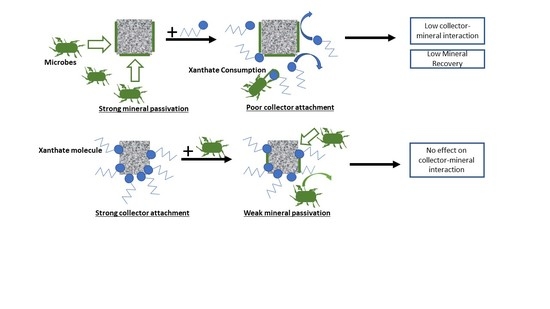
Investigating the Electrochemical Interaction of a Thiol Collector with Chalcopyrite and Galena in the Presence of a Mixed Microbial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Mineral Preparation
2.1.2. Reagents
2.1.3. Microbial Cell Growth and Preparation
2.2. Zeta Potential Measurements
2.3. Rest Potential Measurements
2.4. Microflotation Tests
3. Results and Discussion
3.1. Zeta Potential Measurements on Sulphide Minerals in the Presence and Absence of Microbes
3.2. Rest Potential Measurements for Sulphide Minerals in the Presence of Microbes
3.2.1. Rest Potential Measurements for Chalcopyrite in the Presence and Absence of Microbes
3.2.2. Rest Potential Measurements for Galena in the Presence and Absence of Microbes
3.2.3. Summary of Interaction Mechanisms in the Presence of Microbes
3.3. Microflotation Tests
3.3.1. Microflotation Tests on Chalcopyrite in the Presence of Microbes
3.3.2. Microflotation Tests on Galena in the Presence of Microbes
3.3.3. General Discussion of Microflotation Test Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubel, J.; Smith, R.W.; Misra, M.; Chen, S. Microorganisms as Chemical Reagents: The Hematite System. Miner. Eng. 1992, 5, 547–556. [Google Scholar] [CrossRef]
- Attia, Y.A.; Elzeky, M.; Ismail, M. Enhanced Separation of Pyrite from Oxidized Coal by Froth Flotation Using Biosurface Modification. Int. J. Miner. Process 1993, 37, 61–71. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ohmura, N.; Saiki, H. A Novel Mineral Processing by Flotation Using Thiobacillus Ferrooxidans. Process. Met. 1999, 9, 335–342. [Google Scholar] [CrossRef]
- Farahat, M.; Hirajima, T.; Sasaki, K. Adhesion of Ferroplasma Acidiphilum onto Pyrite Calculated from the Extended DLVO Theory Using the Van Oss-Good-Chaudhury Approach. J. Colloid Interface Sci. 2010, 349, 594–601. [Google Scholar] [CrossRef]
- Slatter, K.; Plint, N.; Cole, M.; Dilsook, V.; de Vaux, D.; Palm, N.; Oostendorp, B. Water Management in Anglo Platinum Process Operations: Effects of Water Quality on Process Operations. In Proceedings of the International Mine Water Management Conference, Pretoria, South Africa, 19–23 October 2009; pp. 46–55. [Google Scholar]
- Forssberg, K.S.; Hallin, M.I. Process water recirculation in a lead-zinc plant and Oter sulphide flotation plants. In Challenges in Mineral Processing; Sastry, K.V., Fuerstenau, M.C., Eds.; Society for Mining, Metallurgy and Exploration: Littleton, CO, USA, 1989; pp. 452–466. [Google Scholar]
- Levay, G.; Schumann, R. A Systematic Approach to Water Quality Management in the Minerals Processing Industry. In Proceeding of Water in Mining Conference, Brisbane, Australia, 14–16 November 2006; pp. 277–287. [Google Scholar]
- Cwalina, B.; Steindor, K. The Influence of Flotation Reagents on Sulfur-Oxidizing Bacteria: Acidithiobacilus Thiooxidans. Physicochem. Probl. Miner. Process. 2008, 42, 37–46. [Google Scholar]
- Levay, G.; Smart, R.; Skinner, W. The Impact of Water Quality on Flotation Performance. J. S. Afr. Inst. Min. Metall. 2001, 1, 69–76. [Google Scholar]
- Evdokimova, G.; Gershenkop, A.S.; Fokina, N. The Impact of Bacteria of Circulating Water on Apatite-Nepheline Ore Flotation. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2012, 43, 398–404. [Google Scholar] [CrossRef]
- Africa, C.J.; Van Hille, R.P.; Harrison, S.T.L. Attachment of Acidithiobacillus Ferrooxidans and Leptospirillum Ferriphilum Cultured under Varying Conditions to Pyrite, Chalcopyrite, Low-Grade Ore and Quartz in a Packed Column Reactor. Appl. Microbiol. Biotechnol. 2013, 97, 1317–1324. [Google Scholar] [CrossRef]
- Sanhueza, A.; Ferrer, I.J.; Vargas, T.; Amils, R.; Sánchez, C. Attachment of Thiobacillus Ferrooxidans on Synthetic Pyrite of Varying Structural and Electronic Properties. Hydrometallurgy 1999, 51, 115–129. [Google Scholar] [CrossRef]
- Yu, R.; Ou, Y.; Tan, J.; Wu, F.; Sun, J.; Miao, L.; Zhong, D. Effect of EPS on Adhesion of Acidithiobacillus Ferrooxidans on Chalcopyrite and Pyrite Mineral Surfaces. Trans. Nonferrous Met. Soc. China 2011, 21, 407–412. [Google Scholar] [CrossRef]
- Crundwell, F.K. How Do Bacteria Interact with Minerals? Hydrometallurgy 2003, 71, 75–81. [Google Scholar] [CrossRef]
- Leduc, D.; Leduc, L.G.; Ferroni, G.D. Quantification of Bacterial Populations Indigenous to Acidic Drainage Streams. Water. Air. Soil Pollut 2002, 135, 1–21. [Google Scholar] [CrossRef]
- Deo, N.; Natarajan, K.A.; Somasundaran, P. Mechanisms of Adhesion of Paenibacillus Polymyxa onto Hematite, Corundum and Quartz. Int. J. Miner. Process 2001, 62, 27–39. [Google Scholar] [CrossRef]
- Blake, R.C.; Shute, E.A.; Howard, G.T. Solubilization of Minerals by Bacteria: Electrophoretic Mobility of Thiobacillus Ferrooxidans in the Presence of Iron, Pyrite, and Sulfur. Appl. Environ. Microbiol. 1994, 60, 3349–3357. [Google Scholar] [CrossRef]
- Solari, J.A.; Huerta, G.; Escobar, B.; Vargas, T.; Badilla-Ohlbaum, R.; Rubio, J. Interfacial Phenomena Affecting the Adhesion of Thiobacillus Ferrooxidans to Sulphide Mineral Surface. Colloids Surf. 1992, 69, 159–166. [Google Scholar] [CrossRef]
- Rao, M.K.; Somasundaran, P.; Schilling, K.M.; Carson, B.; Ananthapadmanabhan, K.P. Bacterial Adhesion onto Apatite Minerals—Electrokinetic Aspects. Colloids Surf. A Physicochem. Eng. Asp. 1993, 79, 293–300. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.J.; Vink, S. Mechanism Study of the Impact of Water-Borne Bacteria on Flotation. Int. J. Miner. Process. 2013, 123, 39–45. [Google Scholar] [CrossRef]
- Zobell, C.E. The Effect of Solid Surfaces upon Bacterial Activity1. J. Bacteriol. 1943, 46, 39–56. [Google Scholar] [CrossRef]
- Woods, R. The Oxidation of Ethyl Xanthate on Platinum, Gold, Copper, and Galena Electrodes. Relation to the Mechanism of Mineral Flotation. J. Phys. Chem. 1971, 75, 354–362. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, W.; Wang, D. Electrochemistry of Flotation of Sulphide Minerals; Springer: Beijing, China, 2009; pp. 63–111. [Google Scholar]
- Bozkurt, V.; Xu, Z.; Finch, J.A. Pentlandite/Pyrrhotite Interaction and Xanthate Adsorption. Int. J. Miner. Process. 1998, 52, 203–214. [Google Scholar] [CrossRef]
- Trahar, W.J.; Guy, P.J. The effects of oxidation and mineral interaction of sulphide flotation. In Flotation of Sulphide Minerals; Forssberg, K.S., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 1985; pp. 91–110. [Google Scholar]
- Leja, J. Surface Chemistry of Froth Flotation; Plenum Press: New York, NY, USA, 1982; pp. 341–432. [Google Scholar]
- Rao, S. Collector Mechanism I. Thiol collectors in sulfide minerals. In Surface Chemistry of Froth Flotation; Springer: Boston, MA, USA, 2004; pp. 479–526. [Google Scholar]
- Buswell, A.M.; Bradshaw, D.J.; Harris, P.J.; Ekmekci, Z. The Use of Electrochemical Measurements in the Flotation of a Platinum Group Minerals (PGM) Bearing Ore. Miner. Eng. 2002, 15, 395–404. [Google Scholar] [CrossRef]
- Allison, S.A.; Goold, L.A.; Nicol, M.J.; Granville, A. Determination of the Products of Reaction between Various Sulfide Minerals and Aqueous Xanthate Solution, and a Correlation of the Products with Electrode Rest Potentials. Met. Trans. 1972, 3, 2613–2618. [Google Scholar] [CrossRef]
- Pesic, B.; Oliver, D.J.; Wichlacz, P. An Electrochemical Method of Measuring the Oxidation Rate of Ferrous to Ferric Iron with Oxygen in the Presence of Thiobacillus Ferrooxidans. Biotechnol. Bioeng. 1989, 33, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.R. The Mechanism of Bacterial Action in the Leaching of Pyrite by Thiobacillus Ferrooxidans. An Electrochemical Study. J. Electrochem. Soc. 1999, 146, 2906. [Google Scholar] [CrossRef]
- Fowler, T.A.; Holmes, P.R.; Crundwell, F.K. On the Kinetics and Mechanism of the Dissolution of Pyrite in the Presence of Thiobacillus Ferrooxidans. Hydrometallurgy 2001, 59, 257–270. [Google Scholar] [CrossRef]
- Taguta, J.; O’Connor, C.T.; McFadzean, B. The Effect of the Alkyl Chain Length and Ligand Type of Thiol Collectors on the Heat of Adsorption and Floatability of Sulphide Minerals. Miner. Eng. 2017, 110, 145–152. [Google Scholar] [CrossRef]
- Grano, S.R.; Prestidge, C.A.; Ralston, J. Solution Interaction of Ethyl Xanthate and Sulphite and Its Effect on Galena Flotation and Xanthate Adsorption. Int. J. Miner. Process 1997, 52, 161–186. [Google Scholar] [CrossRef]
- Wiese, J.; Harris, P.; Bradshaw, D. The Influence of the Reagent Suite on the Flotation of Ores from the Merensky Reef. Miner. Eng. 2005, 18, 189–198. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The Influence of Electrolytes Present in Process Water on the Flotation Behaviour of a Cu-Ni Containing Ore. Miner. Eng. 2016, 96–97, 99–107. [Google Scholar] [CrossRef]
- Tadie, M.; Corin, K.C.; Wiese, J.G.; O’Connor, C.T. Electrochemical Interactions of Platinum Group Minerals with Copper Sulphate. Miner. Eng. 2017, 112, 43–49. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Connor, C.T. Measurement of the Sub-Process of Bubble Loadin in Flotation. Miner. Eng. 1996, 9, 443–448. [Google Scholar] [CrossRef]
- Elmahdy, A.M.; El-Mofty, S.E.; Abdel-Khalek, N.A.; El-Midany, A.A. Impact of the Adsorption of Corynebacterium Diphtheriae Intermedius Bacteria on Enhancing the Separation Selectivity of Dolomite and Apatite. Adsorpt. Sci. Technol. 2011, 29, 47–58. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rao, K.H.; Forssberg, K.S.E.; Natarajan, K.A. Surface Chemical Characterisation of Paenibacillus Polymyxa before and after Adaptation to Sulfide Minerals. Int. J. Miner. Process 2001, 62, 3–25. [Google Scholar] [CrossRef]
- Gu, G.H.; Hu, K.T.; Li, S.K. Surface Characterization of Chalcopyrite Interacting with Leptospirillum Ferriphilum. Trans. Nonferrous Met. Soc. China 2014, 24, 1898–1904. [Google Scholar] [CrossRef]
- Santhiya, D.; Subramanian, S.; Natarajan, K.A. Surface Chemical Studies on Galena and Sphalerite in the Presence of Thiobacillus Thiooxidans with Reference to Mineral Beneficiation. Miner. Eng. 2000, 13, 747–763. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.M.; Norde, W.; Lyklema, J.; Zehnder, A.J.B. The Isoelectric Point of Bacteria as an Indicator for the Presence of Cell Surface Polymers That Inhibit Adhesion. Colloids Surf. B Biointerfaces 1995, 4, 191–197. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rao, K.H. Role of a Heterotrophic Paenibacillus Polymyxa Bacteria in the Bioflotation of Some. Sulfide Miner. 2000, 16, 35–41. [Google Scholar]
- Tadie, M.; Corin, K.C.; Wiese, J.G.; Nicol, M.; O’Connor, C.T. An Investigation into the Electrochemical Interactions between Platinum Group Minerals and Sodium Ethyl Xanthate and Sodium Diethyl Dithiophosphate Collectors: Mixed Potential Study. Miner. Eng. 2015, 83, 44–52. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Chander, S.; Woods, R. Froth flotation. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G., Yoon, R., Eds.; Society for Mining, Metallurgy and Exploration: Littleton, CO, USA, 2007; pp. 425–465. [Google Scholar]
- Bowden, J.L.; Young, C.A. Xanthate Chemisorption at Copper and Chalcopyrite Surfaces. J. S. Afr. Inst. Min. Met. 2016, 116, 503–508. [Google Scholar] [CrossRef][Green Version]
- Mielczarski, J.A.; Mielczarski, E.; Cases, J.M. Interaction of Amyl Xanthate with Chalcopyrite, Tetrahedrite, and Tennantite at Controlled Potentials. Simulation and Spectroelectrochemical Results for Two-Component Adsorption Layers. Langmuir 1996, 12, 6521–6529. [Google Scholar] [CrossRef]
- Chandraprabha, M.N.; Natarajan, K.A. Surface Chemical and Flotation Behaviour of Chalcopyrite and Pyrite in the Presence of Acidithiobacillus Thiooxidans. Hydrometallurgy 2006, 83, 146–152. [Google Scholar] [CrossRef]
- Somasundaran, P.; Nagaraj, D.R. Chemistry and Applications of Chelating Agents in Flotation and Flocculation. In Reagents in Mineral Industry; Jones, M.J., Oblatt, R., Eds.; Springer: London, UK, 1984; pp. 209–219. [Google Scholar]
- Galaction, A.I.; Cascaval, D.; Oniscu, C.; Turnea, M. Prediction of Oxygen Mass Transfer Coefficients in Stirred Bioreactors for Bacteria, Yeasts and Fungus Broths. Biochem. Eng. J. 2004, 20, 85–94. [Google Scholar] [CrossRef]
- Manev, E.; Pugh, R.J. Frother/Collector Interactions in Thin Froth Films and Flotation. Colloids Surf. A Physicochem. Eng. Asp. 1993, 70, 289–295. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Harris, P.J.; O’Connor, C.T. Synergistic Interactions between Reagents in Sulphide Flotation. J. S. Afr. Inst. Min. Metall. 1998, 98, 187–192. [Google Scholar]
- Ekmekçi, Z.; Demirel, H. Effects of Galvanic Interaction on Collectorless Flotation Behaviour of Chalcopyrite and Pyrite. Int. J. Miner. Process. 1997, 52, 31–48. [Google Scholar] [CrossRef]
- Rao, Y.M.K.; Natarajan, K.A. Electrochemical Effects of Mineral-Mineral Interactions on the Flotation of Chalcopyrite and Sphalerite. Int. J. Miner. Process 1989, 27, 279–293. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.J.; Vink, S. Impact of Chalcopyrite Depression by Water-Borne Bacteria in Pure and Combined Mineral Systems. Int. J. Miner. Process. 2013, 123, 18–24. [Google Scholar] [CrossRef]






| Mineral | BET Surface Area (m2/g) |
|---|---|
| Chalcopyrite | 0.2327 |
| Galena | 0.0688 |
| Ion Concentrations (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Na+ | Cl− | SO42− | NO3− | CO32− | pH | Temp °C |
| 80 | 70 | 153 | 287 | 240 | 176 | 17 | 9–9.5 | 25–35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhonde, N.; Smart, M.; Corin, K.; Schreithofer, N. Investigating the Electrochemical Interaction of a Thiol Collector with Chalcopyrite and Galena in the Presence of a Mixed Microbial Community. Minerals 2020, 10, 553. https://doi.org/10.3390/min10060553
Mhonde N, Smart M, Corin K, Schreithofer N. Investigating the Electrochemical Interaction of a Thiol Collector with Chalcopyrite and Galena in the Presence of a Mixed Microbial Community. Minerals. 2020; 10(6):553. https://doi.org/10.3390/min10060553
Chicago/Turabian StyleMhonde, Ngoni, Mariette Smart, Kirsten Corin, and Nora Schreithofer. 2020. "Investigating the Electrochemical Interaction of a Thiol Collector with Chalcopyrite and Galena in the Presence of a Mixed Microbial Community" Minerals 10, no. 6: 553. https://doi.org/10.3390/min10060553
APA StyleMhonde, N., Smart, M., Corin, K., & Schreithofer, N. (2020). Investigating the Electrochemical Interaction of a Thiol Collector with Chalcopyrite and Galena in the Presence of a Mixed Microbial Community. Minerals, 10(6), 553. https://doi.org/10.3390/min10060553