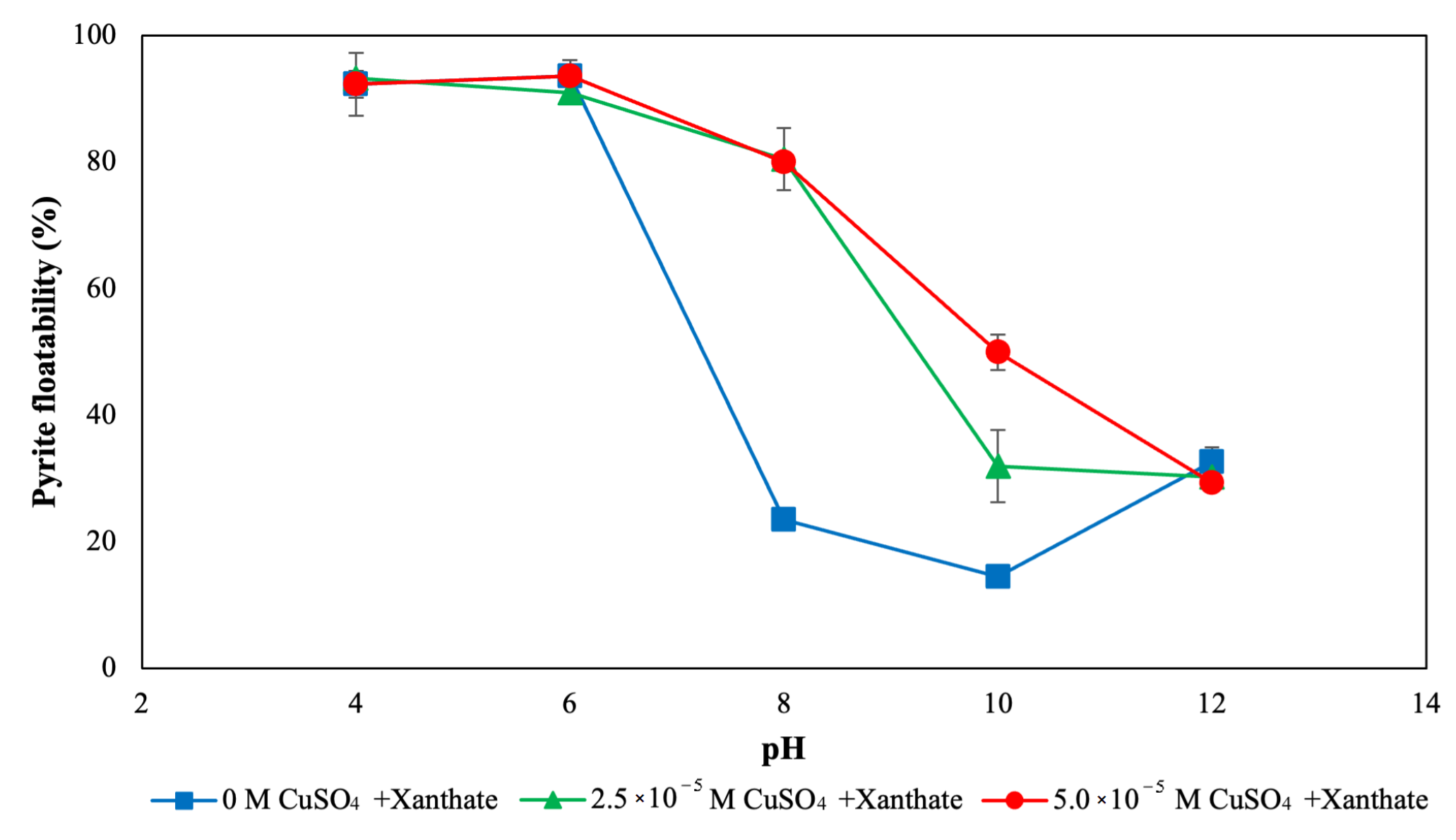
3.1. Flotation and Bioflotation of Cu-Activated Pyrite
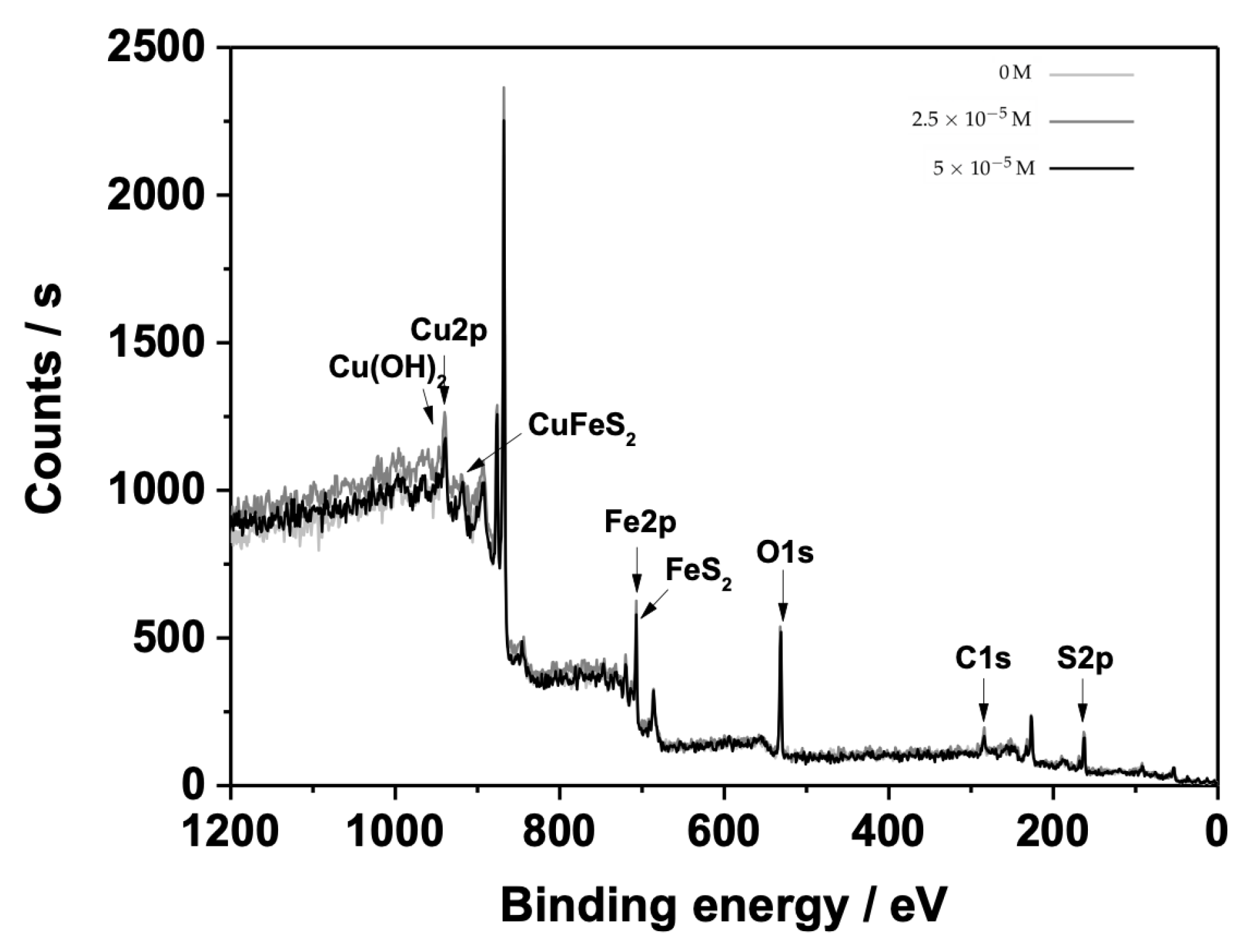
3.2. X-ray Photoelectron Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, J.R.; Woods, R. Electrochemical investigation of contact angle and of flotation in the presence of alkylxanthates. II. Galena and pyrite surfaces. Aust. J. Chem. 1977, 30, 981–991. [Google Scholar] [CrossRef]
- Leppinen, J.O. FTIR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals. Int. J. Miner. Process. 1990, 30, 245–263. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, Z.; Bozkurt, V.; Finch, J.A. Pyrite flotation in the presence of metal ions and sphalerite. Int. J. Miner. Process. 1997, 52, 187–201. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]
- Weisener, C.; Gerson, A. An investigation of the Cu (II) adsorption mechanism on pyrite by ARXPS and SIMS. Miner. Eng. 2000, 13, 1329–1340. [Google Scholar] [CrossRef]
- Comisión Chilena del Cobre. Consumo de agua en la Minería del Cobre al 2019; Gobierno de Chile: Santiago, Chile, 2020. [Google Scholar]
- Mu, Y.; Peng, Y. The effect of saline water on copper activation of pyrite in chalcopyrite flotation. Miner. Eng. 2019, 131, 336–341. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ohmura, N.; Saiki, H. A novel mineral flotation process using Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 3588–3593. [Google Scholar] [CrossRef] [Green Version]
- Chandraprabha, M.; Natarajan, K.; Modak, J.M. Selective separation of pyrite and chalcopyrite by biomodulation. Colloids Surf. B Biointerfaces 2004, 37, 93–100. [Google Scholar] [CrossRef]
- Chandraprabha, M.; Natarajan, K.; Somasundaran, P. Selective separation of pyrite from chalcopyrite and arsenopyrite by biomodulation using Acidithiobacillus ferrooxidans. Int. J. Min. Process. 2005, 75, 113–122. [Google Scholar] [CrossRef]
- Hosseini, T.; Kolahdoozan, M.; Tabatabaei, Y.; Oliazadeh, M.; Noaparast, M.; Eslami, A.; Manafi, Z.; Alfantazi, A. Bioflotation of Sarcheshmeh copper ore using Thiobacillus ferrooxidans bacteria. Miner. Eng. 2005, 18, 371–374. [Google Scholar] [CrossRef]
- Misra, M.; Bukka, K.; Chen, S. The effect of growth medium of Thiobacillus ferrooxidans on pyrite flotation. Miner. Eng. 1996, 9, 157–168. [Google Scholar] [CrossRef]
- Mehrabani, J.; Mousavi, S.; Noaparast, M. Evaluation of the replacement of NaCN with Acidithiobacillus ferrooxidans in the flotation of high-pyrite, low-grade lead–zinc ore. Sep. Purif. Technol. 2011, 80, 202–208. [Google Scholar] [CrossRef]
- Ohmura, N.; Kitamura, K.; Saiki, H. Selective adhesion of Thiobacillus ferrooxidans to pyrite. Appl. Environ. Microbiol. 1993, 59, 4044–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San Martín, F.; Kracht, W.; Vargas, T. Biodepression of pyrite using Acidithiobacillus ferrooxidans in seawater. Miner. Eng. 2018, 117, 127–131. [Google Scholar] [CrossRef]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of artificial seawater. Limnol. Oceanogr. 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Ohmura, N.; Kitamura, K.; Saiki, H. Mechanism of microbial flotation using Thiobacillus ferrooxidans for pyrite suppression. Biotechnol. Bioeng. 1993, 41, 671–676. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 188–189. [Google Scholar]
- Hackl, R.P.; Dreisinger, D.B.; Peters, E.; King, J.A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy 1995, 39, 25–48. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Morales-Gallardo, M.V.; Ayala, A.M.; Pal, M.; Jacome, M.C.; Antonio, J.T.; Mathews, N.R. Synthesis of pyrite FeS2 nanorods by simple hydrothermal method and its photocatalytic activity. Chem. Phys. Lett. 2016, 660, 93–98. [Google Scholar] [CrossRef]



| Reagent | Chemical Formula | Molecular Weight | Concentration (g/L) |
|---|---|---|---|
| Ammonium sulfate | (NH4)2SO4 | 132.14 | 0.4 |
| Potassium hydrogen phosphate trihydrate | K2HPO4·3H2O | 228.23 | 0.056 |
| Magnesium sulfate heptahydrate | MgSO4·7H2O | 246.48 | 0.4 |
| Iron sulfate heptahydrate | FeSO4·7H2O | 278.02 | 14.85 |
| Salt | Molecular Weight | Concentration (g/kg of Distilled Water) |
|---|---|---|
| NaCl | 58.44 | 23.926 |
| Na2SO4 | 142.04 | 4.008 |
| KCl | 74.56 | 0.677 |
| NaHCO3 | 84.00 | 0.196 |
| KBr | 119.01 | 0.098 |
| H3BO3 | 61.83 | 0.026 |
| NaF | 41.99 | 0.003 |
| MgCl2·6H2O | 203.33 | 10.83 |
| CaCl2·2H2O | 147.03 | 1.52 |
| SrCl2·6H2O | 266.64 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Martín, F.; Valles, I.; Kracht, W.; Vargas, T.; Aguilar, C. Biodepression of Copper-Activated Pyrite with Acidithiobacillus ferrooxidans in Flotation with Fresh and Seawater. Minerals 2021, 11, 1039. https://doi.org/10.3390/min11101039
San Martín F, Valles I, Kracht W, Vargas T, Aguilar C. Biodepression of Copper-Activated Pyrite with Acidithiobacillus ferrooxidans in Flotation with Fresh and Seawater. Minerals. 2021; 11(10):1039. https://doi.org/10.3390/min11101039
Chicago/Turabian StyleSan Martín, Francisca, Ignacio Valles, Willy Kracht, Tomás Vargas, and Claudio Aguilar. 2021. "Biodepression of Copper-Activated Pyrite with Acidithiobacillus ferrooxidans in Flotation with Fresh and Seawater" Minerals 11, no. 10: 1039. https://doi.org/10.3390/min11101039
APA StyleSan Martín, F., Valles, I., Kracht, W., Vargas, T., & Aguilar, C. (2021). Biodepression of Copper-Activated Pyrite with Acidithiobacillus ferrooxidans in Flotation with Fresh and Seawater. Minerals, 11(10), 1039. https://doi.org/10.3390/min11101039







