Manganese Nodules in Chile, an Alternative for the Production of Co and Mn in the Future—A Review
Abstract
1. Introduction
- Increased production costs;
- Drive toward increasing production levels to compensate for the drop in grades;
- Diversifying the extraction in consideration of other elements and obtaining byproducts from the main element to be exploited (an example is the enormous growth of the molybdenum industry, which is obtained as a by-product from copper flotation processes);
- Changing the costs of essential resources, such as water and electricity.
2. Manganese Nodules
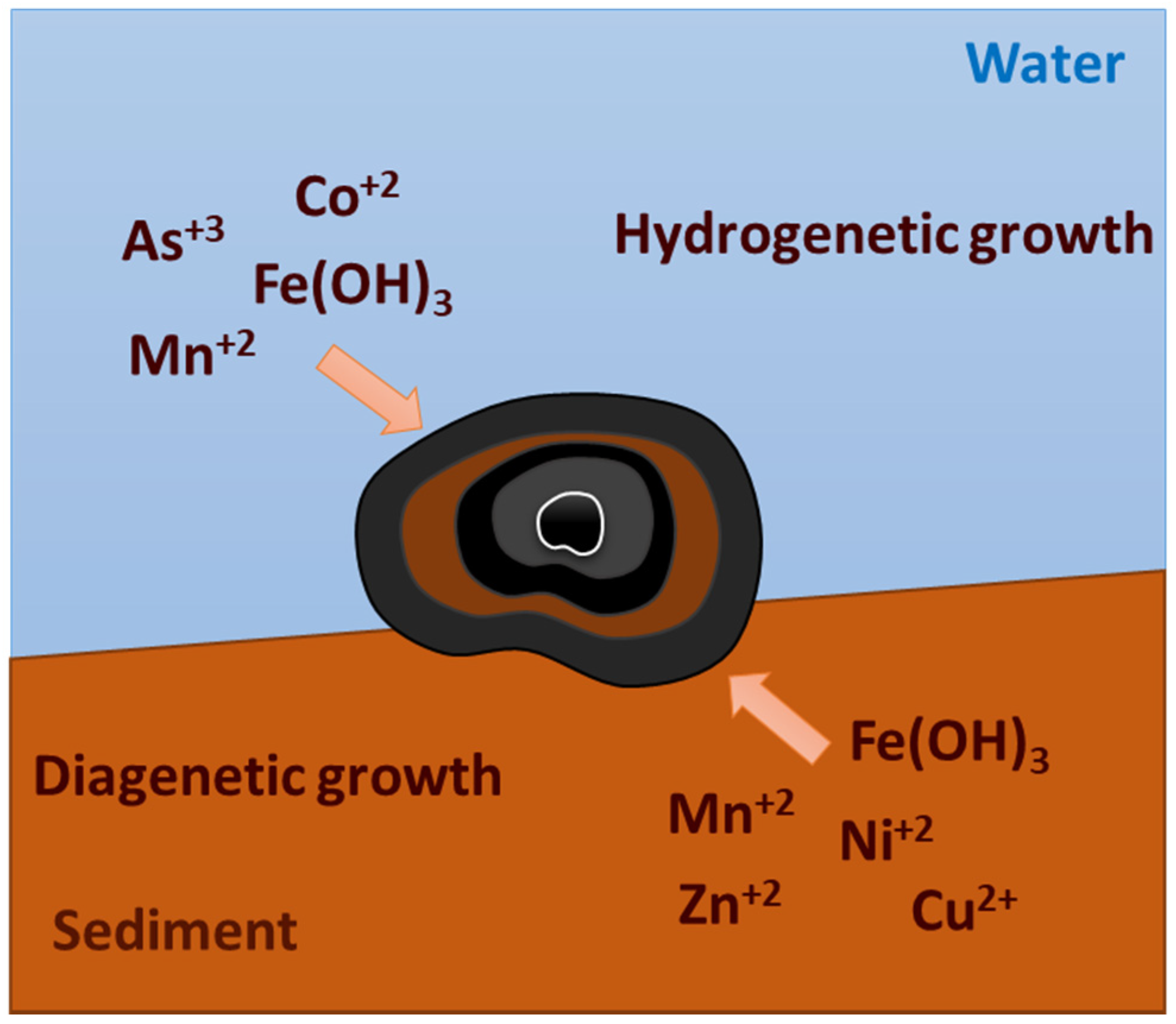
2.1. Formation and Growth
- Hydrogenetic nodules are minerals accumulate by precipitation directly from seawater [15,16]. They are polynuclear and irregular, with smooth and finely porous textures, in which the predominant Mn mineral is vernadite (δ-MnO2). This type of nodule is believed to have a similar content of Fe and Mn and a relatively high degree of nickel, copper, and cobalt.
- Diagenetic nodules are formed due to the precipitation of elements from interstitial water within the sediment or on the sediment surface [15,16]. They are commonly ellipsoidal to discoidal with porous and sandy surfaces, with Todorokite being the dominant mineral. Such nodules are rich in manganese but poor in Fe, Ni, Cu, and Co.
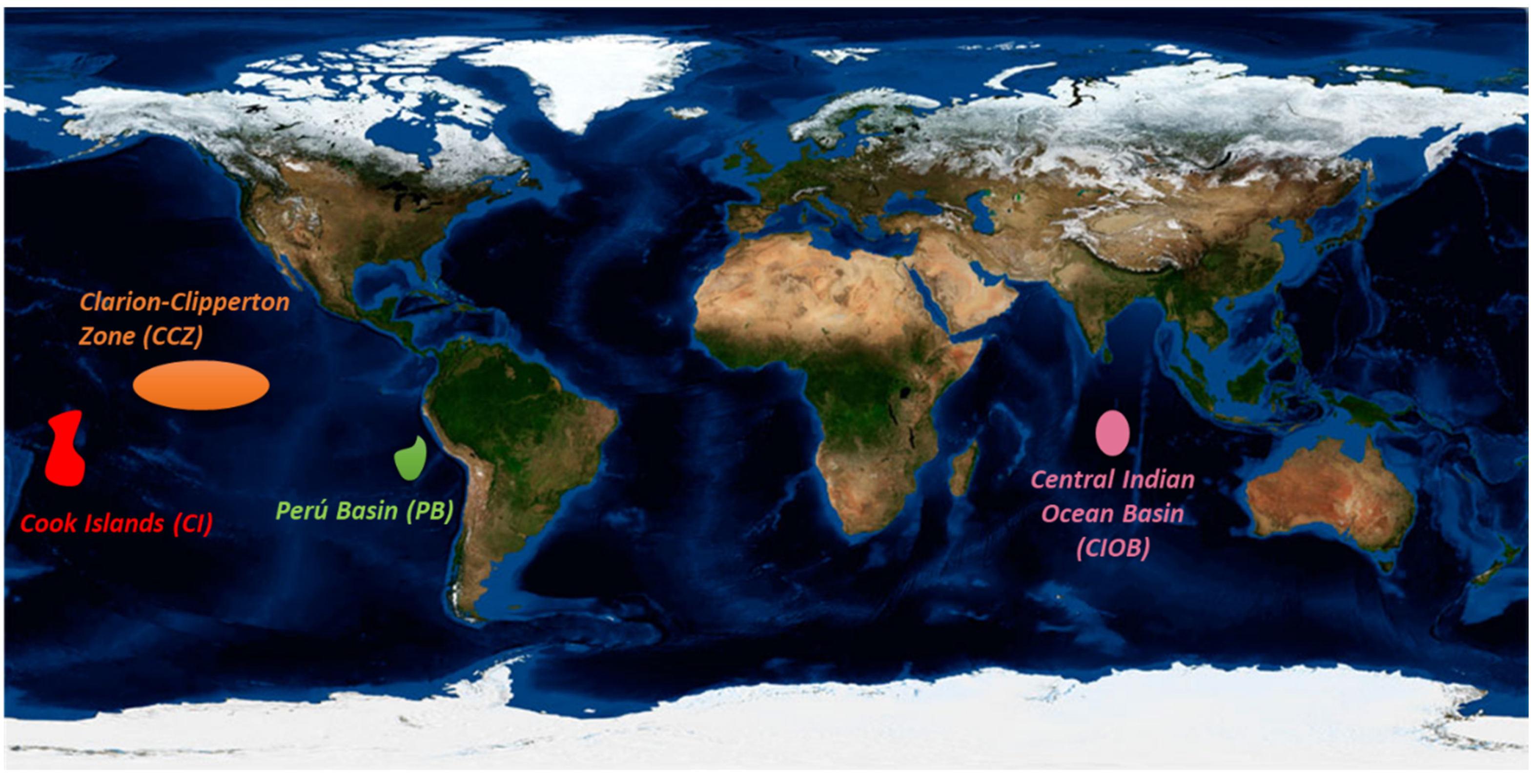
2.2. Distribution in the World
2.2.1. Clarion-Clipperton Zone (CCZ)
2.2.2. Peru Basin (PB)
2.2.3. The Central Indian Ocean Basin (CIOB)
2.2.4. Cook Islands (CI)
2.2.5. Other Areas
2.3. Chemical and Mineralogical Composition
2.4. Collection Mechanisms
- IKS Germany extracted manganese nodules using a collector mounted on a powered track; the extraction system includes a flexible hose for the hydraulic transport of solids with a high-pressure inlet. There is a satellite extraction system consisting of several small mining units and a mothership.
- DORD (Japan) tested recovering manganese nodules with a collector system supported by five sleds and equipped with four units of suction and intake devices between sledges.
- COMRA, in China, has worked on a special system to collect manganese nodules, and it is a hybrid collector based on hydraulic principles. It consists of a catchment device, double jets and deflector plates, a coanda nozzle and a transport channel, and an outlet with a grid to separate the sediments from the nodules. It is reported to have a high collection rate and low sediment content.
- KORDI, Korea, in order to collect the Mn nodules, developed a collection device consisting of a hydraulic lift and a mechanical conveyor. The detachment and separation of the nodules (from the sediments) is carried out by the joint action of a pair of water jets and deflector plates. A rotating fin system draws the nodules to the collector.
- Department of Ocean Development (India) extraction equipment consists of a crawler vehicle, a mechanical screw harvesting head, a bucket elevator to transport the nodule to the hopper, a crusher and a pump for transporting the mixture of nodules and water to the vertical module. Once the nodules are tracked, the screw transports (from the collector head) sweep the material of interest dispersed to the elevator, pass to the hopper where they are crushed (to 10 mm), and they finally reach the vertical module.
- Atlantis Deep Sea, in the USA, developed a nodule recovery kit that has two parts—A surface subsystem and an ocean floor subsystem. The upper part includes a ship for operational control and maintenance support for the lower subsystem. The ocean floor subsystem has a mobile, maneuverable, and self-propelled mining vehicle that collects, handles, washes, and crushes nodules. It includes a shock absorber that temporarily stores the crushed material and serves to isolate the mining vehicle from the dynamics of a pipe that extends downward from the surface of the ship. The global system has sensors that detect the location of the mining vehicle and show the topography of the ocean floor where it is applied.
2.5. Resource Quantification
3. Manganese Nodules in Chile
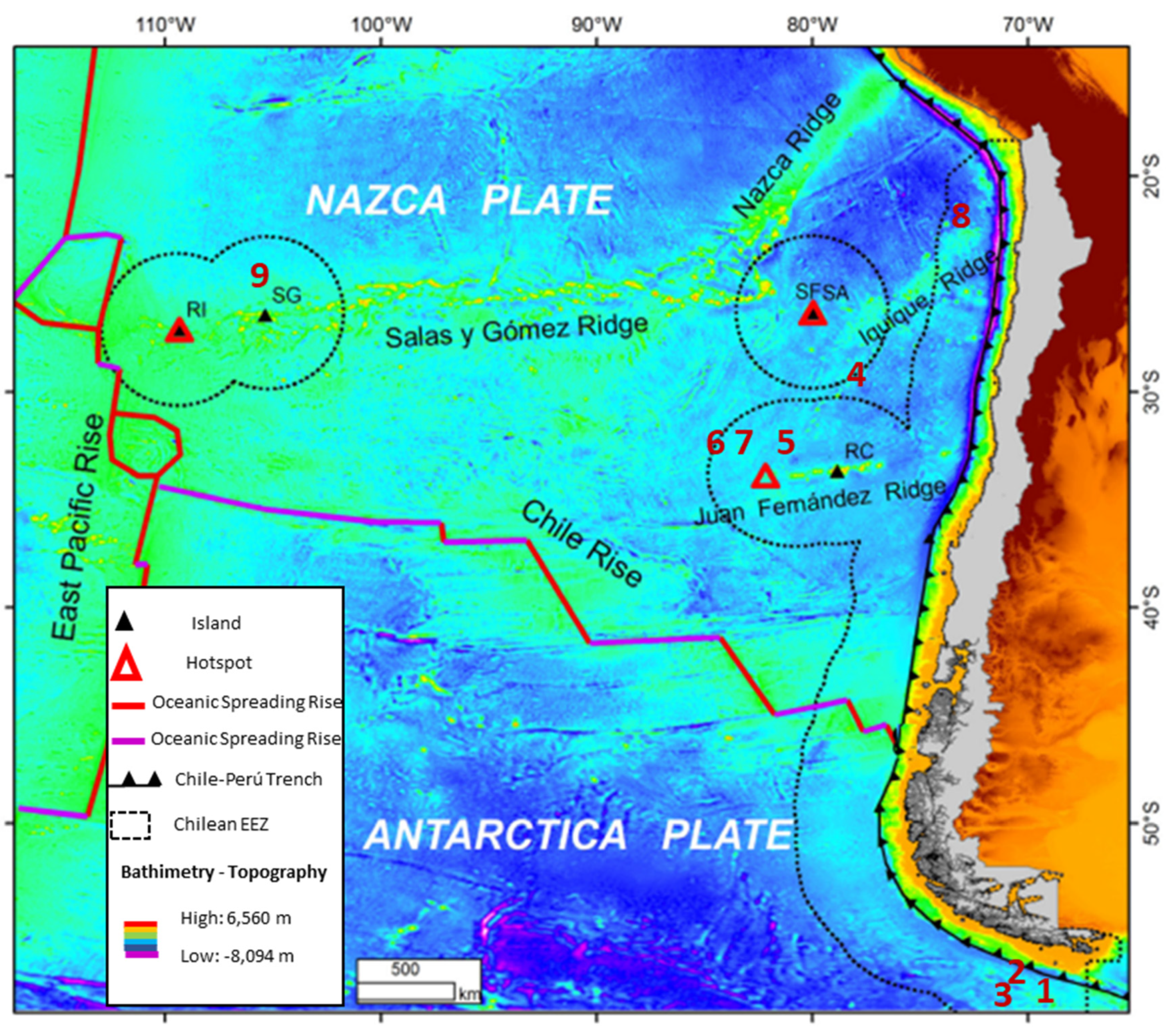
- Manganese nodules—the known reserves within Chile are as follows: Robinson Crusoe Island, at 3820 m depth, with a nodule concentration of 4–5 kg/m2, with a combined Ni + Co + Cu grade of 0.87%. At the mouth of the Rio Loa, 4332 m deep, there are fields with a Cu + Ni content of 1.38% (Site 7 of Table 2). Surrounding the Juan Fernandez ridge there is a zone of manganese nodules that is between 3650 m and 3873 m deep, having a combined Cu + Ni grade between 0.25% and 0.96%. Surrounding the San Francisco and San Antonio Islands, grades of up to 19.8% Fe, 14.638% Mn, and 0.305% Co have been observed (Site 4 of Table 2). In Northern Chile, at a depth of 4332 m, nodules have been sampled which contain an average of 0.65% Cu, 0.73% Ni and 0.81% Zn (Site 8 of Table 2). Close to the Salas and Gomez Islands, at a depth of 2890 m, nodule samples having an Fe grade of 18.8% and combined Cu+Ni grades of 0.6% have been collected (Site 9 of Table 2). In Southern Chile, south of the Magallanes, samples have been collected at depths ranging from 3767 m to 4007 m, with Mn grades that vary between 7.993% and 16.63%, Cu + Ni grades between 0.3% and 0.57%, and Co grades between 0.163% and 0.532% (Sites 1, 2, and 3 of Table 2) [12,14,57]
- Ferromanganese crusts—within the Chilean territory, the most promising area regarding ferromanganese crusts is found in the volcanic cord of which the Salas and Gómez and San Félix and San Ambrosio islands are part, as are the sedimentary basins that surround the Island Easter. The metallic content in these areas is relatively low in terms of copper and nickel concentration (between 0.25% and 0.30%), but it has a significant manganese content (10%) [12,14].
- Submarine polymetallic sulphides—To date, no deposit of massive sulphides from the seabed in Chile has been systematically sampled. However, the German Geometep program did explorations in the Eastern Pacific Ridge, to the north and south of Easter Island, where black chimneys and smokers were photographed and sampled at high temperatures at a depth of 2550 m. Based on the studies carried out in this exploration, it was estimated that these deposits could contain 300–500 g/t silver, 5–10 g/t gold, 10–20% zinc, 5–10% lead, and 1–10% copper [12,58].
- Sediments—in the national territory, there are sediments with a significant amount of marine phosphorites (P2O5), where the two main deposits are found on the Mejillones Peninsula, where it is estimated that the average phosphate grade is between 6% and 7%, and the Caldera Basin with an average grade of 18% of P2O5 [12,14]. Also, there are submarine gas hydrates, where there is a huge natural gas field (7500 km2), just 50 km from the Chilean coast, which extends from the city of Valparaíso to Puerto Montt [59,60].
3.1. Exploitation of Submarine Deposits in Chile
- Decree Law No. 3525/1980: National Service of Geology and Mining;
- Law No. 18.097/1982: Organic Constitutional Law on Mining Concessions;
- Law No. 18,248/1983: Mining Code;
- Decree No. 1/1987: Mining Code Regulations;
- Decree with Force of Law No. 1/1987: Creation of the Chilean Copper Commission;
- Decree No. 132/2004: Mining Safety Regulations;
- Law No. 20,551/2011: Closure of Mining Works and Installations;
- Decree No. 41/2012: Regulation Closing Works and Mining Facilities;
- Decree No. 100/2013: Creation of the International Mining Advisory Council.
3.2. Challenges to the Exploration and Exploitation of Manganese Nodules
- The duration of commercial mining operations is long;
- It is difficult to predict the climatic changes over a sufficiently long timeframe;
- There is a lack of data, and examples of large-scale operations to serve as benchmarks;
- It is difficult for equipment to be well maintained over time, especially in systems that are under such extreme hydrostatic pressure.
3.3. Environmental Issues
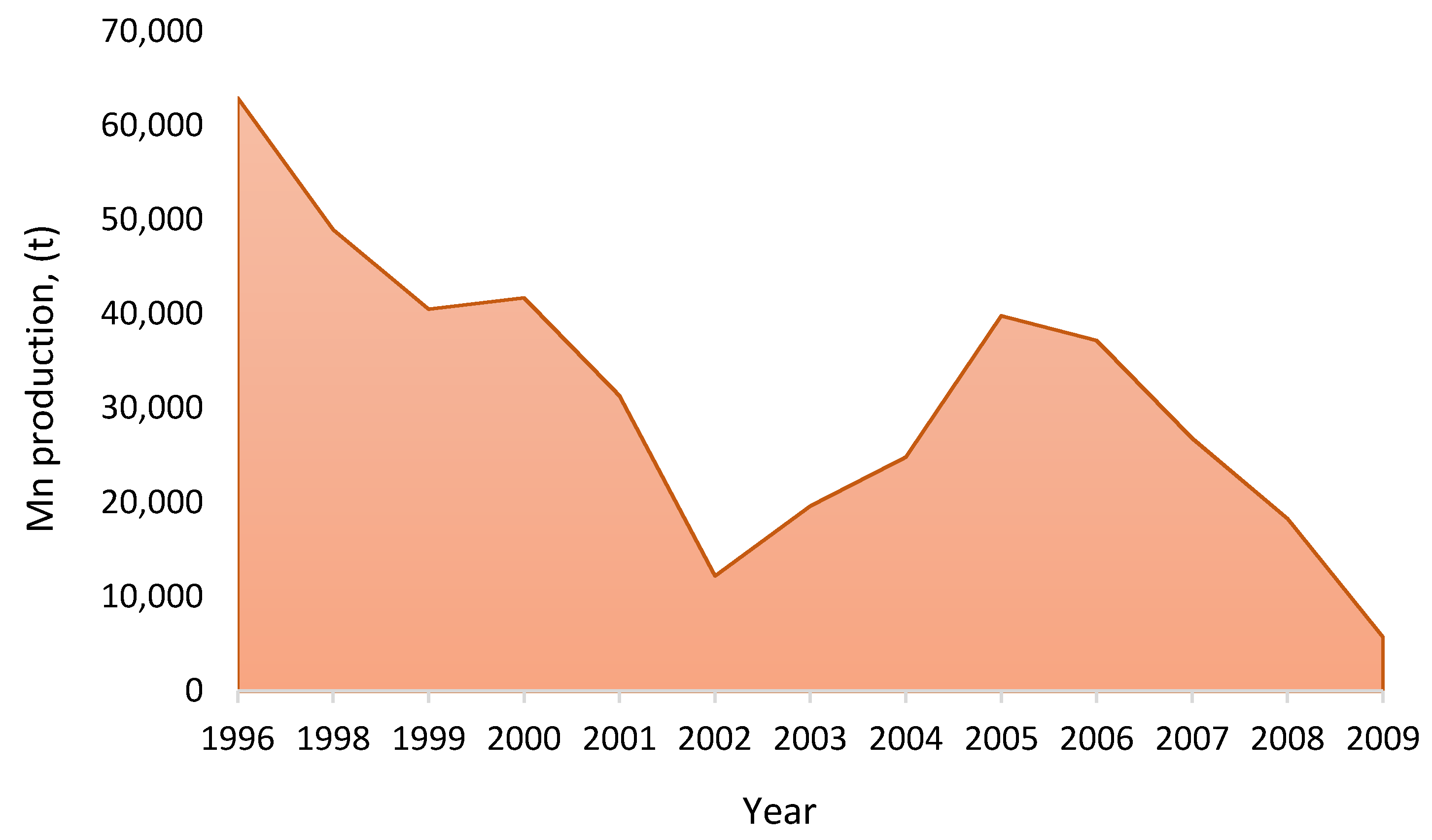
3.4. Context of the Manganese and Cobalt Market in Chile
4. Co and Mn Extractive Metallurgical Processes from Manganese Nodules
- Pyro-hydrometallurgical method for processing manganese nodules. This technology has a pyrometallurgical stage that allows the selective reduction of non-ferrous metals. A polymetallic alloy (Fe-Cu-Ni-Co-Mn) and a SiMn slag are formed. Subsequently, the complex alloy goes into a hydrometallurgical stage.
- Hydrometallurgical method of selective dissolution or by autoclaving of manganese nodules. They are based on leaching in an acidic or alkaline medium with or without the presence of reducing agents. In the case of the autoclave method, the process is exposed to a medium under high pressure and temperature.
4.1. Pyro-Hydrometallurgical Treatments
4.1.1. Segregation by Roasting in the Presence of Chlorination Agents
4.1.2. Metal Extraction by Sulfating Roasting and Leaching
4.1.3. Reduction of Manganese Nodules and Selective Ammonia Leaching
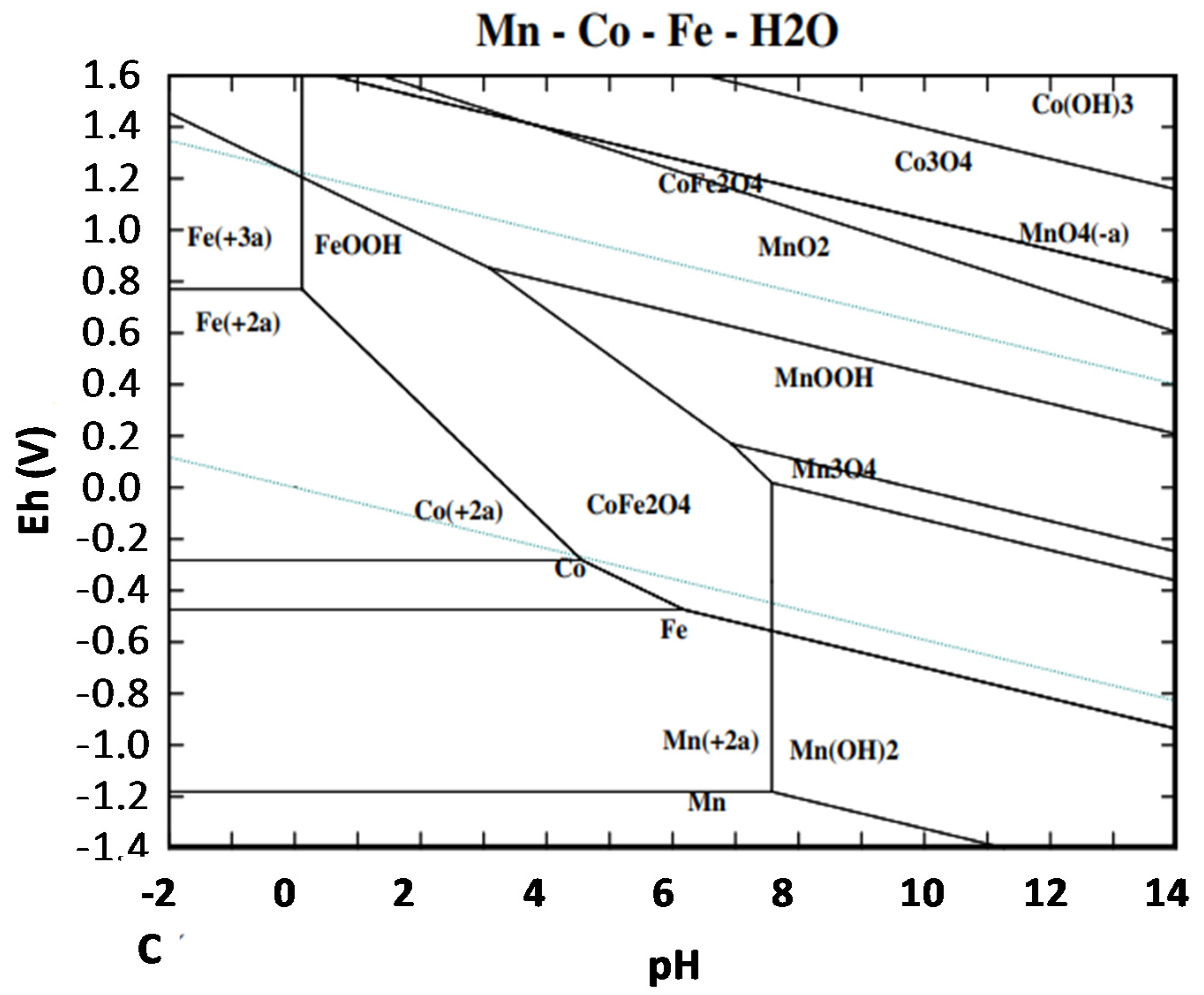
4.2. Hydrometallurgical Treatments
4.3. Tailings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ylä-Mella, J.; Pongrácz, E. Drivers and Constraints of Critical Materials Recycling: The Case of Indium. Resources 2016, 5, 34. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Sen, P.K. Metals and materials from deep sea nodules: An outlook for the future. Int. Mater. Rev. 2010, 55, 364–391. [Google Scholar] [CrossRef]
- Marchetti, P. Las opciones de Chile para explotar cobalto, reafirmar su liderazgo minero y mover US$1.000 millones más. Available online: http://www.emol.com/noticias/Economia/2017/08/25/872507/Las-opciones-de-Chile-para-explotar-cobalto-reafirmar-su-liderazgo-minero-y-mover-US1000-mas.html (accessed on 1 June 2020).
- Consejo Minero. Reporte Annual. 2018, pp. 227–249. Available online: https://consejominero.cl/newsletter/2019/Reporte-Anual-2018.pdf (accessed on 1 June 2020).
- Toro, N. Optimization of Parameters for the Extraction of Elements from Minerals in Acid Media; Technical University of Cartagena: Cartagena, Spain, 2020. [Google Scholar]
- Pak, S.-J.; Seo, I.; Lee, K.-Y.; Hyeong, K. Rare Earth Elements and Other Critical Metals in Deep Seabed Mineral Deposits: Composition and Implications for Resource Potential. Minerals 2018, 9, 3. [Google Scholar] [CrossRef]
- Wall, F.; Rollat, A.; Pell, R.S. Responsible Sourcing of Critical Metals. Elements 2017, 13, 313–318. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- USGS, U.S. Geological Survey; Lisa A. Corathers: Reston, VI, USA, 2020; pp. 98–99. Available online: https://www.usgs.gov/centers/nmic/manganese-statistics-and-information (accessed on 1 June 2020).
- Sharma, R. Deep-Sea Mining: Resource Potential, Technical and Environmental Considerations; Springer: Berlin, Germany, 2017; pp. 1–535. [CrossRef]
- Vergara Cortés, H. Recursos Minerales Oceánicos y Áreas potenciales dela ZEE de Chile. Su exploración y formación de recursos humanos. Rev. Mar. 1999, 116, 479–583. [Google Scholar]
- Glasby, G.P.; Li, J.; Sun, Z. Deep-Sea Nodules and Co-rich Mn Crusts. Mar. Georesources Geotechnol. 2014, 33, 72–78. [Google Scholar] [CrossRef]
- Pérez, K.; Villegas, Á.; Saldaña, M.; Jeldres, R.I.; González, J.; Toro, N. Initial investigation into the leaching of manganese from nodules at room temperature with the use of sulfuric acid and the addition of foundry slag—Part II. Sep. Sci. Technol. 2020, 1–6. [Google Scholar] [CrossRef]
- Konstantinova, N.; Cherkashov, G.; Hein, J.R.; Mirão, J.; Dias, L.; Madureira, P.M.; Kuznetsov, V.; Maksimov, F. Composition and characteristics of the ferromanganese crusts from the western Arctic Ocean. Ore Geol. Rev. 2017, 87, 88–99. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Castillo, J.; Higuera, F.; Acosta, R. Leaching of Manganese from Marine Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Tailings. Miner. 2019, 9, 289. [Google Scholar] [CrossRef]
- Nautilus Minerals Deep Sea Mining. Available online: http://www.nautilusminerals.com/IRM/content/default.aspx (accessed on 1 October 2019).
- Hein, J.R. Manganese Nodules. Encyclopedia of Geoarchaeology; Springer: New York, NY, USA, 2014; Part 2; pp. 1–7. [Google Scholar] [CrossRef]
- Low-Pfeng, A.; Peters-Recagno, E. La Frontera Final: El Oceáno Profundo; SEMARNAT-INECC: Mexico City, Mexico, 2014; ISBN 9786078246700. [Google Scholar]
- Hein, J.R.; Koschinsky, A.; Kuhn, T. Deep-ocean polymetallic nodules as a resource for critical materials. Nat. Rev. Earth Environ. 2020, 1, 158–169. [Google Scholar] [CrossRef]
- Hein, J.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 273–291. [Google Scholar]
- Wegorzewski, A.V.; Kuhn, T.; Dohrmann, R.; Wirth, R.; Grangeon, S. Mineralogical characterization of individual growth structures of Mn-nodules with different Ni+Cu content from the central Pacific Ocean. Am. Miner. 2015, 100, 2497–2508. [Google Scholar] [CrossRef]
- Marino, E.; Blasco, I.; Blanco, L.; González, F.J.; Somoza, L.; Medialdea, T. Llega la era de la Minería Submarina; Tierra y Tecnología: Madrid, Spain, 2017. [Google Scholar]
- Conrad, T.; Hein, J.R.; Paytan, A.; Clague, D.A. Formation of Fe-Mn crusts within a continental margin environment. Ore Geol. Rev. 2017, 87, 25–40. [Google Scholar] [CrossRef]
- Pattan, J.; Parthiban, G. Do manganese nodules grow or dissolve after burial? Results from the Central Indian Ocean Basin. J. Asian Earth Sci. 2007, 30, 696–705. [Google Scholar] [CrossRef]
- Cronan, D.S. Chapter 2 Deep-Sea Nodules: Distribution and Geochemistry. Elsevier Oceanogr. Ser. 1977, 15, 11–44. [Google Scholar] [CrossRef]
- Freepik Mapa Mundial con las Fronteras. Available online: https://www.freepik.es/vector-premium/ilustracion-mapa-mundial-fronteras_4319571.html (accessed on 1 June 2020).
- ISA. A Geological Model of Polymetallic Nodule Deposits in the Clarion-Clipperton Fracture Zone; International Seabed Authority: Kingston, Jamaica, 2010. [Google Scholar]
- Marchig, V.; Reyss, J. Diagenetic mobilization of manganese in Peru Basin sediments. Geochim. et Cosmochim. Acta 1984, 48, 1349–1352. [Google Scholar] [CrossRef]
- Koschinsky, A. Heavy metal distributions in Peru Basin surface sediments in relation to historic, present and disturbed redox environments. Deep. Sea Res. Part II: Top. Stud. Oceanogr. 2001, 48, 3757–3777. [Google Scholar] [CrossRef]
- Reyss, J.; Lemaitre, N.; Ku, T.; Marchig, V.; Southon, J.; Nelson, D.; Vogel, J. Growth of a manganese nodule from Peru Basin: A radiochemical anatomy. Geochim. Cosmochim. Acta 1985, 49, 2401–2408. [Google Scholar] [CrossRef]
- Vineesh, T.C.; Nath, B.N.; Banerjee, R.; Jaisankar, S.; Lekshmi, V. Manganese nodule morphology as indicators for oceanic processes in the Central Indian Basin. Int. Geol. Rev. 2009, 51, 27–44. [Google Scholar] [CrossRef]
- Pushcharovsky, Y.M. Tectonic types of deepwater basins in the Indian Ocean. Geotectonology 2007, 41, 355–367. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Ghosh, A.K. Dynamics of formation of ferromanganese nodules in the Indian Ocean. J. Asian Earth Sci. 2010, 37, 394–398. [Google Scholar] [CrossRef][Green Version]
- Banerjee, R.; Mukhopadhyay, R. Nature and distribution of manganese nodules from three sediment domains of the Central Indian Basin, Indian Ocean. Geo Marine Lett. 1991, 11, 39–43. [Google Scholar] [CrossRef]
- Valsangkar, A.; Khadge, N.; Desa, J.E. Geochemistry of polymetallic nodules from the Central Indian Ocean Basin. Mar. Geol. 1992, 103, 361–371. [Google Scholar] [CrossRef]
- Cronan, D.S.; Rothwell, G.; Croudace, I.W. An ITRAX Geochemical Study of Ferromanganiferous Sediments from the Penrhyn Basin, South Pacific Ocean. Mar. Georesources Geotechnol. 2010, 28, 207–221. [Google Scholar] [CrossRef]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Baker, E.; Beaudoin, Y. Manganese Nodules A Physical, Biological, Environmental, and Technical Review; Secretariat of the Pacific Community: New Caledonia, Australia, 2013; pp. 1–52. [Google Scholar]
- Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Kuhn, T.; Madureira, P.; Wegorzewski, A.V.; Mirão, J.; Medialdea, T.; Oeser, M.; Miguel, C.; Reyes, J.; et al. Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods. Minerals 2019, 9, 439. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Lunar, R.; Martínez-Frías, J.; Rubí, J.A.M.; Torres, T.; Ortíz, J.E.; Díaz-Del-Río, V. Fe-Mn nodules associated with hydrocarbon seeps: A new discovery in the Gulf of Cadiz (eastern central Atlantic). Episodes 2007, 30, 187–196. [Google Scholar] [CrossRef]
- González, F.; Somoza, L.; Lunar, R.; Martinez, E.S.; Rubi, J.A.M.; De Torres, T.; Ortiz, J.; Del Río, V.D.; Pinheiro, L.; Magalhães, V.; et al. Hydrocarbon-derived ferromanganese nodules in carbonate-mud mounds from the Gulf of Cadiz: Mud-breccia sediments and clasts as nucleation sites. Mar. Geol. 2009, 261, 64–81. [Google Scholar] [CrossRef]
- González, J.; Somoza, L.; Lunar, R.; Martínez, G.; Rubi, J.A.M.; Torres, T.; Ortiz, J.; Diaz-Del-Rio, V. Internal features, mineralogy and geochemistry of ferromanganese nodules from the Gulf of Cadiz: The role of the Mediterranean Outflow Water undercurrent. J. Mar. Syst. 2010, 80, 203–218. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; León, R.; Medialdea, T.; De Torres, T.; Ortiz, J.E.; Lunar, R.; Martinez-Frias, J.; Merinero, R. Ferromanganese nodules and micro-hardgrounds associated with the Cadiz Contourite Channel (NE Atlantic): Palaeoenvironmental records of fluid venting and bottom currents. Chem. Geol. 2012, 310, 56–78. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Hein, J.R.; Medialdea, T.; León, R.; Urgorri, V.; Reyes, J.; Martín-Rubí, J.A. Phosphorites, Co-rich Mn nodules, and Fe-Mn crusts from Galicia Bank, NE Atlantic: Reflections of Cenozoic tectonics and paleoceanography. Geochem. Geophys. Geosystems 2016, 17, 346–374. [Google Scholar] [CrossRef]
- Canet, C.; Prol-Ledesma, R.M.; Bandy, W.L.; Schaaf, P.; Linares, C.; Camprubí, A.; Tauler, E.; Gutierrez, C.A.Q.M. Mineralogical and geochemical constraints on the origin of ferromanganese crusts from the Rivera Plate (western margin of Mexico). Mar. Geol. 2008, 251, 47–59. [Google Scholar] [CrossRef]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.M.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Gálvez, E.; Cánovas, M.; Trigueros, E.; Castillo, J.; Hernández, P. Optimization of Parameters for the Dissolution of Mn from Manganese Nodules with the Use of Tailings in An Acid Medium. Minerals 2019, 9, 387. [Google Scholar] [CrossRef]
- Miller, K.A.; Thompson, K.F.; Johnston, P.; Santillo, D. An Overview of Seabed Mining Including the Current State of Development, Environmental Impacts, and Knowledge Gaps. Front. Mar. Sci. 2018, 4, 4. [Google Scholar] [CrossRef]
- ISA. Workshop on Polymetallic Nodule Mining Technology—Current Status and Challenges Ahead; International Seabed Authority: Chennai, India, 2008. [Google Scholar]
- Welling, C.G.; Davenport, G.H.; Reichert, G.; Snyder, C.M.; Harrold, M.C.; Donze, S.H.; Larsen, F. Ocean Mining System and Process. US Patent 4, 232, 903 (US 973854 (781228)), 11 November 1980. [Google Scholar]
- Zhao, G.; Xiao, L.; Peng, T.; Zhang, M. Experimental Research on Hydraulic Collecting Spherical Particles in Deep Sea Mining. Energies 2018, 11, 1938. [Google Scholar] [CrossRef]
- Halbach, P.; Marchig, V.; Scherhag, C. Regional variations in Mn, Ni, Cu, and Co of ferromanganese nodules from a basin in the Southeast Pacific. Mar. Geol. 1980, 38, M1–M9. [Google Scholar] [CrossRef]
- Ministerio de Defensa Nacional. Ministerio de Defensa Nacional Libro de la Defensa Nacional 1a Parte: El Estado de Chile; Ministerio de Defensa Nacional: Santiago, Chile, 2010; Volume 1, pp. 24–75. [Google Scholar]
- Völker, D.; Koschinsky, A. Recursos geoquímicos del fondo marino. Geol. Mar. Chile 2010, 60–65. [Google Scholar]
- Flückiger, M.; Valenzuela, E. Impacto de la minería submarina en la producción minera de Chile. En: Actas del VII Congreso Geológico Chileno; Universidad de Concepción: Concepción, Chile, 1994; Volume 1, pp. 286–289. [Google Scholar]
- Velásquez, M. Los Hidratos de Gas Submarinos Una esperanza energética y la contribución de la Armada. Rev. Mar. 2004, 121, 66–74. [Google Scholar]
- MundoMarítimo Gigantesco Yacimiento de Gas Natural en Chile. Available online: https://www.mundomaritimo.cl/noticias/gigantesco-yacimiento-de-gas-natural-en-chile (accessed on 1 June 2020).
- García, M.; Correa, J.; Maksaev, V.; Townley, B. Potential mineral resources of the Chilean offshore: An overview. Andean Geol. 2020, 47, 1–13. [Google Scholar] [CrossRef]
- Consejo Minero Legislación Minera. Available online: https://consejominero.cl/chile-pais-minero/legislacion-minera/ (accessed on 1 June 2020).
- Townley, B.; Díaz, A.; Luca, R. Estado del arte y Potenciales Recursos Co y Mn en Chile. 2017, p. 55. Available online: https://www.corfo.cl/sites/Satellite?blobcol=urldatablobkey=idblobtable=MungoBlobsblobwhere=1475166637562ssbinary=true (accessed on 1 June 2020).
- COCHILCO Inversión en la minería chilena—Cartera de proyectos 2017–2026. 2017. Available online: https://www.cochilco.cl/Listado%20Temtico/2017%2008%2010%20Cartera%20de%20proyectos%202017%20-%202026%20vf.pdf (accessed on 1 June 2020).
- Cifuentes, C. Chile, minería más allá del Cobre. 2019. Available online: https://www.cochilco.cl/Presentaciones/20190822Chile-mineríamásalládelcobre_CCG.pdf (accessed on 1 June 2020).
- COCHILCO Anuario de Estadísticas del Cobre y Otros Minerales (1997–2016). 2017. Available online: https://www.cochilco.cl/Lists/Anuario/Attachments/17/Anuario-avance7-10-7-17.pdf (accessed on 1 June 2020).
- Jana, R.; Singh, D.; Roy, S. Alcohol-modified hydrochloric acid leaching of sea nodules. Hydrometallurgy 1995, 38, 289–298. [Google Scholar] [CrossRef]
- Randhawa, N.S.; Hait, J.; Jana, R.K. A brief overview on manganese nodules processing signifying the detail in the Indian context highlighting the international scenario. Hydrometallurgy 2016, 165, 166–181. [Google Scholar] [CrossRef]
- Abramovski, T.; Stefanova, V.P.; Causse, R.; Romanchuk, A. Technologies for the processing of polymetallic nodules from Clarion Clipperton Zone in the Pacific Ocean. J. Chem. Technol. Metall. 2017, 52, 258–269. [Google Scholar]
- Fuerstenau, D.W.; Han, K.N. Metallurgy and Processing of Marine Manganese Nodules. Miner. Process. Extr. Met. Rev. 1983, 1, 1–83. [Google Scholar] [CrossRef]
- Thoumsin, F.J.; Coussement, R. Fluid-bed roasting reactions of copper and cobalt sulfide concentrates. JOM 1964, 16, 831–834. [Google Scholar] [CrossRef]
- Theys, L.F.; Lee, L.V. Sulfate roasting copper-cobalt sulfide concentrates. JOM 1958, 10, 134–136. [Google Scholar] [CrossRef]
- Hoover, M.; Han, K.; Fuerstenau, D. Segregation roasting of nickel, copper and cobalt from deep-sea manganese nodules. Int. J. Miner. Process. 1975, 2, 173–185. [Google Scholar] [CrossRef]
- Di Yorio, C.; Betancourt, E.; Vivas, R.; Rus, J. Ni, Co recovery study and Fe by acid leaching in columns. Rev. de Met. 2006, 42, 41–48. [Google Scholar] [CrossRef]
- Pahlman, J.E.; Khalafalla, S.E. Selective Recovery of Nickel, Cobalt, Manganese from Sea Nodules with Sulfurous Acid. U.S. Patent US 4, 138, 465 (US 860249 (771213)), 6 February 1979. [Google Scholar]
- Kawahara, M.; Tokikawa, K.; Mitsuo, T. Sulfating Roasting of Manganese Nodules and Selective Leaching of Roasted Ore. Shigen-to-Sozai. J. Min. Mater. Process. Inst. Jpn. 1991, 107, 305–309. [Google Scholar] [CrossRef]
- Wilder, T.C. Two Stage Selective Leaching of Copper and Nickel from Complex Ore. US Patent US 3 736 125 (US 55306 (700716), 29 May 1973. [Google Scholar]
- Elodino, M. Procesos Siderugicos. Universidad Autónoma Metropolitana. Amacalli Editores; Universidad Autónoma Metropolitana: Mexico City, Mexico, 1992. [Google Scholar]
- Jana, R.K.; Akerkar, D.D. Studies of the Metal—Ammonia—Carbon Dioxide—Water System in Extraction Metallurgy of Polymetallic Sea Nodules. Hydrometallurgy 1989, 22, 363–378. [Google Scholar] [CrossRef]
- Kanungo, S.; Jena, P. Studies on the dissolution of metal values in manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1988, 21, 23–39. [Google Scholar] [CrossRef]
- Khalafalla, S.E.; Pahlmann, J.E. Selective extraction of metals from Pacific sea nodules with dissolved sulphur dioxide. J. Metals 1981, 33, 37–42. [Google Scholar]
- Han, K.N.; Fuertenau, D.W. Extraction behavior of metallic elements from deep-sea manganese nodules in reducing medium. Marine Mining 1986, 2, 155–169. [Google Scholar]
- Momade, F.; Momade, Z. Reductive leaching of manganese oxide ore in aqueous methanol–sulphuric acid medium. Hydrometallurgy 1999, 51, 103–113. [Google Scholar] [CrossRef]
- Pankratova, A.B.; Nevskaya, E.Y.; Kutepov, A.M.; Gorichev, I.G.; Izotov, A.D.; Zaitsev, B.E. Dissolution Kinetics of Manganese (III, IV) Oxides in Sulfuric Acid in the Presence of Ethylenediaminetetraacetic Acid. Theor. Found. Chem. Eng. 2001, 35, 168–174. [Google Scholar] [CrossRef]
- Sahoo, R.; Naik, P.; Das, S. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- Senanayake, G. A mixed surface reaction kinetic model for the reductive leaching of manganese dioxide with acidic sulfur dioxide. Hydrometallurgy 2004, 73, 215–224. [Google Scholar] [CrossRef]
- Torres, D.; Ayala, L.; Saldaña, M.; Cánovas, M.; Jeldres, R.I.; Nieto, S.; Castillo, J.; Robles, P.; Toro, N. Leaching Manganese Nodules in an Acid Medium and Room Temperature Comparing the Use of Different Fe Reducing Agents. Metals 2019, 9, 1316. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules—A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Lee, Y. Ferroalloys: Production and use in Steel-making. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3039–3044. [Google Scholar]

| Element | CCZ Nodules | Peru Basin Nodules | Indian Ocean Nodules | Cook Island Nodules |
|---|---|---|---|---|
| Mean | Mean | Mean | Mean | |
| Fe (wt.%) | 6.16 | 6.12 | 7.14 | 16.1 |
| Mn | 28.4 | 34.2 | 24.4 | 15.9 |
| Si | 6.55 | 4.82 | 10.02 | 8.24 |
| Al | 2.36 | 1.5 | 2.92 | 3.25 |
| Ti | 0.32 | 0.16 | 0.42 | 1.15 |
| Bi (ppm) | 8.8 | 3.3 | - | 11 |
| Co | 2.098 | 475 | 1111 | 4116 |
| Cu | 10,704 | 5988 | 10,406 | 2290 |
| Li | 131 | 311 | 110 | 61 |
| Mo | 590 | 547 | 600 | 262 |
| Nb | 22 | 13 | 98 | 92 |
| Ni | 13,002 | 13,008 | 11,010 | 3756 |
| Pb | 338 | 121 | 731 | 2000 |
| Pt | 0.13 | 0.04 | - | 0.21 |
| Te | 3.6 | 1.7 | 40 | 23 |
| Th | 15 | 6.9 | 76 | 34 |
| Tl | 199 | 129 | 347 | 138 |
| V | 445 | 431 | 497 | 920 |
| W | 62 | 75 | 92 | 59 |
| Zn | 1366 | 1845 | 1207 | 516 |
| Zr | 307 | 325 | 752 | 468 |
| REE | 813 | 403 | 1039 | 1707 |
| Nodule Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Latitude S | 59.017 | 57.983 | 57,083 | 30.392 | 33.05 | 33.042 | 33.033 | 21.338 | 26.2 |
| Longitude W | 70.75 | 70.883 | 70.983 | 78.98 | 82.05 | 83.942 | 84.05 | 73.908 | 106.3 |
| Depth (m) | 3797 | 4007 | 3767 | 3820 | 3881 | 3650 | 3873 | 4332 | 2890 |
| Element (wt.%) | |||||||||
| Fe | 24.56 | 17.957 | 18.02 | 19.802 | 16.375 | 19.25 | 6.7 | 6.7 | 18.8 |
| Mn | 16.63 | 15.183 | 7.993 | 14.638 | 17.067 | - | 10.1 | 10.1 | 10.6 |
| Ni | 0.2 | 0.435 | 0.357 | 0.295 | 0.518 | 0.285 | 0.5 | 0.73 | 0.43 |
| Cu | 0.1 | 0.135 | 0.153 | 0.238 | 0.3 | 0.175 | 0.23 | 0.65 | 0.17 |
| Co | 0.24 | 0.532 | 0.163 | 0.305 | 0.108 | 0.105 | 0.14 | 0.04 | 0.14 |
| Zn | 0.07 | 0.084 | 0.068 | 0.052 | 0.08 | 0.065 | 0.07 | 0.81 | - |
| Pb | 0.28 | 0.11 | 0.07 | 0.1 | - | - | 0.06 | 0.03 | - |
| Mo | - | - | - | 0.026 | - | 0.048 | - | - | - |
| Cu + Ni | 0.3 | 0.57 | 0.51 | 0.533 | 0.818 | 0.46 | 0.73 | 1.38 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro, N.; Jeldres, R.I.; Órdenes, J.A.; Robles, P.; Navarra, A. Manganese Nodules in Chile, an Alternative for the Production of Co and Mn in the Future—A Review. Minerals 2020, 10, 674. https://doi.org/10.3390/min10080674
Toro N, Jeldres RI, Órdenes JA, Robles P, Navarra A. Manganese Nodules in Chile, an Alternative for the Production of Co and Mn in the Future—A Review. Minerals. 2020; 10(8):674. https://doi.org/10.3390/min10080674
Chicago/Turabian StyleToro, Norman, Ricardo I. Jeldres, Javier A. Órdenes, Pedro Robles, and Alessandro Navarra. 2020. "Manganese Nodules in Chile, an Alternative for the Production of Co and Mn in the Future—A Review" Minerals 10, no. 8: 674. https://doi.org/10.3390/min10080674
APA StyleToro, N., Jeldres, R. I., Órdenes, J. A., Robles, P., & Navarra, A. (2020). Manganese Nodules in Chile, an Alternative for the Production of Co and Mn in the Future—A Review. Minerals, 10(8), 674. https://doi.org/10.3390/min10080674








