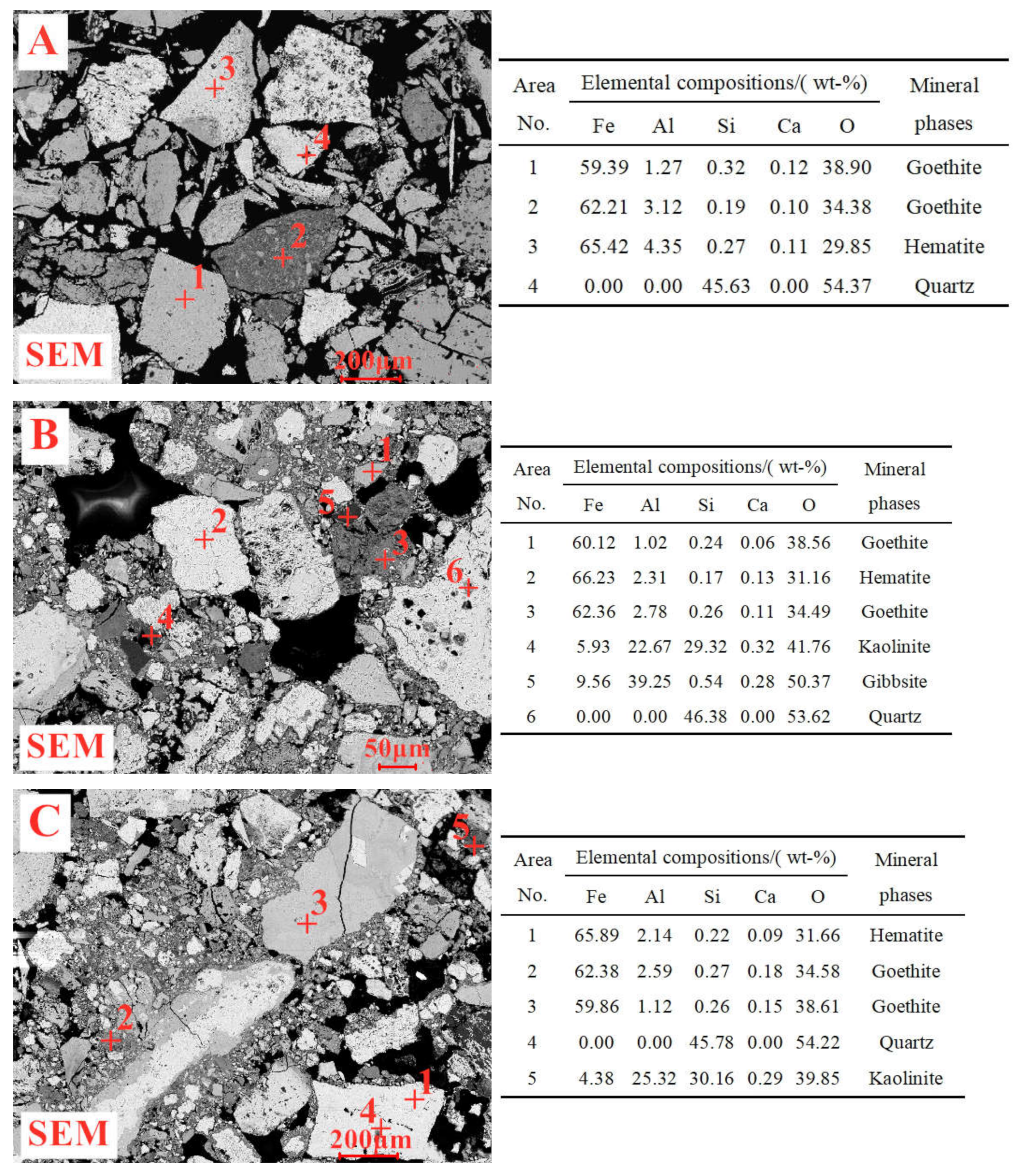
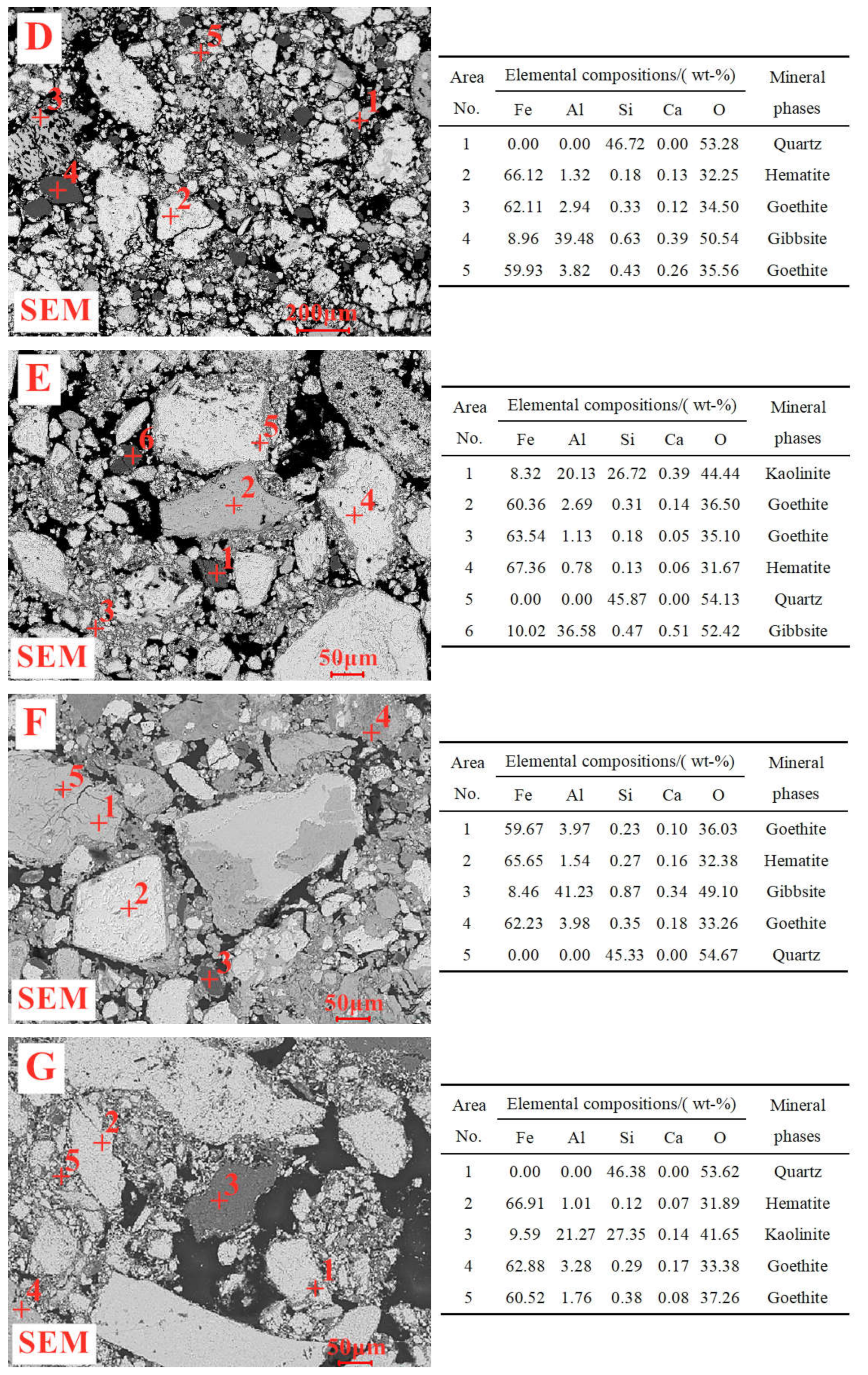
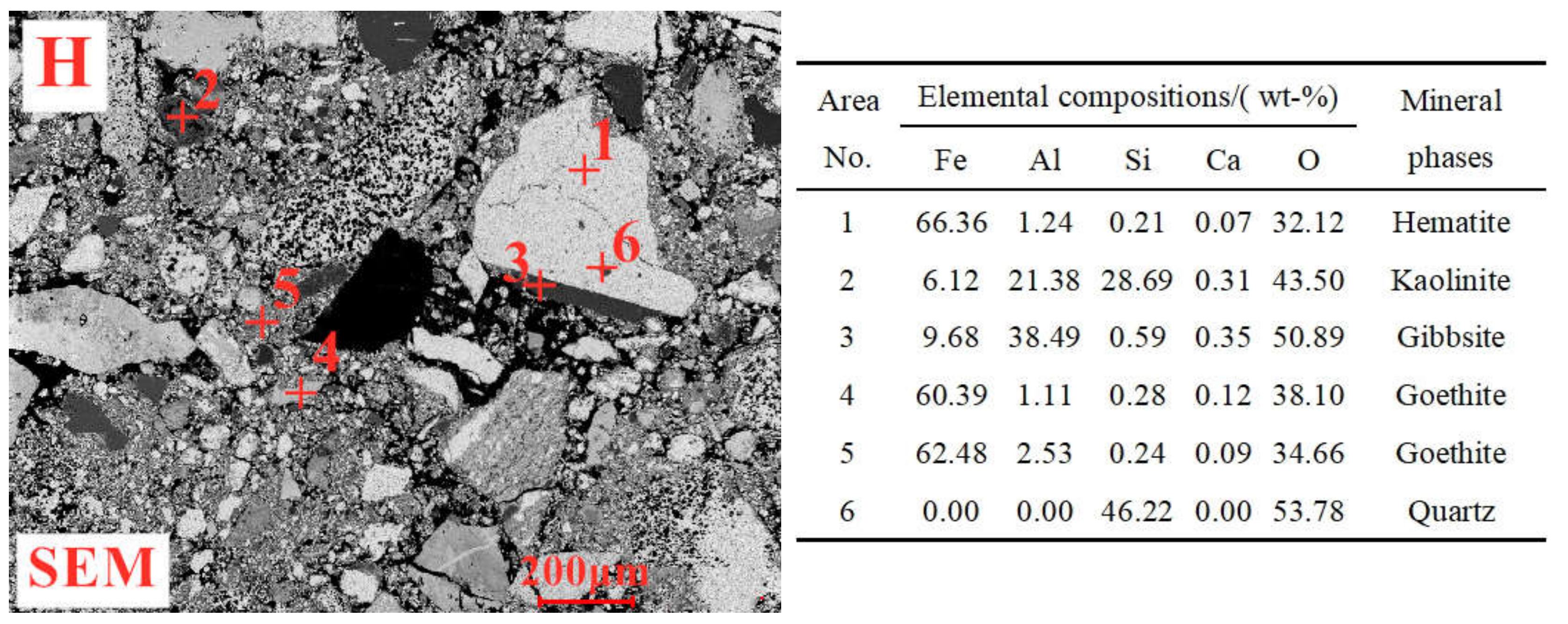
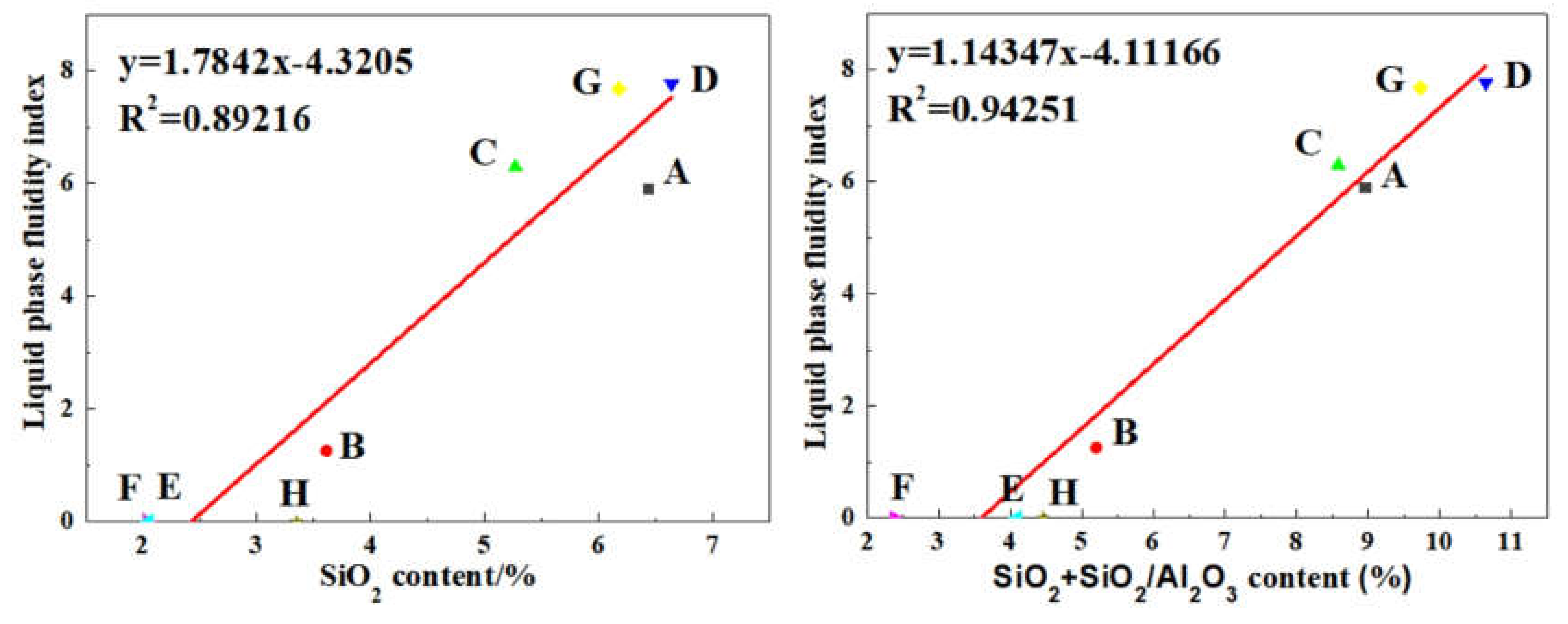
1. Introduction
Due to the huge steel production and high iron ores mining cost in China, over 80% of the iron ores are imported from Australia, Brazil, South Africa, and India to meet the growing needs of steel production in China according to the data of National Bureau of Statistics. However, with the depletion of high-grade iron ore resources, alumina concentration of the imported iron ores, particularly for Indian iron ores, is increasing gradually leading to the increment in Al
2O
3 content of iron ore sinter, which constitutes a major proportion of blast furnace burden [
1,
2,
3]. This would lead to the deterioration of gas permeability in blast furnace, the decrease of slag fluidity and reducibility of sinter and the increase of coke consumption and sulphur load [
4,
5,
6]. It is not only deleterious to the blast furnace smelting process but also has a negative impact on steel quality in the subsequent steelmaking process. Hence, it is essential to effectively utilize the abundant high-alumina iron ores with low iron grade.
As confirmed in the literatures [
7,
8,
9,
10,
11,
12], the increase of Al
2O
3 content is expected to be detrimental to the sintering performance of iron ores due to the low reactivity of alumina bearing minerals and high viscosity of primary melts. Therefore, it is necessary to investigate the action mechanism of alumina on iron ore sintering. As is well known, high-temperature characteristics of iron ores are identified as the basic indexes to evaluate the impact of iron ore properties on the sintering process as well as the output and quality of sinter through the interaction and consolidation between iron ores and fluxes, which mainly include assimilability, liquid phase fluidity, and characteristics of the bonding matrix [
13,
14,
15,
16,
17]. The assimilability is identified as the ability of iron ore powders reacting with CaO during sintering, indicating the difficulty of liquid phase formation in the sintering process [
18,
19]. Liquid phase fluidity represents the effective bonding behavior of liquid phase [
20,
21]. Characteristics of the bonding matrix act as the generative properties of the bonding matrix mainly including its morphology, component, and formation amount [
22,
23,
24]. The product sinter with high strength and good reducibility is desired for blast furnace production. The iron ore sintering process is mainly subject to the bonding phases which can effectively bond with solid phases such as hematite and magnetite to form the product sinter with good strength. In addition, it is well known that sinter strength is determined by the self-strength of the bonding phases and consolidation behavior of solid phases by liquid phases [
25]. In addition, silico-ferrite of calcium and alumina (SFCA) is regarded as the most desirable bonding phase formed from a partly liquid state due to its high self-strength and good reducibility [
26]. Thus, the formation ability and fluidity of liquid phase and characteristics of the bonding matrix are the key factors on iron ore sintering. Great importance should be given to the influence of alumina on high-temperature characteristics of iron ores.
Many researches on high-temperature characteristics of iron ores with various alumina concentration have been carried out [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. It is found that high Al
2O
3 content tends to promote the lowest assimilation temperature and namely weakens the assimilability [
18,
19]. Increased alumina concentration increases the viscosity and decreases the fluidity of liquid phase [
20,
21,
27,
28]. According to the previous studies [
22,
23,
24,
29], the increasing Al
2O
3 content contributes to the formation of SFCA, leading to a higher proportion of SFCA in sinter. However, the excessive increase of Al
2O
3 content (>1.0%) is expected to result in a drastic deterioration in the properties of SFCA. Namely, the amount of sheet-like, columnar, and blocky SFCA with lower mechanical strength is increased significantly, while a substantial reduction in the amount of dendritic and eutectic SFCA with higher strength is detected [
30,
31,
32,
33]. Taking into account the impact of alumina concentration on high-temperature characteristics and the different alumina types in iron ores, considerable emphasis has therefore been placed on the influence of alumina types on high-temperature characteristics recently. As indicated in the reference [
34], Sierra Leone ore, a high alumina iron ore containing alumina in the form of alumogoethite and gibbsite, possessed poor assimilability and liquid phase fluidity. This was further supported by the investigations [
35,
36]. On this basis, it was proved that gibbsite (Al(OH)
3) was more conducive to assimilability than kaolinite (Al
2O
3·2SiO
2·2H
2O) [
37]. In addition, gibbsite gangues yielded low-Al
2O
3-SFCA with high liquid phase fluidity, while kaolinite gangues produced high-Al
2O
3-SFCA with low liquid phase fluidity. Furthermore, the relative effect of gibbsite, kaolinite, and aluminous goethite ([Al
xFe
(1−x)]OOH) as alumina sources on formation mechanisms of SFCA was studied [
38]. It was demonstrated that kaolinite was favorable to maximize the formation of higher strength SFCA followed by aluminous goethite and gibbsite in turn, attributed to the different reactivity of various types of alumina.
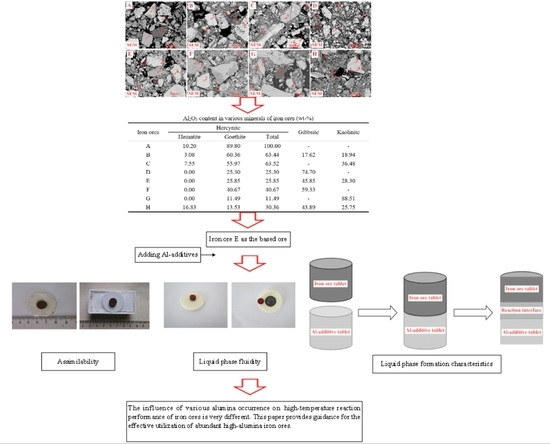
However, there are still many problems to be settled. On the one hand, although some researchers are paying attention to the influence of alumina type recently, they always expound the action mechanism of different alumina types by adopting some kinds of alumina-rich iron ores. This would lead to ambiguous results due to that the used iron ores generally possess several types of alumina such as kaolinite, gibbsite, and hercynite. Hence, as researchers aim at the influence of one type of alumina, other types of alumina could cause interference and it would be hard to accurately clarify the influence of specific alumina type. On the other hand, many investigations are mainly about the alumina types of kaolinite, hercynite, or gibbsite while other alumina types such as free state alumina and diaspore are rarely involved. The influence of alumina concentration and type on assimilability, liquid phase fluidity, and characteristics of the bonding matrix has not been simultaneously investigated. The previous studies generally focus on the influence of alumina concentration and type on one or two high-temperature characteristics. Comprehensive investigations have not been conducted to clearly elucidate the influence of all alumina types on high-temperature characteristics of iron ores. In order to solve the problems above, a new research methodology has been proposed in this paper to investigate the influence of alumina concentration and type on the high-temperature characteristics of iron ores by five Al-additives substituting for various types of alumina including free state alumina, gibbsite, kaolinite, hercynite, and diaspore, respectively. Each Al-additive only contains one type of alumina, which contributes to clearly distinguish the influence of different types of alumina. Thus, we can identify the alumina types which are more beneficial for iron ore sintering. This would also be favorable to select the appropriate alumina-rich iron ores for sinter production.
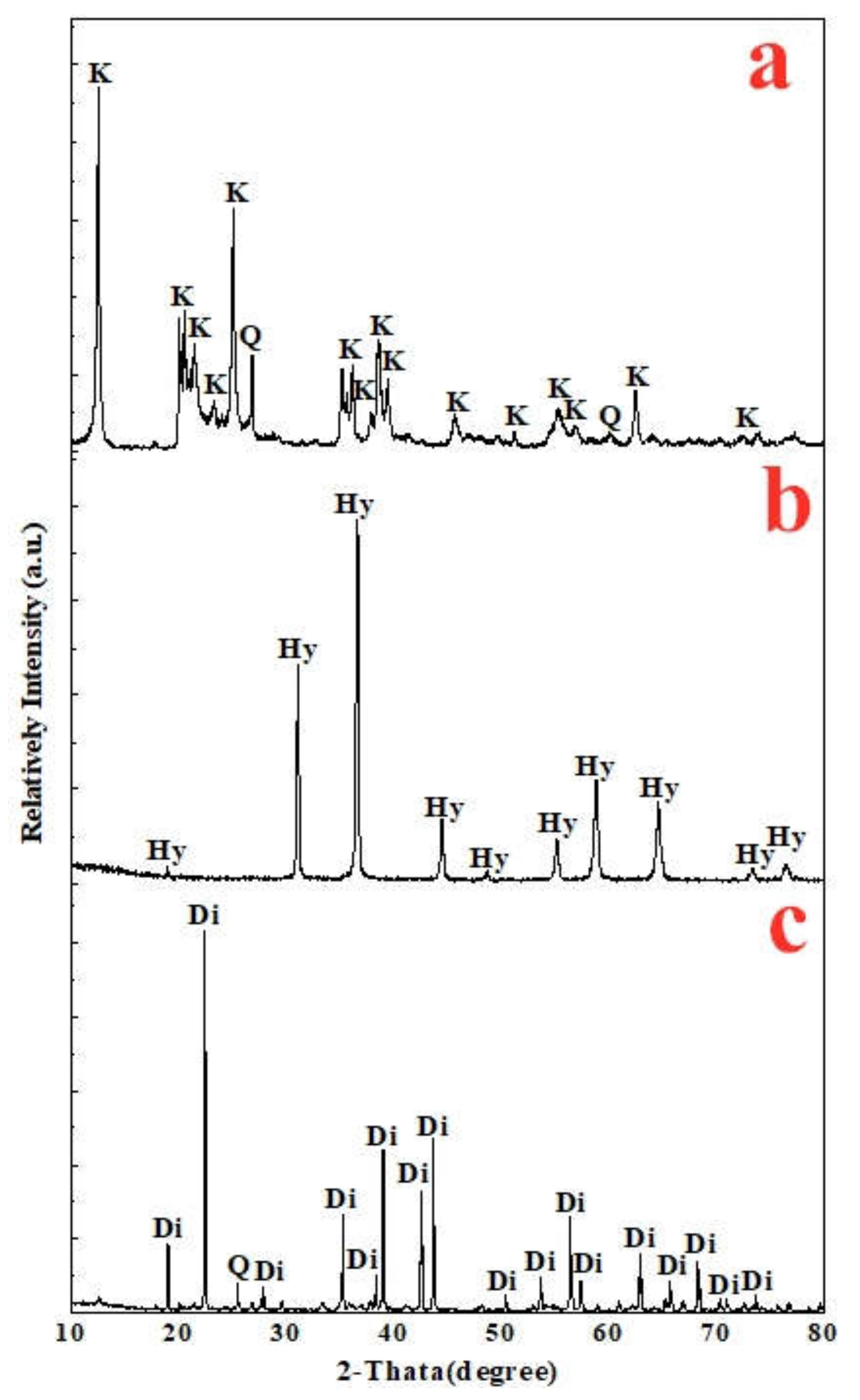
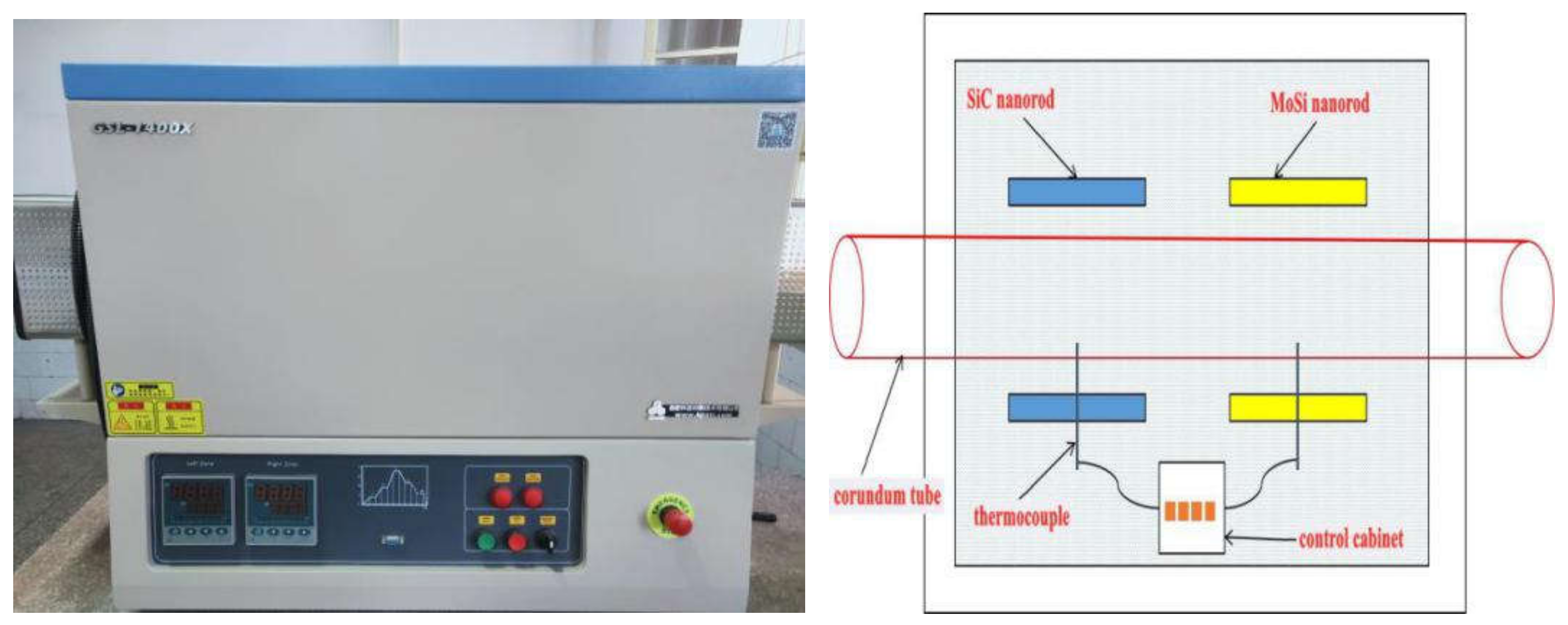
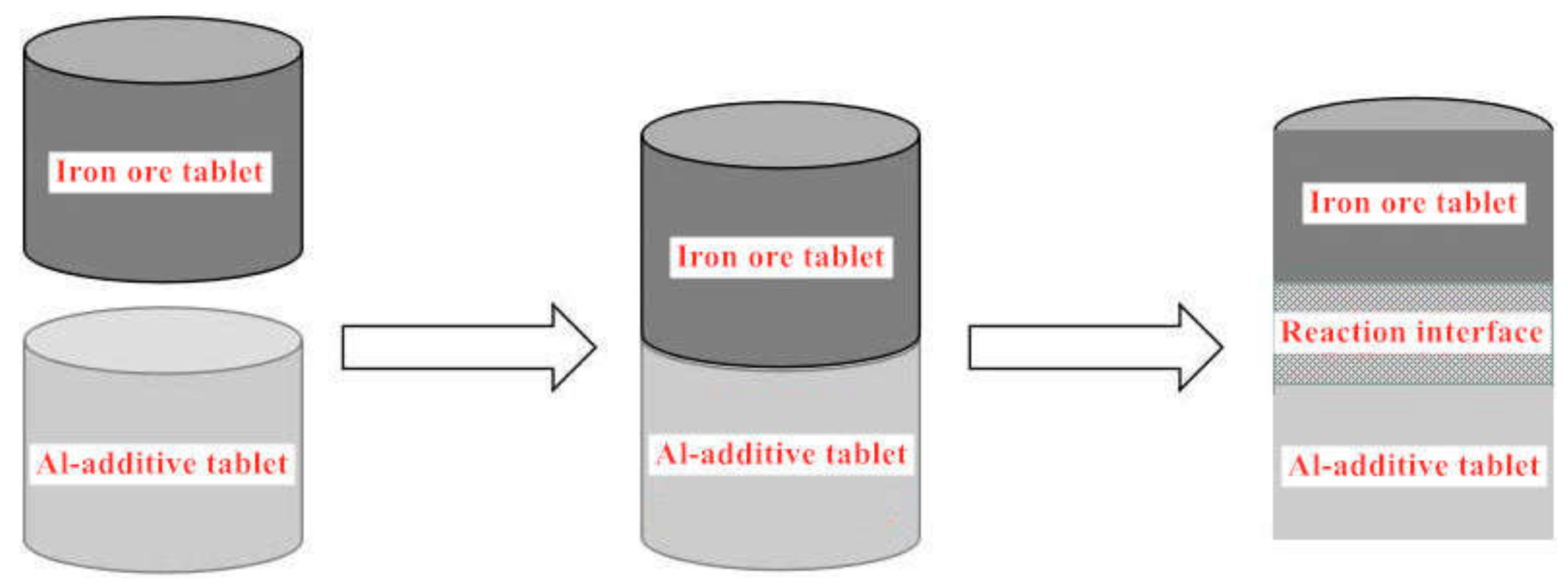
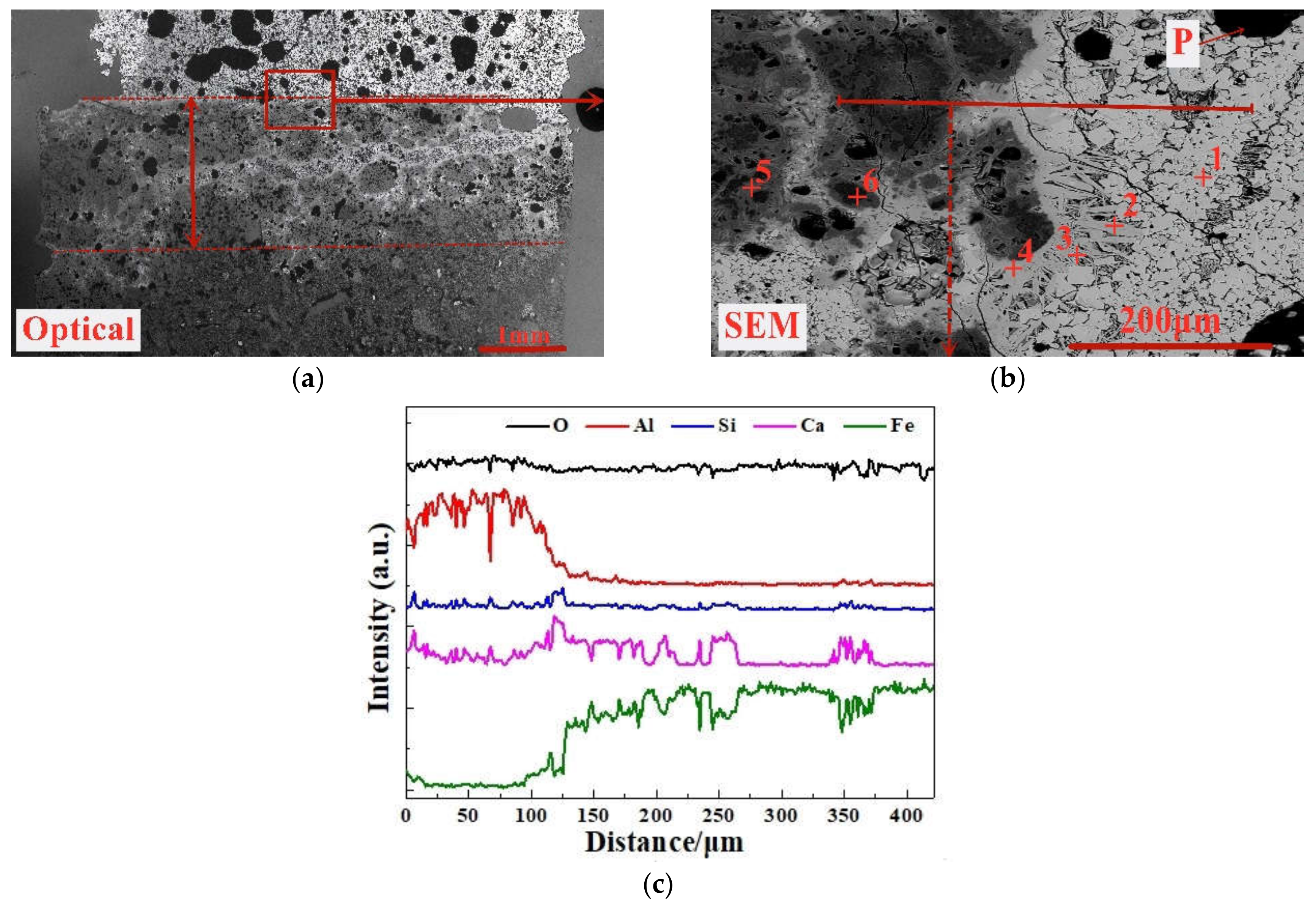
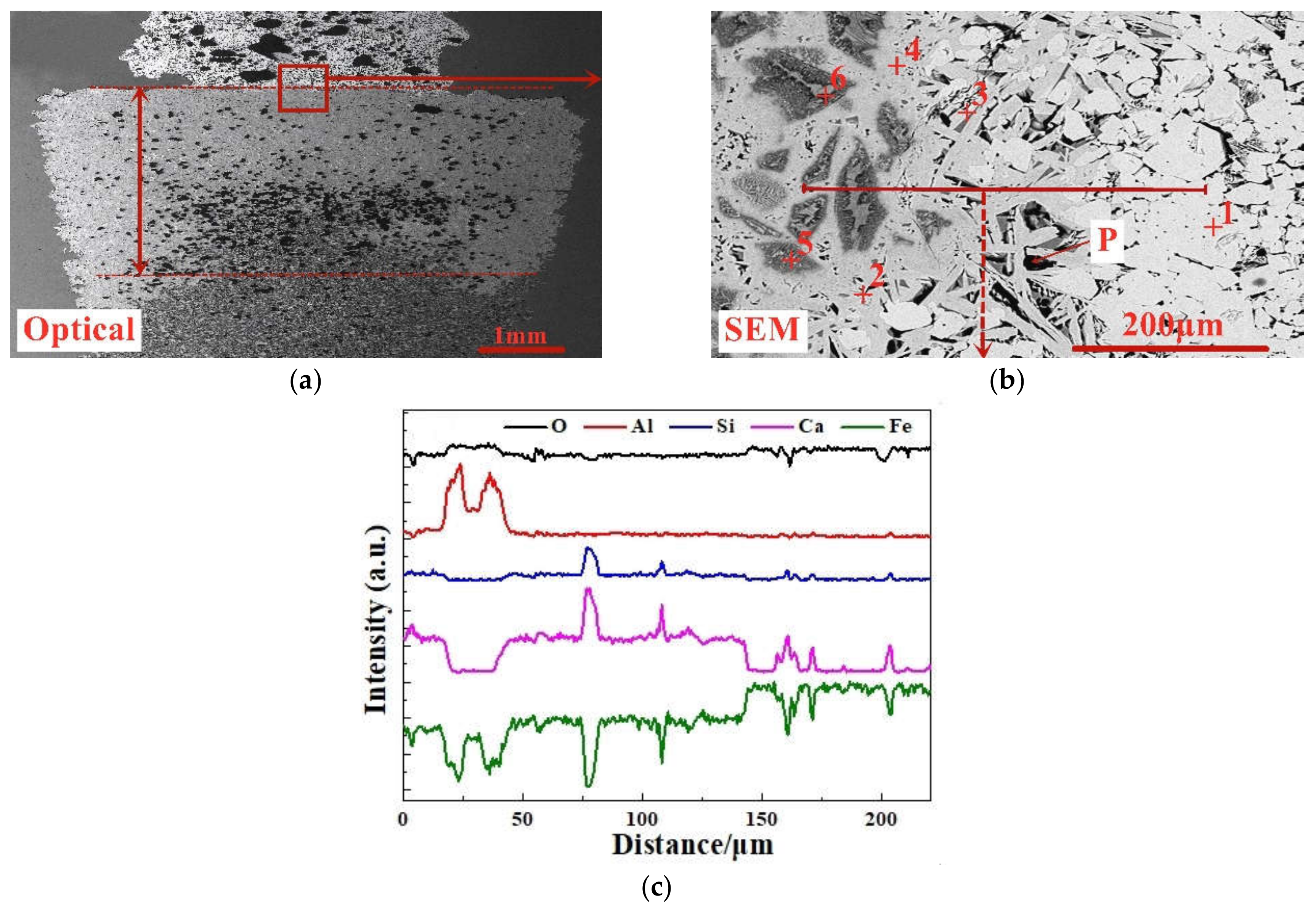
In this paper, based on the analyses of eight imported iron ores widely used in the steel industry of China, the differences of high-temperature characteristics of the eight iron ores including assimilability, liquid phase fluidity, and characteristics of the bonding matrix were ascertained, and the influence of five aluminum types, i.e., free state alumina, gibbsite, kaolinite, hercynite, and diaspore, on high-temperature characteristics was investigated comprehensively via analogue sintering tests conducted in the horizontal tube furnace to provide good guidance for the rational utilization of the abundant high-alumina iron ores.
4. Conclusions
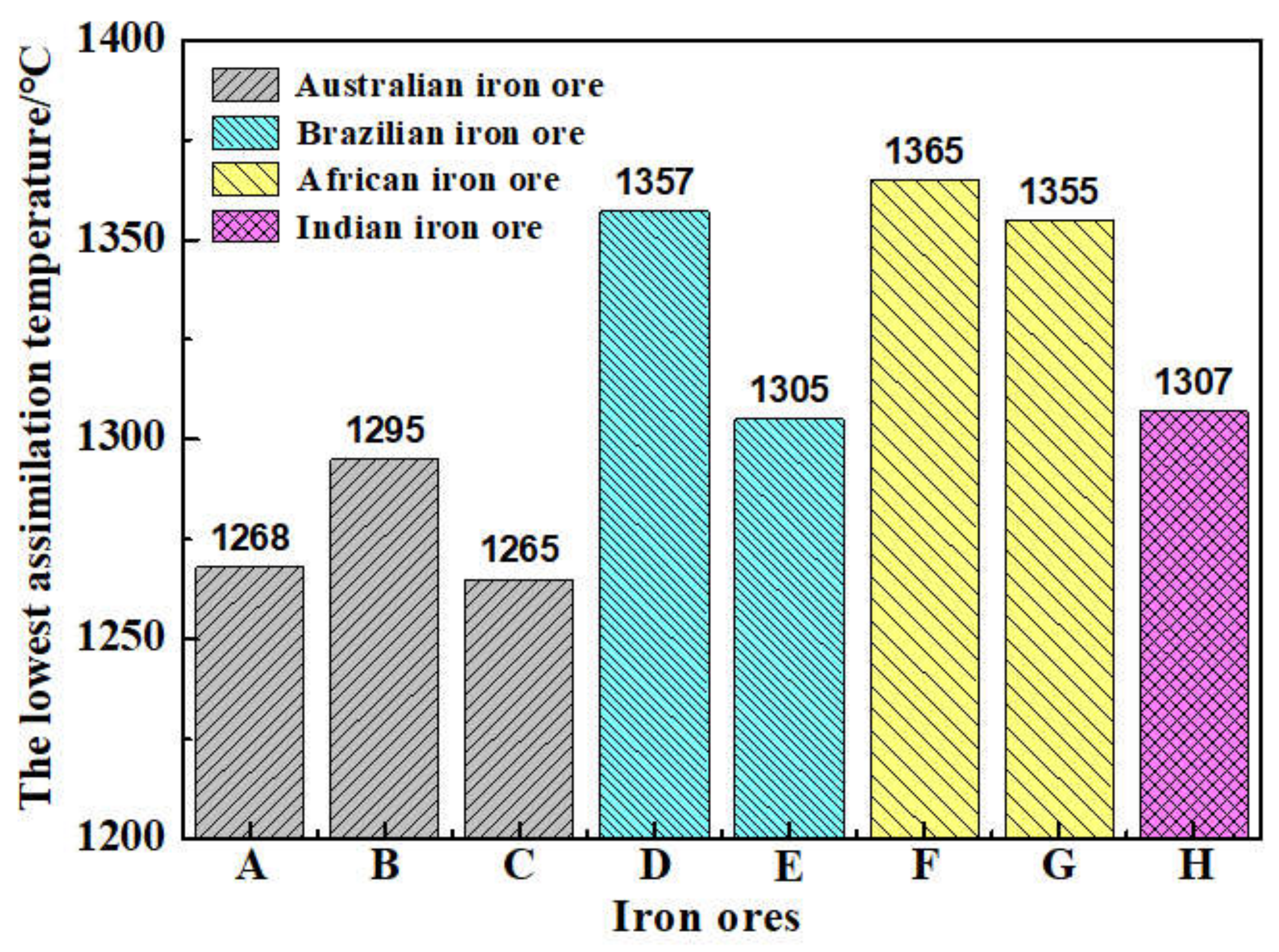
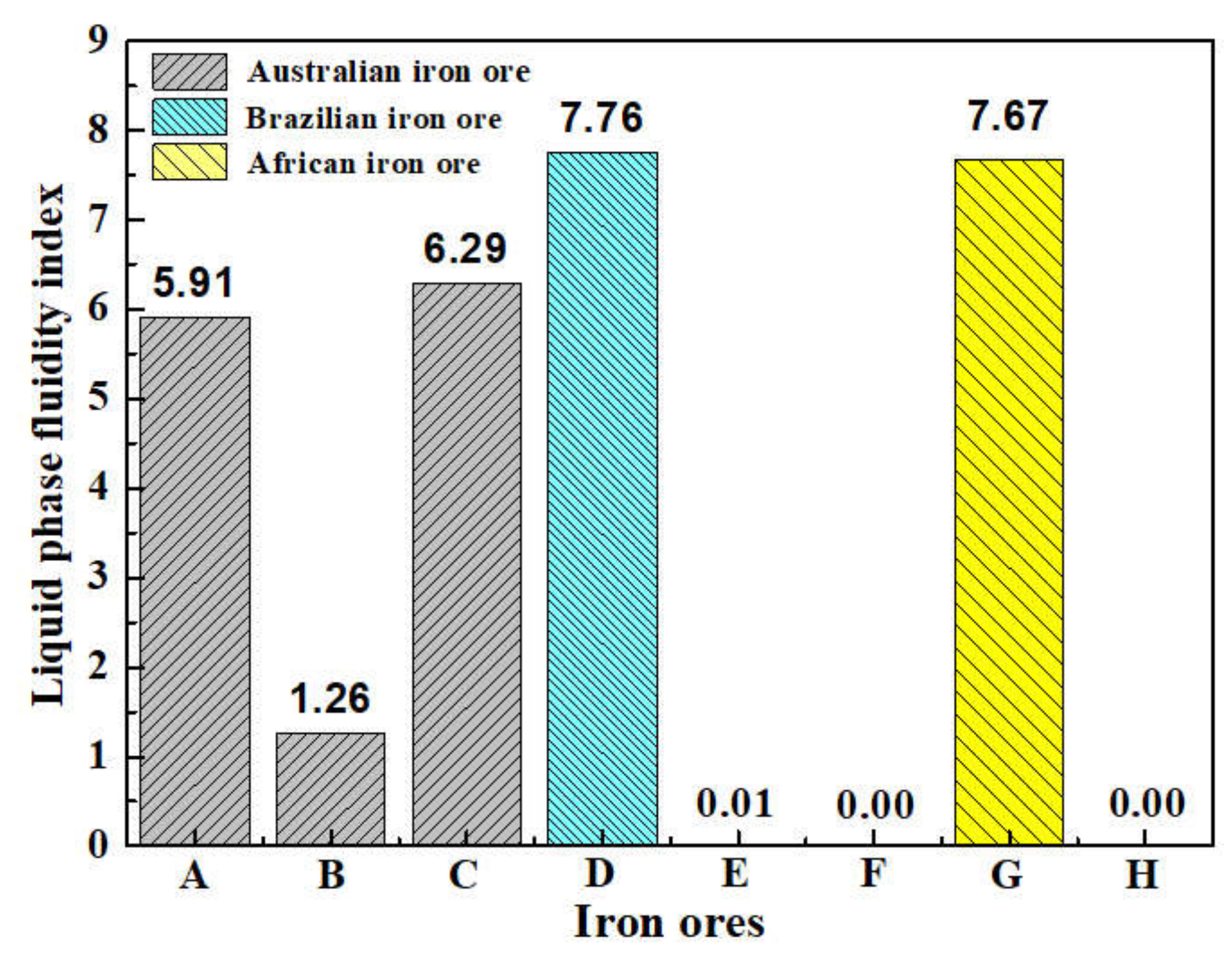
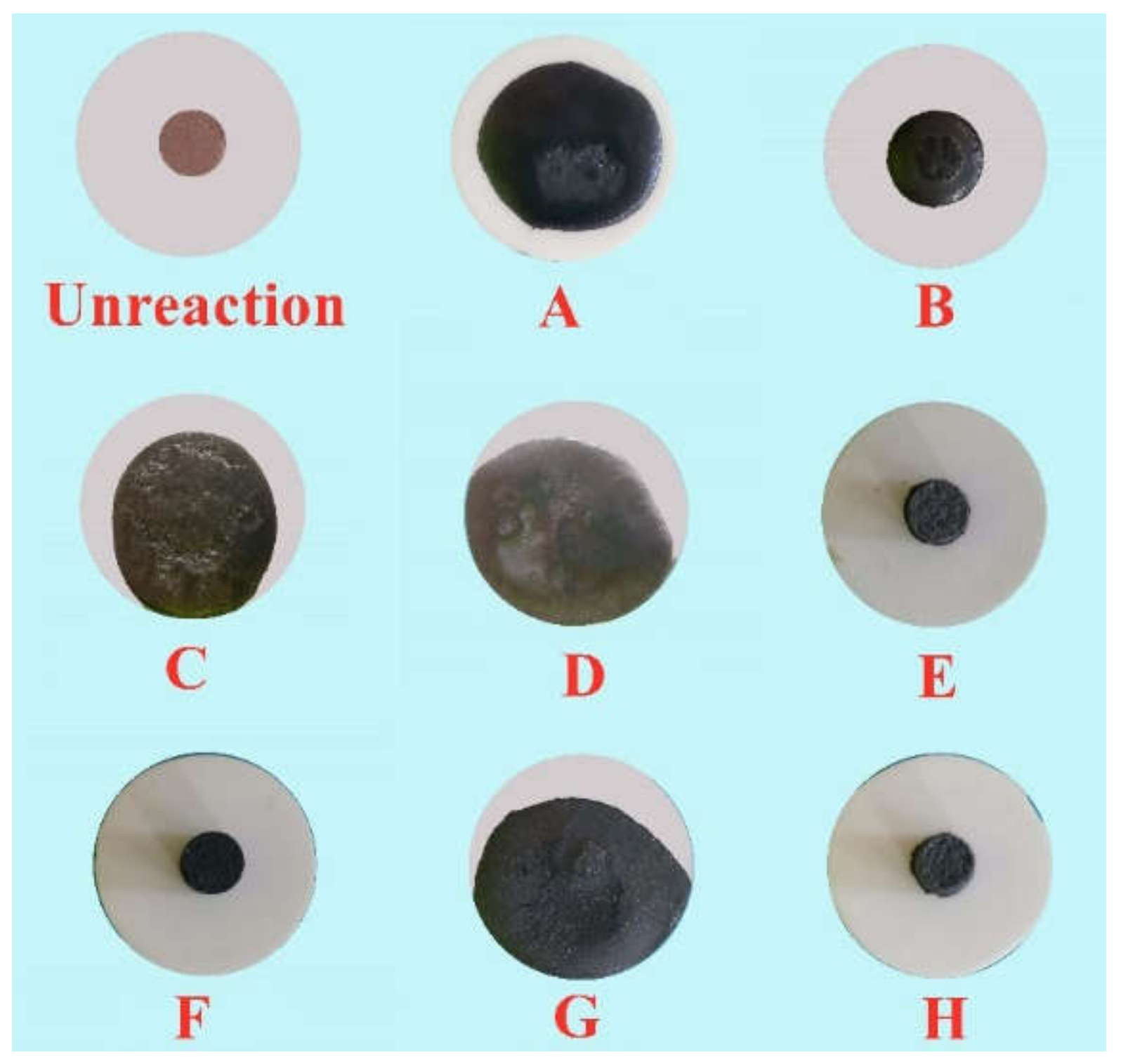
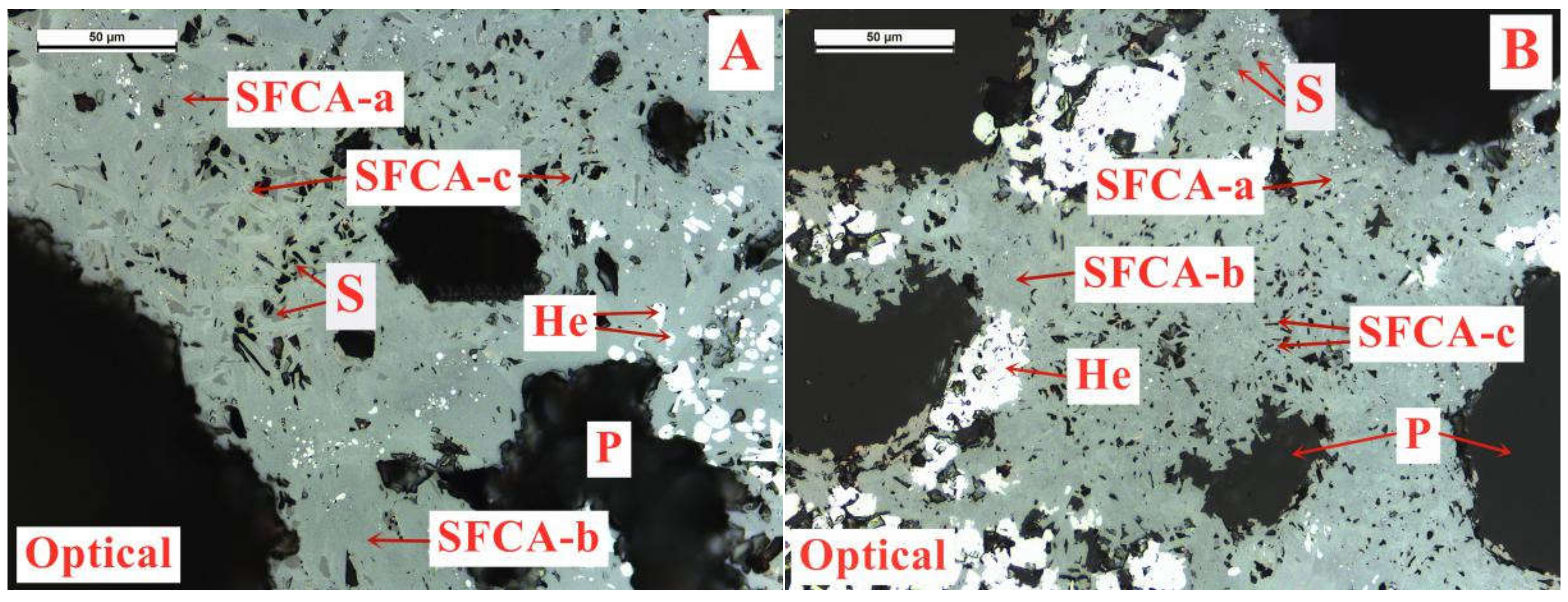
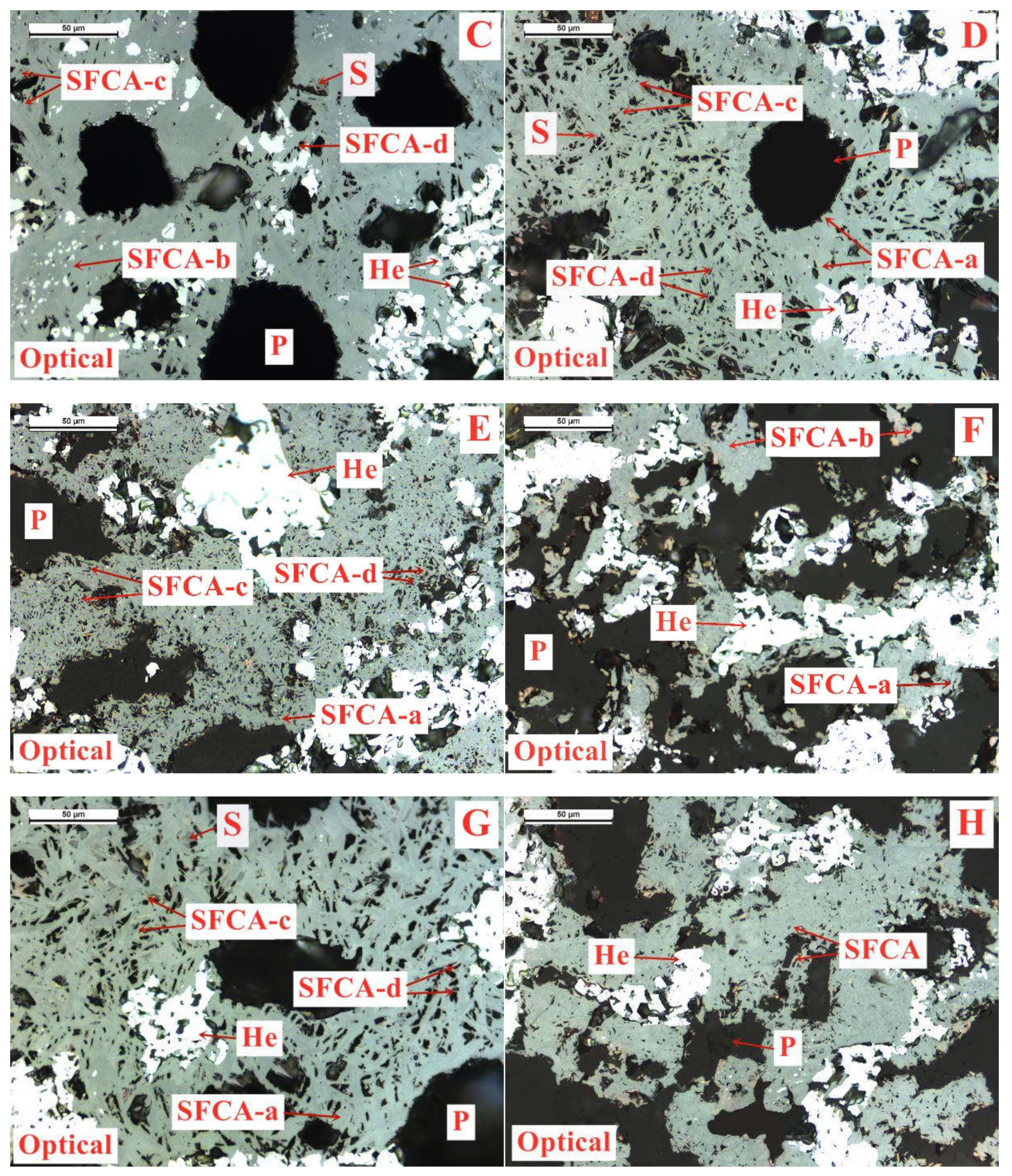
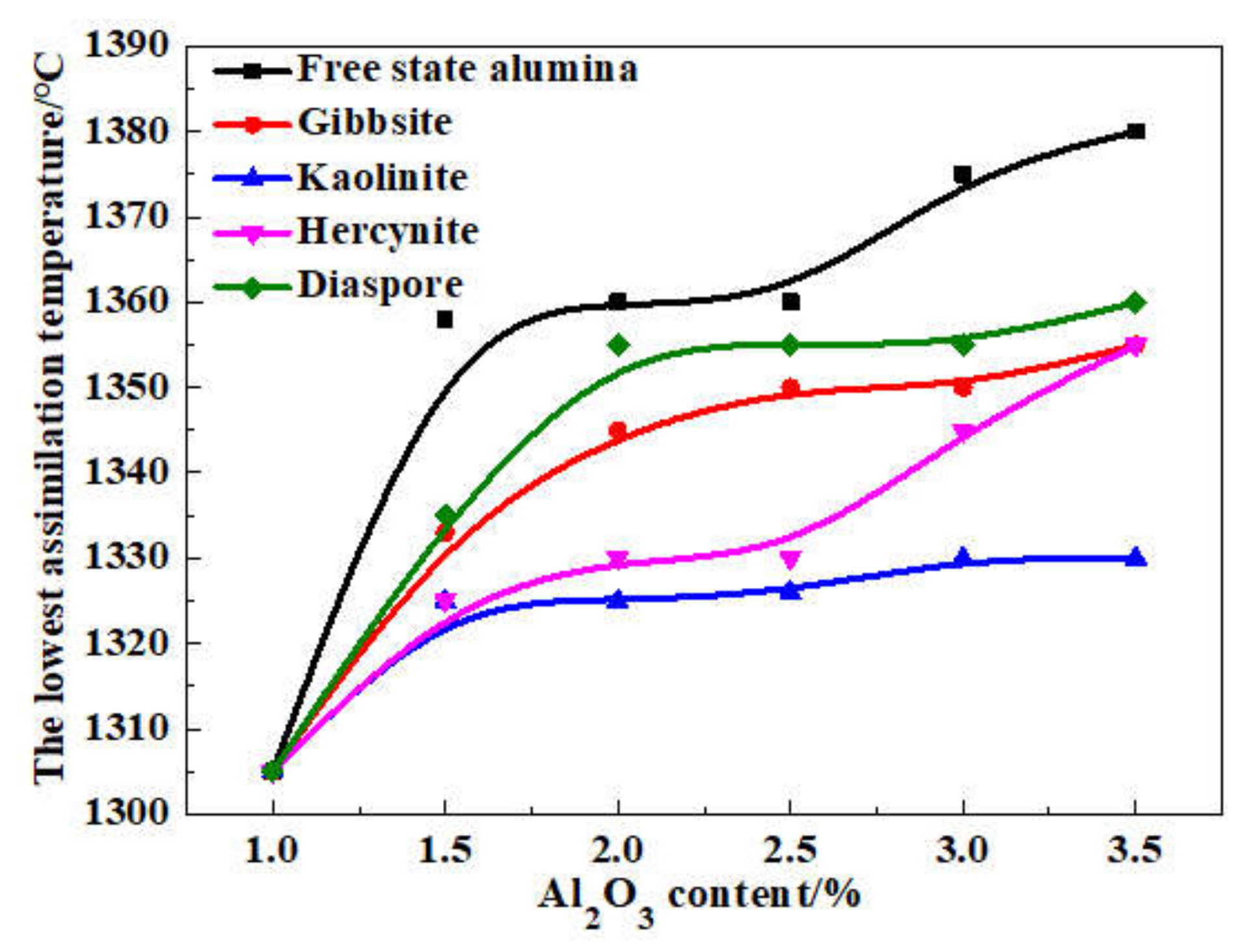
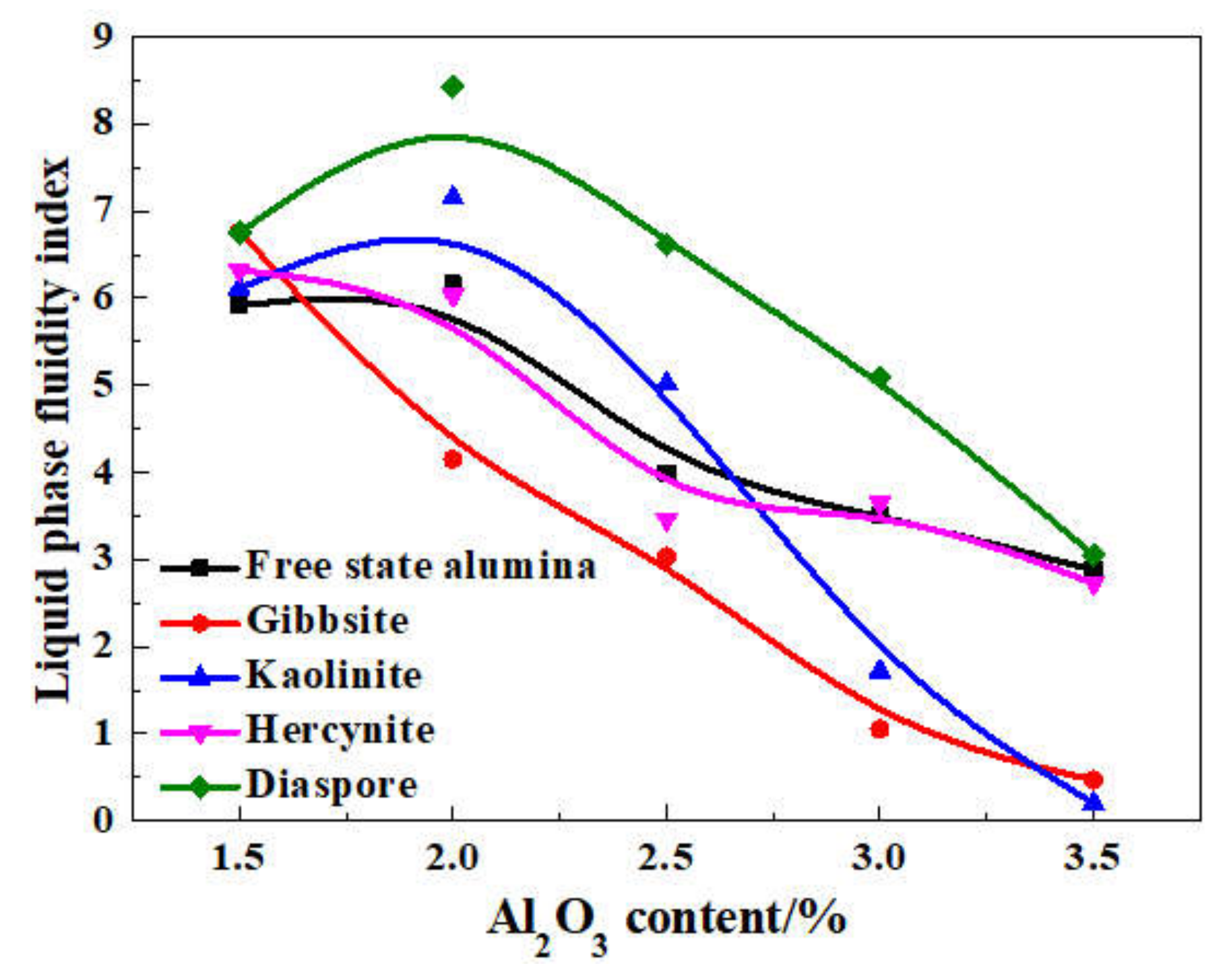
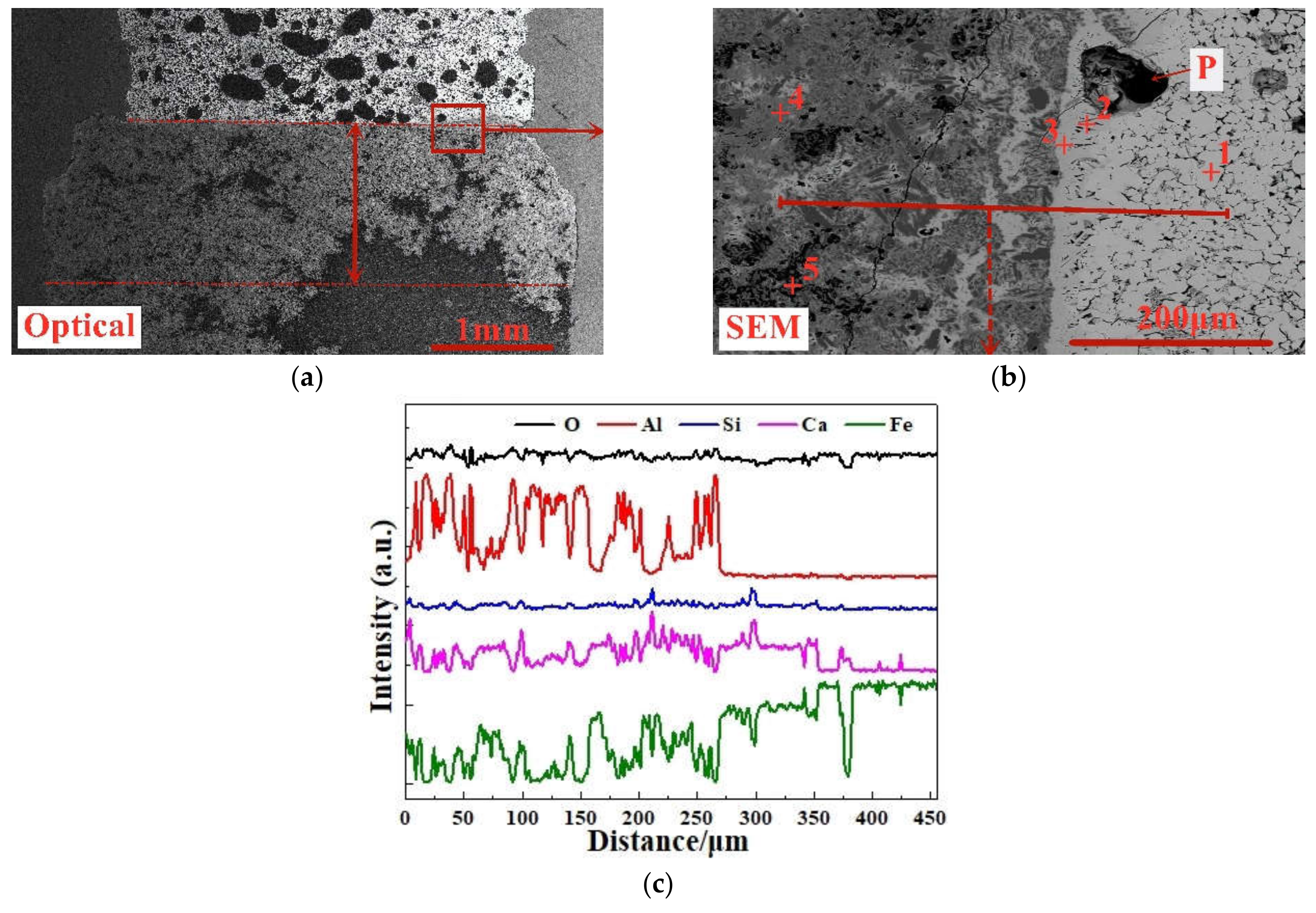
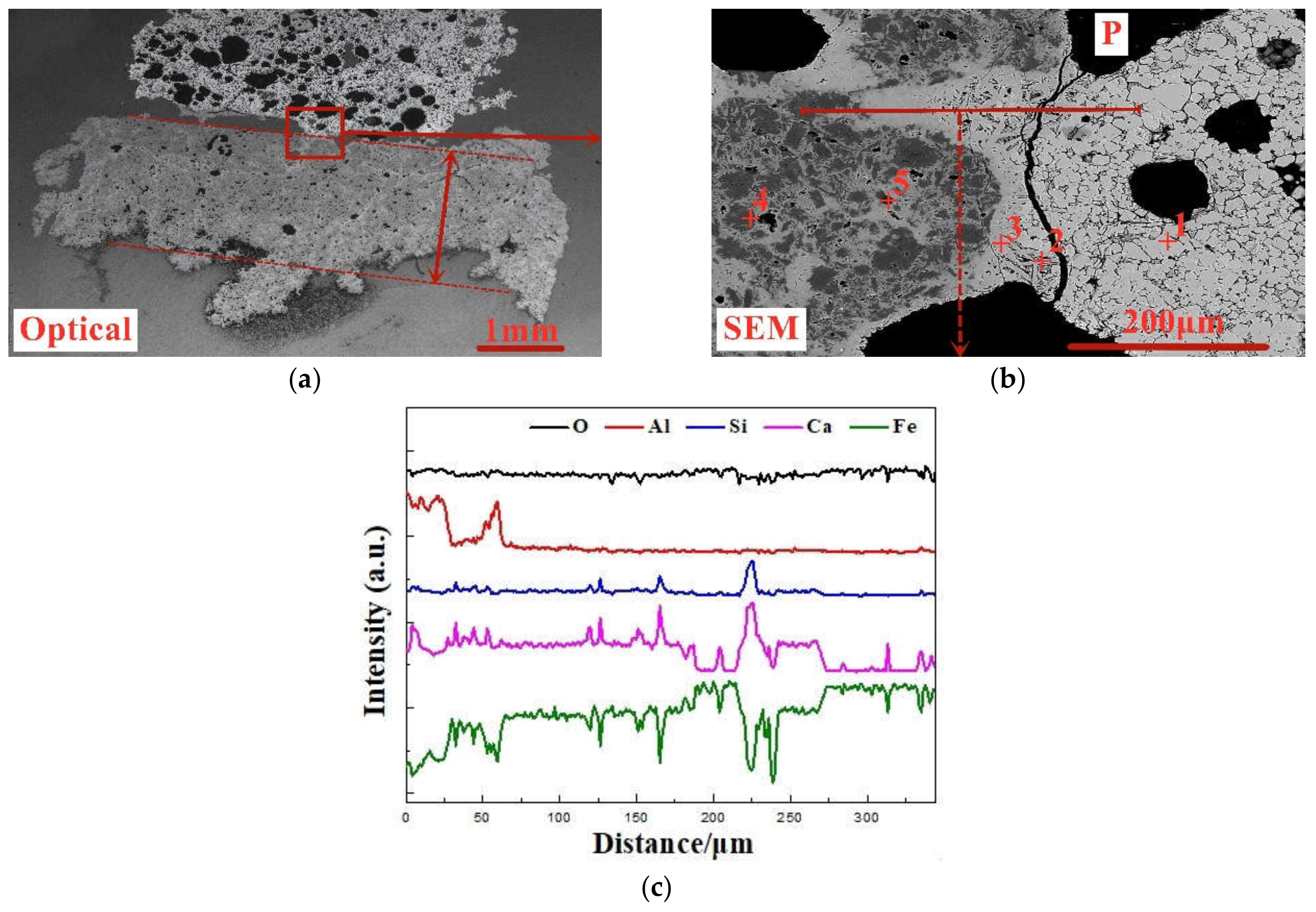
On the basis of the investigations of alumina type of typical iron ores and its possible influence on high-temperature characteristics, the relevant different impacts of various alumina types and concentrations are revealed. Iron ores possess different high-temperature characteristics not only attributed to the difference of chemical compositions but also owing to their various alumina types. It is found that the assimilability of iron ores is weakened with the increase of Al2O3 content. However, the adverse influence extents of different types of alumina are quite different. Kaolinite leads to relatively better assimilability (<1330 °C), followed by hercynite, gibbsite, and diaspore, while free state alumina is extremely deleterious to assimilability. Moreover, the influence of alumina type on liquid phase fluidity of iron ores is comparatively complicated with the Al2O3 content varying. Higher Al2O3 content (≥1.5%) is confirmed harmful to the liquid phase fluidity, but the influence degrees are obviously different. Diaspore contributes to correspondingly higher liquid phase fluidity (>3), followed by kaolinite, free state alumina, hercynite, and gibbsite in turn. Remarkably, as the Al2O3 content is over 2.70%, the harmfulness of kaolinite on the liquid phase fluidity is increased dramatically to only next to that of gibbsite. Furthermore, kaolinite and hercynite have much more positive effect on the formation of higher strength bonding phases such as dendritic or acicular SFCA due to the better reactivity. Extensive silicate is also observed in the reaction interface between the iron ore and kaolinite owing to the higher SiO2 content of kaolinite. Gibbsite and diaspore are instrumental in forming relatively lower strength lamellar or tabular SFCA, while disseminated SCFA with a rather poorer strength is massively formed in the reaction interface with alumina in the form of free state alumina. Overall, kaolinite and hercynite are more beneficial for high-temperature characteristics of iron ores, followed by diaspore and gibbsite, while free state alumina possesses the most adverse impact. Combined with the impact of alumina concentration and type on high-temperature characteristics, the relevant influence on the sintering performance of iron ores will be elucidated in the future studies for better achieving the effective utilization of the abundant high-alumina iron ores.