Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore
Abstract
1. Introduction
1.1. Water Recovery, Froth Stability, and Entrainment
1.2. Methods to Quantify Entrainment
1.3. Seawater Flotation
2. Materials and Methods
2.1. Samples and Reagents
2.2. Quantifying Entrainment
3. Results
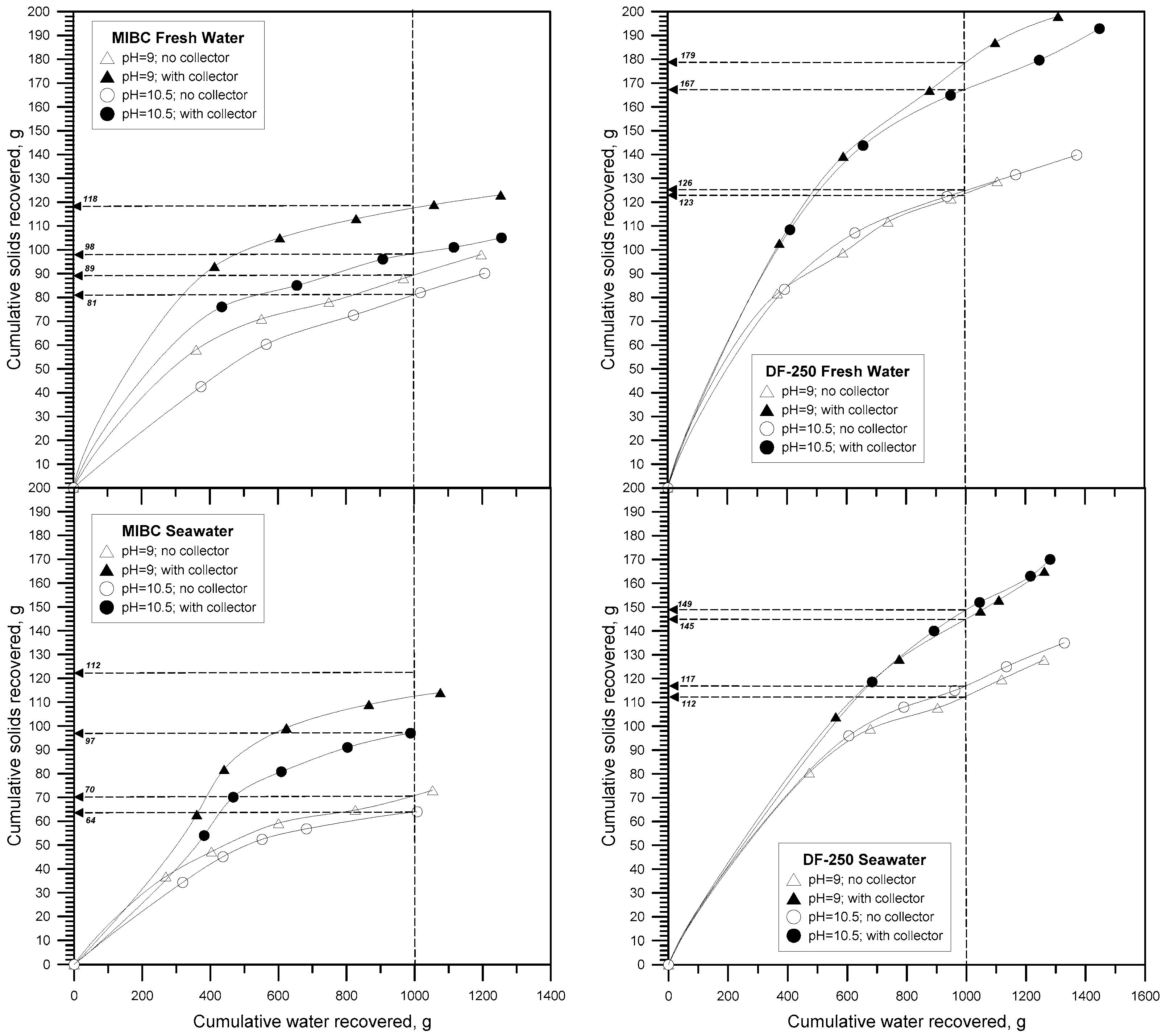
3.1. Results Method 1
3.2. Results Method 2
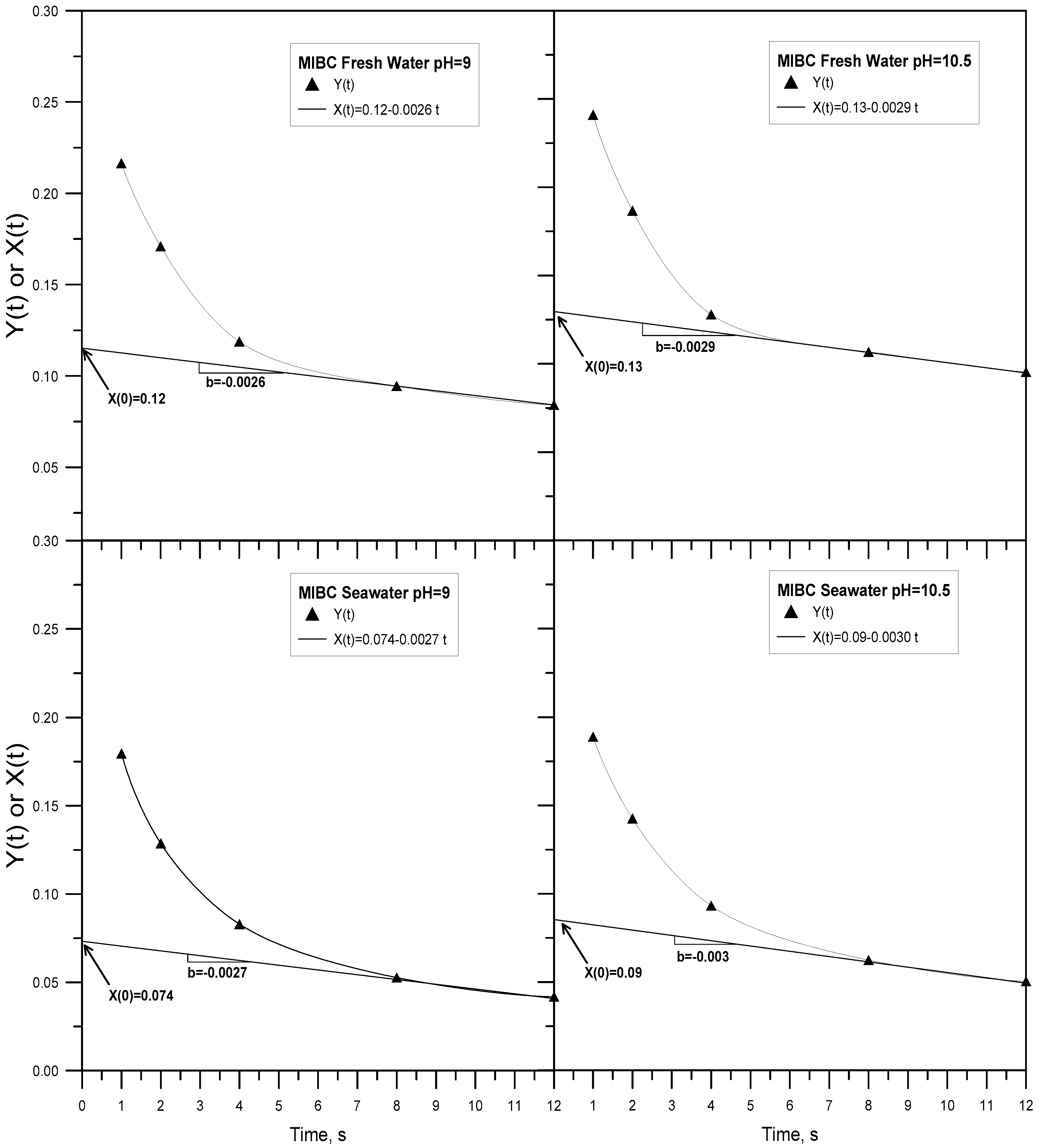
3.3. Results Method 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.G.; Warren, L.J. Entrainment of particles into flotation froths. Miner. Process. Extr. Metall. Rev. 1989, 5, 123–145. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K.; Bradshaw, D. A review of entrainment: Mechanisms, contributing factors and modelling in flotation. Miner. Eng. 2015, 70, 77–91. [Google Scholar] [CrossRef]
- Savassi, O.N.; Alexander, D.J.; Franzidis, J.P.; Manlapig, E.V. An empirical model for entrainment in industrial flotation plants. Miner. Eng. 1998, 11, 243–256. [Google Scholar] [CrossRef]
- Melo, F.; Laskowski, J.S. Fundamental properties of flotation frothers and their effect on flotation. Miner. Eng. 2006, 19, 766–773. [Google Scholar] [CrossRef]
- Melo, F.; Laskowski, J.S. Effect of frothers and solid particles on the rate of water transfer to froth. Int. J. Miner. Process. 2007, 84, 33–40. [Google Scholar] [CrossRef]
- Kracht, W.; Orozco, Y.; Acuña, C. Effect of surfactant type on the entrainment factor and selectivity of flotation at laboratory scale. Miner. Eng. 2016, 92, 216–220. [Google Scholar] [CrossRef]
- Neethling, S.J.; Cilliers, J.J. The entrainment factor in froth flotation: Model for particle size and other operating parameter effects. Int. J. Miner. Process. 2009, 93, 141–148. [Google Scholar] [CrossRef]
- Neethling, S.J.; Lee, H.T.; Cilliers, J.J. The recovery of liquid from flowing foams. J. Phys. Condens. Matter 2003, 15, 1563–1576. [Google Scholar] [CrossRef]
- Neethling, S.J.; Lee, H.T.; Cilliers, J.J. Simple relationships for predicting the recovery of liquid from flowing foams and froths. Miner. Eng. 2003, 16, 1123–1130. [Google Scholar] [CrossRef]
- Neethling, S.J.; Brito-Parada, P. Predicting flotation behavior—The interaction between froth stability and performance. Miner. Eng. 2018, 120, 60–65. [Google Scholar] [CrossRef]
- Neethling, S.J.; Cilliers, J.J.; Woodburn, E.T. Prediction of the water distribution in a flowing foam. Chem. Eng. Sci. 2000, 55, 4021–4028. [Google Scholar] [CrossRef]
- Nguyen, A.V. Liquid drainage in single Plateau borders of foam. J. Colloid Interface Sci. 2002, 249, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P. Dimensional analysis of foam drainage. Chem. Eng. Sci. 2006, 61, 4503–4510. [Google Scholar] [CrossRef]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Pugh, R.J. Experimental techniques for studying the structure of foams and froths. Adv. Colloid Interface Sci. 2005, 114–115, 239–251. [Google Scholar] [CrossRef]
- Savassi, O.N. Direct Estimation of the Degree of Particles in Industrial Flotation Cells. Ph.D. Thesis, School of Engineering, The University of Queensland, Brisbane, Australia, 1998. [Google Scholar]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Warren, L.J. Determination of the contributions of true flotation and entrainment in batch flotation tests. Int. J. Miner. Process. 1985, 14, 33–44. [Google Scholar] [CrossRef]
- Ross, V.E. Flotation and entrainment of particles during batch flotation tests. Miner. Eng. 1990, 3, 245–256. [Google Scholar] [CrossRef]
- Uribe, L.; Gutierrez, L.; Jerez, O. The depressing effect of clay minerals on the floatability of chalcopyrite. Miner. Process. Extr. Metall. Rev. 2016, 37, 227–235. [Google Scholar] [CrossRef]
- Uribe, L.M. Efecto del Agua de Mar en la Recuperación de Minerales de Cobre Molibdeno por Procesos de Flotación. Ph.D. Thesis, Universidad de Concepcion, Concepción, Chile, 2017. [Google Scholar]
- Rebolledo, E.; Laskowski, J.S.; Gutierrez, L.; Castro, S. Use of dispersants in flotation of molybdenite in seawater. Miner. Eng. 2017, 100, 71–74. [Google Scholar] [CrossRef]
- Yepsen, R.; Gutierrez, L.; Laskowski, J.S. Flotation behavior of enargite in the process of flotation using seawater. Miner. Eng. 2019, 142, 105897. [Google Scholar] [CrossRef]
- Ramirez, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Alvarez, A.; Gutierrez, L.; Laskowski, J.S. Use of polyethylene oxide to improve flotation of fine molybdenite. Miner. Eng. 2018, 127, 232–237. [Google Scholar] [CrossRef]
- Castro, S. Proceso Para Pre-Tratar Agua de Mar y Otras Aguas Salinas Para su Utilización en Procesos Industriales. Universidad de Concepcion. Chilean Patent No. 00475/INAPI, 12 May 2010. [Google Scholar]
- Castro, S. Challenges in flotation of Cu-Mo sulfide ores in sea water. In Water in Mineral Processing; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2012; pp. 29–40. [Google Scholar]
- Castro, S.; Rioseco, P.; Laskowski, J.S. Depression of molybdenite in sea water. In Proceedings of the 26th International Mineral Processing Congress, New Delhi, India, 24–28 September 2012; pp. 739–752. [Google Scholar]
- Castro, S.; Uribe, L.; Laskowski, J.S. Depression of inherently hydrophobic minerals by hydrolysable metal cations: Molybdenite depression in seawater. In Proceedings of the XXVII International Mineral Processing Congress-IMPC, Santiago, Chile, 20–24 October 2014; pp. 20–24. [Google Scholar]
- Cho, Y.S.; Laskowski, J.S. Effect of flotation frothers on bubble size and foam stability. Int. J. Miner. Process. 2002, 64, 69–80. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Cho, Y.S.; Ding, K. Effect of frothers on bubble size and foam stability in potash ore flotation systems. Can. J. Chem. Eng. 2003, 81, 63–69. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S.; Heiskanen, K. Effect of frothers on bubble size. Int. J. Miner. Process. 2005, 76, 225–233. [Google Scholar] [CrossRef]
- Grau, R.A.; Laskowski, J.S. Role of frothers in bubble generation and coalescence in a mechanical flotation cell. Can. J. Chem. Eng. 2006, 84, 170–182. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Quinn, J.J.; Maldonado, M.; Gomez, C.O.; Finch, J.A. Experimental study on the shape-velocity relationship of an ellipsoidal bubble in inorganic salt solutions. Miner. Eng. 2014, 55, 5–10. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S.; Ramos, O. Effect of seawater main components on frothability in the flotation of cu-mo sulfide ore. Physicochem. Probl. Miner. Process. 2014, 50, 17–29. [Google Scholar]
- Ramos, O.; Castro, S.; Laskowski, J.S. Copper-molybdenum ores flotation in sea water: Floatability and frothability. Miner. Eng. 2013, 53, 108–112. [Google Scholar] [CrossRef]
- Iskra, J.; Laskowski, J.S. Frothers and frothing. In Frothing in Flotation II; Laskowski, J.S., Woodburn, E.T., Eds.; Gordon and Breach: New York, NY, USA, 1969; pp. 1–50. [Google Scholar]

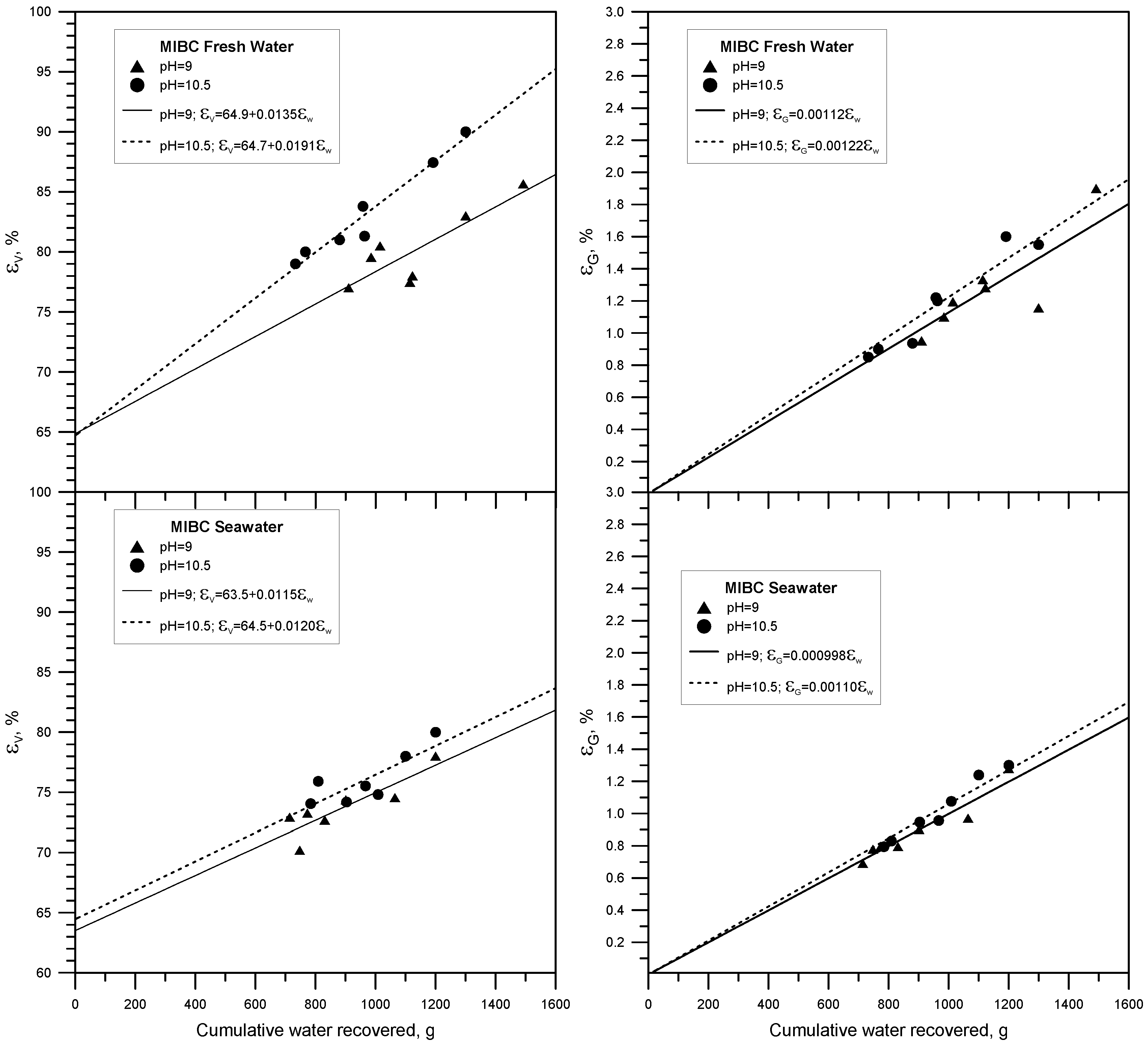


| Frother | Solution | pH | εtrue | eV | eG |
| MIBC | Fresh Water | 9.0 | 64.9 | 0.0135 | 0.00112 |
| MIBC | Fresh Water | 10.5 | 64.7 | 0.0191 | 0.00122 |
| MIBC | Seawater | 9.0 | 63.5 | 0.0115 | 0.00100 |
| MIBC | Seawater | 10.5 | 64.5 | 0.0120 | 0.00110 |
| Frother | Solution | pH | εtrue | eV | eG |
| DF-250 | Fresh Water | 9.0 | 68.3 | 0.0162 | 0.00233 |
| DF-250 | Fresh Water | 10.5 | 69.8 | 0.0157 | 0.00284 |
| DF-250 | Seawater | 9.0 | 67.6 | 0.0145 | 0.00200 |
| DF-250 | Seawater | 10.5 | 67.1 | 0.0140 | 0.00259 |
| Frother | Solution | pH | X(0) | b |
| MIBC | Fresh Water | 9.0 | 0.12 | −0.0026 |
| MIBC | Fresh Water | 10.5 | 0.13 | −0.0029 |
| MIBC | Seawater | 9.0 | 0.074 | −0.0027 |
| MIBC | Seawater | 10.5 | 0.09 | −0.0030 |
| Frother | Solution | pH | X(0) | b |
| DF-250 | Fresh Water | 9.0 | 0.18 | −0.0038 |
| DF-250 | Fresh Water | 10.5 | 0.21 | −0.0047 |
| DF-250 | Seawater | 9.0 | 0.13 | −0.0035 |
| DF-250 | Seawater | 10.5 | 0.14 | −0.0024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, L.; Betancourt, F.; Uribe, L.; Maldonado, M. Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore. Minerals 2020, 10, 615. https://doi.org/10.3390/min10070615
Gutierrez L, Betancourt F, Uribe L, Maldonado M. Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore. Minerals. 2020; 10(7):615. https://doi.org/10.3390/min10070615
Chicago/Turabian StyleGutierrez, Leopoldo, Fernando Betancourt, Lina Uribe, and Miguel Maldonado. 2020. "Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore" Minerals 10, no. 7: 615. https://doi.org/10.3390/min10070615
APA StyleGutierrez, L., Betancourt, F., Uribe, L., & Maldonado, M. (2020). Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore. Minerals, 10(7), 615. https://doi.org/10.3390/min10070615






