Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Samples
2.3. Characterization
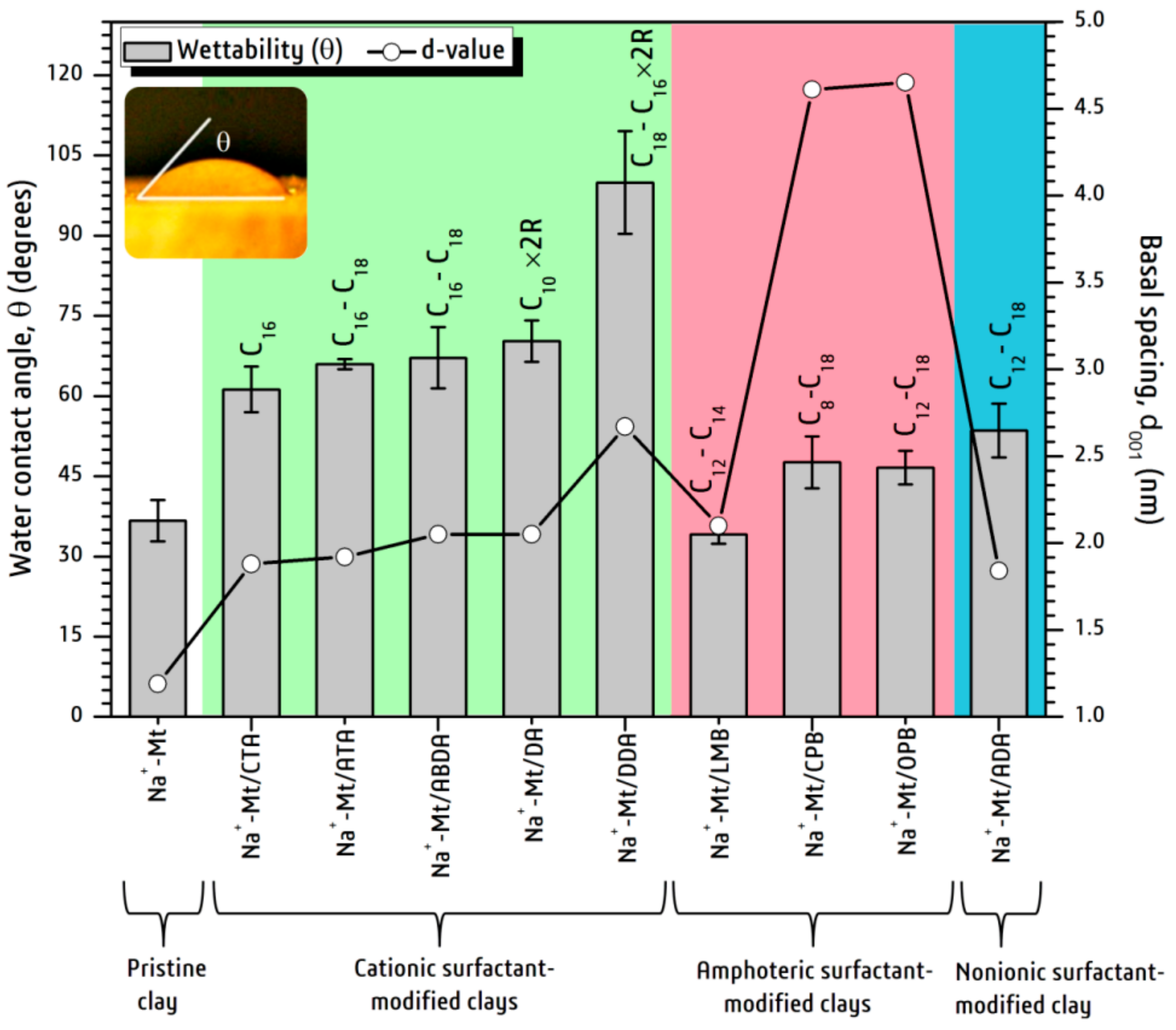
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral-organic interactions. In Developments in Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Kruglikov, A.; Vasilchenko, A.; Kasprzhitskii, A.; Lazorenko, G. Atomic-level understanding of interface interactions in a halloysite nanotubes-PLA nanocomposite. RSC Adv. 2019, 9, 39505–39514. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- Murtaza, M.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M.; Syed, N.A. Quaternary ammonium gemini surfactants having different spacer length as clay swelling inhibitors: Mechanism and performance evaluation. J. Mol. Liq. 2020, 308, 113054. [Google Scholar] [CrossRef]
- Ghasemi, M.; Moslemizadeh, A.; Shahbazi, K.; Mohammadzadeh, O.; Zendehboudi, S.; Jafari, S. Primary evaluation of a natural surfactant for inhibiting clay swelling. J. Petrol. Sci. Eng. 2019, 178, 878–891. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Khakiev, Z.; Yavna, V. Dynamic behavior and stability of soil foundation in heavy haul railway tracks: A review. Constr. Build. Mater. 2019, 205, 111–136. [Google Scholar] [CrossRef]
- Estabragh, A.R.; Babalar, M.; Javadi, A.A.; Afsari, E. Impacts of heating and surfactant treatments on the geotechnical properties of a cohesive soil. Int. J. Mech. Sci. 2018, 144, 909–918. [Google Scholar] [CrossRef]
- Shah, M.V.; Pandya, H.J.; Shukla, A.D. Influence of Chemical Additives on Shrinkage and Swelling Characteristics of Bentonite Clay. Procedia Eng. 2017, 189, 932–937. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Jaber, M. Organoclays used as colloidal and rheological additives in oil-based drilling fluids: An overview. Appl. Clay Sci. 2019, 177, 63–81. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.R.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Wang, C.-C.; Juang, L.-C.; Lee, C.-K.; Hsu, T.-C.; Lee, J.-F.; Chao, H.-P. Effects of exchanged surfactant cations on the pore structure and adsorption characteristics of montmorillonite. J. Colloid Interface Sci. 2004, 280, 27–35. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhou, Q.; Martens, W.N.; Kloprogge, T.J.; Yuan, P.; Xi, Y.; Zhu, J.; Frost, R.L. Microstructure of HDTMA+-modified montmorillonite and its influence on sorption characteristics. Clays Clay Miner. 2006, 54, 689–696. [Google Scholar] [CrossRef]
- He, H.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Xi, Y.; Kloprogge, J.T. Changes in the morphology of organoclays with HDTMA+ surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef]
- He, H.; Ma, Y.; Zhu, J.; Yuan, P.; Qing, Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl. Clay Sci. 2010, 48, 67–72. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Garfias-Mesías, L.F.; Paul, D.R. Thermal degradation of commercially available organoclays studied by TGA-FTIR. Thermochim. Acta. 2007, 457, 92–102. [Google Scholar] [CrossRef]
- Calderon, J.U.; Lennox, B.; Kamal, M.R. Thermally stable phosphonium-montmorillonite organoclays. Appl. Clay Sci. 2008, 40, 90–98. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Yavna, V. Synthesis and structural characterization of betaine- and imidazoline-based organoclays. Chem. Phys. Lett. 2018, 692, 264–270. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; de Oliveira, V.R.L.; dos Santos, F.K.G.; de Barros Neto, E.L.; de Lima Leite, R.H.; Aroucha, E.M.M.; de Oliveira Silva, K.N. Influence of the ionic and nonionic surfactants mixture in the structure and properties of the modified bentonite clay. J. Mol. Liq. 2018, 272, 990–998. [Google Scholar] [CrossRef]
- Shah, K.J.; Mishra, M.K.; Shukla, A.D.; Imae, T.; Shah, D.O. Controlling wettability and hydrophobicity of organoclays modified with quaternary ammonium surfactants. J. Colloid Interface Sci. 2013, 407, 493–499. [Google Scholar] [CrossRef]
- Mermut, A.R.; Cano, A.F. Baseline studies of the Clay Minerals Society source clays: Chemical analyses of major elements. Clays Clay. Miner. 2001, 49, 381–386. [Google Scholar] [CrossRef]
- Borden, D.; Giese, R.F. Baseline studies of the Clay Minerals Society source clays: Cation exchange capacity measurements by the ammonia-electrode method. Clays Clay. Miner. 2001, 49, 444–445. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Khater, A.; Yavna, V. Mid-infrared spectroscopic assessment of plasticity characteristics of clay soils. Minerals 2018, 8, 184. [Google Scholar] [CrossRef]
- McLauchlin, A.R.; Thomas, N.L. Preparation and characterization of organoclays based on an amphoteric surfactant. J. Colloid Interface Sci. 2008, 321, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, M.; Wold, S.; Jonsso, M. Porosity investigation of compacted bentonite using XRD profile modeling. J. Contam. Hydrol. 2012, 128, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Açışlı, Ö.; Karaca, S.; Gürses, A. Investigation of the alkyl chain lengths of surfactants on their adsorption by montmorillonite (Mt) from aqueous solutions. Appl. Clay Sci. 2017, 142, 90–99. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L. Structures of OTMA- and DODMA-bentonite and their sorption characteristics towards organic compounds. J. Colloid Interface Sci. 2009, 331, 8–14. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Zhou, Q.; Frost, R.L.; He, H.; Xi, Y. Changes in the surfaces of adsorbed p-nitrophenol on methyltrioctadecylammonium bromide organoclay-An XRD, TG, and infrared spectroscopic study. J. Colloid Interface Sci. 2007, 314, 405–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
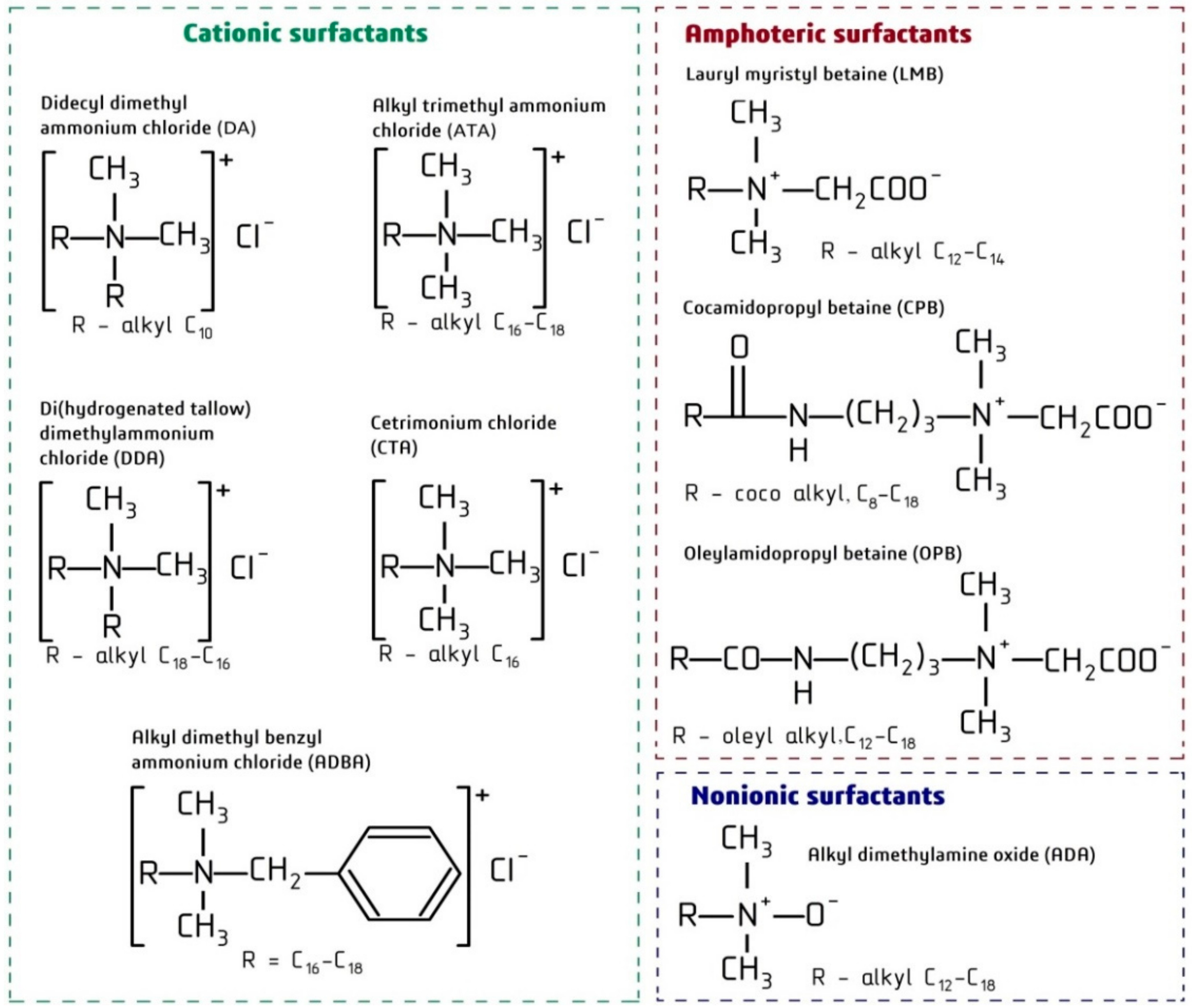
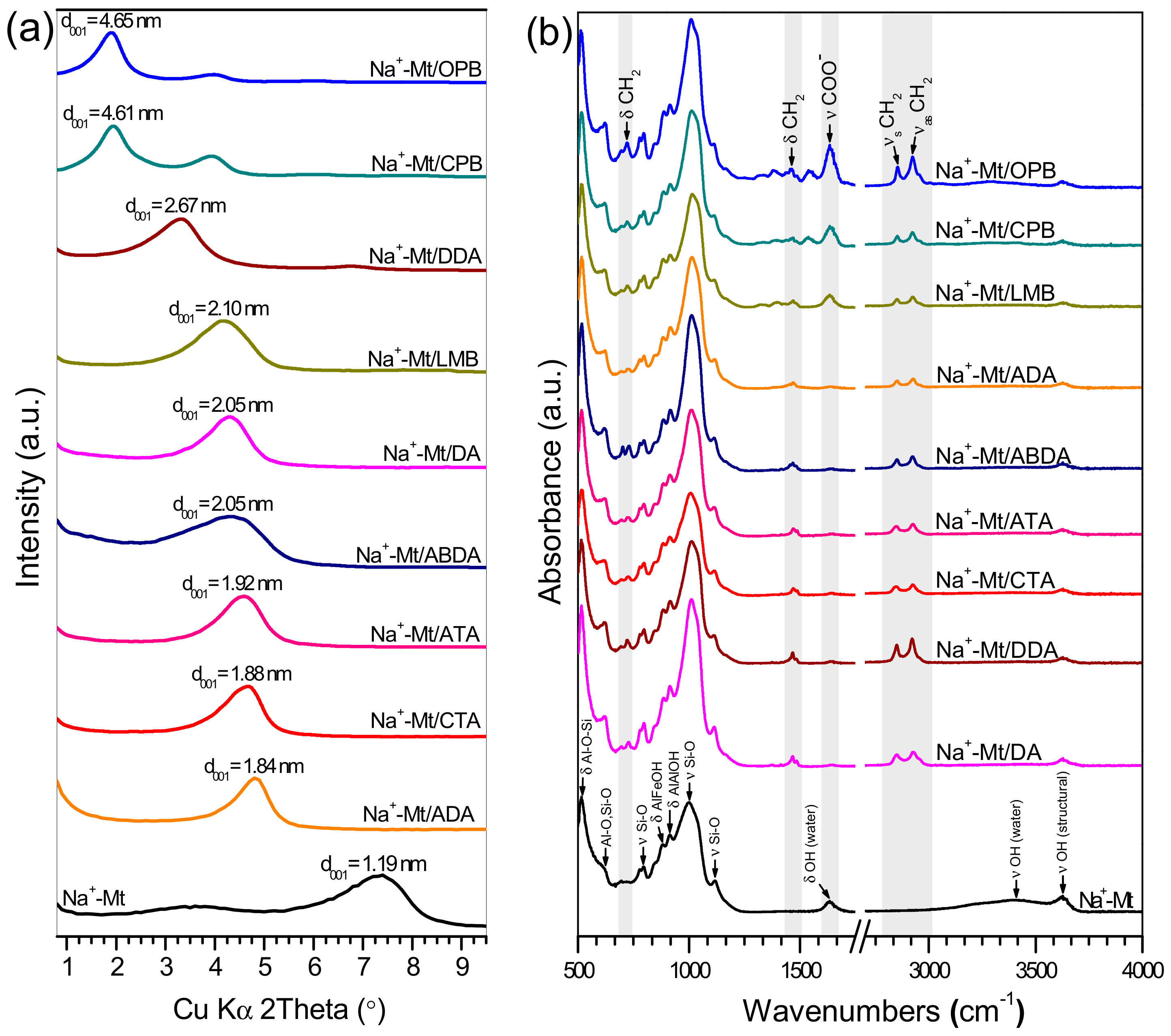
| Abbreviation | Full Name | Impurities | Molecular Weight (g/mol) | CAS Number |
|---|---|---|---|---|
| DA | Didecyl dimethyl ammonium chloride | 30% ethanol | 362.1 | 7173-51-5 |
| DDA | Di(hydrogenated tallow) dimethylammonium chloride | 25% isopropanol | 558.4 | 61789-80-8 |
| CTA | Cetrimonium chloride | 50% ethanol | 320.0 | 112-02-7 |
| ATA | Alkyl trimethyl ammonium chloride | 50% ethanol | N.R. | 61788-78-1 |
| ADBA | Alkyl dimethyl benzyl ammonium chloride | 50% ethanol | 311.9 | 63449-41-2 |
| LMB | Lauryl myristyl betaine | 70% water | 271.4 | 66455-29-6 |
| CPB | Cocamidopropyl betaine | 63% water | 342.5 | 61789-40-0 |
| OPB | Oleylamidopropyl betaine | 70% water | 424.7 | 6436555 |
| ADA | Alkyl dimethylamine oxide | 70% water | 271.5 | 68955-55-5 |
| Assignment | Wavenumber (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na+-Mt/DA | Na+-Mt/DDA | Na+-Mt/CTA | Na+-Mt/ATA | Na+-Mt/ABDA | Na+-Mt/ADA | Na+-Mt/LMB | Na+-Mt/CPB | Na+-Mt/OPB | |
| Antisymmetric CH2 stretching | 2927 | 2922 | 2925 | 2925 | 2923 | 2927 | 2924 | 2923 | 2923 |
| Symmetric CH2 stretching | 2851 | 2850 | 2847 | 2848 | 2850 | 2851 | 2850 | 2853 | 2853 |
| COO− stretching | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | 1634 | 1364 | 1634 |
| CH2 bending | 1467 | 1467 | 1471 | 1471 | 1468 | 1471 | 1470 | 1468 | 1463 |
| CH2 rocking | 728 | 720 | 727 | 727 | 730 | 726 | 723 | 721 | 721 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazorenko, G.; Kasprzhitskii, A.; Yavna, V. Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants. Minerals 2020, 10, 732. https://doi.org/10.3390/min10090732
Lazorenko G, Kasprzhitskii A, Yavna V. Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants. Minerals. 2020; 10(9):732. https://doi.org/10.3390/min10090732
Chicago/Turabian StyleLazorenko, Georgy, Anton Kasprzhitskii, and Victor Yavna. 2020. "Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants" Minerals 10, no. 9: 732. https://doi.org/10.3390/min10090732
APA StyleLazorenko, G., Kasprzhitskii, A., & Yavna, V. (2020). Comparative Study of the Hydrophobicity of Organo-Montmorillonite Modified with Cationic, Amphoteric and Nonionic Surfactants. Minerals, 10(9), 732. https://doi.org/10.3390/min10090732








