Simultaneous Partitioning of Divalent Metal Ions between Alabandite and 1 mol/L (Ni, Mg, Co, Zn, Fe)Cl2 Aqueous Solutions under Supercritical Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental System
2.2. Solid Starting Material
2.3. Reaction Solution
2.4. Experimental Procedure
3. Results and Discussion
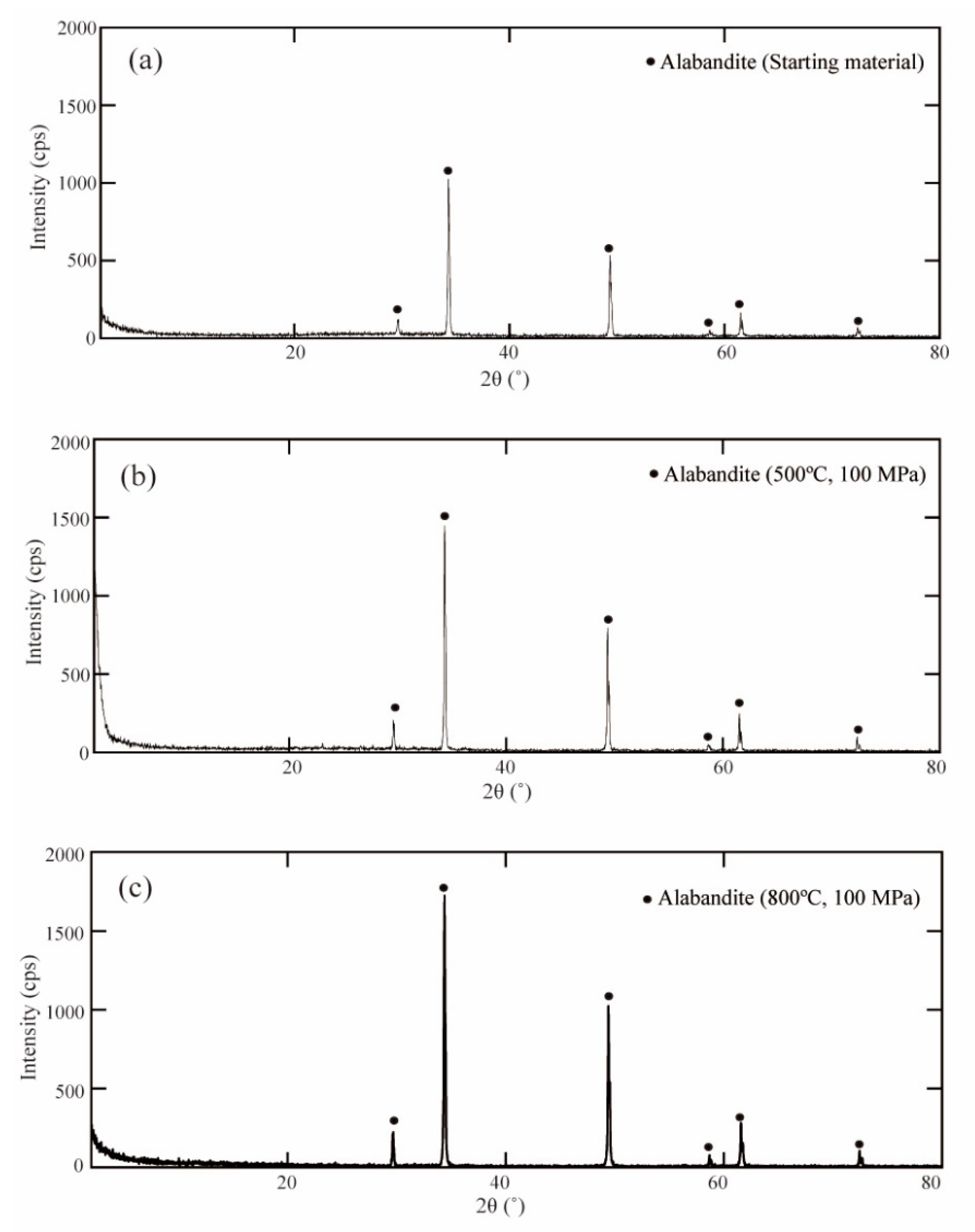
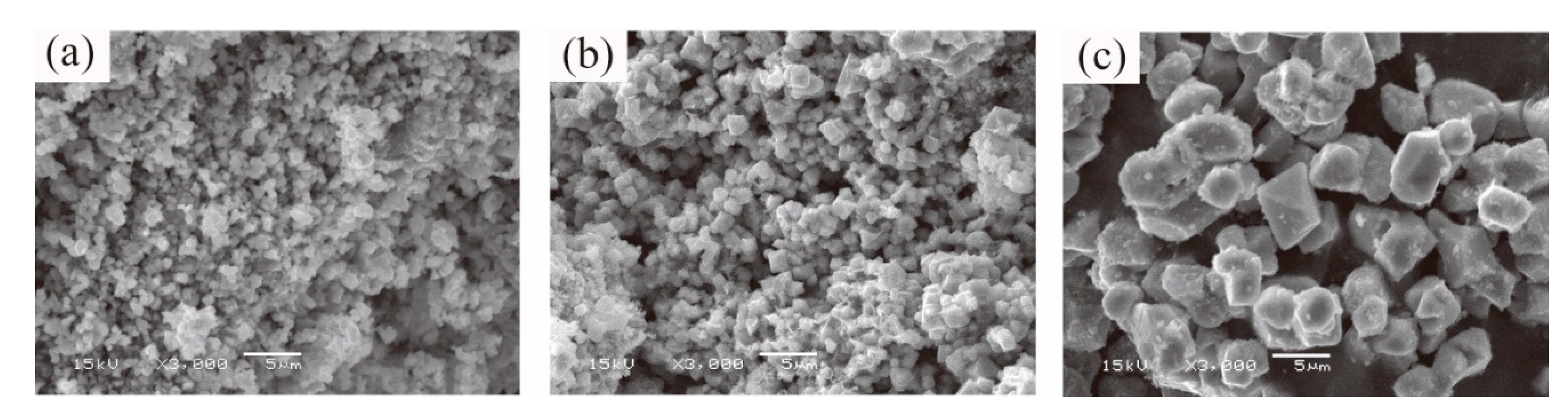
3.1. Solid and Liquid Products
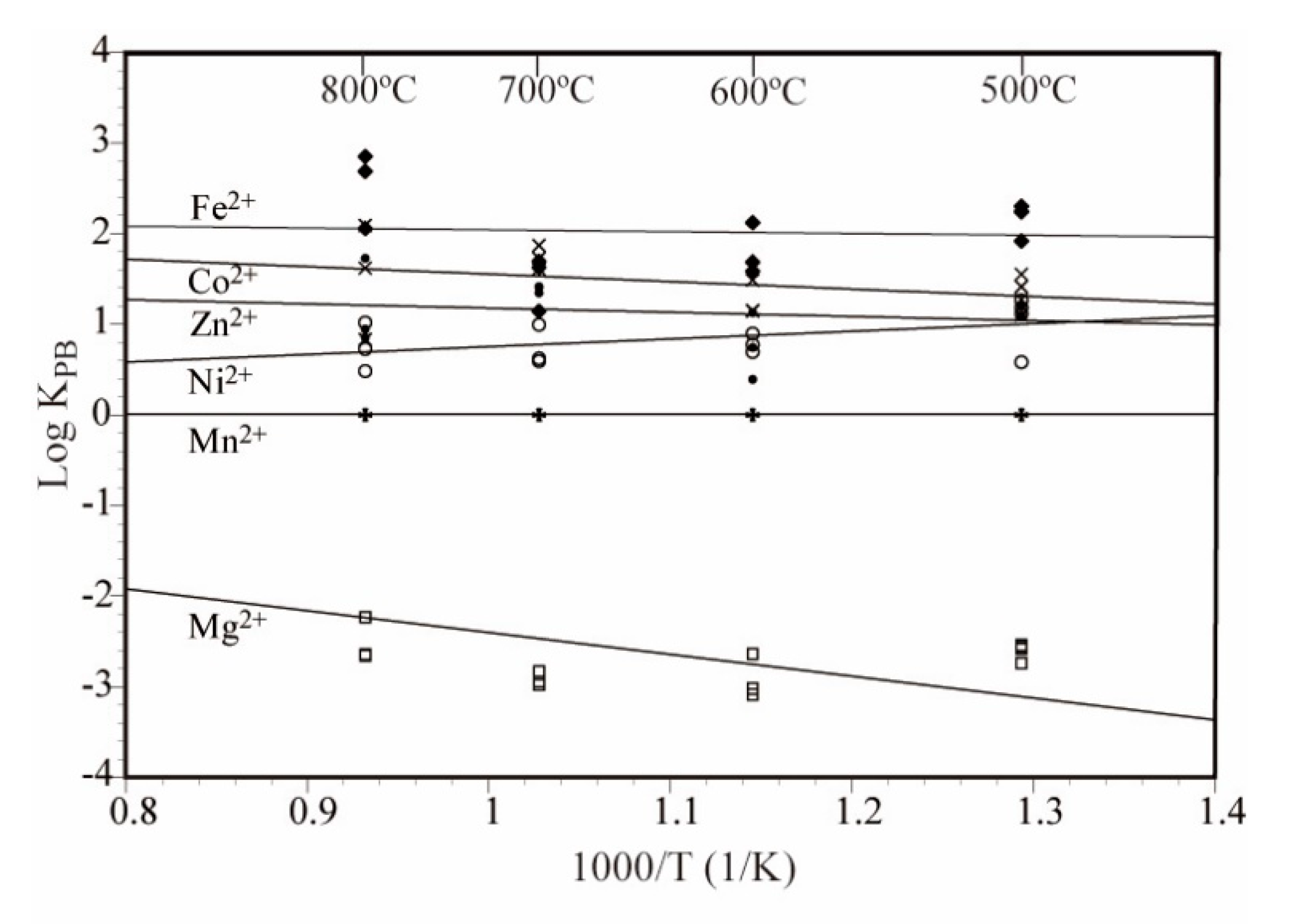
3.2. Element Partitioning
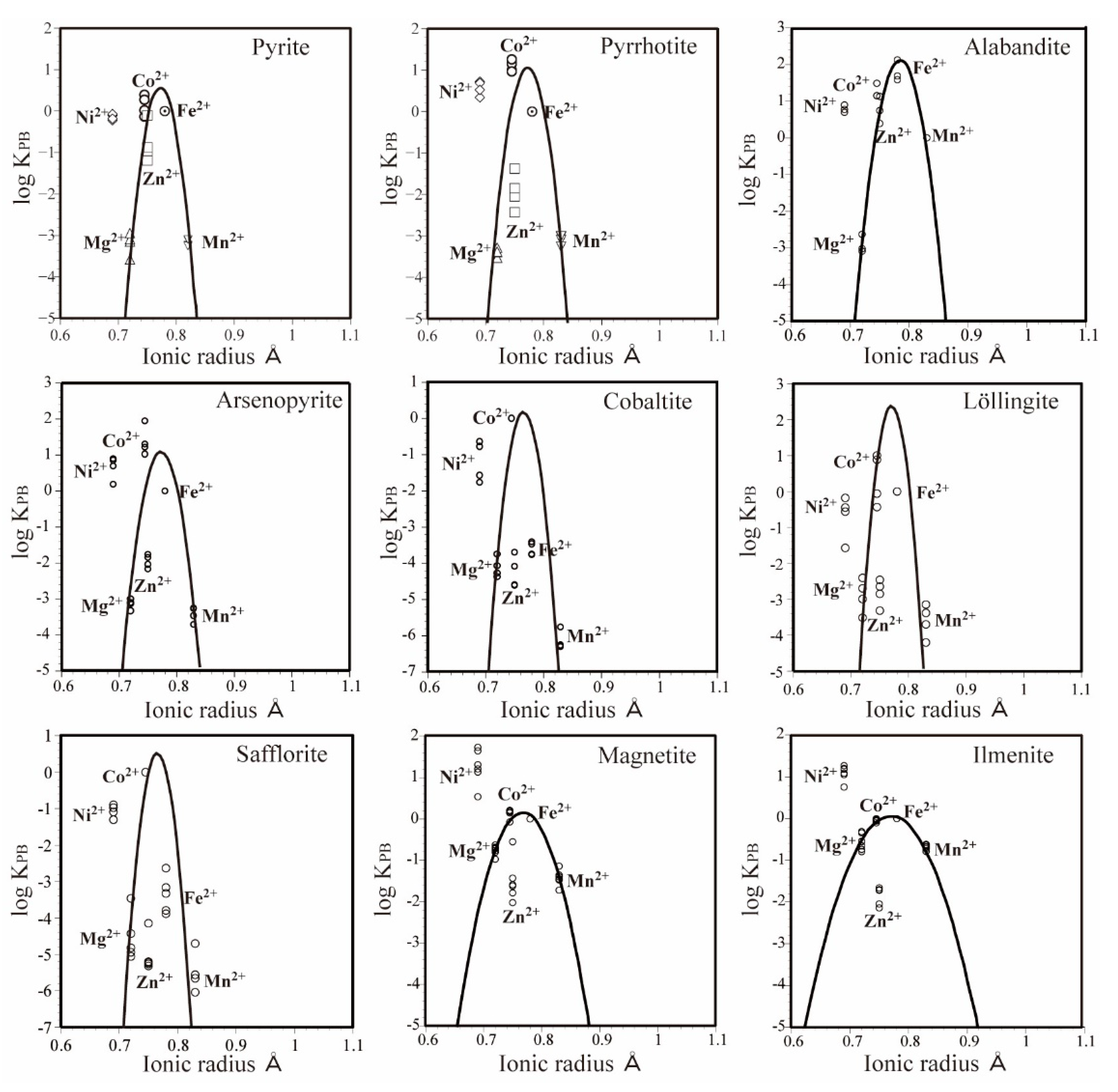
3.3. Partitioning Behavior of the Elements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roedder, E. Fluid inclusions. Reviews in Mineralogy. Mineral. Soc. Amer. 1984, 12, 644. [Google Scholar]
- Uchida, E.; Hata, Y.; Uemura, H. Experiments on the simultaneous partitioning of divalent cations between pyrite or pyrrhotite and 2M aqueous chloride solution under supercritical conditions. Jpn. Mag. Miner. Petrol. Sci. 2017, 46, 124–134. (In Japanese) [Google Scholar]
- Uchida, E.; Izumi, Y.; Watanabe, H. Experiments on the simultaneous partitioning of divalent cations between arsenopyrite or cobaltite and hydrothermal chloride solution under supercritical conditions. Jpn. Mag. Miner. Petrol. Sci. 2019, 48, 103–112. (In Japanese) [Google Scholar] [CrossRef]
- Uchida, E.; Sugino, Y.; Yokoyama, H. Experimental investigation of the simultaneous partitioning of divalent cations between löllingite or safflorite and 2 mol/L aqueous chloride solutions under supercritical conditions. J. Miner. Petrol. Sci. 2020, 115. [Google Scholar] [CrossRef]
- Gaines, R.V.; Skinner, H.C.W.; Foord, E.E.; Mason, B.; Rosenzweig, A.R. Dana’s New Mineralogy, 8th ed.; John Willey & Sons, Inc.: New York, NY, USA, 1997; 1819p. [Google Scholar]
- Staffansson, L.I. On the Mn-MnS phase diagram. Met. Trans. B 1976, 7, 131–134. [Google Scholar] [CrossRef]
- Mann, G.S.; Vlack, L.H. FeS-MnS phase relationships in the presence of excess iron. Met. Trans. B 1976, 7, 469–475. [Google Scholar] [CrossRef]
- Uchida, E.; Tsutsui, K. Cation Leaching from the basalt JB-1a by 2M NaCl hydrothermal solutions. Resour. Geol. 2000, 50, 93–102. [Google Scholar] [CrossRef]
- Uchida, E.; Goryozono, Y.; Naito, M. Aqueous speciation of magnesium, strontium, nickel and cobalt chlorides in hydrothermal solutions at 600 °C and 1 kbar. Geochem. J. 1996, 30, 99–109. [Google Scholar] [CrossRef]
- Onuma, N.; Higuchi, H.; Wakita, H.; Nagasawa, H. Trace element partition between pyroxenes and the host lava. Earth Planet. Sci. Lett. 1968, 5, 47–51. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Revised values of effective ionic radii. Acta Crystal. 1970, 26, 1046–1048. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Prediction of crystal-melt partition coefficients from elastic moduli. Nature 1994, 372, 452–454. [Google Scholar] [CrossRef]
- Blundy, J.; Wood, B. Partitioning of trace elements between crystals and melts. Earth Planet. Sci. Lett. 2003, 210, 383–397. [Google Scholar] [CrossRef]
- Matsui, Y.; Onuma, N.; Nagasawa, H.; Higuchi, H.; Banno, S. Crystal structure control in trace element partitioning between crystal and magma. Bull. Soc. Franç. Minéral. Cristal. 1977, 100, 315–324. [Google Scholar]
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: New York, NY, USA, 1960; 644p. [Google Scholar]
- Uchida, E.; Takahashi, C.; Ohta, N. Experiments on the simultaneous partitioning of divalent metal ions between spinel group minerals (spinel and magnetite) and 2M aqueous chloride solutions under supercritical conditions. Jpn. Mag. Mineral. Petrol. Sci. 2004, 33, 1–11. (In Japanese) [Google Scholar]
- Uchida, E.; Tokuda, Y.; Sakamori, T. Experiments on the element distribution between ilmenite and aqueous chloride solution under supercritical conditions. J. Miner. Petrol. Econ. Geol. 2000, 95, 12–22. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Matsui, Y. Behavior of Elements in Igneous Activity. In Earth Sciences 4, Material Science of the Earth III; Matsui, Y., Banno, S., Eds.; Iwanami Shoten: Tokyo, Japan, 1979; pp. 165–189. (In Japanese) [Google Scholar]
- Burns, R.G. Mineralogical Applications of Crystal Theory; Cambridge University Press: London, UK, 1970; 224p. [Google Scholar]

| Run No. | Duration | Temperature | Pressure | Solid | Fluid |
|---|---|---|---|---|---|
| Days | (°C) | (kb) | (mg) | (μL) | |
| 510 | 14 | 500 | 1 | 30 | 25 2 |
| 511 | 14 | 500 | 1 | 31.3 | 25 2 |
| 512 | 14 | 500 | 1 | 31.6 | 25 2 |
| 513 | 14 | 500 | 1 | 29.9 | 25 2 |
| 610 | 7 | 600 | 1 | 29.8 | 20 1 |
| 602 | 7 | 600 | 1 | 30.3 | 20 1 |
| 604 | 7 | 600 | 1 | 29.7 | 20 1 |
| 709 | 5 | 600 | 1 | 29.8 | 20 1 |
| 710 | 5 | 700 | 1 | 30.3 | 20 1 |
| 713 | 5 | 700 | 1 | 29.9 | 20 1 |
| 806 | 3 | 800 | 1 | 30.3 | 20 1 |
| 808 | 3 | 800 | 1 | 30.3 | 20 1 |
| 812 | 3 | 800 | 1 | 31.1 | 20 2 |
| T (°C), P (kb) | Run No. | * | Mg | Mn | Fe | Co | Ni | Zn |
|---|---|---|---|---|---|---|---|---|
| 500 °C, 1 kb | 510 | Alanbandite | 0.0014 | 0.9775 | 0.0155 | 0.0039 | 0.0025 | 0.0013 |
| Fluid (Metotal) | 0.44409 | 0.55527 | 0.00004 | 0.00017 | 0.00037 | 0.00005 | ||
| Fluid (MeCl2aq) | 0.27243 | 0.40423 | 0.00003 | 0.00014 | 0.00350 | 0.00004 | ||
| log KPB | −2.7470 | 0.0000 | 2.3427 | 1.1150 | 0.5841 | 1.1694 | ||
| log KPN | −2.6726 | 0.0000 | 2.3297 | 1.0614 | −0.5296 | 1.1284 | ||
| 511 | Alanbandite | 0.0019 | 0.9712 | 0.0148 | 0.0070 | 0.0065 | 0.0019 | |
| Fluid (Metotal) | 0.40561 | 0.59376 | 0.00005 | 0.00023 | 0.00026 | 0.00009 | ||
| Fluid (MeCl2aq) | 0.23955 | 0.44578 | 0.00004 | 0.00019 | 0.00025 | 0.00007 | ||
| log KPB | −2.5431 | 0.0000 | 2.2576 | 1.2697 | 1.1842 | 1.1108 | ||
| log KPN | −2.4388 | 0.0000 | 2.2300 | 1.2282 | 1.0768 | 1.0955 | ||
| 512 | Alanbandite | 0.0019 | 0.9662 | 0.0155 | 0.0083 | 0.0073 | 0.0021 | |
| Fluid (Metotal) | 0.41885 | 0.58059 | 0.00005 | 0.00019 | 0.00024 | 0.00008 | ||
| Fluid (MeCl2aq) | 0.25067 | 0.43135 | 0.00004 | 0.00016 | 0.00023 | 0.00007 | ||
| log KPB | −2.5645 | 0.0000 | 2.2702 | 1.4191 | 1.2619 | 1.1979 | ||
| log KPN | −2.4706 | 0.0000 | 2.2380 | 1.3647 | 1.1514 | 1.1269 | ||
| 513 | Alanbandite | 0.0016 | 0.9665 | 0.0158 | 0.0082 | 0.0083 | 0.0005 | |
| Fluid (Metotal) | 0.38257 | 0.61672 | 0.00012 | 0.00015 | 0.00040 | 0.00003 | ||
| Fluid (MeCl2aq) | 0.22067 | 0.47144 | 0.00011 | 0.00013 | 0.00038 | 0.00003 | ||
| log KPB | −2.5737 | 0.0000 | 1.9244 | 1.5426 | 1.1219 | 1.0267 | ||
| log KPN | −2.4514 | 0.0000 | 1.8455 | 1.4881 | 1.0275 | 0.9101 | ||
| 600 °C, 1 kb | 601 | Alanbandite | 0.0013 | 0.9731 | 0.0108 | 0.0066 | 0.0066 | 0.0016 |
| Fluid (Metotal) | 0.61177 | 0.38723 | 0.00011 | 0.00019 | 0.00044 | 0.00025 | ||
| Fluid (MeCl2aq) | 0.50321 | 0.28106 | 0.00010 | 0.00015 | 0.00042 | 0.00020 | ||
| log KPB | −3.0728 | 0.0000 | 1.5918 | 1.1406 | 0.7759 | 0.4060 | ||
| log KPN | −3.1272 | 0.0000 | 1.4941 | 1.1041 | 0.6569 | 0.3637 | ||
| 602 | Alanbandite | 0.0014 | 0.9735 | 0.0117 | 0.0061 | 0.0061 | 0.0011 | |
| Fluid (Metotal) | 0.60330 | 0.39585 | 0.00010 | 0.00017 | 0.00050 | 0.00008 | ||
| Fluid (MeCl2aq) | 0.49384 | 0.28871 | 0.00009 | 0.00014 | 0.00047 | 0.00006 | ||
| log KPB | −3.0252 | 0.0000 | 1.6774 | 1.1641 | 0.6956 | 0.7475 | ||
| log KPN | −3.0753 | 0.0000 | 1.5861 | 1.1113 | 0.5854 | 0.7354 | ||
| 604 | Alanbandite | 0.0033 | 0.9713 | 0.0120 | 0.0064 | 0.0051 | 0.0019 | |
| Fluid (Metotal) | 0.58970 | 0.40985 | 0.00004 | 0.00009 | 0.00028 | 0.00006 | ||
| Fluid (MeCl2aq) | 0.47890 | 0.30126 | 0.00004 | 0.00007 | 0.00026 | 0.00005 | ||
| log KPB | −2.6268 | 0.0000 | 2.1024 | 1.4772 | 0.8857 | 1.1259 | ||
| log KPN | −2.6701 | 0.0000 | 1.9687 | 1.4527 | 0.7842 | 1.0714 | ||
| 700 °C, 1 kb | 709 | Alanbandite | 0.0017 | 0.9798 | 0.0087 | 0.0039 | 0.0026 | 0.0033 |
| Fluid (Metotal) | 0.59927 | 0.40045 | 0.00007 | 0.00006 | 0.00011 | 0.00004 | ||
| Fluid (MeCl2aq) | 0.56046 | 0.36115 | 0.00007 | 0.00006 | 0.00011 | 0.00004 | ||
| log KPB | −2.9358 | 0.0000 | 1.7058 | 1.4243 | 0.9850 | 1.5279 | ||
| log KPN | −2.9515 | 0.0000 | 1.6610 | 1.3795 | 0.9401 | 1.4830 | ||
| 710 | Alanbandite | 0.0020 | 0.9750 | 0.0104 | 0.0060 | 0.0038 | 0.0029 | |
| Fluid (Metotal) | 0.58230 | 0.41685 | 0.00032 | 0.00011 | 0.00038 | 0.00004 | ||
| Fluid (MeCl2aq) | 0.54226 | 0.37700 | 0.00031 | 0.00010 | 0.00037 | 0.00004 | ||
| log KPB | −2.8331 | 0.0000 | 1.1429 | 1.3677 | 0.6310 | 1.4913 | ||
| log KPN | −2.8458 | 0.0000 | 1.1130 | 1.3655 | 0.5989 | 1.4477 | ||
| 713 | Alanbandite | 0.0015 | 0.9775 | 0.0110 | 0.0061 | 0.0023 | 0.0017 | |
| Fluid (Metotal) | 0.59807 | 0.40146 | 0.00011 | 0.00010 | 0.00024 | 0.00002 | ||
| Fluid (MeCl2) | 0.55863 | 0.36219 | 0.00011 | 0.00009 | 0.00024 | 0.00002 | ||
| log KPB | −2.9871 | 0.0000 | 1.6135 | 1.3989 | 0.5950 | 1.5429 | ||
| log KPN | −3.0022 | 0.0000 | 1.5688 | 1.3999 | 0.5503 | 1.4982 | ||
| 800 °C, 1 kb | 806 | Alanbandite | 0.0120 | 0.8813 | 0.0474 | 0.0230 | 0.0134 | 0.0228 |
| Fluid (Metotal) | 0.69846 | 0.30074 | 0.00002 | 0.00019 | 0.00044 | 0.00015 | ||
| Fluid (MeCl2aq) | 0.68507 | 0.28734 | 0.00002 | 0.00018 | 0.00044 | 0.00015 | ||
| log KPB | −2.2319 | 0.0000 | 2.9078 | 1.6160 | 1.0167 | 1.7149 | ||
| log KPN | −2.2433 | 0.0000 | 2.8880 | 1.6197 | 0.9969 | 1.6951 | ||
| 808 | Alanbandite | 0.0046 | 0.9059 | 0.0493 | 0.0203 | 0.0089 | 0.0109 | |
| Fluid (Metotal) | 0.69191 | 0.30660 | 0.00003 | 0.00006 | 0.00098 | 0.00042 | ||
| Fluid (MeCl2aq) | 0.67836 | 0.29308 | 0.00003 | 0.00006 | 0.00097 | 0.00041 | ||
| log KPB | −2.6478 | 0.0000 | 2.7452 | 2.0588 | 0.4877 | 0.9437 | ||
| log KPN | −2.6588 | 0.0000 | 2.7256 | 2.0393 | 0.4725 | 0.9345 | ||
| 812 | Alanbandite | 0.0038 | 0.8866 | 0.0848 | 0.0109 | 0.0037 | 0.0101 | |
| Fluid (Metotal) | 0.61576 | 0.38301 | 0.00032 | 0.00036 | 0.00030 | 0.00026 | ||
| Fluid (MeCl2aq) | 0.60045 | 0.36774 | 0.00032 | 0.00035 | 0.00030 | 0.00025 | ||
| log KPB | −2.5741 | 0.0000 | 2.0587 | 1.1166 | 0.7266 | 1.2248 | ||
| log KPN | −2.5809 | 0.0000 | 2.0411 | 1.1112 | 0.7089 | 1.2242 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, E.; Murasugi, M.; Okuda, S. Simultaneous Partitioning of Divalent Metal Ions between Alabandite and 1 mol/L (Ni, Mg, Co, Zn, Fe)Cl2 Aqueous Solutions under Supercritical Conditions. Minerals 2020, 10, 696. https://doi.org/10.3390/min10080696
Uchida E, Murasugi M, Okuda S. Simultaneous Partitioning of Divalent Metal Ions between Alabandite and 1 mol/L (Ni, Mg, Co, Zn, Fe)Cl2 Aqueous Solutions under Supercritical Conditions. Minerals. 2020; 10(8):696. https://doi.org/10.3390/min10080696
Chicago/Turabian StyleUchida, Etsuo, Motoki Murasugi, and Shuichi Okuda. 2020. "Simultaneous Partitioning of Divalent Metal Ions between Alabandite and 1 mol/L (Ni, Mg, Co, Zn, Fe)Cl2 Aqueous Solutions under Supercritical Conditions" Minerals 10, no. 8: 696. https://doi.org/10.3390/min10080696
APA StyleUchida, E., Murasugi, M., & Okuda, S. (2020). Simultaneous Partitioning of Divalent Metal Ions between Alabandite and 1 mol/L (Ni, Mg, Co, Zn, Fe)Cl2 Aqueous Solutions under Supercritical Conditions. Minerals, 10(8), 696. https://doi.org/10.3390/min10080696





