Rare Earth Elements and Sr Isotope Ratios of Large Apatite Crystals in Ghareh Bagh Mica Mine, NW Iran: Tracing for Petrogenesis and Mineralization
Abstract
1. Introduction
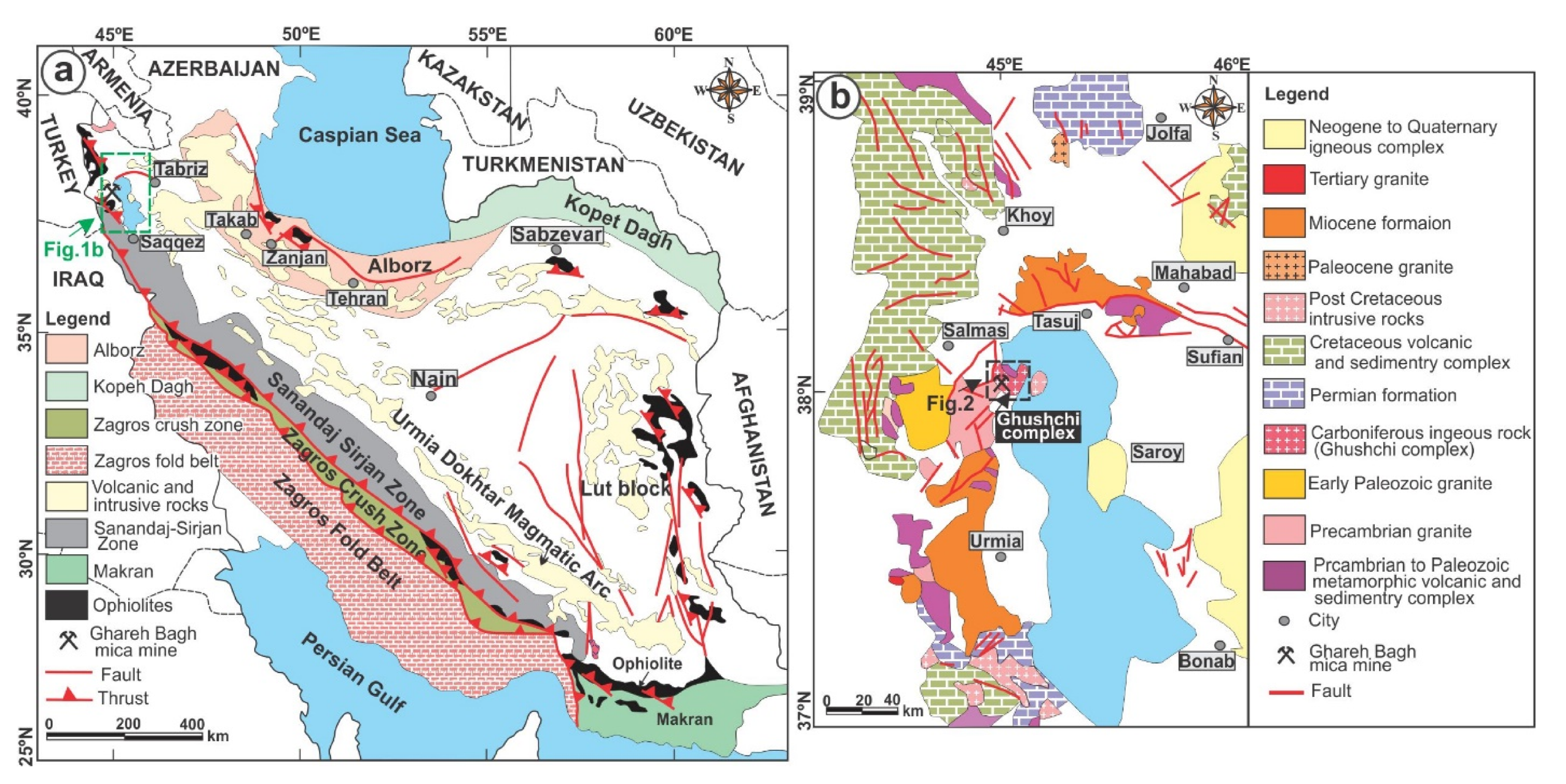
2. Geological Setting and Petrographic Studies
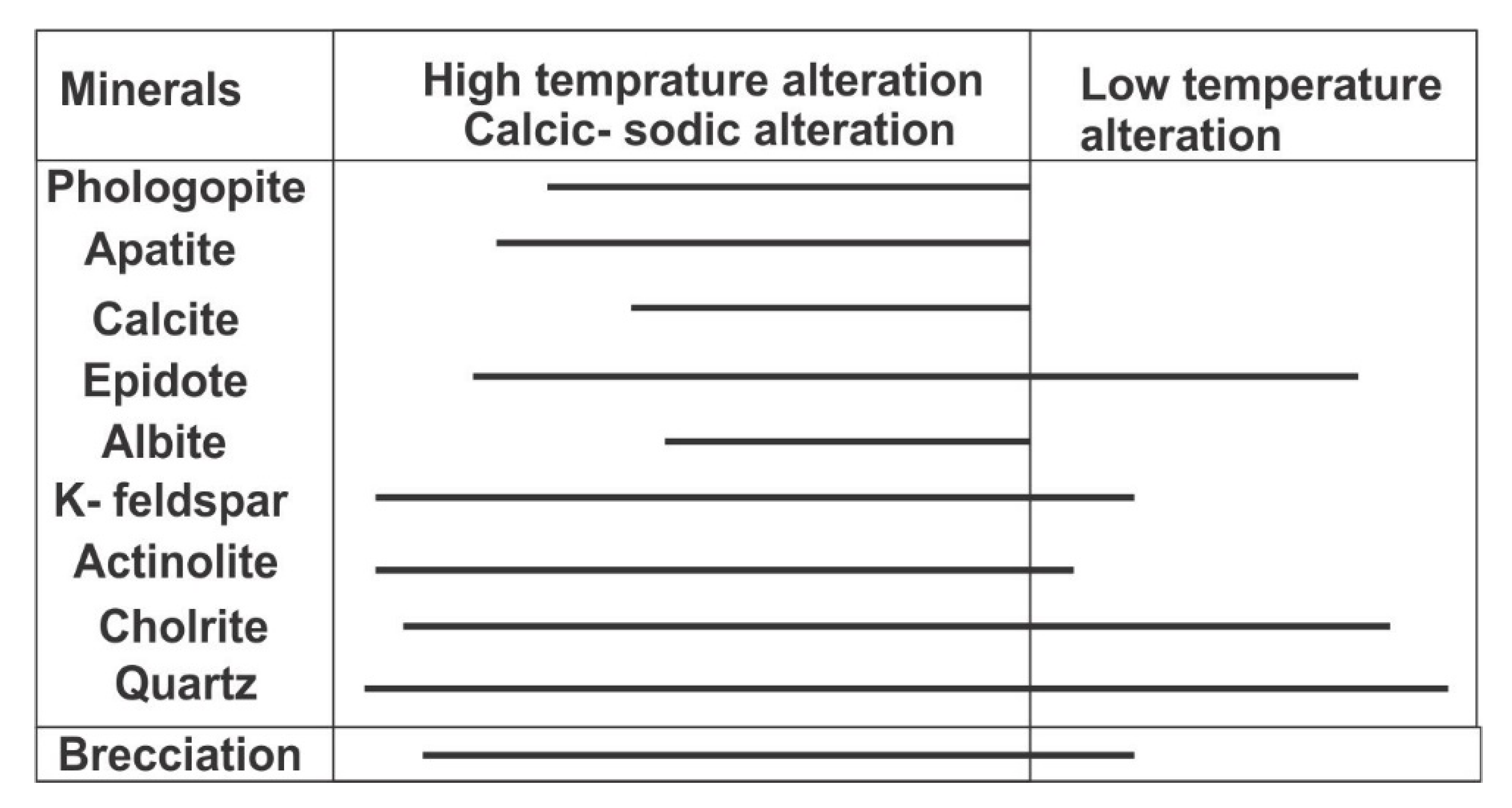
3. Ore Deposit Mineralogy
4. Analytical Techniques
5. Results
5.1. Apatite Chemistry
5.2. 87Sr/86Sr Ratios
6. Discussion
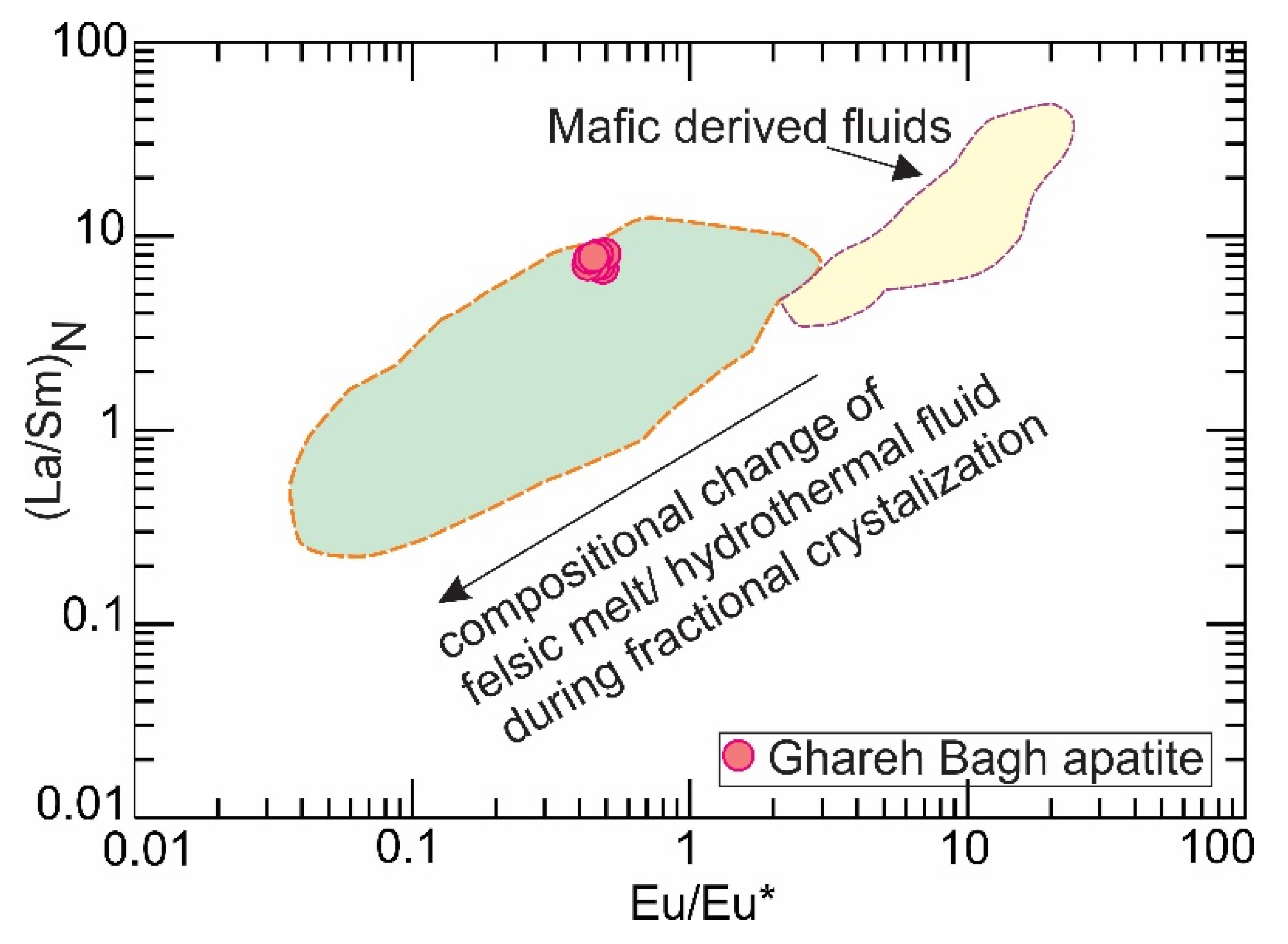
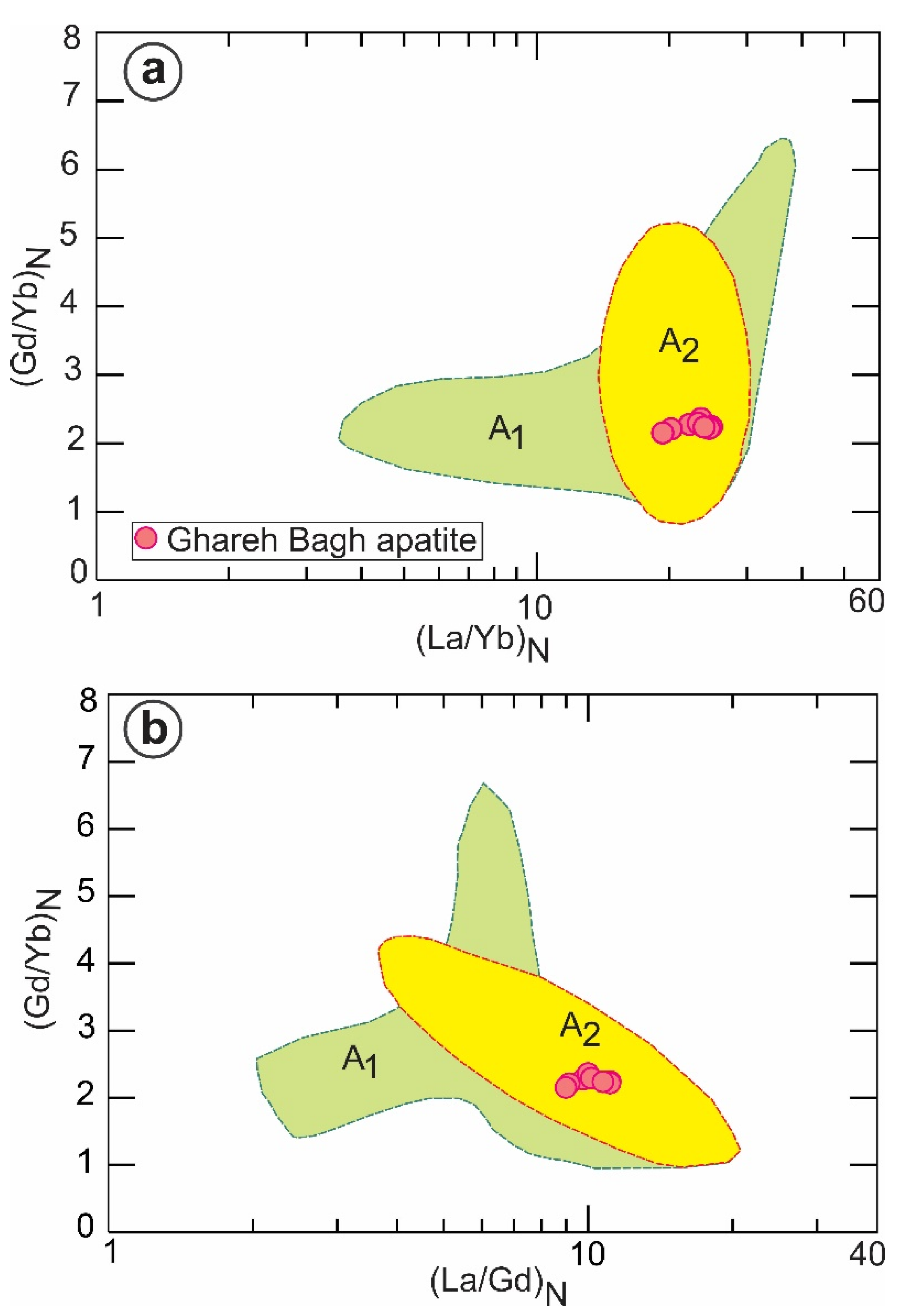
6.1. Apatite Genesis
6.2. Apatite and Its Relation to the Host Granite
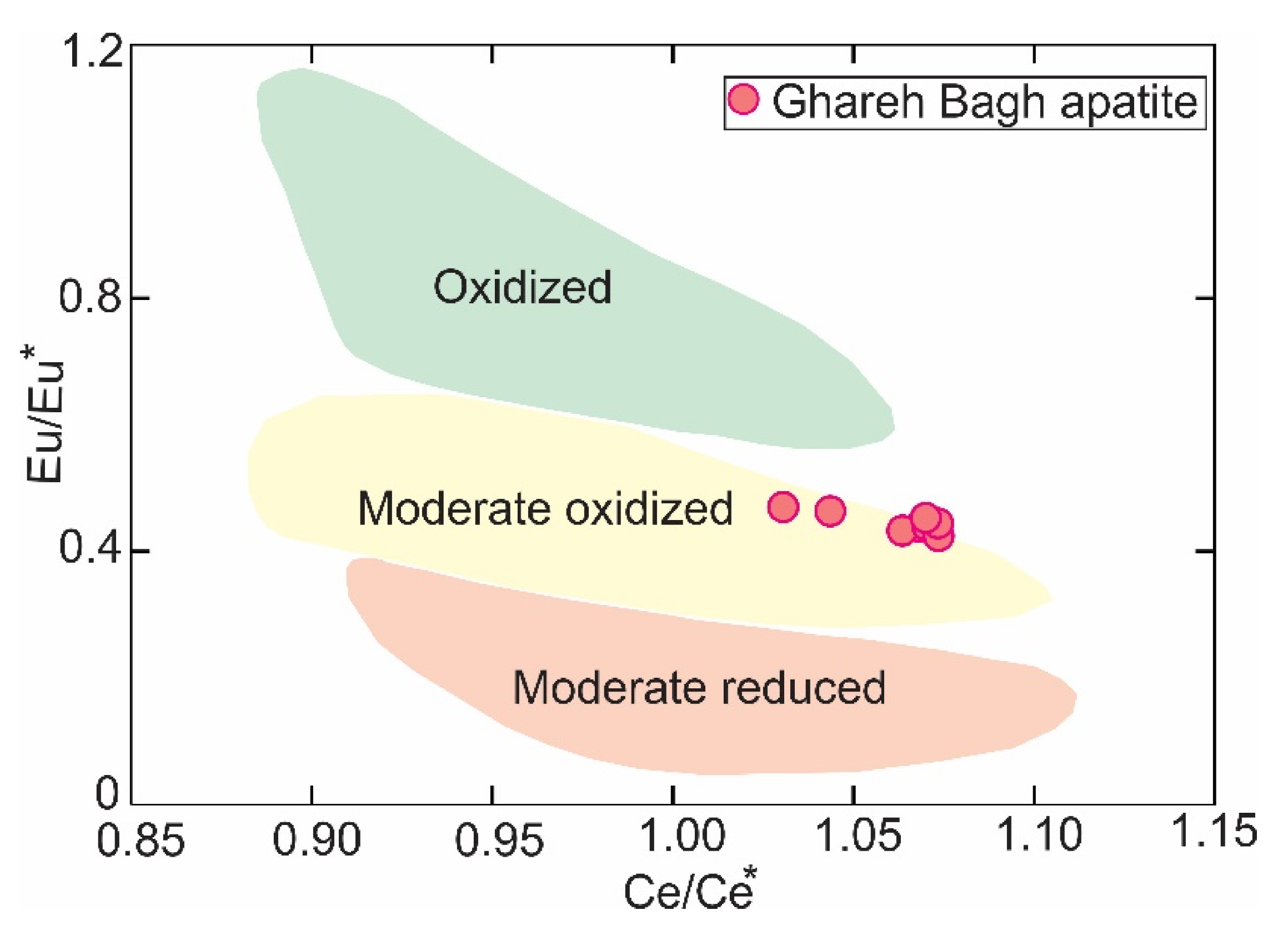
6.3. Eu Contents in the Apatite and Oxygen Fugacity
6.4. 87Sr/86Sr Ratios in the Apatite Granis
6.5. Mica–Apatite Deposit Mineralization
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoskin, P.W.O.; Kinny, P.D.; Wyborn, D.; Chappell, B.W. Identifying accessory mineral saturation during differentiation in granitoid magmas: An integrated approach. J. Petrol. 2000, 41, 1365–1396. [Google Scholar] [CrossRef]
- Belousova, E.A.; Griffin, W.L.; O’Reilly, S.Y.; Fisher, N.I. Apatite as an indicator mineral for mineral exploration: Trace-element compositions and their relationship to host rock type. J. Geochem. Explor. 2002, 76, 45–69. [Google Scholar] [CrossRef]
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite trace element compositions: A robust new tool for mineral exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Bouzari, F.; Hart, C.J.R.; Bissig, T.; Barker, S. Hydrothermal alteration revealed by apatite luminescence and chemistry: A potential indicator mineral for exploring covered porphyry copper deposits. Econ. Geol. 2016, 111, 1397–1410. [Google Scholar] [CrossRef]
- Dill, H.G. Can REE patterns and U-Th variations be used as a tool to determine the origin of apatite in clastic rocks? Sediment. Geol. 1994, 92, 175–196. [Google Scholar] [CrossRef]
- Morton, A.; Yaxley, G. Detrital apatite geochemistry and its application in provenance studies. Spec. Pap. Geol. Soc. Am. 2007, 420, 319–344. [Google Scholar]
- Morton, A.C.; Hallsworth, C. Chapter 7 Stability of Detrital Heavy Minerals During Burial Diagenesis. In Heavy Minerals in Use; Mange, M.A., Wright, D.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 58, pp. 215–245. [Google Scholar]
- Abdullin, F.; Solé, J.; Solari, L.; Shchepetilnikova, V.; Meneses-Rocha, J.J.; Pavlinova, N.; Rodríguez-Trejo, A. Single-grain apatite geochemistry of Permian-Triassic granitoids and Mesozoic and Eocene sandstones from Chiapas, southeast Mexico: Implications for sediment provenance. Int. Geol. Rev. 2016, 58, 1132–1157. [Google Scholar] [CrossRef]
- Sha, L.K.; Chappell, B.W. Apatite chemical composition, determined by electron microprobe and laser-ablation inductively coupled plasma mass spectrometry, as a probe into granite petrogenesis. Geochim. Cosmochim. Acta 1999, 63, 3861–3881. [Google Scholar] [CrossRef]
- Chu, M.F.; Wang, K.L.; Griffin, W.L.; Chung, S.L.; O’Reilly, S.Y.; Pearson, N.J.; Iizuka, Y. Apatite composition: Tracing petrogenetic processes in Transhimalayan granitoids. J. Petrol. 2009, 50, 1829–1855. [Google Scholar] [CrossRef]
- Ismail, R.; Ciobanu, C.L.; Cook, N.J.; Teale, G.S.; Giles, D.; Mumm, A.S.; Wade, B. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide-copper-gold deposit, Yorke Peninsula, South Australia. Lithos 2014, 184–187, 456–477. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Azadbakht, Z.; Lentz, D.R.; Mcfarlane, C.R.M. Apatite Chemical Compositions from Acadian-Related Granitoids of New Brunswick, Canada: Implications for Petrogenesis and Metallogenesis. Minerals 2018, 8, 598. [Google Scholar] [CrossRef]
- Cao, M.; Li, G.; Qin, K.; Seitmuratova, E.Y.; Liu, Y. Major and trace element characteristics of apatites in granitoids from central Kazakhstan: Implications for petrogenesis and mineralization. Resour. Geol. 2012, 62, 63–83. [Google Scholar] [CrossRef]
- Webster, J.D.; Piccoli, P.M. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Mercer, C.N.; Watts, K.E.; Gross, J. Apatite trace element geochemistry and cathodoluminescent textures—A comparison between regional magmatism and the Pea Ridge IOAREE and Boss IOCG deposits, southeastern Missouri iron metallogenic province, USA. Ore Geol. Rev. 2020, 116, 103129. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Davidson, G.J.; Mehrabi, B.; Meffre, S.; Ghazban, F. Significance of apatite REE depletion and monazite inclusions in the brecciated Se-Chahun iron oxide-apatite deposit, Bafq district, Iran: Insights from paragenesis and geochemistry. Chem. Geol. 2011, 281, 253–269. [Google Scholar] [CrossRef]
- Creaser, R.A.; Gray, C.M. Preserved initial 87Sr/86Sr in apatite from altered felsic igneous rocks: A case study from the Middle Proterozoic of South Australia. Geochim. Cosmochim. Acta 1992, 56, 2789–2795. [Google Scholar] [CrossRef]
- Tsuboi, M. The use of apatite as a record of initial 87Sr/86Sr ratios and indicator of magma processes in the Inagawa pluton, Ryoke belt, Japan. Chem. Geol. 2005, 221, 157–169. [Google Scholar] [CrossRef]
- Tsuboi, M.; Suzuki, K. Heterogeneity of initial 87Sr/86Sr ratios within a single pluton: Evidence from apatite strontium isotopic study. Chem. Geol. 2003, 199, 189–197. [Google Scholar] [CrossRef]
- Farver, J.R.; Giletti, B.J. Oxygen and strontium diffusion kinetics in apatite and potential applications to thermal history determinations. Geochim. Cosmochim. Acta 1989, 53, 1621–1631. [Google Scholar] [CrossRef]
- Nabatian, G.; Rastad, E.; Neubauer, F.; Honarmand, M.; Ghaderi, M. Iron and Fe–Mn mineralisation in Iran: Implications for Tethyan metallogeny. Aust. J. Earth Sci. 2015, 62, 211–241. [Google Scholar] [CrossRef]
- Mokhtari, M.A.A.; Zadeh, G.H.; Emami, M.H. Genesis of iron-apatite ores in Posht-e-Badam Block (Central Iran) using REE geochemistry. J. Earth Syst. Sci. 2013, 122, 795–807. [Google Scholar] [CrossRef]
- Azizi, H.; Mehrabi, B.; Akbarpour, A. Genesis of tertiary magnetite-apatite deposits, Southeast of Zanjan, Iran. Resour. Geol. 2009, 59, 330–341. [Google Scholar] [CrossRef]
- Alipour, S. Mine, Report of Semi-quantitative Exploration in Ghareh Bagh Mica; Industries and Mines Company of Western Azarbayjan: Urmia, Iran, 1985. [Google Scholar]
- Ghafarzadeh, A. Geological Report of Ghare Bagh Mica Mine, Western Azarbayjan; Campany of Exploration Servies: Tehran, Iran, 1988. [Google Scholar]
- Moghadam, H.S.; Li, X.-H.; Ling, X.-X.; Stern, R.J.; Khedr, M.Z.; Chiaradia, M.; Ghorbani, G.; Arai, S.; Tamura, A. Devonian to Permian evolution of the Paleo-Tethys Ocean: New evidence from U-Pb zircon dating and Sr-Nd-Pb isotopes of the Darrehanjir–Mashhad “ophiolites”, NE Iran. Gondwana Res. 2015, 28, 781–799. [Google Scholar] [CrossRef]
- Badr, A.; Davoudian, A.R.; Shabanian, N.; Azizi, H.; Asahara, Y.; Neubauer, F.; Dong, Y.; Yamamoto, K. A- and I-type metagranites from the North Shahrekord Metamorphic Complex, Iran: Evidence for Early Paleozoic post-collisional magmatism. Lithos 2018, 300, 86–104. [Google Scholar] [CrossRef]
- Azizi, H.; Chung, S.-L.; Tanaka, T.; Asahara, Y. Isotopic dating of the Khoy metamorphic complex (KMC), northwestern Iran: A significant revision of the formation age and magma source. Precambrian Res. 2011, 185, 87–94. [Google Scholar] [CrossRef]
- Daneshvar, N.; Maanijou, M.; Azizi, H.; Asahara, Y. Petrogenesis and geodynamic implications of an Ediacaran (550 Ma) granite complex (metagranites), southwestern Saqqez, northwest Iran. J. Geodyn. 2019, 132, 101669. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Stockli, D.F.; Horton, B.K.; Axen, G.J.; Stockli, L.D.; Grove, M.; Schmitt, A.K.; Walker, J.D. U-Pb zircon geochronology of late Neoproterozoic–Early Cambrian granitoids in Iran: Implications for paleogeography, magmatism, and exhumation history of Iranian basement. Tectonophysics 2008, 451, 71–96. [Google Scholar] [CrossRef]
- Azizi, H.; Kazemi, T.; Asahara, Y. A-type granitoid in Hasansalaran complex, northwestern Iran: Evidence for extensional tectonic regime in northern Gondwana in the Late Paleozoic. J. Geodyn. 2017, 108, 56–72. [Google Scholar] [CrossRef]
- Moghadam, H.S.; Li, X.-H.; Ling, X.-X.; Stern, R.J.; Santos, J.F.; Meinhold, G.; Ghorbani, G.; Shahabi, S. Petrogenesis and tectonic implications of Late Carboniferous A-type granites and gabbronorites in NW Iran: Geochronological and geochemical constraints. Lithos 2015, 212, 266–279. [Google Scholar] [CrossRef]
- Ahankoub, M.; Jahangiri, A.; Asahara, Y.; Moayyed, M. Petrochemical and Sr-Nd isotope investigations of A-type granites in the east of Misho, NW Iran. Arab. J. Geosci. 2013, 6, 4833–4849. [Google Scholar] [CrossRef]
- Azizi, H.; Jahangiri, A. Cretaceous subduction-related volcanism in the northern Sanandaj-Sirjan Zone, Iran. J. Geodyn. 2008, 45, 178–190. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Corfu, F.; Masoudi, F.; Mehrabi, B.; Mohajjel, M. U-Pb dating and emplacement history of granitoid plutons in the northern Sanandaj-Sirjan Zone, Iran. J. Asian Earth Sci. 2011, 41, 238–249. [Google Scholar] [CrossRef]
- Mazhari, S.A.; Bea, F.; Amini, S.; Ghalamghash, J.; Molina, J.F.; Montero, P.; Scarrow, J.H.; Williams, I.S. The Eocene bimodal Piranshahr massif of the Sanandaj-Sirjan Zone, NW Iran: A marker of the end of the collision in the Zagros orogen. J. Geol. Soc. London. 2009, 166, 53–69. [Google Scholar] [CrossRef]
- Stöcklin, J. Structural history and tectonics of Iran1: A review. Am. Assoc. Pet. Geol. Bull. 1968, 52, 1229–1258. [Google Scholar]
- Eftekhar Nezhad, J.; Gorashi, M.; Mehrparto, M.; Arshadi, S.; Zohrehbakhsh, A.; Bolouchi, M.H.; Siaidi, A. The Tabriz-Poldasht Quadrangle Map (Scale: 1: 250,000): Geological Survey and Mineral Exploration of Iran 1989, No. NJ 38-7; Geological Survey of Iran: Tehran, Iran, 1989. [Google Scholar]
- Eftekharnejad, J. The Mahabad Quadrangle Map (Scale 1: 250,000): Geological Survey and Mineral Exploration of Iran 1973, No. NJ 38-15; Geological Survey of Iran: Tehran, Iran, 1973. [Google Scholar]
- Khodabandeh, A.A.; Amini-Fazl, A. Geological Map of Tasuj Sheet (1: 100,000), No. 5066; Geological Survey of Iran: Tehran, Iran, 1993. [Google Scholar]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and A Global Perspective. In Non-Renewable Resource Issues: Geoscientific and Societal Challenges; Sinding-Larsen, R., Wellmer, F., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 131–155. ISBN 9789048186792. [Google Scholar]
- McDonough, W.F.; Sun, S.-S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Li, Z.; Duan, D.; Jiang, S.; Ma, Y.; Yuan, H. In situ Analysis of Major Elements, Trace Elements and Sr Isotopic Compositions of Apatite from the Granite in the Chengchao Skarn-Type Fe Deposit, Edong Ore District: Implications for Petrogenesis and Mineralization. J. Earth Sci. 2018, 29, 295–306. [Google Scholar] [CrossRef]
- Bédard, J.H. Parental magmas of the Nain Plutonic Suite anorthosites and mafic cumulates: A trace element modelling approach. Contrib. Mineral. Petrol. 2001, 141, 747–771. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M.; Drake, M.J. Ion microprobe study of plagioclase-basalt partition experiments at natural concentration levels of trace elements. Geochim. Cosmochim. Acta 1998, 62, 1175–1193. [Google Scholar] [CrossRef]
- Ayers, J.C.; Watson, E.B. Apatite/fluid partitioning of rare-earth elements and strontium: Experimental results at 1.0 GPa and 1000 °C and application to models of fluid-rock interaction. Chem. Geol. 1993, 110, 299–314. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Li, H.; Ding, X.; Wu, K.; Guo, J.; Liu, J.Q.; Sun, W.D. Formation of A-type granites in the Lower Yangtze River Belt: A perspective from apatite geochemistry. Lithos 2018, 304–307, 125–134. [Google Scholar] [CrossRef]
- Keppler, H. Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature 1996, 380, 237–240. [Google Scholar] [CrossRef]
- Reed, M.J.; Candela, P.A.; Piccoli, P.M. The distribution of rare earth elements between monzogranitic melt and the aqueous volatile phase in experimental investigations at 800 °C and 200 MPa. Contrib. Mineral. Petrol. 2000, 140, 251–262. [Google Scholar] [CrossRef]
- Borchert, M.; Wilke, M.; Schmidt, C.; Cauzid, J.; Tucoulou, R. Partitioning of Ba, La, Yb and Y between haplogranitic melts and aqueous solutions: An experimental study. Chem. Geol. 2010, 276, 225–240. [Google Scholar] [CrossRef]
- Adlakha, E.; Hanley, J.; Falck, H.; Boucher, B. The origin of mineralizing hydrothermal fluids recorded in apatite chemistry at the Cantung W–Cu skarn deposit, NWT, Canada. Eur. J. Mineral. 2018, 30, 1095–1113. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Rare Earth Element Behaviour in Apatite from the Olympic Dam Cu–U–Au–Ag Deposit, South Australia. Minerals 2017, 7, 135. [Google Scholar] [CrossRef]
- Chen, M.; Bagas, L.; Liao, X.; Zhang, Z.; Li, Q. Hydrothermal apatite SIMS Th–Pb dating: Constraints on the timing of low-temperature hydrothermal Au deposits in Nibao, SW China. Lithos 2019, 324, 418–428. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Advay, M.; Ashraphi, N. K/Ar dating of Ghareh-Bagh and Yarim-Ghayeh mica mines (NW Iran), with reference to their origin. J. basic Sci. Islam. Azad Univ. 2015, 25, 71–79. [Google Scholar]
- Lukanin, O.A.; Dernov-Pegarev, V.F. Partitioning of rare earth elements between an aqueous chloride fluid phase and melt during the decompression-driven degassing of granite magmas. Geochem. Int. 2010, 48, 961–978. [Google Scholar] [CrossRef]
- Fleischer, M.; Altschuler, Z.S. The lanthanides and yttrium in minerals of the apatite group - an analysis of the available data. Neues Jahrb. Fuer Mineral. Monatshefte 1986, 18, 467–480. [Google Scholar]
- Hammerli, J.; Kemp, A.I.S.; Spandler, C. Neodymium isotope equilibration during crustal metamorphism revealed by in situ microanalysis of REE-rich accessory minerals. Earth Planet. Sci. Lett. 2014, 392, 133–142. [Google Scholar] [CrossRef]
- Henrichs, I.A.; O’Sullivan, G.; Chew, D.M.; Mark, C.; Babechuk, M.G.; McKenna, C.; Emo, R. The trace element and U-Pb systematics of metamorphic apatite. Chem. Geol. 2018, 483, 218–238. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Hoboken, NJ, USA, 1985; Volume 1, 312p. [Google Scholar]
- Uher, P.; Ondrejka, M.; Bačík, P.; Broska, I.; Konečný, P. Britholite, monazite, REE carbonates, and calcite: Products of hydrothermal alteration of allanite and apatite in A-type granite from Stupné, Western Carpathians, Slovakia. Lithos 2015, 236, 212–225. [Google Scholar] [CrossRef]
- Eby, G.N. Chemical subdivision of the A-type granitoids: Petrogenetic and tectonic implications. Geology 1992, 20, 641–644. [Google Scholar] [CrossRef]
- Lesnov, F.P. Rare Earth Elements in Ultramafic and Mafic Rocks and Their Minerals: Minor and Accessory Minerals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 324. [Google Scholar]
- Ravindran, A.; Mezger, K.; Balakrishnan, S.; Kooijman, E.; Schmitt, M.; Berndt, J. Initial 87Sr/86Sr as a sensitive tracer of Archaean crust-mantle evolution: Constraints from igneous and sedimentary rocks in the western Dharwar Craton, India. Precambrian Res. 2020, 337, 105523. [Google Scholar] [CrossRef]
- Zafar, T.; Leng, C.B.; Zhang, X.C.; Rehman, H.U. Geochemical attributes of magmatic apatite in the Kukaazi granite from western Kunlun orogenic belt, NW China: Implications for granite petrogenesis and Pb-Zn (-Cu-W) mineralization. J. Geochemical Explor. 2019, 204, 256–269. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Zhou, M.-F.; Gao, J.-F.; Li, X.-C.; Li, J.-W. In situ Sr isotope analysis of apatite by LA-MC-ICPMS: Constraints on the evolution of ore fluids of the Yinachang Fe-Cu-REE deposit, Southwest China. Miner. Depos. 2015, 50, 871–884. [Google Scholar] [CrossRef]
- Betkowski, W.B.; Harlov, D.E.; Rakovan, J.F. Hydrothermal mineral replacement reactions for an apatite-monazite assemblage in alkali-rich fluids at 300–600 °C and 100 MPa. Am. Mineral. 2016, 101, 2620–2637. [Google Scholar] [CrossRef]
- Palma, G.; Barra, F.; Reich, M.; Valencia, V.; Simon, A.C.; Vervoort, J.; Leisen, M.; Romero, R. Halogens, trace element concentrations, and Sr-Nd isotopes in apatite from iron oxide-apatite (IOA) deposits in the Chilean iron belt: Evidence for magmatic and hydrothermal stages of mineralization. Geochim. Cosmochim. Acta 2019, 246, 515–540. [Google Scholar] [CrossRef]
- Elliott, H.A.L.; Wall, F.; Chakhmouradian, A.R.; Siegfried, P.R.; Dahlgren, S.; Weatherley, S.; Finch, A.A.; Marks, M.A.W.; Dowman, E.; Deady, E. Fenites associated with carbonatite complexes: A review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Van Gosen, B.S.; Seal II, R.R.; McCafferty, A.E. A Deposit Model for Carbonatite and Peralkaline Intrusion-Related Rare Earth Element Deposits: Chapter J in Mineral Deposit Models for Resource Assessment; U.S. Geological Survey: Reston, VA, USA, 2014; p. 58.
- Bonin, B. A-type granites and related rocks: Evolution of a concept, problems and prospects. Lithos 2007, 97, 1–29. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Mineral. Petrol. 1987, 95, 407–419. [Google Scholar] [CrossRef]
- Webster, J.D.; Mandeville, C.W. Fluid Immiscibility in Volcanic Environments. Rev. Mineral. Geochem. 2007, 65, 313–362. [Google Scholar] [CrossRef]
- La Cruz, N.L.; Simon, A.C.; Wolf, A.S.; Reich, M.; Barra, F.; Gagnon, J.E. The geochemistry of apatite from the Los Colorados iron oxide–apatite deposit, Chile: Implications for ore genesis. Miner. Depos. 2019, 54, 1143–1156. [Google Scholar] [CrossRef]
- Hautmann, S.; Witham, F.; Christopher, T.; Cole, P.; Linde, A.T.; Sacks, I.S.; Sparks, R.S.J. Strain field analysis on Montserrat (W.I.) as tool for assessing permeable flow paths in the magmatic system of Soufrière Hills Volcano. Geochem. Geophys. Geosystems 2014, 15, 676–690. [Google Scholar] [CrossRef]
- Aoki, K.; Ishiwaka, K.; Kanisawa, S. Fluorine geochemistry of basaltic rocks from continental and oceanic regions and petrogenetic application. Contrib. Mineral. Petrol. 1981, 76, 53–59. [Google Scholar] [CrossRef]
- Clemens, J.D.; Holloway, J.R.; White, A.J.R. Origin of an A-type granite; experimental constraints. Am. Mineral. 1986, 71, 317–324. [Google Scholar]
- Edgar, A.D.; Lloyd, F.E.; Vukadinovic, D. The role of fluorine in the evolution of ultrapotassic magmas. Mineral. Petrol. 1994, 51, 173–193. [Google Scholar] [CrossRef]
- Sallet, R. Fluorine as a tool in the petrogenesis of quartz-bearing magmatic associations: Applications of an improved F–OH biotite–apatite thermometer grid. Lithos 2000, 50, 241–253. [Google Scholar] [CrossRef]






| Sample Name. | AB1 | AB2 | AB3 | AB4 | AS1 | AS2 | AS3 | AS4 | Average |
|---|---|---|---|---|---|---|---|---|---|
| Y | 301 | 415 | 497 | 273 | 270 | 260 | 236 | 250 | 313 |
| La | 1070 | 1270 | 1458 | 982 | 1053 | 977 | 935 | 953 | 1087 |
| Ce | 1993 | 2277 | 2586 | 1824 | 1946 | 1811 | 1721 | 1759 | 1990 |
| Pr | 191 | 219 | 253 | 175 | 184 | 171 | 161 | 166 | 190 |
| Nd | 626 | 739 | 849 | 563 | 587 | 557 | 520 | 544 | 623 |
| Sm | 88.1 | 110 | 129 | 78.3 | 81.2 | 77.1 | 71.0 | 75.3 | 88.7 |
| Eu | 11.9 | 15.8 | 18.7 | 10.4 | 10.6 | 10.1 | 9.46 | 10.2 | 12.1 |
| Gd | 77.2 | 98.0 | 114 | 69.2 | 66.8 | 67.8 | 59.4 | 62.6 | 76.9 |
| Tb | 9.31 | 12.6 | 15.0 | 8.49 | 8.21 | 8.06 | 7.43 | 7.68 | 9.60 |
| Dy | 52.7 | 71.6 | 86.6 | 47.9 | 45.7 | 46.5 | 40.8 | 43.3 | 54.4 |
| Ho | 10.2 | 14.3 | 17.3 | 9.46 | 9.25 | 8.93 | 7.97 | 8.41 | 10.7 |
| Er | 28.3 | 39.2 | 47.9 | 25.8 | 25.1 | 24.3 | 22.1 | 23.2 | 29.5 |
| Tm | 3.66 | 5.16 | 6.43 | 3.54 | 3.19 | 3.20 | 2.97 | 3.01 | 3.90 |
| Yb | 22.2 | 29.0 | 34.8 | 19.2 | 19.5 | 19.4 | 17.5 | 18.3 | 22.5 |
| Lu | 2.83 | 3.52 | 4.24 | 2.68 | 2.51 | 2.53 | 2.35 | 2.49 | 2.89 |
| ∑REE | 4186 | 4904 | 5619 | 3819 | 4042 | 3784 | 3579 | 3676 | 4201 |
| La/Nd | 1.71 | 1.72 | 1.72 | 1.74 | 1.80 | 1.75 | 1.80 | 1.75 | 1.75 |
| Ce/YbN | 23.6 | 20.6 | 19.5 | 24.9 | 26.2 | 24.6 | 25.8 | 25.2 | 23.8 |
| (La/Yb)N | 32.7 | 29.7 | 28.5 | 34.7 | 36.7 | 34.3 | 36.3 | 35.3 | 33.5 |
| (La/Sm)N | 7.58 | 7.22 | 7.07 | 7.83 | 8.10 | 7.91 | 8.23 | 7.90 | 7.73 |
| (La/Gd)N | 11.6 | 10.9 | 10.7 | 11.9 | 13.2 | 12.1 | 13.2 | 12.8 | 12.1 |
| (Gd/Yb)N | 2.81 | 2.73 | 2.66 | 2.92 | 2.77 | 2.83 | 2.74 | 2.76 | 2.78 |
| LREE | 3968 | 4615 | 5274 | 3622 | 3851 | 3593 | 3409 | 3497 | 3979 |
| HREE | 218 | 289 | 345 | 197 | 191 | 191 | 170 | 179 | 223 |
| HREE/LREE | 18.2 | 16.0 | 15.3 | 18.4 | 20.2 | 18.8 | 20.1 | 19.5 | 18.3 |
| Eu/Eu*(1) | 0.44 | 0.46 | 0.47 | 0.43 | 0.44 | 0.42 | 0.44 | 0.45 | 0.45 |
| Ce/Ce*(2) | 1.07 | 1.04 | 1.03 | 1.06 | 1.07 | 1.07 | 1.07 | 1.07 | 1.06 |
| Y/Y*(3) | 0.95 | 0.96 | 0.95 | 0.95 | 0.97 | 0.94 | 0.96 | 0.96 | 0.95 |
| Sample | AB1 | AB2 | AB3 | AB4 | AS1 | AS2 | AS3 | AS4 |
|---|---|---|---|---|---|---|---|---|
| 87Sr/86Sr(p) | 0.709182 | 0.709449 | 0.709501 | 0.709244 | 0.709250 | 0.709211 | 0.709170 | 0.709184 |
| 1 sigma error | 0.000006 | 0.000006 | 0.000006 | 0.000008 | 0.000006 | 0.000006 | 0.000006 | 0.000007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshvar, N.; Azizi, H.; Asahara, Y.; Tsuboi, M.; Hosseini, M. Rare Earth Elements and Sr Isotope Ratios of Large Apatite Crystals in Ghareh Bagh Mica Mine, NW Iran: Tracing for Petrogenesis and Mineralization. Minerals 2020, 10, 833. https://doi.org/10.3390/min10090833
Daneshvar N, Azizi H, Asahara Y, Tsuboi M, Hosseini M. Rare Earth Elements and Sr Isotope Ratios of Large Apatite Crystals in Ghareh Bagh Mica Mine, NW Iran: Tracing for Petrogenesis and Mineralization. Minerals. 2020; 10(9):833. https://doi.org/10.3390/min10090833
Chicago/Turabian StyleDaneshvar, Narges, Hossein Azizi, Yoshihiro Asahara, Motohiro Tsuboi, and Mahdi Hosseini. 2020. "Rare Earth Elements and Sr Isotope Ratios of Large Apatite Crystals in Ghareh Bagh Mica Mine, NW Iran: Tracing for Petrogenesis and Mineralization" Minerals 10, no. 9: 833. https://doi.org/10.3390/min10090833
APA StyleDaneshvar, N., Azizi, H., Asahara, Y., Tsuboi, M., & Hosseini, M. (2020). Rare Earth Elements and Sr Isotope Ratios of Large Apatite Crystals in Ghareh Bagh Mica Mine, NW Iran: Tracing for Petrogenesis and Mineralization. Minerals, 10(9), 833. https://doi.org/10.3390/min10090833








