A Novel Pneumatic Planar Magnetic Separator for Magnetite Beneficiation: A Focus on Flowsheet Configuration
Abstract
1. Introduction
- What is the effect of different flowsheet configurations on the performance of the PMS?
- How does the performance of the various PMS flowsheet configurations compare with that of DTR?
- What are the implications of the outcomes of the study on magnetite beneficiation?
2. Materials and Methods
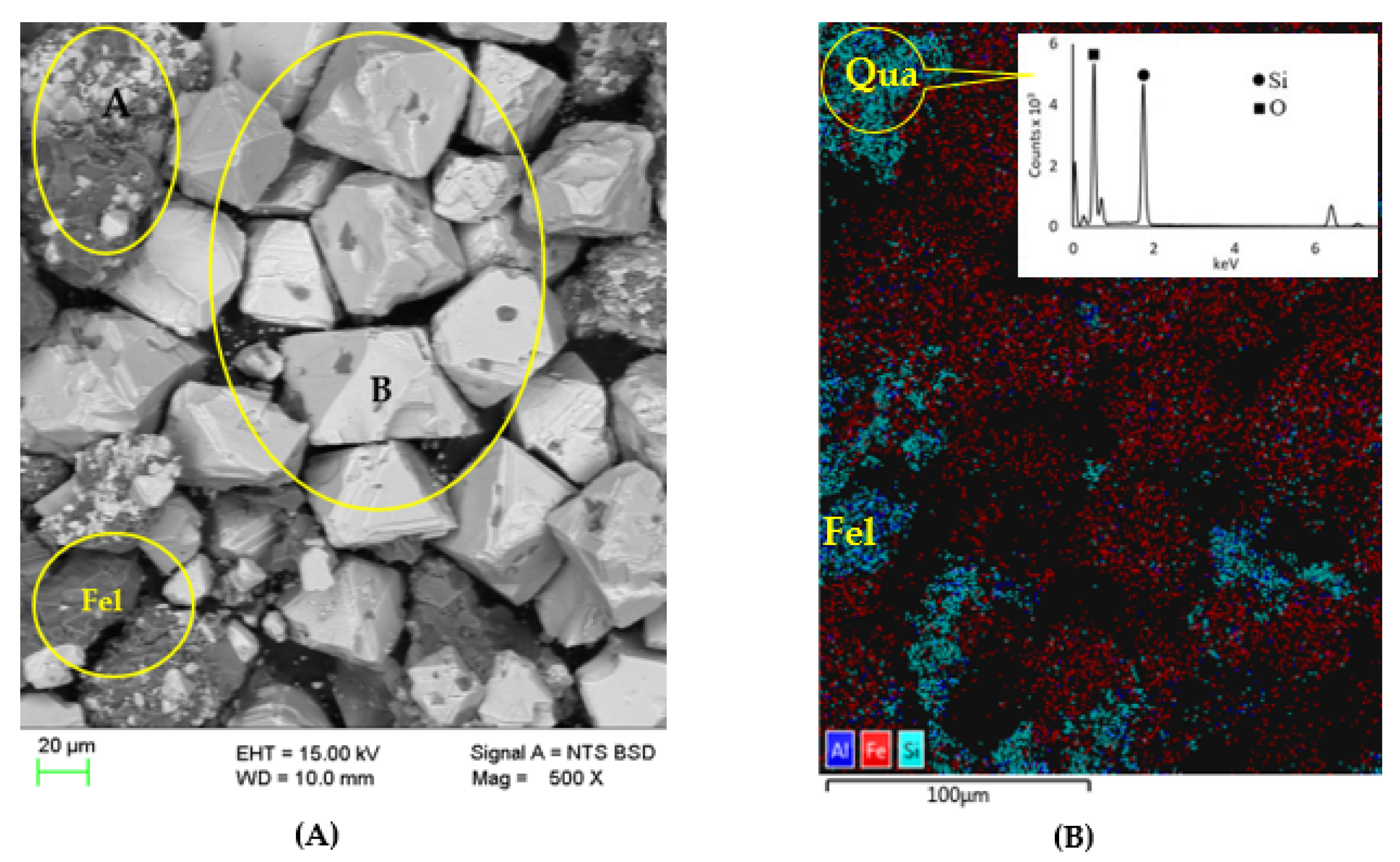
2.1. Materials
2.2. Ore Characterisation Techniques
2.3. Magnetite Liberation Analysis Using QEMSCAN
- Liberated particles were fragments in which more than 80% of the particles were made up of the magnetite.
- High-grade middling particles were the particles in which the magnetite made up between 50% and 80% of the particle.
- Low-grade middling particles were the particles in which between 20% and 50% of the particles were made up of the magnetite.
- Locked particles were those fragments in which the magnetite made up less than 20%.
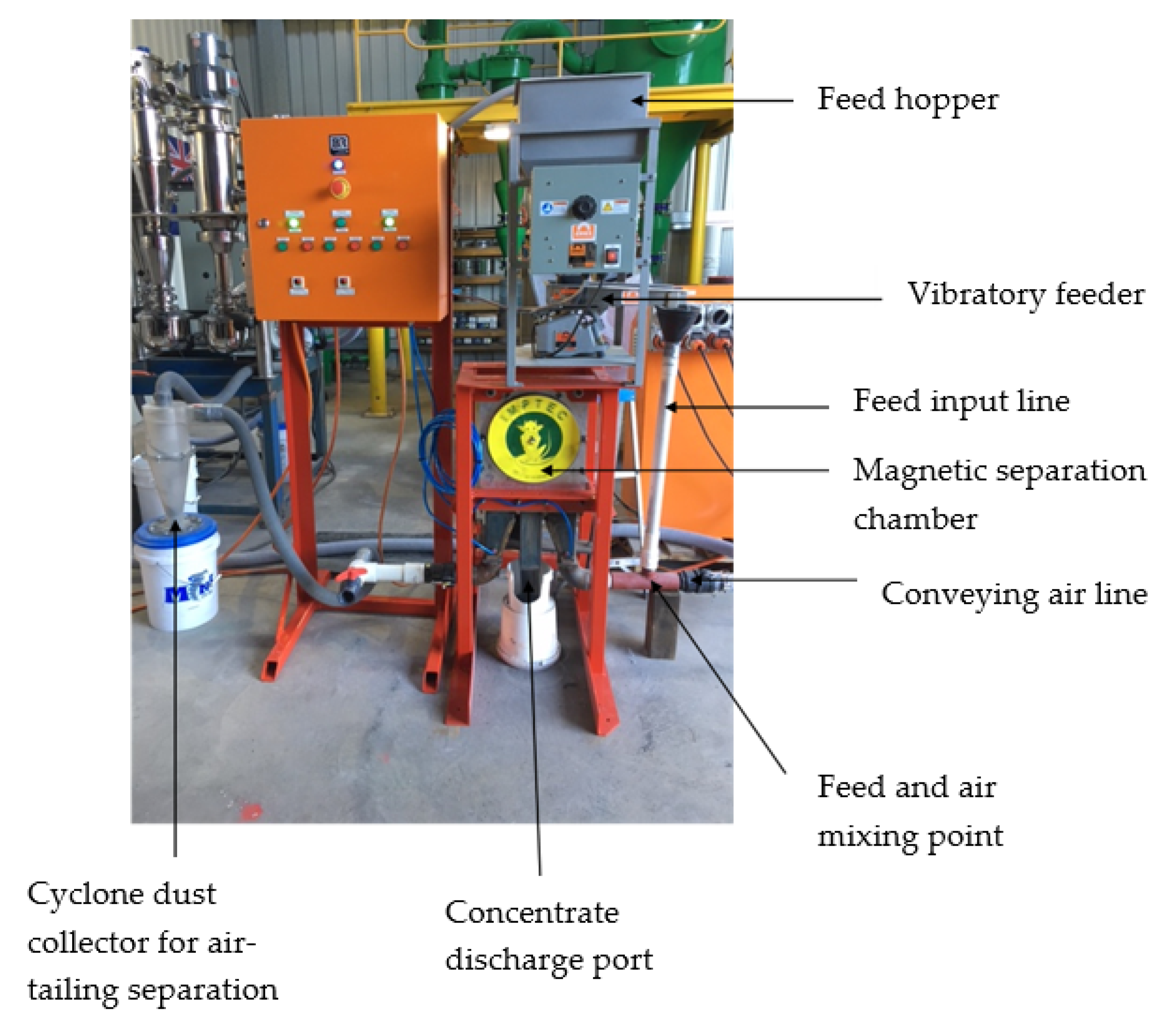
2.4. PMS Experimental Setup
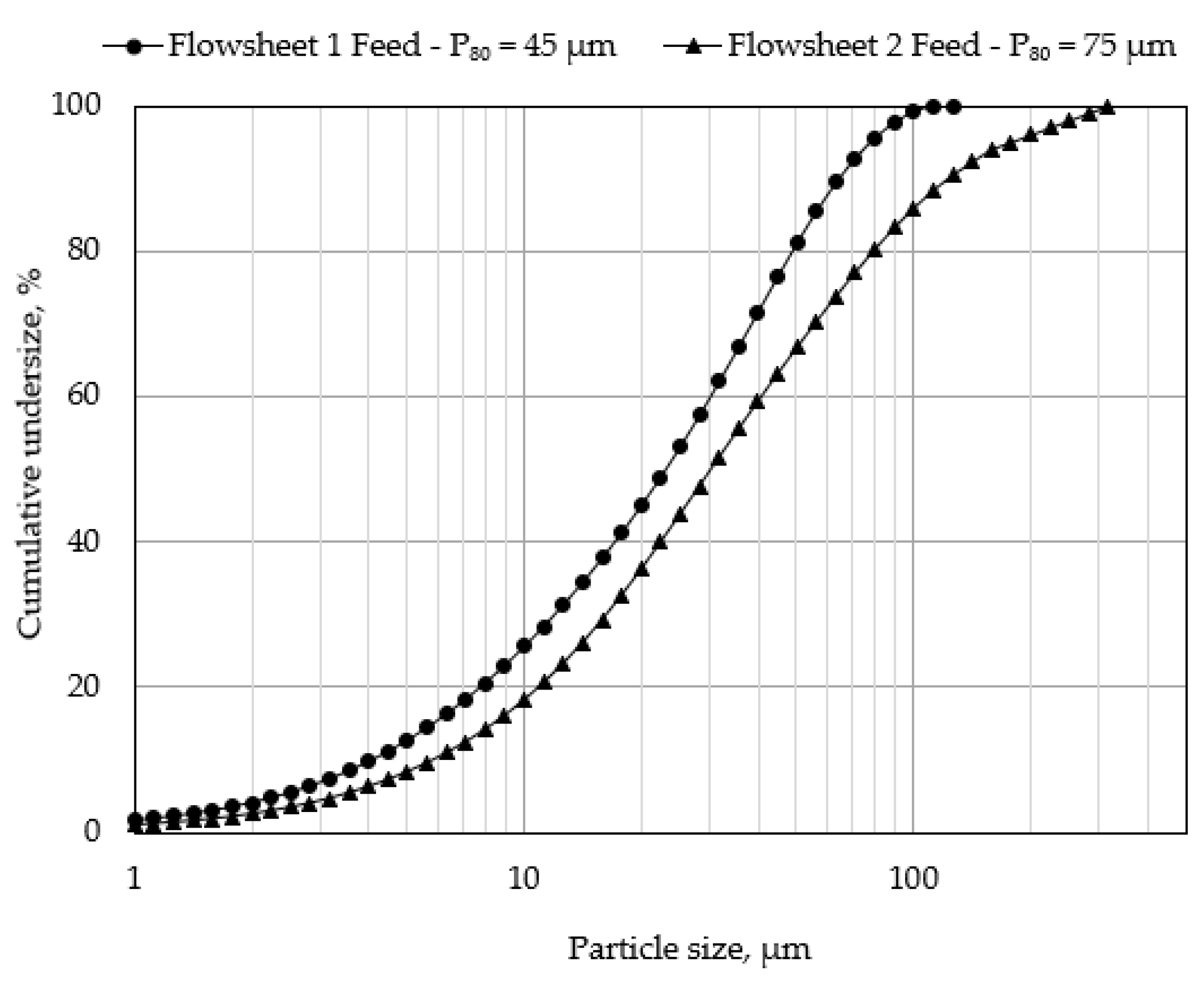
2.4.1. PMS Flowsheet 1
2.4.2. PMS Flowsheet 2
2.5. DTR Experimental Setup
2.6. Evaluation Criteria
3. Results and Discussion
3.1. Ore Characterisation
3.1.1. Elemental and Mineralogical Compositions of the Ore
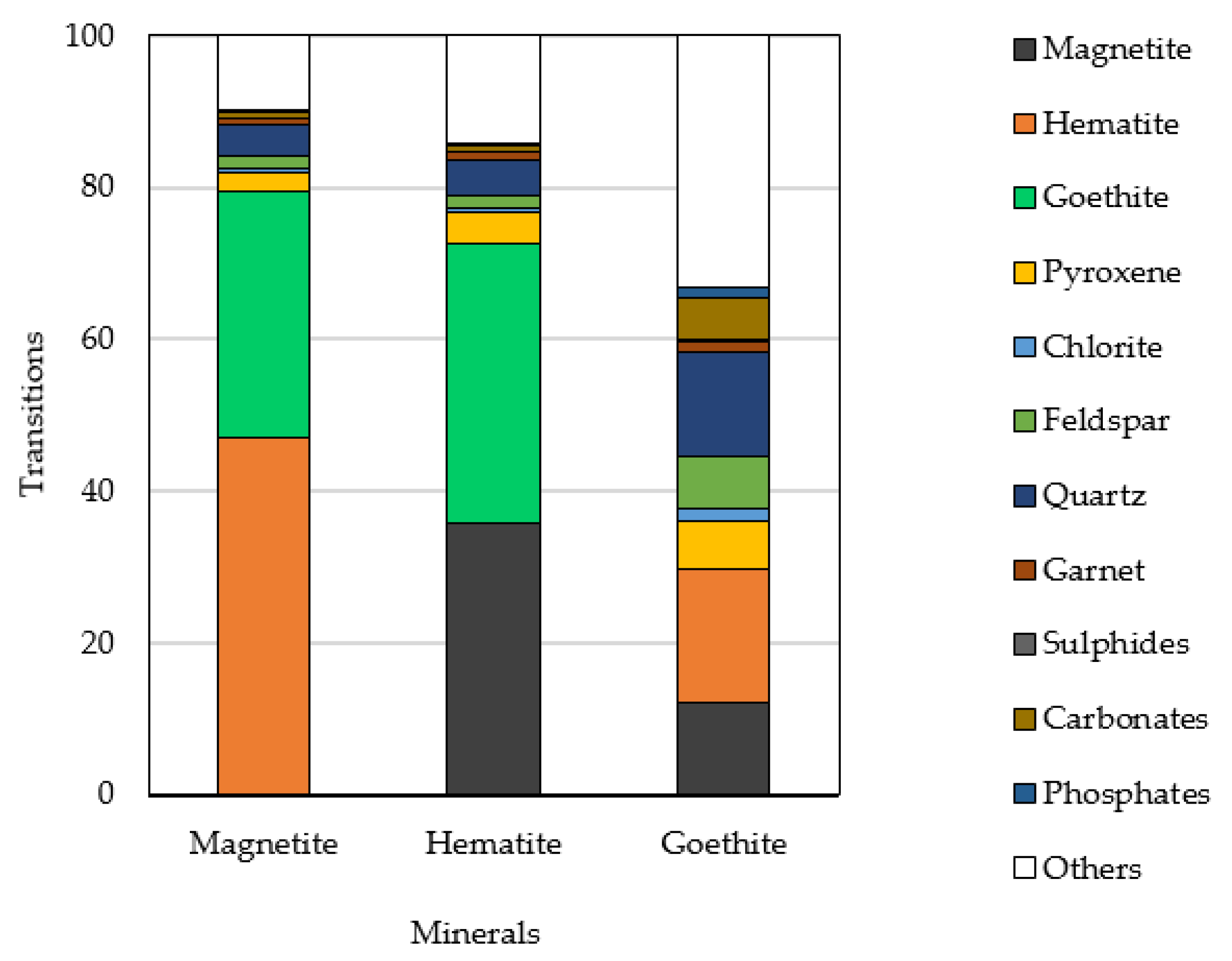
3.1.2. Mineral Association within the Ore
3.1.3. Magnetite Liberation and Interlocking Analysis
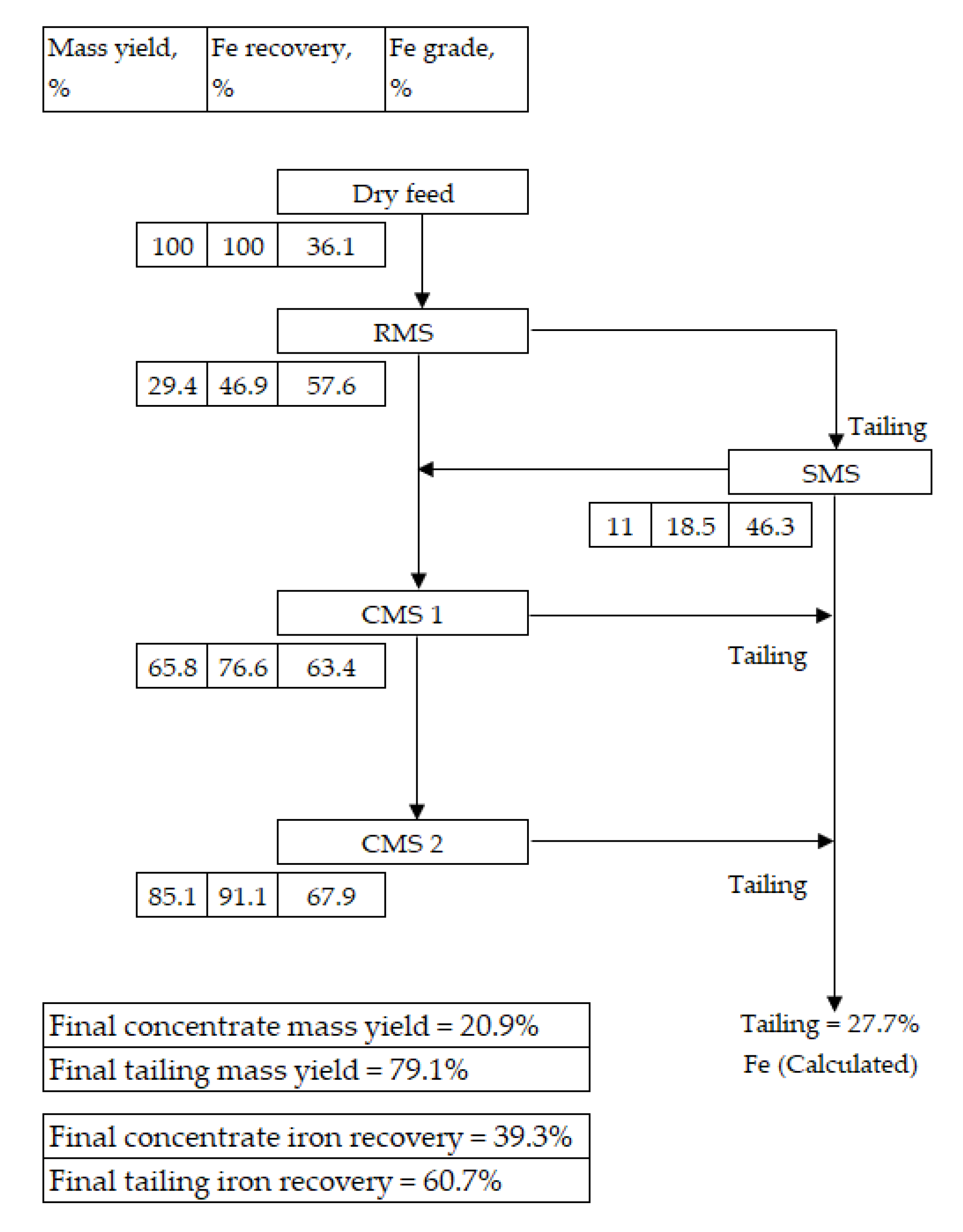
3.2. PMS Flowsheets
3.3. PMS and DTR Comparative Studies
3.4. The Implications of Studies for Magnetite Beneficiation
4. Conclusions
- The dry beneficiation of magnetite ores using the PMS is highly feasible. The PMS generated concentrates with iron grades of more than 68 wt % from the CMS stages of all the studies conducted. For Flowsheet 1, the PMS achieve iron upgrades of 60% and 88% for the RMS stage and CMS stage, respectively. For Flowsheet 2, the PMS achieved an iron upgrade of 56% for the RMS stage, whilst it achieved iron upgrades of 95% and 91% for the final CMS concentrates for Streams 1 and 2, respectively.
- The use of different flowsheet configurations has the potential to improve the performance of the PMS in terms of the early rejection of gangue particles and concentrate purity. For example, in Flowsheet 2, using a relatively coarse feed and introducing a classification unit into the beneficiation circuit resulted in the reduction of the amount of feed that required fine grinding, as in the case of Flowsheet 1. This also resulted in Flowsheet 2 generating concentrate with higher iron grades compared with Flowsheet 1.
- The study also showed that the PMS performance compares well with that of the DTR. For example, for Flowsheet 1, the DTR and PMS achieved mass yields of 27.6% and 29.4%, respectively. After the PMS’ CMS stage, both the DTR and PMS produced concentrate iron grades of 66.2% and 67.9%, respectively. Whilst DTR tests are usually conducted on small sample masses (usually less than 50 g) in batches, PMS tests are conducted on larger samples (usually more than 2 kg) in a continuous process, which ensures sample representativeness compared with the DTR.
- The application of the PMS in the dry beneficiation of magnetite will potentially lower the environmental footprint of magnetite projects, since dry tailing from the magnetite concentrator can be agglomerated and return directly to mining pits, thereby obviating the need for tailing dams.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNab, B.; Jankovic, A.; David, D.; Payne, P. Processing of magnetite iron ores–comparing grinding options. In Proceedings of the Iron Ore Conference, Perth, Australia, 27–29 July 2009; pp. 277–287. [Google Scholar]
- Holmes, R.J.; Lu, L. Introduction: Overview of the global iron ore industry. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 1–42. [Google Scholar]
- Clout, J.M.F.; Manuel, J.R. Mineralogical, chemical, and physical characterisation of iron ore. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 45–84. [Google Scholar]
- Yellishetty, M.; Mudd, G.; Mason, L.; Mohr, S.; Prior, T.; Giurco, D. Iron Resources and Production: Technology, Sustainability and Future Prospects; Prepared for CSIRO Minerals Down Under Flagship; Monash University: Melbourne, Australia; University of Technology: Sydney, Australia, 2012; ISBN 978-1-922173-46-1. [Google Scholar]
- Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. Comparison of the performance of different comminution technologies in terms of energy efficiency and mineral liberation. Miner. Eng. 2020, 156, 106454. [Google Scholar] [CrossRef]
- Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. Assessing the performance of a novel pneumatic magnetic separator for the beneficiation of magnetite ore. Miner. Eng. 2020, 156, 106483. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, L.; Holmes, R. Developments in the physical separation of iron ore: Magnetic separation. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 283–307. [Google Scholar]
- Torii, M.; Lee, T.-Q.; Fukuma, K.; Mishima, T.; Yamazaki, T.; Oda, H.; Ishikawa, N. Mineral magnetic study of the Taklimakan desert sands and its relevance to the Chinese loess. Geophys. J. Int. 2001, 146, 416–424. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Elder, J.; Sherrell, I. Dry versus wet magnetic separation—Horses for courses. In Proceedings of the 8th International Heavy Minerals Conference, Perth, Australia, 5–6 October 2011; pp. 99–106. [Google Scholar]
- Aubrey, W.M. Dry Magnetic Cobbing Separation—Dry Magnetic Cobbing Separation. In Proceedings of the SME Annual Meeting, New York, NY, USA, 16–20 February 1958; pp. 1–13. [Google Scholar]
- Connelly, D.; Yan, D. Trends in Magnetite Ore Processing and Test Work. In Proceedings of the Iron Ore Conference, Perth, Australia, 27–29 July 2009; pp. 231–241. [Google Scholar]
- Svoboda, J. Review of Magnetic Separator. In Magnetic Techniques for the Treatment of Materials; Springer: Dordrecht, The Netherlands, 2004; p. 96. [Google Scholar] [CrossRef]
- Baawuah, E.; Kelsey, C.; Kelly, J.R.; Addai-Mensah, J.; Skinner, W. Dry magnetic separation of magnetite from magnetite-quartz blends using Cyclomag Planar magnetic separator. In Proceedings of the XXIX International Mineral Processing Congress, Moscow, Russia, 17–21 September 2018; pp. 1–10. [Google Scholar]
- Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. Evaluation of the performance of Cyclomag PMS-300 S2. In Proceedings of the Iron Ore Conference, Perth, Australia, 22–24 July 2019; pp. 645–656. [Google Scholar]
- Kelsey, C.G.; Kelly, J.; Skinner, W. Dry Processing of Magnetic Iron Ores—Addressing Cost and Environmental Issues. In Proceedings of the Iron Ore Conference, Perth, Australia, 24–26 July 2017; pp. 215–220. [Google Scholar]
- Kelsey, C.; Kelly, J.R.; Skinner, W. Recasting mineral processing flow-sheets. In Proceedings of the 29th International Mineral Processing Congress, Moscow, Russia, 15–21 September 2018; pp. 1–11. [Google Scholar]
- Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. Advances in superfine crushing—A study on IMPTEC superfine crusher (SFC 160) operating parameters. In Proceedings of the Iron Ore Conference, Perth, Australia, 22–24 July 2019; pp. 633–644. [Google Scholar]
- Aylmore, M.G. Automated mineralogy. In SME Mineral Processing & Extractive Metallurgy Handbook; Dunne, R.C., Kawatra, S.K., Young, C.A., Eds.; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019; Volume 1, pp. 43–68. [Google Scholar]
- Anderson, K.F.E.; Wall, F.; Rollinson, G.K.; Moon, C.J. Quantitative mineralogical and chemical assessment of the Nkout iron ore deposit, Southern Cameroon. Ore Geol. Rev. 2014, 62, 25–39. [Google Scholar] [CrossRef]




| Element | Fe | Si | Al | Mg | Ca | P | Mn |
|---|---|---|---|---|---|---|---|
| wt % | 36.1 | 19.1 | 2.59 | 5.06 | 4.17 | 0.30 | 0.04 |
| Mineral | Chemical Formula | Abundance, wt % | Average Grain Size, µm |
|---|---|---|---|
| Magnetite | Fe3O4 | 21.8 | 14.1 |
| Goethite | FeO.OH | 15.5 | 7.56 |
| Hematite | Fe2O3 | 13.1 | 7.36 |
| Pyroxene (Actinolite and enstatite) | Ca2(Mg,Fe)5Si8O22(OH), Mg2Si2O6 | 3.22 | 5.67 |
| Chlorite (Clinochlore and chamosite) | (Mg,Fe)6(Si,Al)4O10(O)8 | 1.50 | 9.82 |
| Feldspar (Anorthite) | CaAl2Si2O8 | 9.94 | 13.9 |
| Quartz | SiO2 | 12.7 | 14.4 |
| Garnet (Almandine and andradite) | Fe3Al2(SiO4)3, Ca3Fe2(SiO4)3 | 0.86 | 5.29 |
| Other Silicates (Pyrophyllite and olivine) | Al2Si4O10(OH)2, (Mg,Fe)2SiO4 | 8.47 | 6.92 |
| Sulphides (Pyrite and pyrrhotite) | FeS2, Fe2S8 | 0.06 | 5.25 |
| Carbonates (Calcite and dolomite) | CaCO3, CaMg(CO3)2 | 10.7 | 20.1 |
| Phosphates (apatite) | Ca5(PO4)3OH | 1.86 | 8.51 |
| Others (a mixture of unclassified phases) | - | 0.29 | <6.67 |
| Size Fraction. | Fe | Si | Al | Ca | P |
|---|---|---|---|---|---|
| −45 µm | 63.1 | 8.32 | 0.64 | 0.45 | - |
| +45 µm | 46.3 | 16.5 | 1.69 | 1.04 | 0.19 |
| Separation Methods | Concentrate, wt % | ||||||
|---|---|---|---|---|---|---|---|
| Mass Yield | Fe | Si | Al | Ca | P | ||
| DTR | 27.6 | 66.2 | 9.33 | - | - | - | |
| PMS | RMS | 29.4 | 57.6 | 10.2 | 0.74 | 0.52 | 0.08 |
| Final CMS | 20.9 | 67.9 | 6.01 | - | - | - | |
| Separation Methods | Concentrate, wt % | ||||||
|---|---|---|---|---|---|---|---|
| Mass Yield | Fe | Si | Al | Ca | P | ||
| DTR | 36.9 | 57.2 | 13.5 | - | - | - | |
| PMS | RMS | 31.2 | 56.2 | 10.9 | 0.98 | 0.56 | 0.11 |
| Final CMS: Stream 1 | 10.2 | 70.4 | 3.82 | - | - | - | |
| Final CMS: Stream 2 | 11.9 | 69.0 | 5.13 | - | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baawuah, E.; Kelsey, C.; Addai-Mensah, J.; Skinner, W. A Novel Pneumatic Planar Magnetic Separator for Magnetite Beneficiation: A Focus on Flowsheet Configuration. Minerals 2020, 10, 759. https://doi.org/10.3390/min10090759
Baawuah E, Kelsey C, Addai-Mensah J, Skinner W. A Novel Pneumatic Planar Magnetic Separator for Magnetite Beneficiation: A Focus on Flowsheet Configuration. Minerals. 2020; 10(9):759. https://doi.org/10.3390/min10090759
Chicago/Turabian StyleBaawuah, Emmanuel, Christopher Kelsey, Jonas Addai-Mensah, and William Skinner. 2020. "A Novel Pneumatic Planar Magnetic Separator for Magnetite Beneficiation: A Focus on Flowsheet Configuration" Minerals 10, no. 9: 759. https://doi.org/10.3390/min10090759
APA StyleBaawuah, E., Kelsey, C., Addai-Mensah, J., & Skinner, W. (2020). A Novel Pneumatic Planar Magnetic Separator for Magnetite Beneficiation: A Focus on Flowsheet Configuration. Minerals, 10(9), 759. https://doi.org/10.3390/min10090759






