Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill
Abstract
1. Introduction
2. Experimental Materials
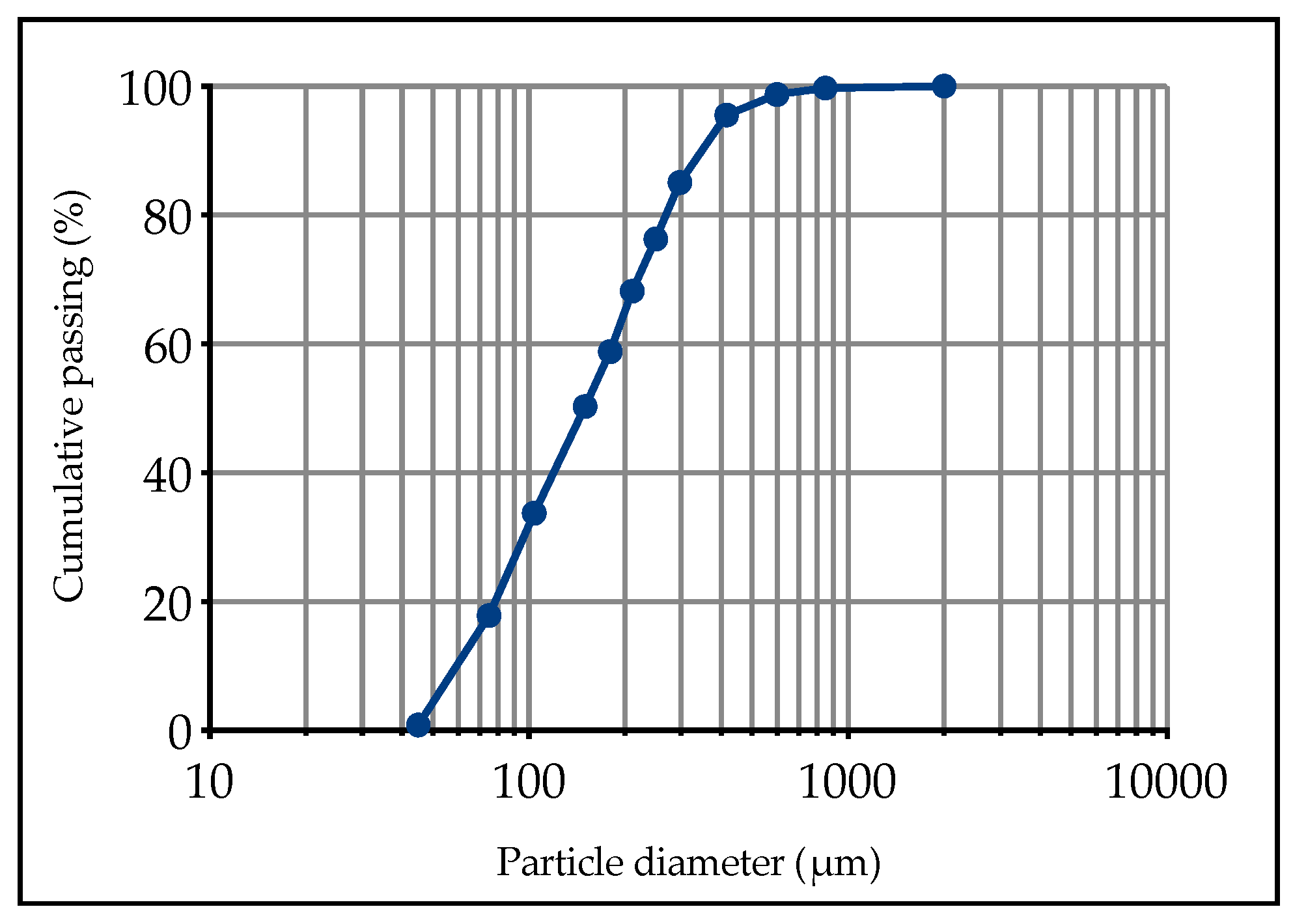
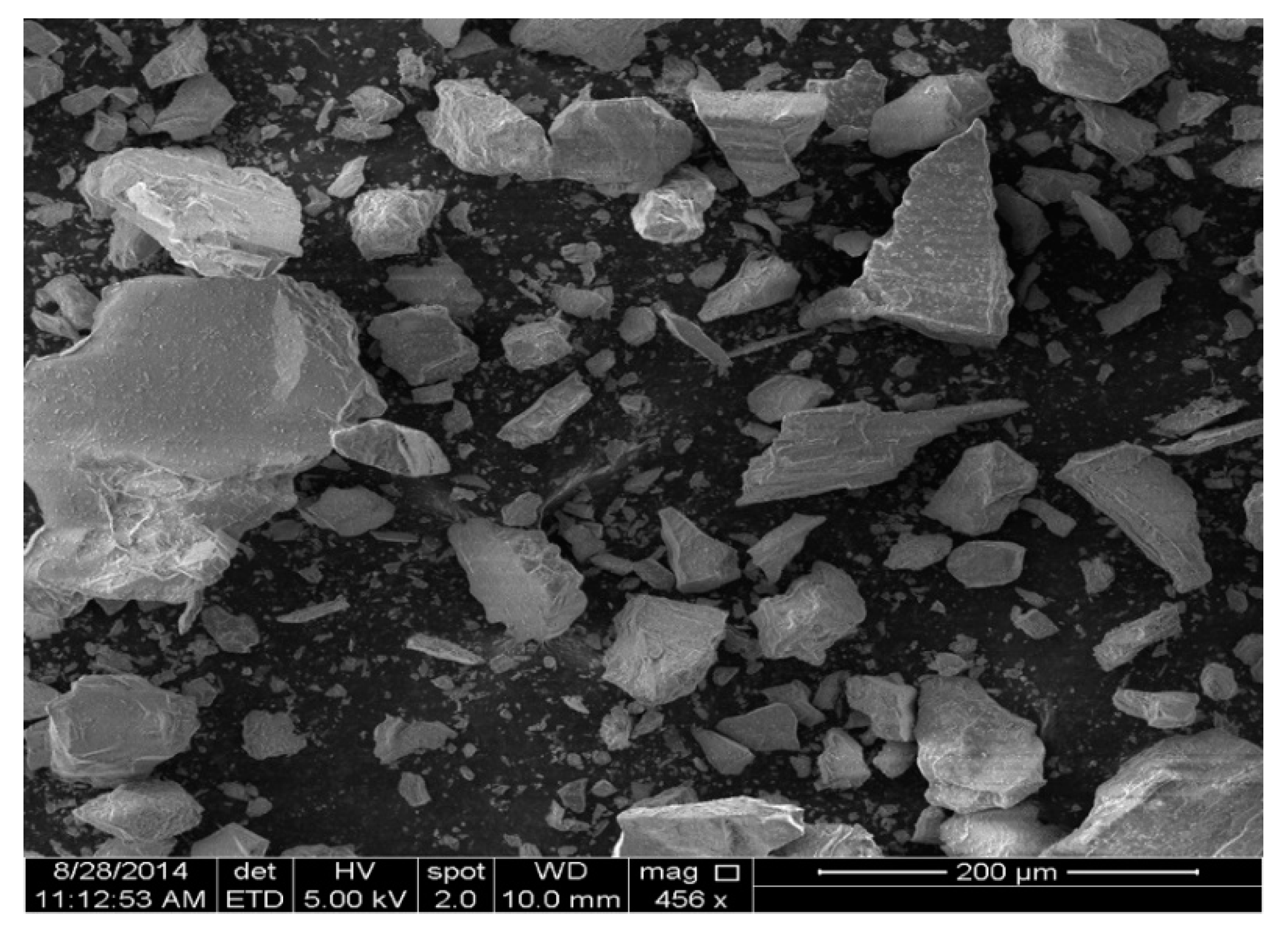
2.1. Mine Tailings
2.2. Binder
2.3. Water
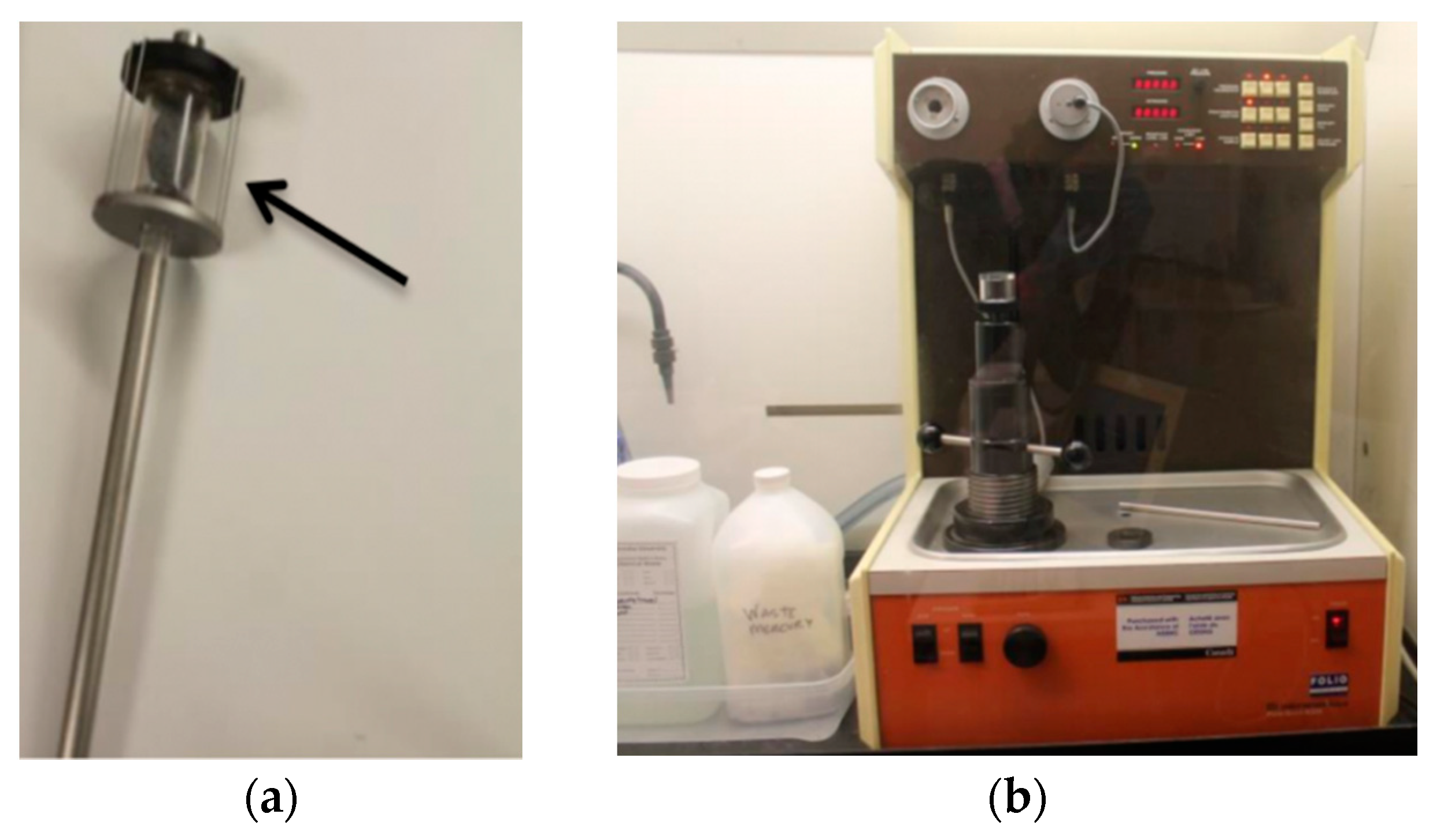
2.4. Foaming Agent and Foam Generator
2.5. FMF Composition
2.6. Experimental Design
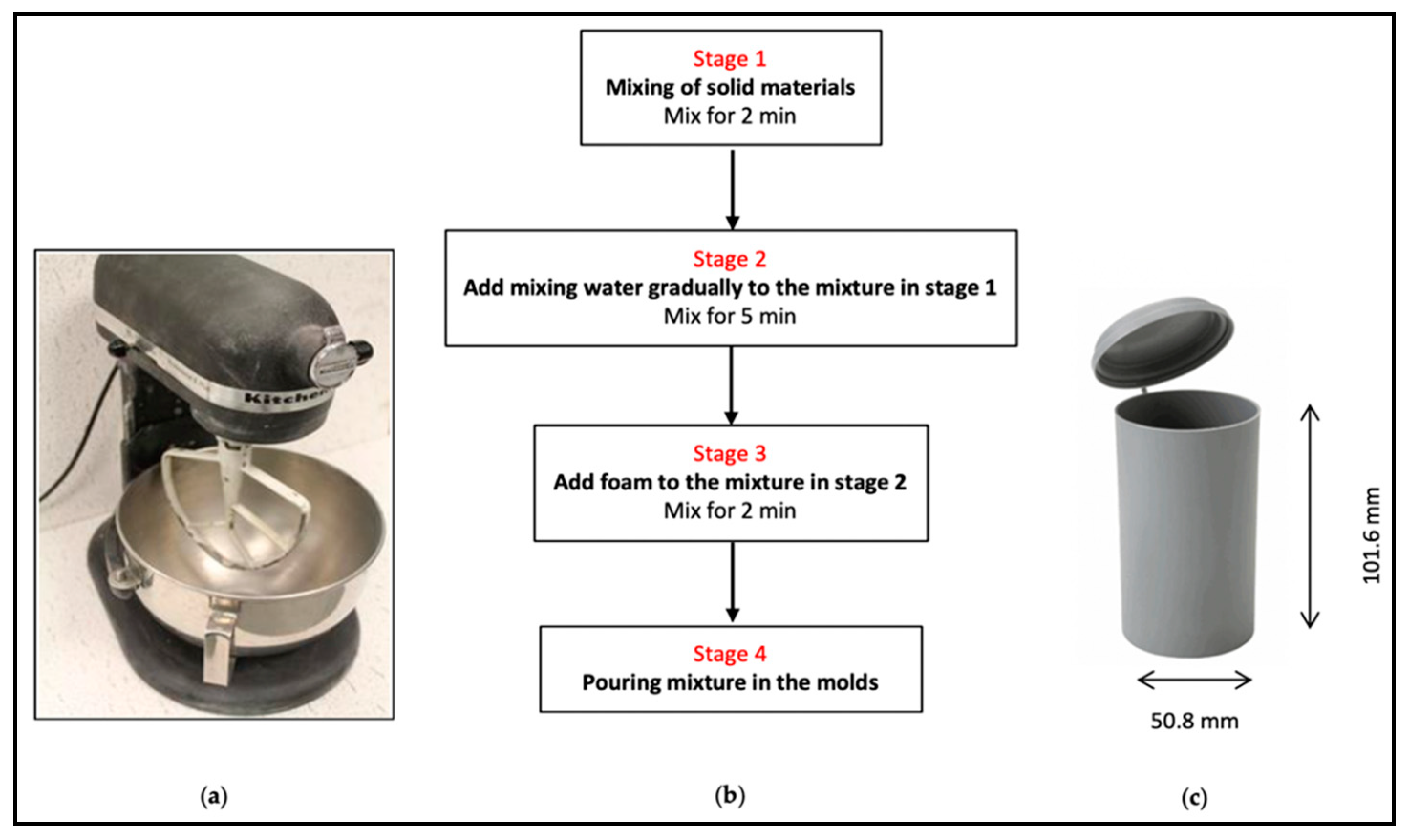
2.7. Sample Preparation
- Tailings and binder were mixed for 2 min at 75 rpm.
- Water was gradually added and the slurry mixed at 75 rpm for 5 min to obtain a homogeneous slurry.
- Premade foam was added to the slurry and mixed for 2 min at 58 rpm. The lower mixing speed prevented the air bubble breakage observed at higher mixing speed.
3. Testing Methods
3.1. Unconfined Compressive Strength Test
3.2. Mercury Intrusion Porosimetry Test
3.3. Dry Density
3.4. Microscopic Analysis
4. Results and Discussion
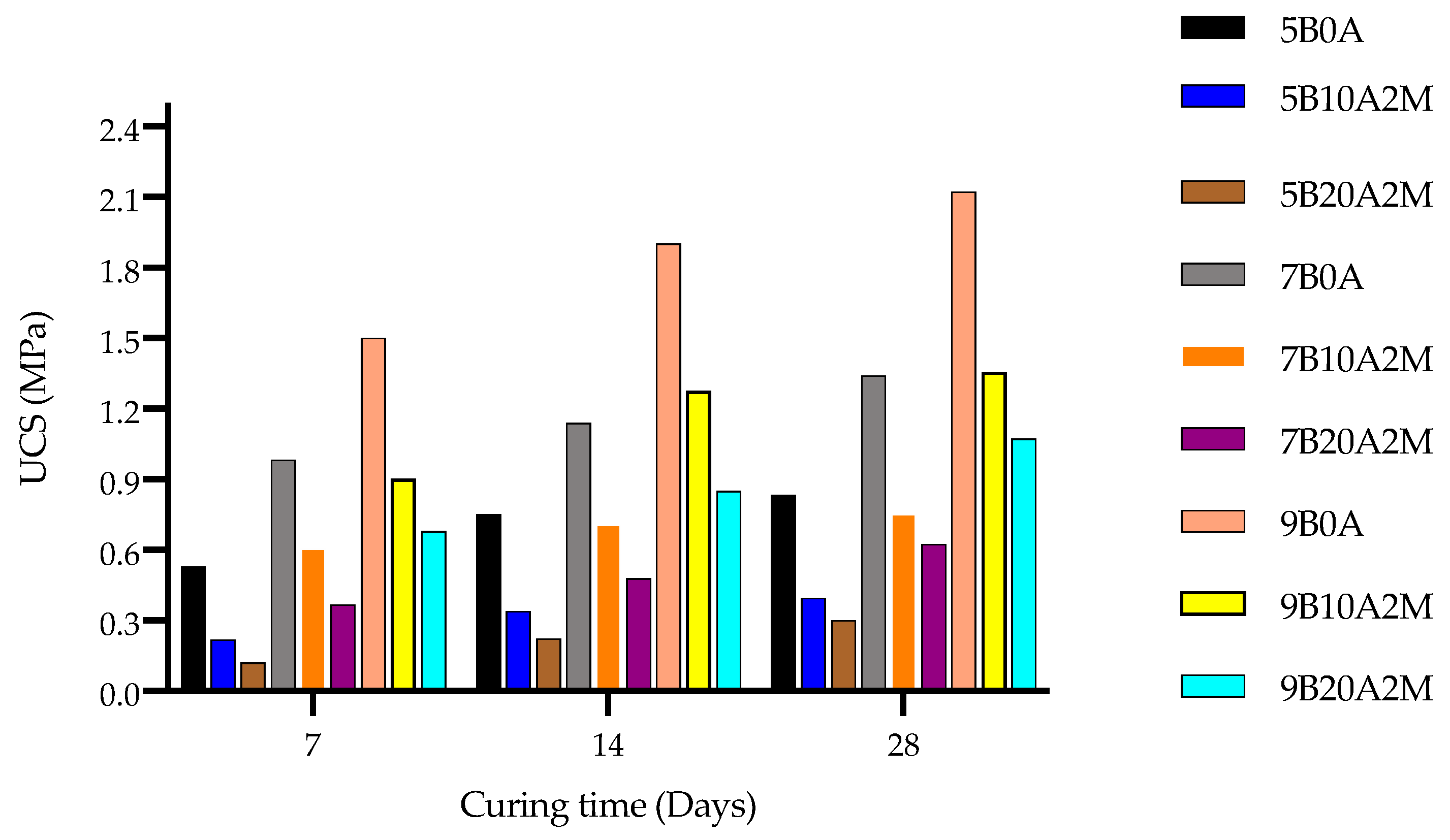
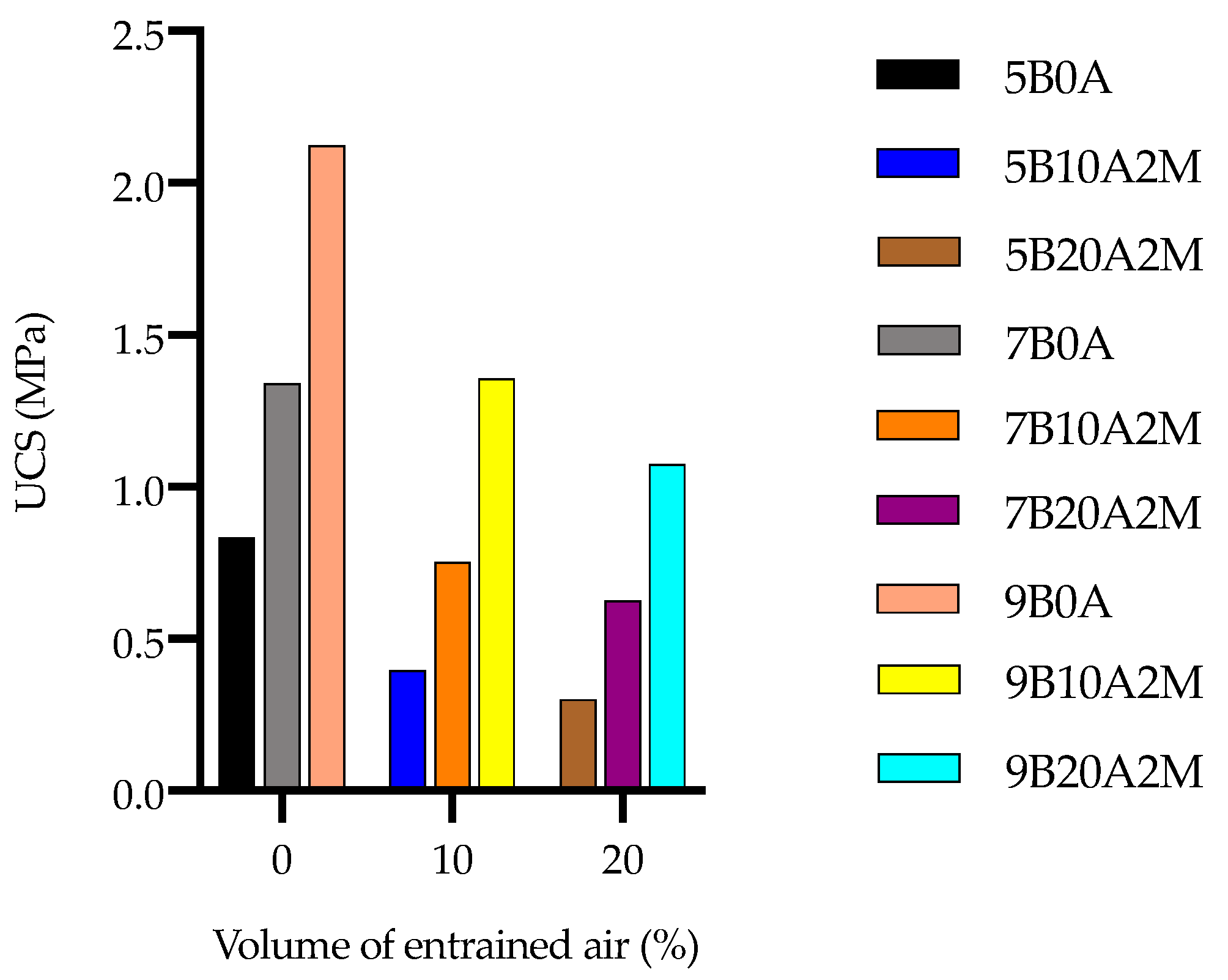
4.1. Unconfined Compressive Strength
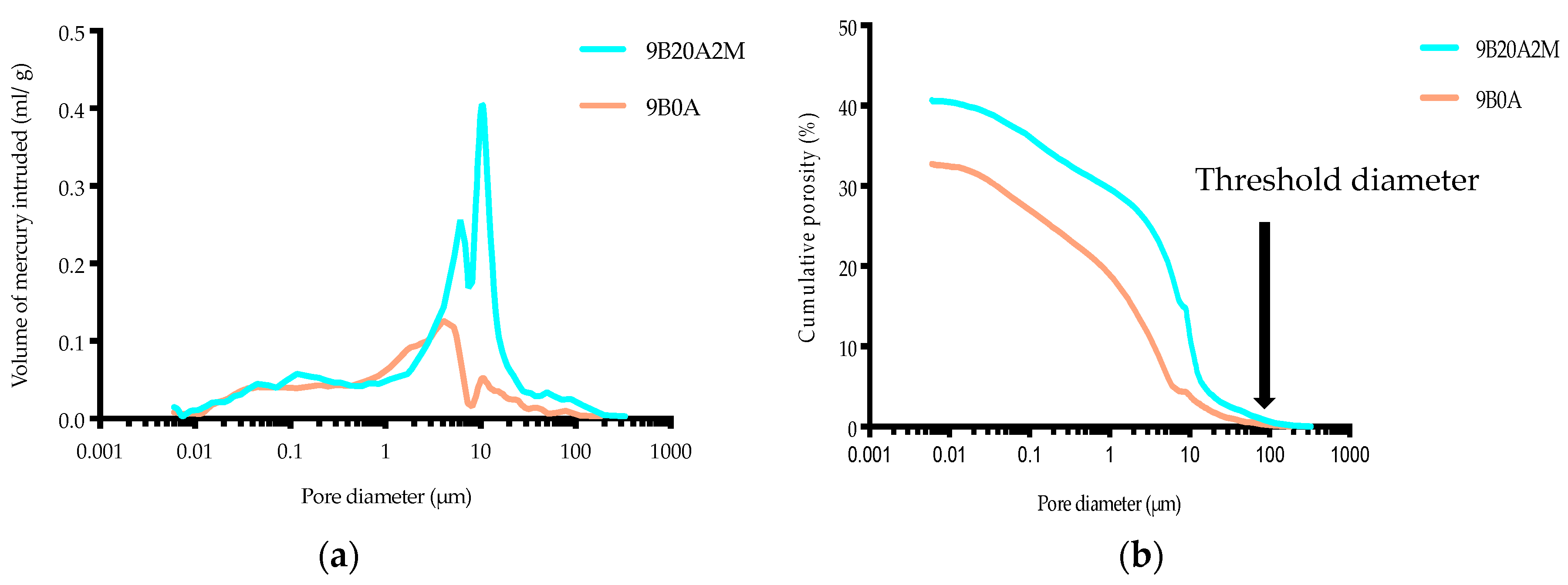
4.2. Porosity
4.3. Dry Densities
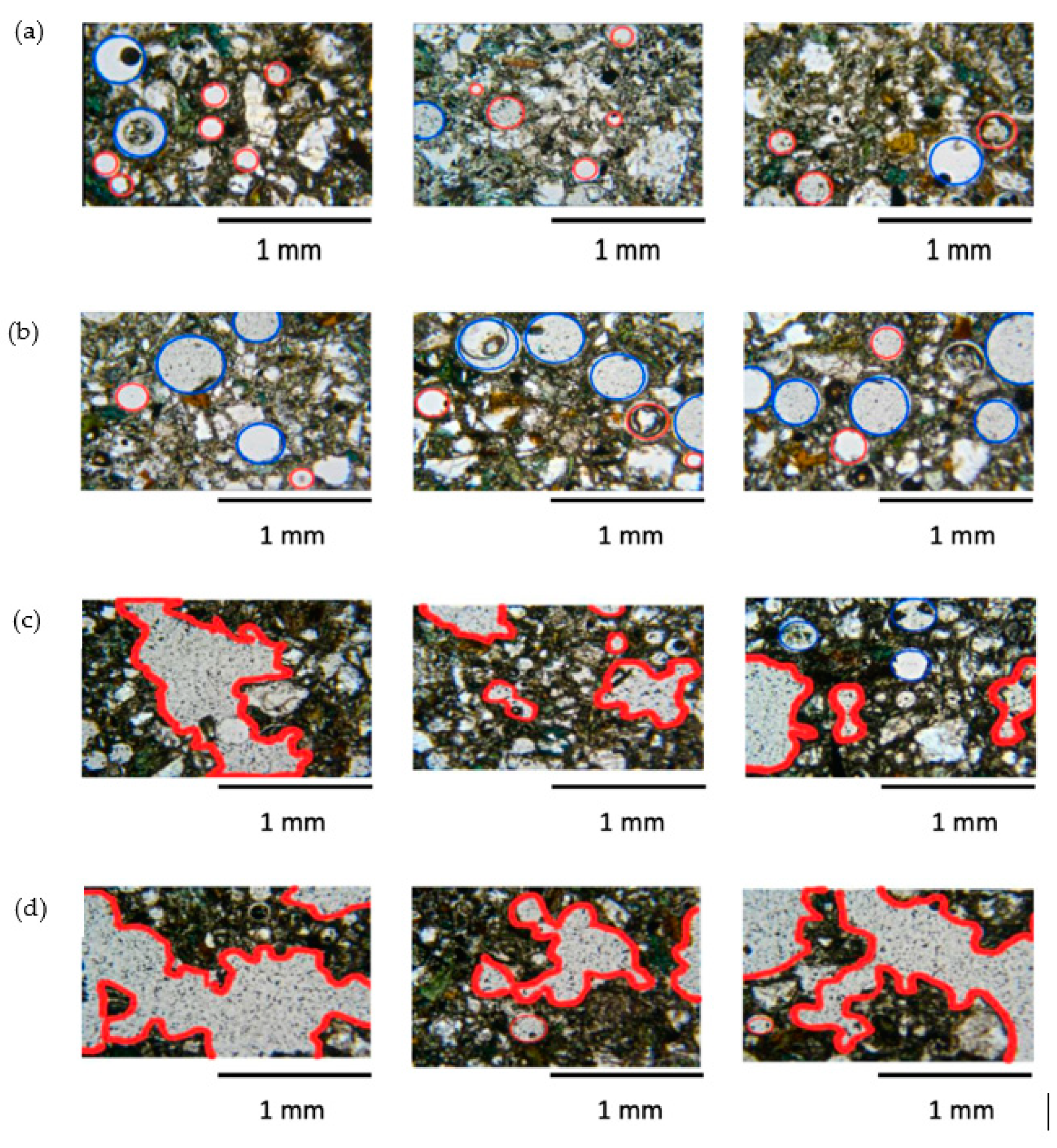
4.4. Bubble Morphology
5. Conclusions
- The solids concentration for the backfill mixture should be optimized to avoid either foam segregation or loss of air bubbles.
- The UCS was strongly negatively influenced by the amount of entrained air. As the amount of air increased from 0 to 10%, the UCS fell by approximately 50%. However, it decreased less sharply (approximately 20%) when increasing the amount of entrained air from 10 to 20%.
- The foaming agent appeared to have a plasticizing effect, which can help enhance the flowability of the filling material. Further investigations are encouraged to investigate the rheological properties of FMF.
- The amount of entrained air strongly influenced the porosity of the filling material. As the amount of air increased, the porosity increased due to the induced air bubbles. The increase in porosity led to a considerable decrease in the dry density. Relatively light FMF can be used to promote a safer working environment, for example, beneath the backfilled stope.
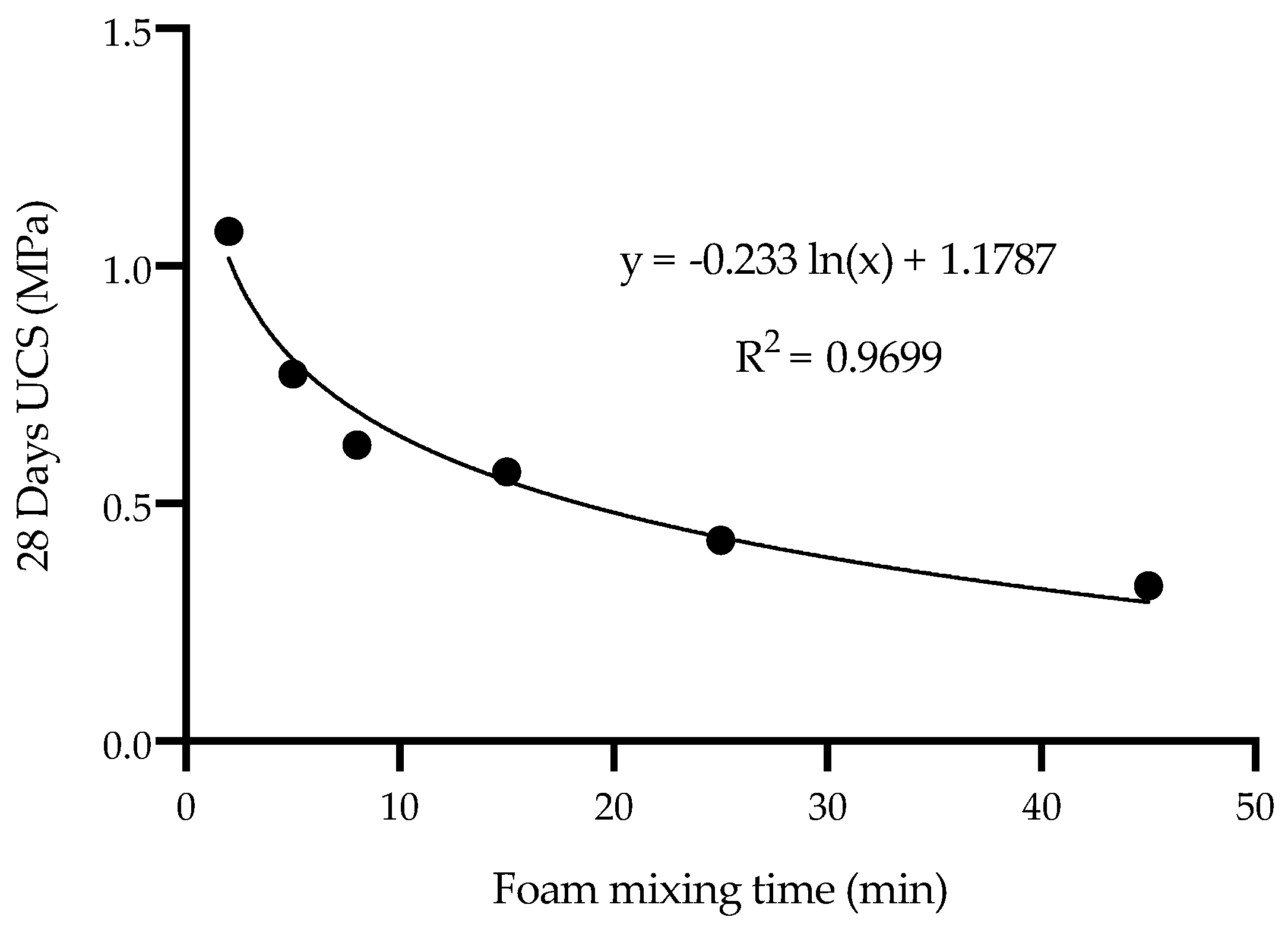
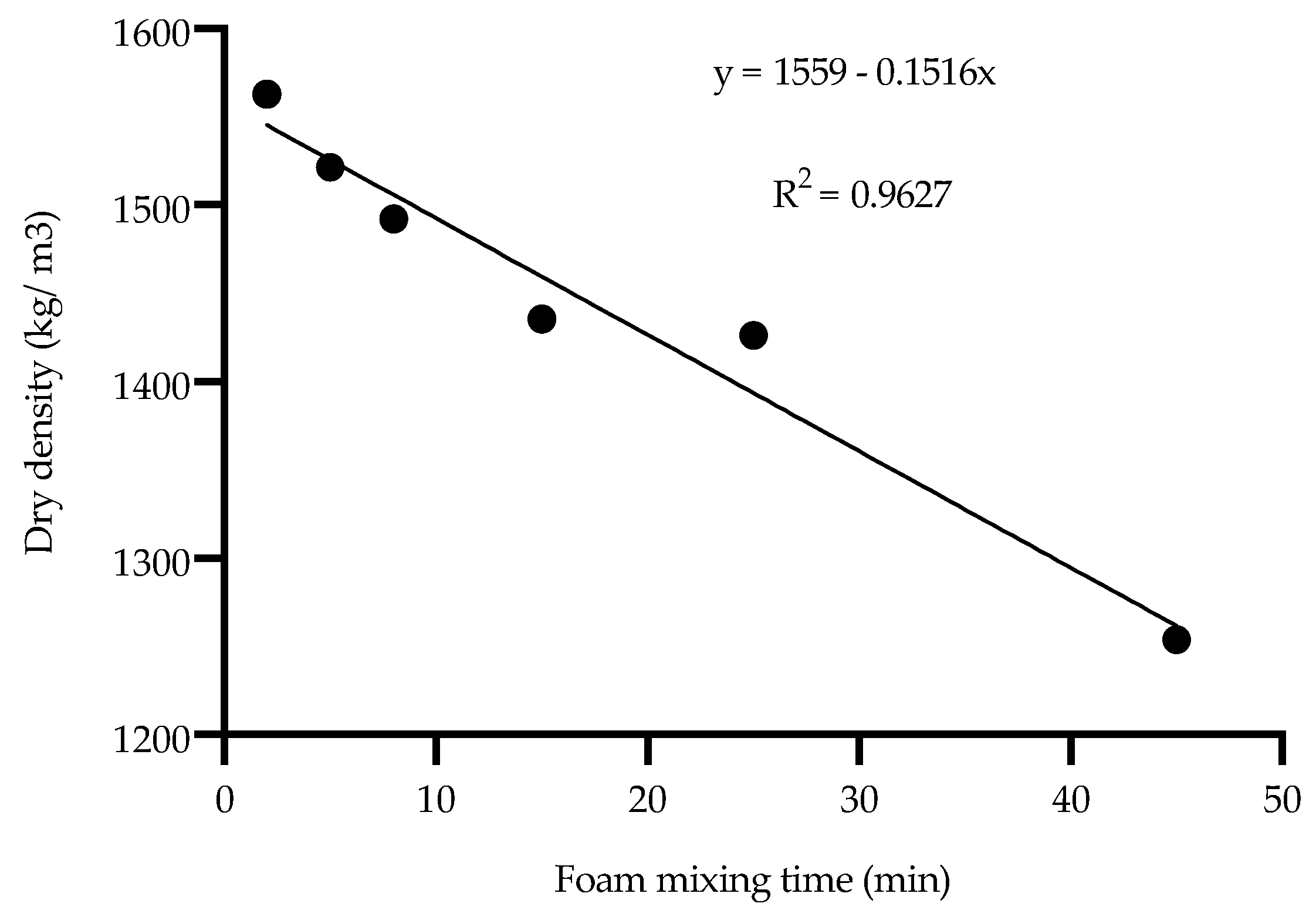
- Foam mixing time must be optimized for the volume of the FMF batch being prepared. Prolonged foam mixing had a significant adverse effect, causing the air bubbles structure to collapse.
- This study indicates that there is a strong potential for FMF to replace HF at the collaborating mine site at a higher solids concentration (78%) than is currently used in the mine (70%). This means more tailings will be used in backfill and less will be stored in storage facilities. Moreover, air bubbles may be used to aid transportation of the backfill and avoid the water drainage requirements of HF. More investigation is recommended in this field.
- One of the advantages of FMF is that it can be used in mines where the mill output of tailings is low and does not meet the backfill production requirement. The volume can be increased by this mechanism of air entrainment.
- Finally, field trials are required to investigate the feasibility of implementing FMF in practice. Further field studies are encouraged, including: (1) Evaluating methods for FMF preparation and placement; (2) quantifying potential air bubble losses during transportation from the preparation plant to the delivery point; (3) optimizing pipeline layout to protect air bubbles, and (4) investigating the potential use of foam as a lubricant layer to reduce pipe wear.
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koohestani, B.; Koubaa, A.; Belem, T.; Bussière, B.; Bouzahzah, H. Experimental investigation of mechanical and microstructural properties of cemented paste backfill containing maple-wood filler. Constr. Build. Mater. 2016, 121, 222–228. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M. An overview on the use of paste backfill technology as a ground support method in cut-and-fill mines. In Proceedings of the 5th Int. Symp. on Ground support in Mining and Underground Construction, Villaescusa & Potvin (eds.), Perth, Australia, 28–30 September 2004. [Google Scholar]
- Peyronnard, O.; Benzaazoua, M. Alternative by-product based binders for cemented mine backfill: Recipes optimisation using Taguchi method. Miner. Eng. 2012, 29, 28–38. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A contribution to understanding the hardening process of cemented pastefill. Miner. Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Sivakugan, N.; Rankine, R.M.; Rankine, K.J.; Rankine, K.S. Geotechnical considerations in mine backfilling in Australia. J. Clean. Prod. 2006, 14, 1168–1175. [Google Scholar] [CrossRef]
- Niroshan, N.; Sivakugan, N.; Veenstra, R.L. Laboratory Study on Strength Development in Cemented Paste Backfills. J. Mater. Civ. Eng. 2017, 29, 04017027. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, I. Utilization of industrial waste products as pozzolanic material in cemented paste backfill of high sulphide mill tailings. J. Hazard. Mater. 2009, 168, 848–856. [Google Scholar] [CrossRef]
- Hassani, F.; Archibald, J. Mine Backfill; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 1998. [Google Scholar]
- Grice, T. Underground Mining with Backfill. In Proceedings of the 2nd Annual Summit—Mine Tailings Disposal Systems, Brisbane, Austrila, 24–25 November 1998; pp. 1–14. [Google Scholar]
- Potvin, Y.; Thomas, E.; Fourie, A. Handbook on Mine Fill; Australian Centre for Geomechanics: Nedlands, Australia, 2005. [Google Scholar]
- Yang, L.; Yilmaz, E.; Li, J.; Liu, H.; Jiang, H. Effect of superplasticizer type and dosage on fluidity and strength behavior of cemented tailings backfill with different solid contents. Constr. Build. Mater. 2018, 187, 290–298. [Google Scholar] [CrossRef]
- Hefni, M.; Hassani, F.P.; Kermani, M.F. A review of the properties of foam mine fill. In Proceedings of the 13th ISRM International Congress of Rock Mechanics, Montreal, QC, Canada, 10–13 May 2015. [Google Scholar]
- Hefni, M.; Hassani, F.; Nokken, M.; Kermani, M.; Vatne, D. Investigation into the development of foam mine fill. In Proceedings of the Eleventh International Symposium on Mining with Backfill, Perth, Australia, 20–22 May 2014; pp. 49–59. [Google Scholar] [CrossRef]
- Hassani, F.; Hefni, M.; Kermani, M.; Vatne, D. Methods and Systems for Foam Mine Fill—Google Patents. AU2015252777A1, 9 September 2019. [Google Scholar]
- Hefni, M.A. An Investigation into the Development and Potential of Foam Minefill; McGill University: Montreal, QC, Canada, 2015. [Google Scholar]
- Du, L.; Folliard, K.J. Mechanisms of air entrainment in concrete. Cem. Concr. Res. 2005, 35, 1463–1471. [Google Scholar] [CrossRef]
- Panesar, D.K. Cellular concrete properties and the effect of synthetic and protein foaming agents. Constr. Build. Mater. 2013, 44, 575–584. [Google Scholar] [CrossRef]
- Khan, Q.S.; Sheikh, M.N.; McCarthy, T.J.; Robati, M.; Allen, M. Experimental investigation on foam concrete without and with recycled glass powder: A sustainable solution for future construction. Constr. Build. Mater. 2019, 201, 369–379. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Farzadnia, N.; Ali, A.A.A. Properties and applications of foamed concrete; A review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Tarasov, A.S.; Kearsley, E.P.; Kolomatskiy, A.S.; Mostert, H.F. Heat evolution due to cement hydration in foamed concrete. Mag. Concr. Res. 2010, 62, 895–906. [Google Scholar] [CrossRef]
- Bing, C.; Zhen, W.; Ning, L. Experimental Research on Properties of High-Strength Foamed Concrete. J. Mater. Civ. Eng. 2012, 24, 113–118. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Nambiar, E.K.; Ranjani, G.I.S. A classification of studies on properties of foam concrete. Cem. Concr. Compos. 2009, 31, 388–396. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of alkali-activated slag as alternative binder on workability and early age compressive strength of cemented paste backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Fall, M.; Célestin, J.C.; Pokharel, M.; Touré, M. A contribution to understanding the effects of curing temperature on the mechanical properties of mine cemented tailings backfill. Eng. Geol. 2010, 114, 397–413. [Google Scholar] [CrossRef]
- Mahlaba, J.S.; Kearsley, E.P.; Kruger, R.A.; Pretorius, P.C. Evaluation of workability and strength development of fly ash pastes prepared with industrial brines rich in SO4= and Cl- to expand brine utilisation. Miner. Eng. 2011, 24, 1077–1081. [Google Scholar] [CrossRef]
- Wu, A.; Wang, Y.; Wang, H.; Yin, S.; Miao, X. Coupled effects of cement type and water quality on the properties of cemented paste backfill. Int. J. Miner. Process. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Castano, G.; Joseph, M. Municipal Drinking Water Produced by Atwater and Charles-J. des Baillets Drinking Water Treatment Plants. Montreal. 2016. Available online: https://ville.montreal.qc.ca/pls/portal/docs/PAGE/EAU_FR/MEDIA/DOCUMENTS/ANNUAL_REPORT_2016_MONTREAL.PDF (accessed on 17 June 2020).
- Mindess, S. Developments in the Formulation and Reinforcement of Roncrete; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Beningfield, N.; Gaimster, R.; Griffin, P. Investigation into the air void characteristics of foamed concrete. In Proceedings of the International Conference on the Use of Foamed Concrete in Construction, Scotland, UK, 5 July 2005. [Google Scholar]
- Ma, H. Mercury intrusion porosimetry in concrete technology: Tips in measurement, pore structure parameter acquisition and application. J. Porous Mater. 2014, 21, 207–215. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273. [Google Scholar] [CrossRef]
- Flatt, R.J. Dispersion forces in cement suspensions. Cem. Concr. Res. 2004, 34, 399–408. [Google Scholar] [CrossRef]
- Lewis, J.A.; Matsuyama, H.; Kirby, G.; Morissette, S.; Young, J.F. Polyelectrolyte effects on the rheological properties of concentrated cement suspensions. J. Am. Ceram. Soc. 2004, 83, 1905–1913. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F.; Bowen, P.; Houst, Y.F. Effect of PCs superplasticizers on the rheological properties and hydration process of slag-blended cement pastes. J. Mater. Sci. 2009, 44, 2714–2723. [Google Scholar] [CrossRef]
- Sakai, E.; Kawakami, A.; Daimon, M. Dispersion mechanisms of comb-type superplasticizers containing grafted polyethylene oxide chains. Macromol. Symp. 2001, 175, 367–376. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T.L. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]






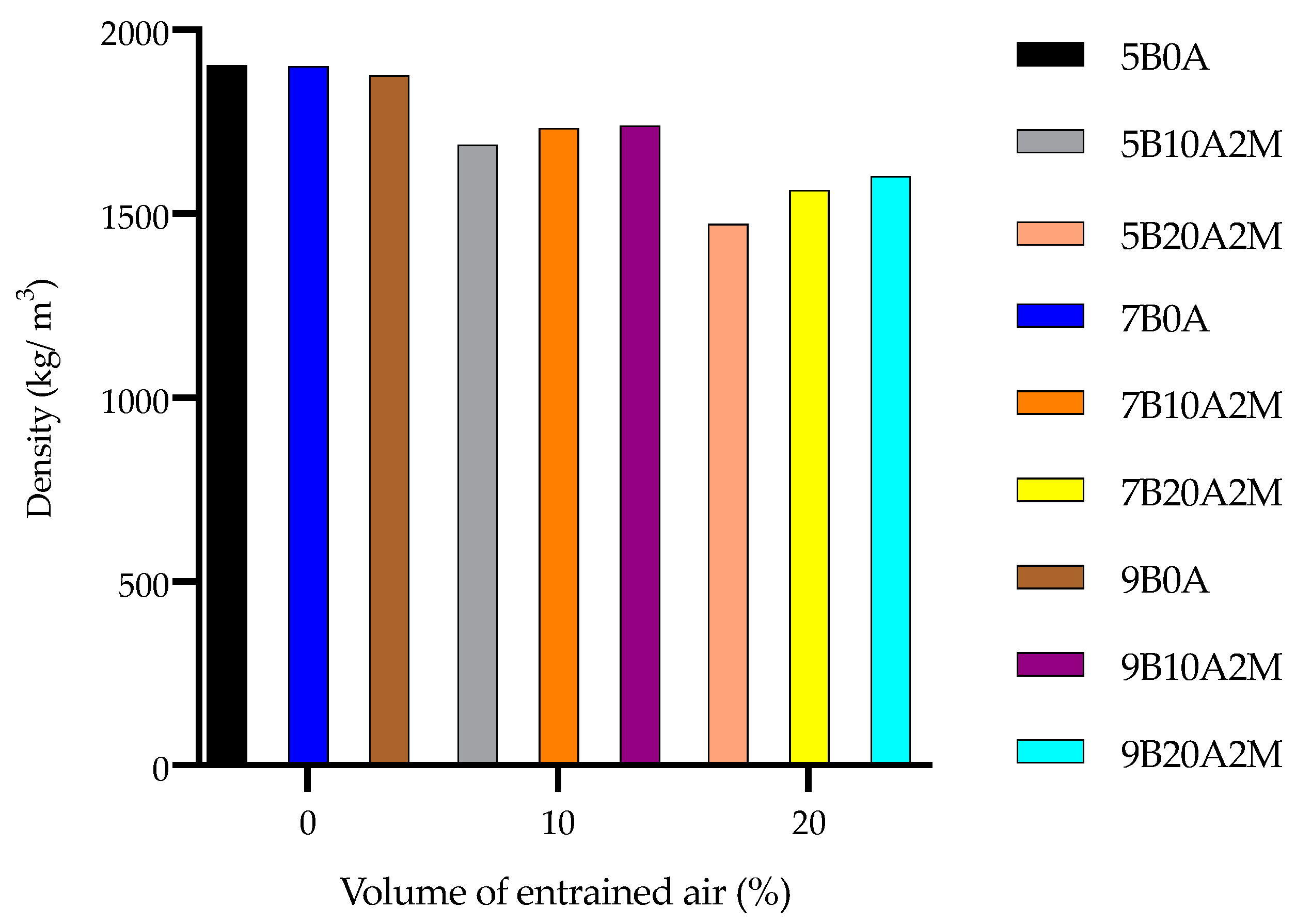
| Quartz | Anorthite | Albite | Actinolite | Biotite | Muscovite | Calcite | Chlorite | Pyrrhotite |
|---|---|---|---|---|---|---|---|---|
| 21.53 | 21.08 | 18.69 | 10.27 | 8.38 | 6.07 | 5.78 | 4.27 | 2.32 |
| Color | Specific Gravity | D10 (µm) | D30 (µm) | D50 (µm) | D60 (µm) | D90 (µm) | Cu 1 | Cc 2 |
|---|---|---|---|---|---|---|---|---|
| Grey | 2.90 | 60.76 | 97.46 | 149.63 | 187.73 | 346.29 | 3.09 | 0.83 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 |
|---|---|---|---|---|---|---|---|
| 19.39 | 4.61 | 2.01 | 61.13 | 3.30 | 0.71 | 2.03 | 2.27 |
| Chlorides Cl | Sulfates SO42− | Nitrites + Nitrates NO2− + NO3− | Sodium Na+ | Calcium Ca2+ | Potassium K+ | Magnesium Mg2+ |
|---|---|---|---|---|---|---|
| 26.88 | 24.15 | 0.26 | 14.57 | 30.97 | 1.50 | 7.88 |
| Solubility in Water | Specific Gravity | Odor | Form | Color | pH |
|---|---|---|---|---|---|
| 100% | 1.1 | Organic | Liquid | Brown | 8 |
| Mixture ID | Solids Concentration (%) | Binder Dosage (%) | Volume of Entrained Air (%) | Foam Mixing Time (Min) |
|---|---|---|---|---|
| 5B0A | 78 | 5 | 0 | - |
| 5B10A-2M | 78 | 5 | 10 | 2 |
| 5B20A-2M | 78 | 5 | 20 | 2 |
| 7B0A | 78 | 7 | 0 | - |
| 7B10A-2M | 78 | 7 | 10 | 2 |
| 7B20A-2M | 78 | 7 | 20 | 2 |
| 9B0A | 78 | 9 | 0 | - |
| 9B10A-2M | 78 | 9 | 10 | 2 |
| 9B20A-2M | 78 | 9 | 20 | 2 |
| Mixture ID | UCS (7 Days) | UCS (14 Days) | UCS (28 Days) | Dry Density (28 Days) | MIP (28 Days) | Microscopic Analysis (28 Days) |
|---|---|---|---|---|---|---|
| 5B0A | 3 | 3 | 3 | 3 | - | - |
| 5B10A-2M | 3 | 3 | 3 | 3 | - | - |
| 5B20A-2M | 3 | 3 | 3 | 3 | - | - |
| 7B0A | 3 | 3 | 3 | 3 | - | - |
| 7B10A-2M | 3 | 3 | 3 | 3 | - | - |
| 7B20A-2M | 3 | 3 | 3 | 3 | - | - |
| 9B0A | 3 | 3 | 3 | 3 | 1 | - |
| 9B10A-2M | 3 | 3 | 3 | 3 | - | - |
| 9B20A-2M | 3 | 3 | 3 | 3 | 1 | 1 |
| 9B20A-5M | 3 | 3 | - | - | ||
| 9B20A-8M | - | - | 3 | 3 | - | 1 |
| 9B20A-15M | - | - | 3 | 3 | - | - |
| 9B20A-25M | - | - | 3 | 3 | - | 1 |
| 9B20A-45M | - | - | 3 | 3 | - | 1 |
| Total | 27 | 27 | 42 | 42 | 2 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefni, M.; Hassani, F. Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill. Minerals 2020, 10, 564. https://doi.org/10.3390/min10060564
Hefni M, Hassani F. Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill. Minerals. 2020; 10(6):564. https://doi.org/10.3390/min10060564
Chicago/Turabian StyleHefni, Mohammed, and Ferri Hassani. 2020. "Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill" Minerals 10, no. 6: 564. https://doi.org/10.3390/min10060564
APA StyleHefni, M., & Hassani, F. (2020). Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill. Minerals, 10(6), 564. https://doi.org/10.3390/min10060564






