Relationship between Textural Parameters of Lamellar Products Obtained by Acid Activation of Pure and Commercial Vermiculites and Their Iron and Water Content
Abstract
1. Introduction
2. Materials and Methods
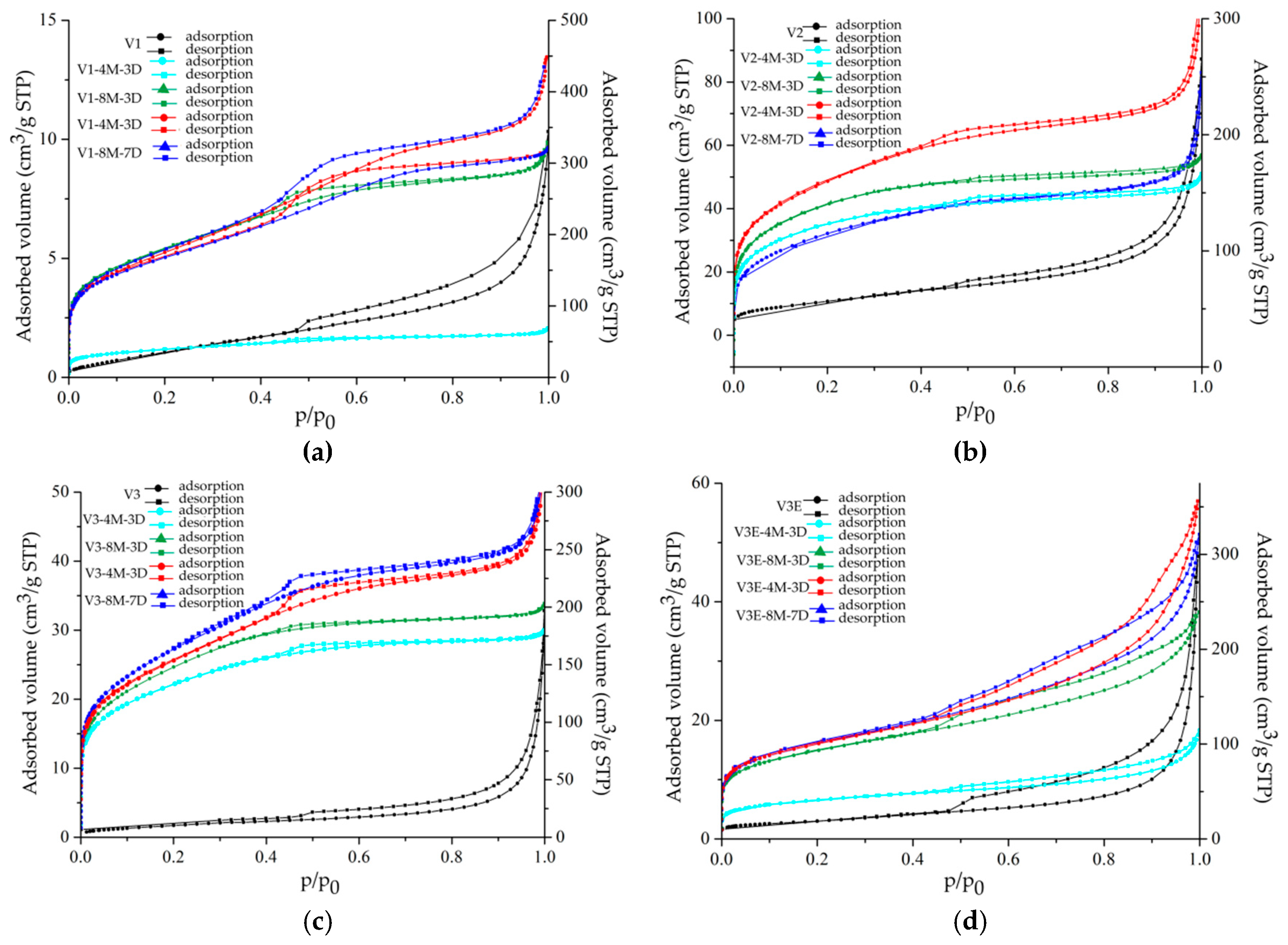
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suzuki, M.; Wada, N.; Hines, D.R.; Whittingham, M.S. Hydration states and phase transitions in vermiculite intercalation compounds. Phys. Rev. B 1987, 36, 2844–2851. [Google Scholar] [CrossRef]
- Walker, G.F. Trioctahedral minerals in the soil clays of North East Scotland. Mineral. Mag. J. Mineral. Soc. 1951, 29, 72–84. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodríguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Myers, J.B. Vermiculite. In Industrial and Minerals Rocks; American Institute of Mechanical Engineers: New York, NY, USA, 1963; pp. 889–895. [Google Scholar]
- Mamina, A.K.; Koteĺnikova, E.N.; Muromtsev, V.A. Influence of the structural per-fection of phlogopite crystals on their cleavability by hydrogen peroxide. Inorg. Mater. 1990, 26, 2104–2107. [Google Scholar]
- Suquet, H.; Chevalier, S.; Marcilly, C.; Barthomeuf, D. Preparation of porous materials by chemical activation of the Llano vermiculite. Clay Miner. 1991, 26, 49–60. [Google Scholar] [CrossRef]
- Üçgül, E.; Girgin, I.Ç. Chemical exfoliation characteristics of Karakoc, phlogopite in hydrogen peroxide solution. Turk. J. Chem. 2002, 26, 431–439. [Google Scholar]
- Obut, A.; Girgin, I.Ç. Hydrogen peroxide exfoliation of vermiculite and phlogopite. Miner. Eng. 2002, 15, 683–687. [Google Scholar] [CrossRef]
- Weiss, Z.; Valášková, M.; Seidlerová, J.; Šupová-Křístková, M.; Šustai, O.; Matějka, V. Preparation of vermiculite nanoparticles using thermal hydrogen peroxide treatment. J. Nano Sci. Nano Technol. 2006, 6, 726–730. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Expansion behaviour of commercial vermiculites at 1000 °C. Appl. Clay Sci. 2010, 48, 492–498. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Expansibility of vermiculites irradiated with microwaves. Appl. Clay Sci. 2011, 51, 33–37. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Exfoliation of vermiculites with chemical treatment using hydrogen peroxide and thermal treatment using microwaves. Appl. Clay Sci. 2014, 87, 219–227. [Google Scholar] [CrossRef]
- Huo, X.; Wu, L.; Liao, L.; Xia, Z.; Wang, L. The effect of interlayer cations on the expansion of vermiculite. Powder Technol. 2012, 224, 241–246. [Google Scholar] [CrossRef]
- Hillier, S.; Marwa, E.M.M.; Rice, C.M. On the mechanism of exfoliation of Vermiculite. Clay Miner. 2013, 48, 563–582. [Google Scholar] [CrossRef]
- Jin, L.; Dai, B. TiO2 activation using acid-treated vermiculite as a support: Characteristics and photoreactivity. Appl. Surf. Sci. 2012, 258, 3386–3392. [Google Scholar] [CrossRef]
- Suquet, H.; Franck, R.; Lambert, J.E.; Marcilly, C.E.E.; Chevalier, S. Catalytic properties of two pre-cracking matrices; a leached vermiculite and a Al-pillared saponite. Appl. Clay Sci. 1994, 8, 349–364. [Google Scholar] [CrossRef]
- Ravichandran, J.; Sivasankar, B. Properties and catalytic activity of acid-modified montmorillonite and vermiculite. Clays Clay Miner. 1997, 45, 854–858. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D. Preparation of porous silica from vermiculite by selective leaching. Appl. Clay Sci. 2003, 22, 187–195. [Google Scholar] [CrossRef]
- Okada, K.; Arimitsu, N.; Kameshima, Y.; Nakajima, A.; MacKenzie, K.J.D. Solid acidity of 2:1 type clay minerals activated by selective leaching. Appl. Clay Sci. 2006, 31, 185–196. [Google Scholar] [CrossRef]
- Maqueda, C.; Romero, A.S.; Morillo, E.; Perez-Rodriguez, J.L. Effect of grinding on the preparation of porous materials by acid-leached vermiculite. J. Phys. Chem. Solids 2007, 68, 1220–1224. [Google Scholar] [CrossRef]
- Maqueda, C.; Perez-Rodriguez, J.L.; Šubrt, J.; Murafa, N. Study of ground and unground leached vermiculite. Appl. Clay Sci. 2009, 44, 178–184. [Google Scholar] [CrossRef]
- Perez-Rodriguez, J.L.; Maqueda, C.; Murafa, N.; Šubrt, J.; Balek, V.; Pulišová, P.; Lančok, A. Study of ground and unground leached vermiculite II. Thermal behaviour of ground acid-treated vermiculite. Appl. Clay Sci. 2011, 51, 274–282. [Google Scholar] [CrossRef]
- Steudel, A.; Batenburg, L.F.; Fischer, H.R.; Weidler, P.G.; Emmerich, K. Alteration of swelling clay minerals by acid activation. Appl. Clay Sci. 2009, 44, 105–115. [Google Scholar] [CrossRef]
- Stawiński, W.; Freitas, O.; Chmielarz, L.; Węgrzyn, A.; Komędera, K.; Mordarski, G.; Figueiredo, S. The influence of acid treatments over vermiculite based material as adsorbent for cationic textile dyestuffs. Chemosphere 2016, 153, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Del Rey-Perez-Caballero, F.J.; Poncelet, G. Microporous 18 angstrom Al-pillared vermiculites: Preparation and characterization. Microporous Mesoporous Mater. 2000, 41, 169–181. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Michalik, M.; Dudek, B.; Piwowarska, Z.; Matusiewicz, A. Acid-activated vermiculites and phlogophites as catalysts for the DeNOx process. Appl. Clay Sci. 2010, 49, 156–162. [Google Scholar] [CrossRef]
- Alves, A.P.M.; Fonseca, M.G.; Wanderley, A.F. Inorganic-organic Hybrids Originating from Organosilane Anchored onto Leached Vermiculite. Mater. Res. 2013, 16, 891–897. [Google Scholar] [CrossRef][Green Version]
- Santos, S.S.G.; Silva, H.R.M.; de Souza, A.G.; Alves, A.P.M.; da Silva Filho, E.C.; Fonseca, M.G. Acid-leached mixed vermiculites obtained by treatment with nitric acid. Appl. Clay Sci. 2015, 104, 286–294. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Stawiński, W.; Freitas, O.; Komędera, K.; Błachowski, A.; Jęczmionek, Ł.; Dańko, T.; Mordarski, G.; Figueiredo, S. Study of adsorptive materials obtained by wet fine milling and acid activation of vermiculite. Appl. Clay Sci. 2018, 155, 37–49. [Google Scholar] [CrossRef]
- Polubesova, T.; Zadaka, D.; Groisman, L.; Nir, S. Water remediation by micelleeclay system: Case study for tetracycline and sulfonamide antibiotics. Water Res. 2006, 40, 2369–2374. [Google Scholar] [CrossRef]
- Silva, H.R.M.; Fonseca, M.G.; Espinola, J.G.P.; Brito, H.F.; Fasustino, W.M.; Teotonio, E.E.S. Luminescent Eu-III complexes immobilized on a vermiculite clay surface. Eur. J. Inorg. Chem. 2014, 11, 1914–1921. [Google Scholar] [CrossRef]
- Justo, A.; Maqueda, C.; Pérez Rodriguez, J.L.; Morillo, E. Expansibility of some vermiculites. Appl. Clay Sci. 1989, 4, 509–519. [Google Scholar] [CrossRef]
- Harraz, H.Z.; Hamdy, M.M. Interstratified vermiculite–mica in the gneiss–metapelite–serpentinite rocks at Hafafit area, Southern Eastern Desert, Egypt: From metasomatism to weathering. J. Afr. Earth Sci. 2010, 58, 305–320. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Marcos, C.; Argüelles, A.; Ruíz-Conde, A.; Sánchez-Soto, P.J.; Blanco, J.A. Study of the dehydration process of vermiculites by applying a vacuum pressure: Formation of interstratified phases. Mineral Mag. 2003, 67, 1253–1268. [Google Scholar] [CrossRef]
- Marcos, C.; Rodriguez, I. Structural changes on vermiculite treated with methanol and ethanol and subsequent microwave irradiation. Appl. Clay Sci. 2016, 123, 304–314. [Google Scholar] [CrossRef]
- Marcos, C. and Rodriguez, I. Effect of propanol and butanol and subsequent microwave irradiation on the structure of commercial vermiculites. Appl. Clay Sci. 2017, 144, 104–114. [Google Scholar] [CrossRef]
- Wypych, F.; Adad, L.B.; Mattoso, N.; Marangon, A.A.S.; Schreiner, W.H. Synthesis and characterization of disordered layered silica obtained by selective leaching of octahedral sheets from chrysotile and phlogopite structures. J. Colloid Interface Sci. 2005, 283, 107–112. [Google Scholar] [CrossRef]
- Chmielarz, L.; Wojciechowska, M.; Rutkowska, M.; Adamski, A.; Węgrzyn, A.; Kowalczyk, A.; Dudek, B.; Boron, P.; Michalik, M.; Matusiewicz, A. Acid activated vermiculites as catalysts of the DeNOx process. Catal. Today 2012, 191, 25–31. [Google Scholar] [CrossRef]
- Yu, X.; Wei, C.; Ke, L.; Wu, H.; Chai, X.; Hu, Y. Preparation of trimethylchlorosilanemodified acid vermiculites for removing diethyl phthalate from water. J. Colloid Interface Sci. 2012, 369, 344–351. [Google Scholar] [CrossRef]
- Ehsani, I.; Turianicová, E.; Baláž, M.; Obut, A. Effects of sulphuric acid dissolution on the physical and chemical properties of a natural and a heated vermiculite. Acta Montanistica Slovaca 2015, 20, 110–115. [Google Scholar]
- Harkonen, M.A.; Keiski, R.A. Porosity and surface area of acid leached phlogopite. The effect of leaching conditions and thermal treatment. Colloids Surf. 1984, 11, 323–339. [Google Scholar] [CrossRef]
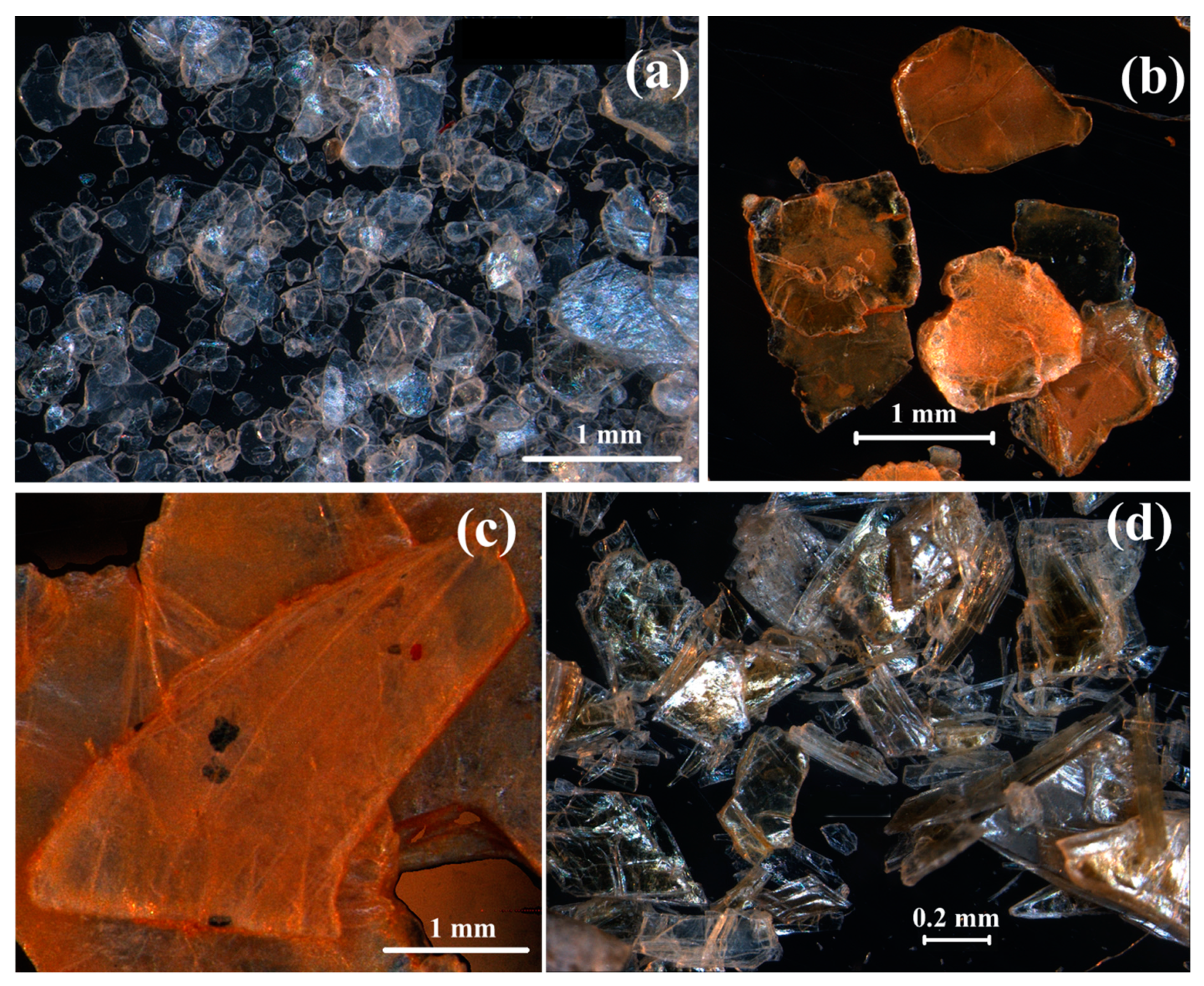
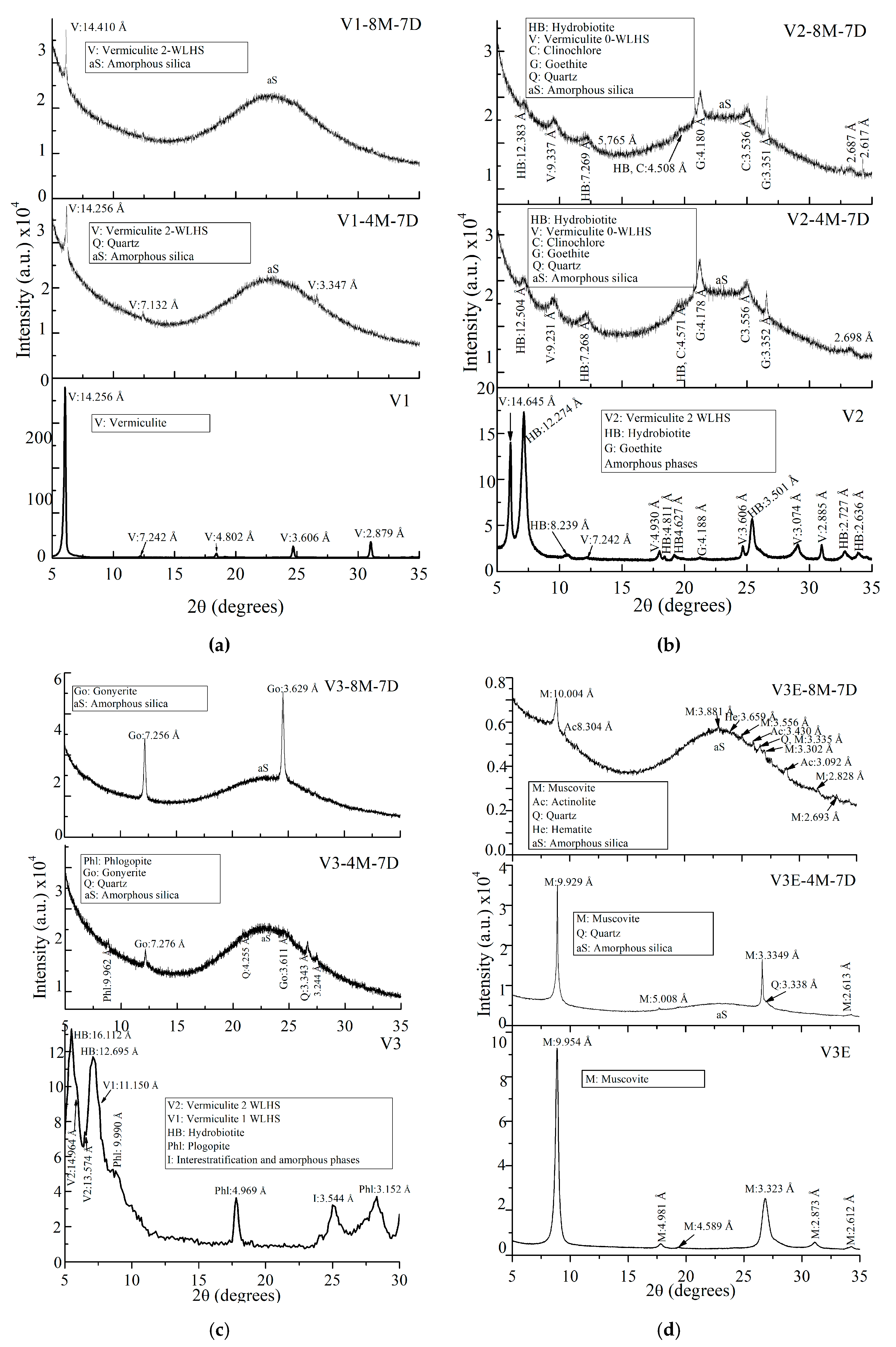
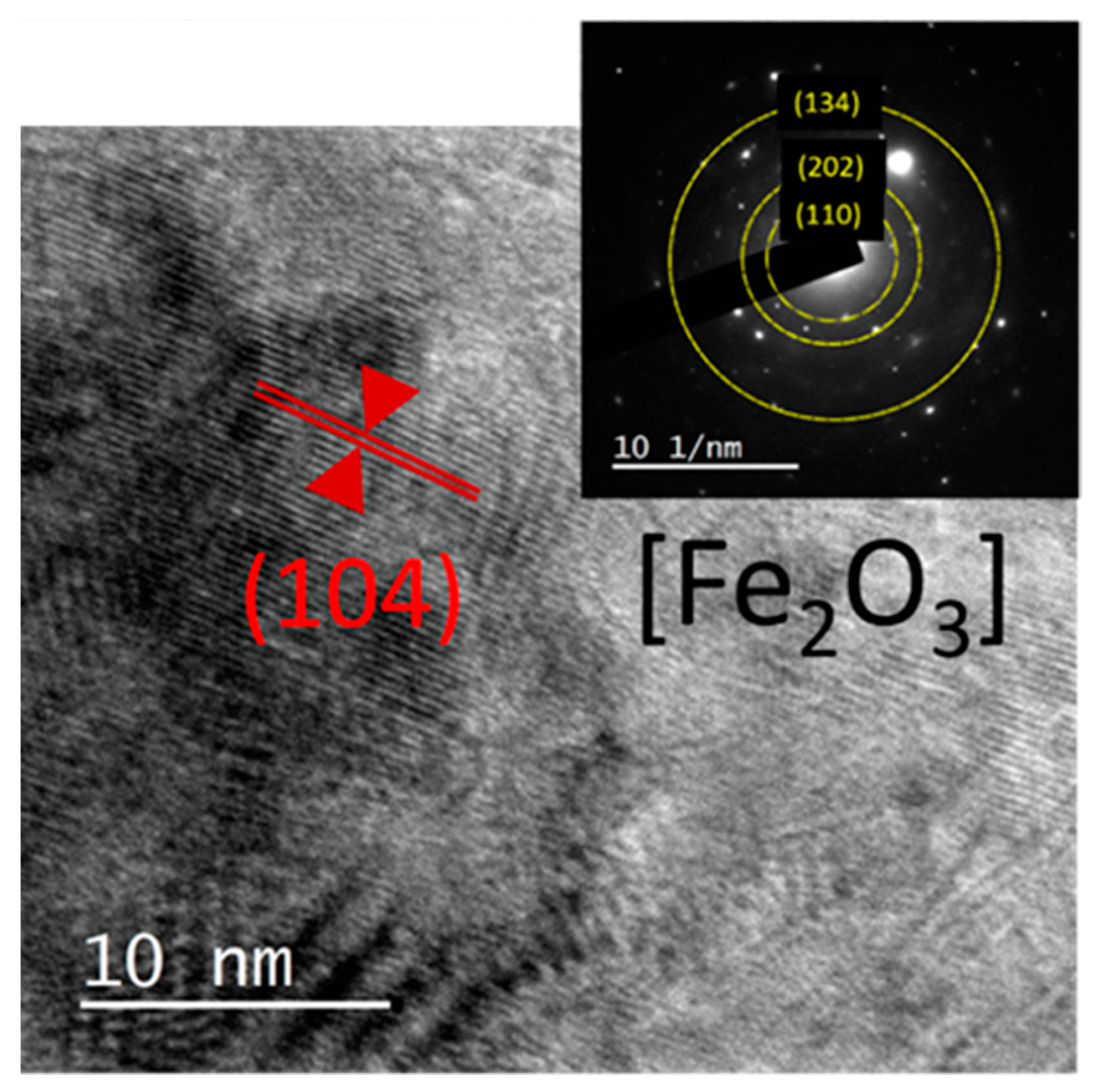
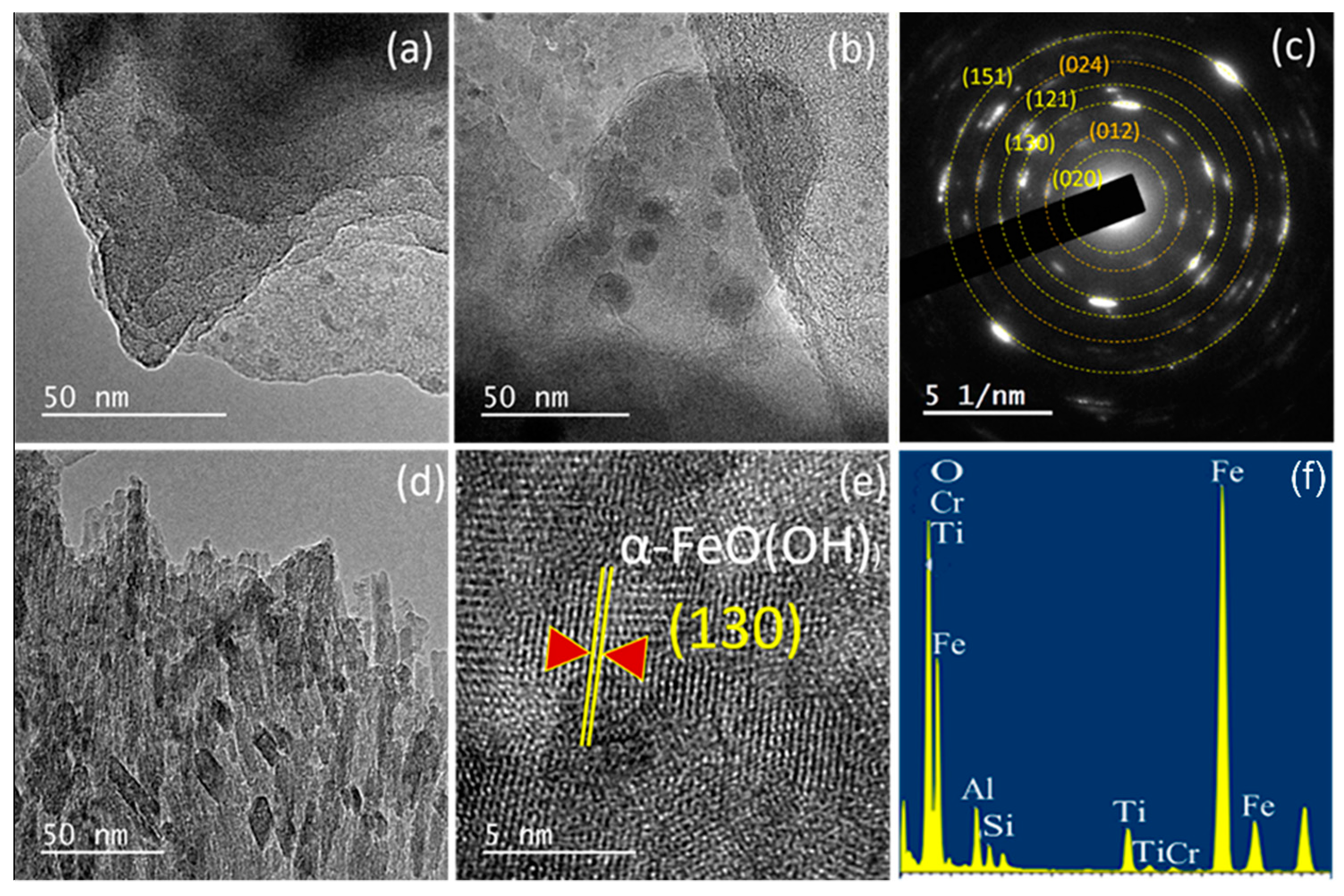
| Oxides | V1 | V2 | V3 | V2E | V3E |
|---|---|---|---|---|---|
| SiO2 | 35.93 | 40.67 | 35.65 | 40.60 | 36.34 |
| Al2O3 | 15.78 | 11.51 | 11.00 | 10.15 | 11.26 |
| MgO | 24.13 | 18.05 | 21.81 | 5.50 | 21.54 |
| FeO | 3.27 | 9.58 | 4.63 | 10.59 | 4.70 |
| TiO2 | 0.33 | 0.79 | 1.16 | 1.30 | 1.35 |
| Cr2O3 | 0.03 | 0.01 | 0.39 | 0.04 | 0.32 |
| K2O | 0.03 | 1.07 | 5.61 | 0.00 | 4.73 |
| CaO | 0.29 | 0.03 | 0.92 | 0.50 | 1.11 |
| Na2O | 0.12 | 0.12 | 3.55 | 0.00 | 1.15 |
| MnO | 0.14 | 0.08 | 0.04 | 0.05 | 0.03 |
| NiO | 0.00 | 0.01 | 0.06 | 0.00 | 0.10 |
| Water content | 25.6 | 13.6 | 12.3 | 5.6 | 7.0 |
| Samples | Weight Loss (%) |
|---|---|
| V1-4M-1D | 70 |
| V1-8M-1D | 75 |
| V1-4M-7D | 72 |
| V1-8M-7D | 70 |
| V2-4M-1D | 12 |
| V2-8M-1D | 14 |
| V2-4M-7D | 47 |
| V2-8M-7D | 47 |
| V3-4M-1D | 22 |
| V3-8M-1D | 27 |
| V3-4M-7D | 53 |
| V3-8M-7D | 52 |
| Sample | O | Si | Mg | Al | Fe | Ti | Cr | Ca | Na | K |
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 69.4 (2.8) | 11.4 (1.1) | 12.1 (0.4) | 5.7 (0.3) | 1.3 (0.9) | 0.1 (0.0) | 0.1 (0.1) | |||
| V1-4M-7D | 73.7 (0.6) | 26.3 (0.6) | ||||||||
| V1-8M-7D | 70.0 (2.8) | 30.0 (2.8) | ||||||||
| V2 | 67.6 (1.5) | 12.7 (0.8) | 9.8 (1.0) | 4.4 (0.3) | 2.8 (0.8) | 0.8 (0.1) | 2.3 (0.4) | |||
| V2-4M-7D | 67.8 (1.8) | 28.8 (3.9) | 2.1 (2.3) | 1.0 (0.3) | 0.4 (0.2) | |||||
| V2-8M-7D | 66.6 (3.3) | 31.2 (3.5) | 0.5 (0.6) | 1.1 (0.2) | 0.5 (0.1) | |||||
| V3 | 66.2 (1.6) | 13.0 (0.7) | 11.8 (0.3) | 4.5 (0.2) | 1.2 (0.2) | 0.3 (0.1) | 0.1 (0.0) | 0.4 (0.1) | 1.0 (0.2) | 1.7 (0.3) |
| V3-4M-7D | 75.1 (4.4) | 24.7 (4.7) | ||||||||
| V3-8M-7D | 77.0 (2.5) | 19.9 (5.5) |
| Sample | SBET (m2/g) | Qm (cm3/g STP) | Vp (cm3/g STP) | Pore Size (nm) | C | R2 |
|---|---|---|---|---|---|---|
| V1 | 3 ± 0 | 0.7 | 0.01 | 2.38 | 61 | 0.99528 |
| V1-4M-3D | 138 ± 0 | 32 | 0.09 | 1.69 | 194 | 0.99992 |
| V1-8M-3D | 598 ± 6 | 137 | 0.54 | 1.69 | 286 | 0.99942 |
| V1-4M-7D | 537 ± 6 | 123 | 0.48 | 1.72 | 401 | 0.99950 |
| V1-8M-7D | 586 ± 4 | 135 | 0.56 | 2.53 | 218 | 0.99970 |
| V2 | 36 ± 0 | 9 | 0.09 | 2.65 | 136 | 0.99963 |
| V2-4M-3D | 411 ± 4 | 94 | 0.08 | 2.56 | 525 | 0.99952 |
| V2-8M-3D | 465 ± 4 | 107 | 0.08 | 2.57 | 437 | 0.99971 |
| V2-4M-7D | 513 ± 9 | 118 | 0.27 | 2.52 | 712 | 0.99869 |
| V2-8M-7D | 389 ± 4 | 89 | 0.28 | 1.80 | 324 | 0.99972 |
| V3 | 7 ± 0 | 1.6 | 0.03 | 2.52 | 28 | 0.99965 |
| V3-4M-3D | 419 ± 6 | 96 | 0.09 | 2.58 | 592 | 0.99949 |
| V3-8M-3D | 505 ± 4 | 116 | 0.13 | 2.54 | 237 | 0.99971 |
| V3-4M-7D | 510 ± 6 | 117 | 0.27 | 2.79 | 361 | 0.99924 |
| V3-8M-7D | 510 ± 6 | 117 | 0.33 | 2.48 | 602 | 0.99891 |
| V3E | 11 ± 0 | 2.5 | 0.07 | 3.08 | 135 | 0.99998 |
| V3E-4M-3D | 148 ± 0 | 34 | 0.07 | 3.09 | 213 | 0.99998 |
| V3E-8M-3D | 284 ± 0 | 65 | 0.29 | 3.08 | 165 | 0.99999 |
| V3E-4M-7D | 358 ± 1 | 82 | 0.44 | 3.11 | 222 | 0.99997 |
| V3E-8M-7D | 331 ± 1 | 77 | 0.26 | 3.08 | 222 | 0.99999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos, C.; Adawy, A.; Rodríguez, I. Relationship between Textural Parameters of Lamellar Products Obtained by Acid Activation of Pure and Commercial Vermiculites and Their Iron and Water Content. Minerals 2020, 10, 661. https://doi.org/10.3390/min10080661
Marcos C, Adawy A, Rodríguez I. Relationship between Textural Parameters of Lamellar Products Obtained by Acid Activation of Pure and Commercial Vermiculites and Their Iron and Water Content. Minerals. 2020; 10(8):661. https://doi.org/10.3390/min10080661
Chicago/Turabian StyleMarcos, Celia, Alaa Adawy, and Irene Rodríguez. 2020. "Relationship between Textural Parameters of Lamellar Products Obtained by Acid Activation of Pure and Commercial Vermiculites and Their Iron and Water Content" Minerals 10, no. 8: 661. https://doi.org/10.3390/min10080661
APA StyleMarcos, C., Adawy, A., & Rodríguez, I. (2020). Relationship between Textural Parameters of Lamellar Products Obtained by Acid Activation of Pure and Commercial Vermiculites and Their Iron and Water Content. Minerals, 10(8), 661. https://doi.org/10.3390/min10080661







