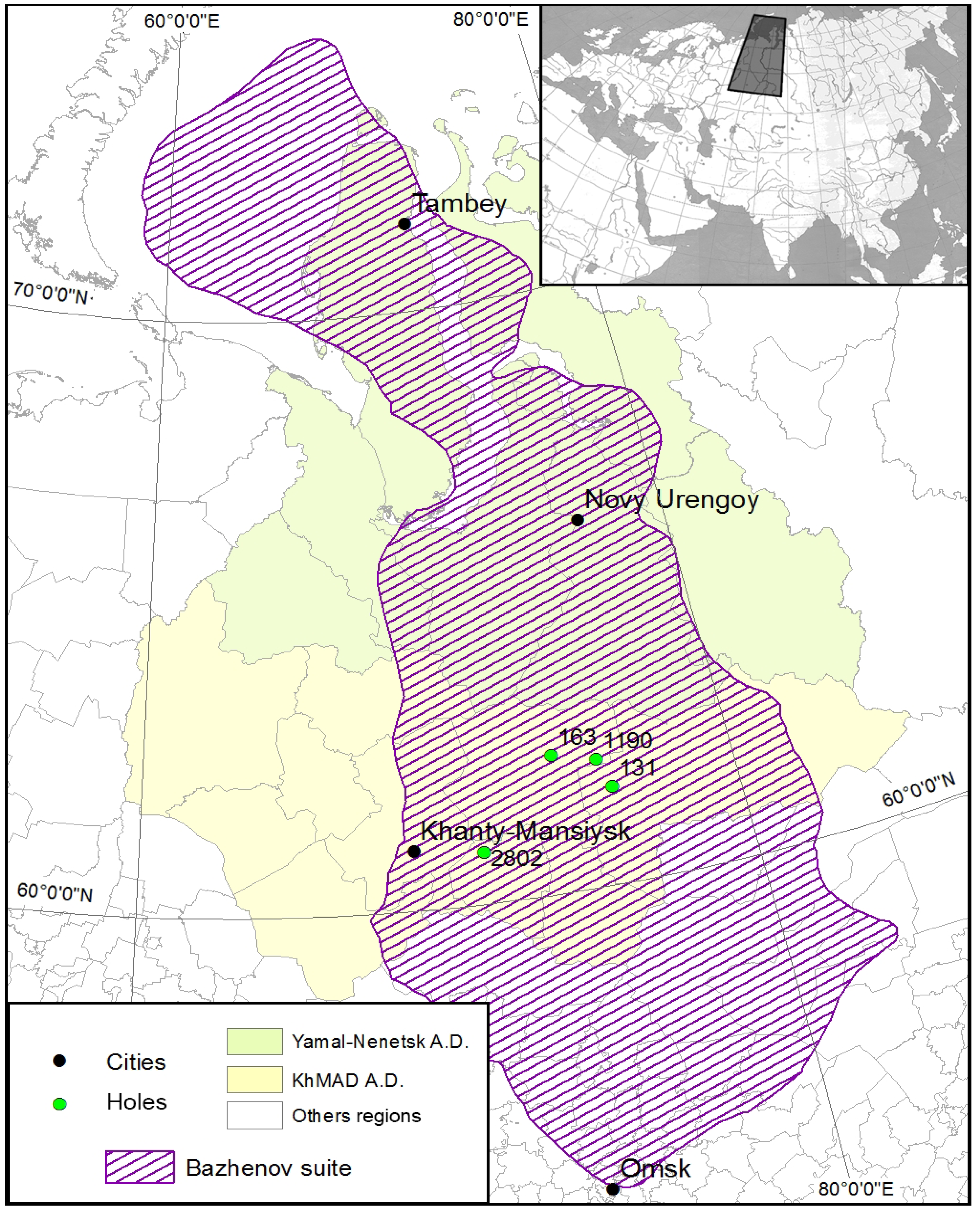
4. Trace Elements of Pyrite Varieties
Framboidal and nodular varieties of pyrite are essentially different from each other and from the host rocks in terms of a spectrum and contents of chemical elements.
Zinс (Zn). Framboidal pyrite from deposits of the Bazhenov Formation is characterized by abnormally high Zn content (up to 8.8 wt. %) This anomalously elevated content point to nano-inclusions of sphalerite in framboidal pyrite. Because the maximum Zn contents are commercially important, it is the dominant element in a spectrum of other related elements. The average Zn content in framboidal pyrite from shale of the Bazhenov Formation is higher (988 ppm) than other global metalliferous shales [
1]. The Zn content in nodular pyrite is characterized by one to two orders of magnitude lower mean and median values (
Table 2).
The correlation analysis of the composition of bituminous sedimentary rocks has revealed a significant positive correlation of Zn with organic matter and high correlation with degree of pyritization against the background of a negative correlation with the clayey material [
1]. In fact, there is a positive correlation between Zn and such organophile elements as V, Mo and Au. In the correlation series of mineralogical and geochemical associations Zn together with Cd ranks first (
Table 3). Based on the results of the correlation analysis of the data on both pyrite varieties, Zn is correlated with elements (Cd, Sn, Hg, Ga, Ge, and In), which are common isomorphic admixtures in the sphalerite structure. This is also confirmed by the mineralogical data [
24]: Zn occurs mainly as sphalerite micro-inclusions in framboidal pyrite. Zn carbonates and aluminosilicates have not yet been determined.
Cadmium (Cd). The Cd content in shales (22.8 ppm after [
1]) of the Bazhenov Formation only slightly exceeds the average worldwide values in black shales (9 ppm after [
27]). The median Cd contents in both pyrite varieties are extremely low, while in Zn-rich samples they reach 0.2 wt. % (see
Table 2). The Cd distribution is non-uniform.
The correlation analysis of sedimentary rocks of the Bazhenov Formation has revealed a significant positive correlation between Cd with sulfide sulfur, organic carbon, degree of pyritization of iron and a significant negative correlation with the clay minerals. Cadmium in both varieties of pyrite shows positive correlation with elements, characteristic of sphalerite (Zn, Hg, Ga, In, Sn), as well as with typical organophilic elements such as V, Mo and Au [
27].
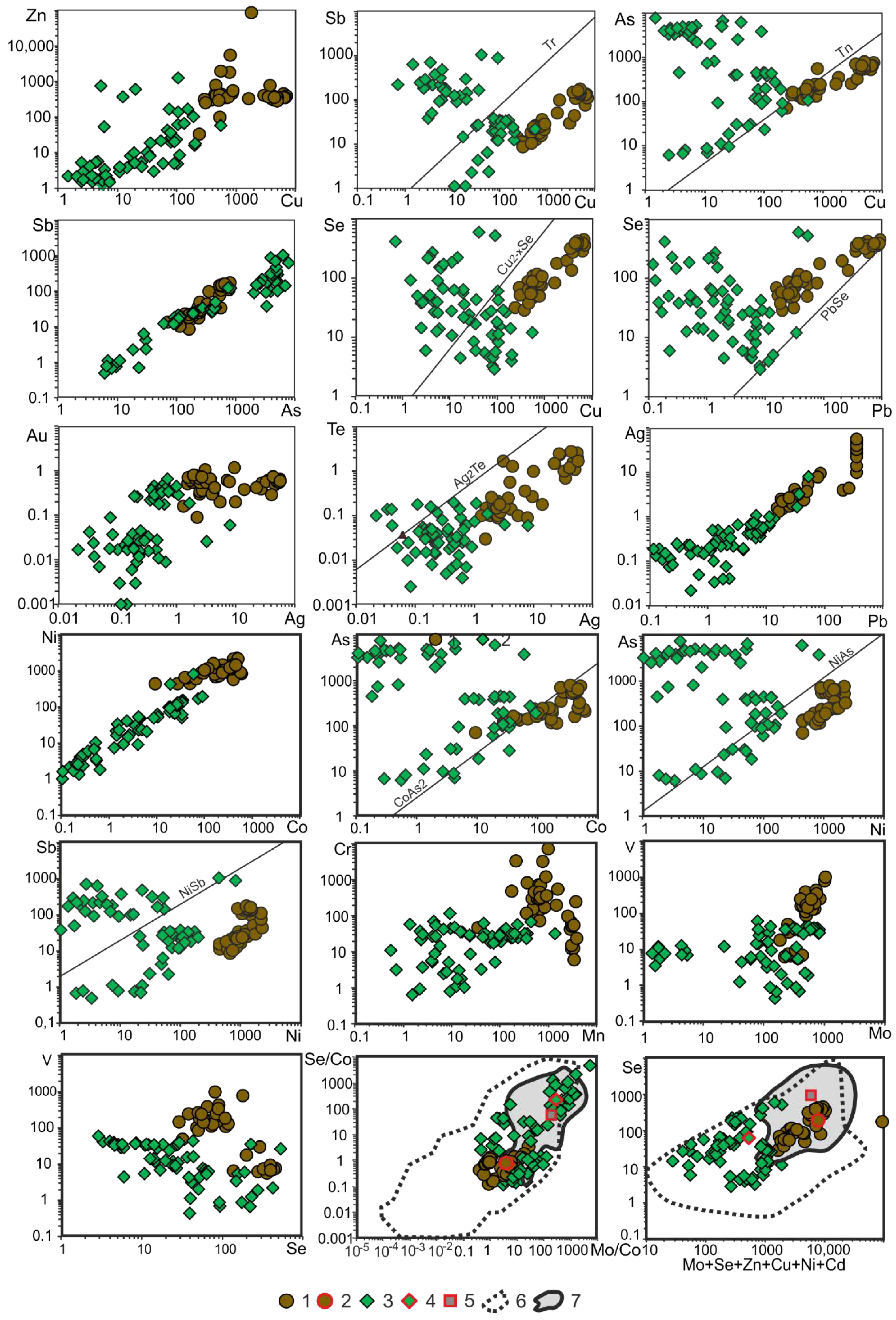
Copper (Cu). Along with Zn, framboidal pyrite from the Bazhenov Formation contains a large amount of Cu (up to 0.7 wt. %). The Cu–Zn diagram shows the direct correlation of contents of these elements (
Figure 3) that is evidence of thin intergrowths of sphalerite and Cu chalcogenides, for example, emulsion intergrowths of chalcopyrite or tennantite in sphalerite. The average Cu concentration in framboidal pyrite are one–three orders of magnitude higher (see
Table 3), than those obtained for pyrite nodules (see
Table 2) and for clayey–siliceous shales (199 ppm after [
1]). The correlation analysis has revealed a significant positive correlation between Cu and organic carbon, sulfide sulfur, and degree of pyritization against the background of negative correlation with the clayey material [
1]. However, as evidenced from the results of the correlation analysis, there is a negative correlation between Cu and typical organophilic elements, such as V and Mo.
Using the maximum correlation path method [
22], it was found that Cu in framboidal pyrite forms a unified series with Sb, Se and Pb (IV in
Table 3). The Cu–Sb association is evidence, at the least, of the presence of nano-inclusions of Cu–Pb sulfosalts or a galena–tennantite paragenesis. The copper in framboidal pyrite is correlated positively with elements, characteristic of tennantite (As, Sb, Ag, Se, Te, Bi) and galena (Pb, Bi, Ag, Sb). The possible occurrence of tennantite is confirmed by directly proportional dependence of Сu, As, and Sb contents in Cu–As, Cu–Sb and Sb–As diagrams (see
Figure 3). The correlation of Cu with Mn is enigmatic for interpretation.
Correlation coefficients, calculated for nodule pyrite, are somewhat different: As and Sb have negative values, probably due to transformation of tennantite into chalcopyrite. The nodular pyrite is characterized by positive relationships of Cu with such organophilic elements as V, Mo and Au, as well as with Hg, Tl, and Ga.
Lead (Pb). The Pb content in framboidal pyrite from the Bazhenov Formation deposits is one-two orders of magnitude higher than that in pyrite nodules (see
Table 2) and host bituminous clayey–siliceous shales (15.7 ppm after [
1]). The maximum Pb contents reach 0.09 wt. %. It was established that Pb was not accumulated in association with organic matter in the Bazhenov deposits [
28,
29]. This conclusion was confirmed by Zanin et al. [
1] based on the correlation analysis results, which showed a significant positive correlation between Pb and the clayey material at a significant negative correlation with sulfide sulfur, organic carbon and degree of pyritization.
However, the correlation analysis of framboidal pyrite has revealed not only higher Pb content than that in the host rocks, but also the close relationships between Pb and Bi, Ag, Te, Sb, and Se, which are typical isomorphic admixtures in galena, and with elements of copper sulfosalts (Cu, As, Sb, Te, Bi, Se), close to tennantite (see
Table 3,
Tables S1 and S2).
In Pb–Se diagram, the line, corresponding to clausthalite, outlines the field of pyrite measurement points from higher Pb contents, than is typical of clausthalite. However, the Pb contents are not so high that clausthalite or galena were easily detected. The Pb contents in framboidal pyrite are only an order of magnitude higher than that in mudstones of the Bazhenov Formation as a whole [
1]. In turn, the Pb content in nodular pyrite in only half of all cases is slightly lower than that in framboidal pyrite (
Table 2,
Figure 3).
Mercury (Hg). The Hg content in deposits of the Bazhenov Formation ranks very low; none of the samples reaching 1 ppm [
1]. At low average Hg content, the maximum values (up to 33 ppm) were detected when analyzing Zn-rich framboidal pyrite. The correlation analysis has revealed the close relationships of Hg with elements, characteristic of sphalerite (Zn, Cd, Sn, Ga, Ge, In) (see
Table S1). At the same time, a positive correlation with such organophilic elements as V, Mo, and Au was established. In nodular pyrite, there is no correlation with Zn, but there is a positive correlation between Hg and Mn, Co, Ni, and Cu and a negative correlation with As and Sb. The latter indicates the appearance of chalcopyrite nano-inclusions in pyrite nodules.
Selenium (Se). On average, the Se content in framboidal pyrite is 5–6 times more (see
Table 2), than that in host black shales (34.7 ppm after [
1]). The average Se content in nodular pyrite is only two times higher than that in host rocks, while maximum contents are higher than those in framboidal pyrite. The correlation analysis of bituminous deposits of the Bazhenov Formation has revealed a significant positive correlation between Se and sulfide sulfur, organic carbon, degree of pyritization and a significant negative correlation with the amount of the clay minerals [
1]. However, in both pyrite varieties, a negative correlation between Se and V does not confirm the relationship of Se with organic matter (See
Table 3). On the contrary, Se is correlated with Mn. A negative correlation between Se and Mo in framboidal pyrite is of specific interest that contradicts modern models of the joint accumulation of these elements [
11].
In general, the Se content is correlated with a wide spectrum of chemical elements, including those in pyrite (Co, Ni), nano-inclusions of galena (Pb, Se, Bi, Sb, Ag, Tl, Te), chalcopyrite and tennantite (Cu, As, Sb, Tl, Se, Te) associations. It is of interest that the correlation between Se and Сo disappears in nodular pyrite. There is a significant negative correlation of Se with Ga.
In the Cu–Se diagram, the field of measurement points of framboidal pyrite is shifted from the Cu
2-xSe line towards the predominance of Cu that is characteristic of chalcopyrite and tennantite nano-inclusions. A significant part of measurement points of nodular pyrite is shifted towards the Se field. It is likely that, in this case, a larger amount of Se enters the structure of granular nodular pyrite than the structure of the framboidal variety. This is also observed in the Pb–Se diagram, where Se/Pb ratio is equal to or greater than in clausthalite. It is known that incorporation of Se into the structure of pyrite is supported by high temperatures of mineral genesis [
30]. In addition to temperature, the higher Se contents may reflect the strong pH-Eh dependence of the dissociation constant for H
2Se [
31]. Se exist as S (IV) or S (VI) in oxic seawater and as elemental Se or reduced selenide Se
2− in anoxic waters [
32]. Selenate and selenite are highly soluble and mobile in contrast to elemental Se and selenides [
33]. A potential sink of Se in marine sediments may be pyrite, which can catalyze Se reduction to selenide and incorporates Se in suboxic sediments [
34].
Tellurium (Te). The Te content in bituminous rocks of the Bazhenov Formation is rarely over 1 ppm [
1]. Only in a single case, the maximum Te content in framboidal pyrite reaches 2.8 ppm. The Te content in framboidal pyrite shows a significant negative correlation with such organophilic elements as V and Mo. Significant positive correlation relationships between Te and elements, characteristic of tennantite (Cu, As, Sb, Ag, Se), galena (Pb, Tl, Sb, Ag, Se) and pyrite (Co, Ni, Mn) were revealed (see
Table S1). However, almost all of these elements can enter into the structure of pyrite in the form of an isomorphic impurity. In nodular pyrite, all these bonds disappear when a positive correlation with Sn and Au appears.
Arsenic (As). The average As content in framboidal pyrite (see
Table 2) is five times higher than that in host black shales of the Bazhenov Formation (67.4 ppm after [
1]). However, the average As content in nodular-like pyrite is even much higher (0.2 wt. %) at the maximum content of 0.8 wt. %. The correlation analysis of the composition of host rocks has revealed a significant positive correlation between As and sulfide sulfur, organic matter, and degree of pyritization of iron against the background of a significant negative correlation with the clayey material [
1]. However, there is no correlation between As and V or Mo in framboidal pyrite, while in the case of nodular pyrite, the correlation is negative.
The positive correlation between As and Sb in nodular pyrite is clearly evident in the As–Sb diagram (see
Figure 3). The Cu–As diagram shows that measurement points are close to the tennantite line (Tn
90Tr
10) (see
Figure 3). However, the field of measurement points with higher As contents is outlined in the same diagram. It is believed that As in black shales is associated usually with pyrite [
35]. Very low dissolved As concentration may influence Cu behavior in sulfidic sediments, where dissolved Cu–As–S complexes determine the bioavailability and mobility of Cu [
36]. A positive correlation of As with Co, Ni and Mn, calculated for framboidal pyrite (see
Table S1), shows that at least a small proportion of As occurs in the composition of pyrite as substitution in the lattice. However, such a correlation is absent in the matrix, calculated for nodular pyrite (see
Table S2).
Antimony (Sb). The average Sb content in host black shales of the Bazhenov Formation is 9.8 ppm after [
1] that is close to the average worldwide value in black shales (8.8 ppm after [
27]). The average Sb contents in both pyrite varieties are 5–15 times higher than the average worldwide value (see
Table 2). The maximum Sb contents (up to 0.1 wt. %) were determined in nodular pyrite. Based on the data of correlation analysis of the composition of deposits of the Bazhenov Formation, the Sb content shows a significant positive correlation with sulfide sulfur, organic carbon, degree of pyritization, as well as a significant negative one with the clayey material [
1]. The correlation analysis of both pyrite varieties has revealed the most significant correlation between Sb and a complex of following trace element—Cu, Ag, As, Se, Pb, Bi, Te, which, most likely, corresponds to the galena–tennantite paragenesis. A correlation between Sb and organophilic elements (V, Mn) is negative or absent. The nodular pyrite shows a positive correlation of Sb with Au and W.
A correlation between Sb and Co, Ni in framboidal pyrite is insignificant, while that in nodular pyrite is noted only for Ni. It is possible that a proportion of Sb can be incorporated isomorphically into not only the structure of tennantite and galena, but also into the pyrite structure as well as As.
Bismuth (Bi). The accumulation of Bi in framboidal pyrite is slightly more intensive than in nodular pyrite (see
Table 2) and host rocks (3.4 ppm after [
1]). The correlation analysis has not revealed a correlation link between Bi and any other element in rocks [
1]. The correlation analysis of framboidal pyrite has revealed various relationships between Bi and elements, typical of galena (Pb, Sb, Ag, Se), sulfosalts (Cu, As, Sb, Se) and, properly, fine-grained pyrite (Co, Ni). A correlation between Bi and V is negative, while between Bi and Mn it is positive. The correlation matrix of nodular pyrite shows relationships between Bi and V, Au, W, Ga, and Hg; there is no correlation between Bi and Ni, while the correlation with As and Sb is negative, while maintaining the close relationships with Сu and Pb, as well as with other aforementioned elements.
Thallium (Tl). The Tl content in framboidal pyrite (see
Table 2) is an order of magnitude higher than that in bituminous clayey–siliceous shales of the Bazhenov Formation (see [
1]). The correlation analysis of the Bazhenov bituminous deposits has revealed an insignificant positive correlation between Tl and sulfur and/or with organic matter and an insignificant negative correlation with the amount of clay minerals [
1]. The calculations, made for framboidal pyrite, have shown positive relationships between Tl and organophilic elements such as V, Mo, Au, as well as non-interpreted relationships with Co and W. These relationships are not observed in nodular pyrite, but there appears a significant positive correlation not only with Co, but also with Ni, Cu, As, Sb, Se, Pb and Ag. These relationships suggest the galena–fahlore paragenesis in association with crystalline-granular pyrite. It is known that Tl can be incorporated isomorphically in both pyrite and in fahlore and galena.
Gold (Au). In terms of the metalliferous potential of pyrite varieties, Au is of interest. The Au content in framboidal pyrite reaches 1.2 ppm, while median values are by almost two orders of magnitude higher, and then average values in siliceous black shales (8.5 ppb after [
27]). It should be noted that the Au content of 0.1 ppm in framboidal pyrite from the black shale association should be considered as high or boundary when assessing the gold potential of black shale sequences [
18].
As seen in the general Ag–Au diagram, constructed for framboidal and nodular pyrite varieties, a directly proportional correlation between these elements is observed, although it is not evident for each pyrite variety separately. The highest Au content is characteristic of framboidal pyrite (0.1–1.2 ppm), while that in some pyrite nodules is also increased (0.1–0.7 ppm). Along with organophilic elements, such as V and Mo, a correlation between Au and other elements is observed. Framboidal pyrite shows the stable positive correlation between Au and elements of the sphalerite association (Zn, Cd, Sn, Hg, Ga, In). A correlation between Au and Tl was revealed only for framboidal pyrite. In general, a correlation between Au and As, characteristic of gold-bearing pyrite is absent or negative (see Apps. I and II). In this case, probably, the incorporation of Tl into the crystal structure contributes to the gold accumulation in pyrite.
Silver (Ag). The Ag content in framboidal pyrite is approximately an order of magnitude higher than that in nodular pyrite (see
Table 2) and in deposits of the Bazhenov Formation (1.4 ppm after [
1]). In most cases, Ag prevails over Au (see
Figure 3). The occurrence of Ag as a biogenic element [
37], as well as Au [
38], in framboidal pyrite can be associated with organic matter. Based on the correlation data, the Ag content has a positive correlation with sulfide sulfur, organic matter, and degree of pyritization, as well as a significant negative one with the clayey material [
1].
However, according to the results of the correlation analysis of framboidal pyrite, the pattern is controversial. There is a significant inverse relationship between Ag and V and hence with organic matter. Silver can be equally ascribed to two geochemical associations: (Cu + Se + Sb + Mn) and (As + Pb + Bi + Te), which, in terms of contents of these elements, most likely belong to the tennantite–galena paragenesis with corresponding isomorphic admixtures in tennantite (Ag, Se, Sb, As, Bi, Te) and galena (Ag, Bi, Sb, Te, Se).
It is most likely that the correlation with Mn indicates initial joint precipitation of Ag with Mn hydroxides under alkaline oxidizing conditions, characteristic of seawater. Such boundary conditions, close to Eh = 0, are typical of formation conditions of framboidal pyrite, which develops after iron monosulfides [
39]. In addition, the relationship of Ag with Ni and Сo is observed in the correlation matrix of nodular pyrite that is evidence that Ag can be incorporated into the crystal structure of pyrite, as noted by many researchers. The disappearance of a significant positive correlation between Ag and Mn argues for the reducing formation conditions of pyrite nodules.
Nickel (Ni). The higher amount of Ni is partitioned between organic matter and pyrite [
11]. In comparison with nodular pyrite, the metalliferous potential of framboidal pyrite is characterized by high Co and Ni contents—elements, which form the unified mineralogical and geochemical association (see
Table 2). Nickel prevails over Сo (Co/Ni < 1–0.1), that is typical of sedimentary pyrite; these two elements can be incorporated isomorphically into the structure of pyrite [
11]. The concentration of As in pyrite also plays a role in the Co/Ni ratio (see
Figure 3). In addition, nickel as a biogenic element, which commonly occurs in organic matter. The presence of nano-inclusions of stibnites is rather less likely.
Vanadium (V). Vanadium in framboidal pyrite occurs in significant amount, reaching 0.1 wt. %. (see
Table 3,
Figure 3). Vanadium is organophilic element under the surface conditions; it enters the composition of oil, bitumens, and forms metal–organic complexes. The V content in framboidal pyrite is the same, as in oil and bitumens [
40]. The V content in nodular pyrite is much less.
The strongest correlation of V is with Mo and Au most probably related to organic matter. There is significant inverse relationship between V and elements, corresponding to nano-inclusions of tennantite (Cu, As, Sb) and galena (Se, Ag, Pb, Bi). In nodular pyrite, on the contrary, there is a directly proportional relationship between V content and all of these elements, as well as with Co and Ni (See
Tables S1 and S2). The relationship between V and Co, Ni, and Mo is typical of oils [
40].
In the correlation series, calculated with the maximum correlation path method for framboidal pyrite, the association of V with Mo, Tl, and Au is clear. The same calculation, performed for nodular pyrite, provides the association of V with Ga, Au, W, and Sn. The stable relationship between V with Au may suggest that Au was extracted from seawater by V-bearing bitumen and then was concentrated in framboidal pyrite during the process of transition of iron monosulfides to disulfides [
38]. This is also confirmed by high maximum Se contents, which is an indicator of increased oxygen content in the atmosphere [
18]. The V–Se diagram shows an inversely proportional relationship in the distribution of these elements in both varieties of pyrite (see
Figure 3).
Molybdenum (Mo). The Mo content in framboidal pyrite occurs in significant amounts, reaching 0.1 wt. %. Framboidal pyrite, as well as a part of nodular pyrite are enriched with this hydrogenic element, characteristic of the Phanerozoic seawater. It is likely that the incorporation of Mo into framboidal pyrite occurred due to sorption processes under the conditions of organic matter oxidation [
38]. Another point of view is that the molybdate ion is converted into reactive thiomolybdate species and is subsequently incorporated into sulfides or scavenged by S-rich organic matter in sulfidic conditions of sediment pore waters or in water column [
3,
5]. Minimal deposition of Mo in organic-rich shales have been attributed to primary low SO
42− (i.e., [
8]). Particle-reactive thiomolibdate in the presence of sufficient H
2S can lead to Mo uptake by Fe-sulfides [
39,
41].
Tungsten (W). The average W content in both pyrite varieties are low (0.2–0.1 ppm) at maximum values of 1.2 ppm. The correlation analysis of the composition of framboidal pyrite has revealed associations, characteristic of tennantite (Cu, As, Sb, Se, Ag), galena (Pb, Sb, Ag, Tl, Te), pyrite (Co, As, Se), as well as Mn. In the correlation matrix (see Apps. 1,2), calculated for nodular pyrite, the positive relationships of W with V, Mo, Au, and Hg and negative ones with As, Sb, and Se are observed. Rutile, captured from the matrix during the growth of pyrite crystals could be a principal source of W. For example, this process took place in black shales of the Kumtor gold-ore deposit [
42].
Manganese (Mn). The Mn content in framboidal pyrite is high (see
Table 2) and an order of magnitude higher than in nodular pyrite. This is in agreement with previously obtained data on the typochemistry of these pyrite varieties: for example, in black shale deposits of pyrite and gold-ore deposits [
42,
43,
44]. Manganese can be incorporated into the structure of pyrite, while it is more likely that Mn can occupy vacant positions. Moreover, Mn can be partly associated with the lithogenic and carbonate component of shales.
Chromium (Cr). The maximum Cr concentration is characteristic of framboidal pyrite (0.7 wt. %), while maximum Cr contents in nodular pyrite is an order of magnitude lower (~0.1 wt. %). The content in black shales is known to be ~100 ppm [
27]. Unlike many other elements, Cr in framboidal pyrite shows only a positive correlation with Ni and a weaker correlation with Bi. At this, the Cr content in nodular pyrite shows only an inversely proportional relationship between Se, As, and Sb (see Apps. I and II). In all cases, Cr occurs at the end of the correlation series (see
Table 3).
Chromium is a biogenic element [
37] and, therefore, should reflect to some extent an amount of organic matter and has a directly proportional relationship with V. However, this is not observed in our data. The biological significance of Cr is not well understood. One cannot rule out that the Cr occurrence is as an isomorphic element in hydromica and chrome spinel, which could be contaminated by a laser beam at the sampling of small pyrite framboids and thinly laminated nodular pyrite, enriched with relic layered aluminosilicate and detrital accessory minerals.
Tin (Sn). The Sn content in both pyrite varieties is low, reaching the maximum values up to 19 ppm in framboidal Zn-bearing pyrite (see
Table 2). An average Sn content in pyrite (see
Table 2) is approximately similar to that in black shales of the Bazhenov Formation: 0.77 ppm [
1]. The correlation analysis of bituminous sedimentary rocks of the Bazhenov Formation has revealed previously a directly proportional relationship between Sn content and the organic matter content and an inverse one between amounts of pyrite and organic matter [
1]. The study of framboidal pyrite has revealed a directly proportional relationship between Sn and isomorphic elements in sphalerite (Zn, Cd, Hg, Ga, Ge, In), as well as organophilic elements such as V, Mo, Au (see
Table 2 and
Table S1). In turn, these relationships are almost completely preserved in nodular pyrite (see
Table 2 and
Table S2); positive relationships between Sn and Co, Ni, Cu, Mn, and W (see
Table 2 and
Table S2) and negative relationships with As and Sb.
Gallium (Ga). In general, the Ga contents in framboidal and nodular pyrites is an order of magnitude lower than the world average contents in black shales (14–20 ppm after [
27]) and bituminous deposits of the Bazhenov Formation (15–27 ppm after [
1]) (see
Table 2). It was previously established that Ga shows a significant positive correlation with the amount of clay minerals and a significant negative correlation with sulfur, organic carbon and degree of pyritization [
1]. However, in our case, Ga in framboidal pyrite shows a positive correlation both with organophilic elements (V, Mo), and with elements of sphalerite isomorphic association (Zn, Cd, Sn, Hg, In, Ge).
In addition, nodular pyrite shows a direct proportional relationship of Ga with Cu and Co, and a negative relationship with As and Sb (see
Tables S1 and S2). Both pyrite varieties are characterized by the presence of a stable Ga–Au association. It should be noted that Ga in other environments often demonstrates a dual character. In particular, Ga in ores of copper sulfide deposits is associated both with hydromica and chlorite, and with sphalerite [
45]. The Ga content in some polymetallic ores reaches 400 ppm [
40]. The decomposition products of aluminosilicates at the silicification of sediments during halmyrolysis could partially be a Ga source in sphalerite from the Bazhenov Formation. Halmyrolysis is the global process of silicification of volcanogenic and terrigenous deposits under slow sedimentation conditions [
46].
Germanium (Ge). The Ge contents in framboidal and nodular varieties of pyrite (see
Table 2) are lower than the world average values and are close to contents in black shales (0.9 ppm after [
1]). The Ge content in framboidal pyrite is somewhat higher than that in nodular one, excluding single maximum Ge contents (up to 83 ppm) in analyses, which yielded high Zn contents. The previously performed correlation analysis of compositions of black shales has revealed a significant positive correlation of Ge with pyrite, organic matter, and degree of pyritization of sediments, as well as a significant negative one with the clayey material [
1]. As for Ge, calculations performed for framboidal pyrite have revealed a positive correlation with both organophilic elements (V, Mo) and elements of the sphalerite isomorphic association (Zn, Cd, Sn, Hg, Ga, In). In nodular pyrite, these relationships are almost completely absent or are extremely weak (V, Sn, Co, In) (see
Tables S1 and S2). Besides renierite, argyrodite, and germanite, the main Ge source in pyrite and stratiform Pb–Zn deposits is sphalerite [
40]. Moreover, feldspars, rutile, magnetite, and organic matter, which were subject to halmyrolysis, could be sources of both Ge and Ga.
Indium (In). The In content is very often below the detection limits (about 0.1–0.01 ppm). However, contents of these chemical elements in Zn-rich framboidal pyrite (Zn 8.8 wt. %) become significant (see see
Tables S1 and S2). Based on correlation relationships, Indium enters the structure of sphalerite.
5. Discussion
The framboidal pyrite and crystalline pyrite nodules are subdivided in Bazhenov Formation by microtextures and trace element concentration. The framboidal pyrite sizes vary from 8 to 32 µm and commonly form clusters. This suggests that the framboids have grown below the seafloor at the oxic-anoxic interface within sediments overlain by oxic water columns rather than in euxinic seawater columns. Framboids developed in euxinic conditions are generally small (up to 5 µm) with a narrow size range because the initial reaction rate is fast and pyrite formation occurs already within the water column [
3,
47,
48,
49]. There is no evidence of pyrite oxidation in seawater in our samples, except in the cases of abundant barite in black shales. Feldspar decay could be the main process of barite concentration in SO
42- enriched seawater. A high concentration of SO
42−, reoxidized from H
2S, on the surface of decayed organic matter reacted with Ba
2+ and then precipitated. The high content of BaSO
4 is therefore an indicator for high bioproductivity [
50,
51]. However formation of modern and Paleozoic stratiform barite at cold methane seeps on continental margins is considered, as well [
52].
The variation from dysoxic to anoxic conditions could be considered as the best condition for pyrite formation and preservation in organic matter rich sediments in the Upper Jurassic period of the West Siberia Sea. This suggestion is confirmed by calculation of the index of syngenetic pyrite (ISPY), as a proportion of oxian-ionic and transition elements [
4]:
where:
This index is used to evaluate a relative amount of syngenetic pyrite. The index should have low values for oxic–dysoxic environments and should be closed to 1 for euxinic environments with a high portion of syngenetic pyrite, but relatively high values are expected also for anoxic conditions with pyrite formed at the seawater/sediment interface [
4]: The ISPY calculated for framboidal pyrite of the Bazhenov Formation displays average value 0.3 with standard deviation 0.14. This value is typical for framboidal pyrite formed in sediments below dysoxic to anoxic seawater. In pyrite of organic-rich mudstone formed in a normal oxygenated marine environment average ISPY is around 0.09 [
4].
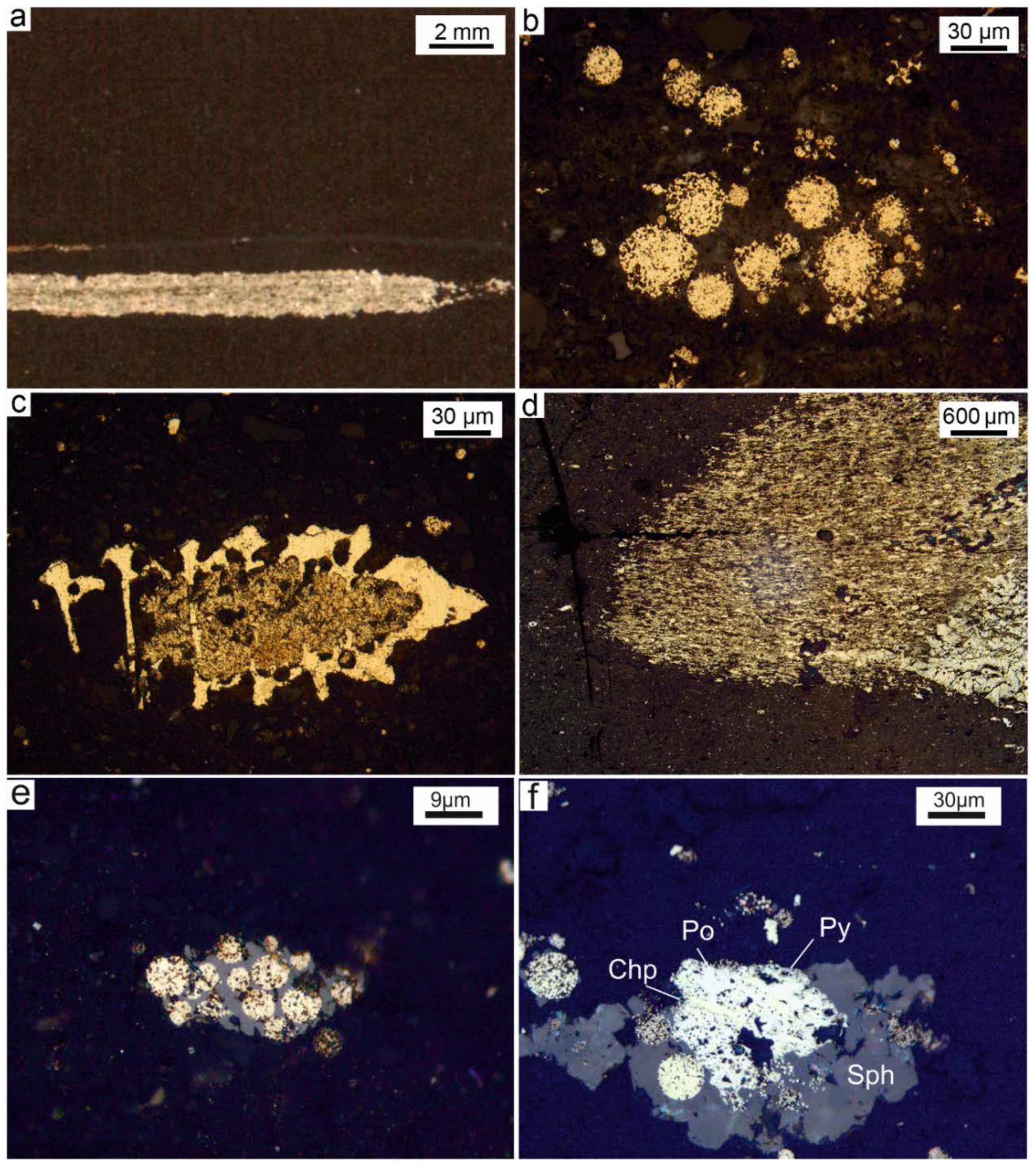
Some nodule-like pyritized bituminous layers and pyrite nodules with fine-grained laminated pyrite are similar by structure to pyritized microbial mat fragments [
26]. Coarse-grained pyrite in the nodules results by crystallization from pore water undersaturated with respect to initial monosulfides toward the end of early diagenesis when pore water sulfide depleted or when the rate of sulfate reduction is low [
53,
54].
The geochemical variations are significant if we compare framboidal and nodular pyrite varieties, scattered in black shales of the Bazhenov Formation. In comparison with nodular pyrite, framboidal pyrite is characterized by the predominance of a wider spectrum of elements. The following series was calculated in descending order from average contents in framboidal pyrite normalized to those in nodular pyrite (ppm): Bi 68 → Pb 48 → Cu 45 → Zn 41 → Ag 29 → Cd 25 → Cr 23 → Co 22 → Ni 18 → Mn 16 → Te 15 → V 8 → Au 4 → Sn 2.8 → Se2.5 → Tl 1.9 → Mo 1.8 → W 1.2 → Sb 0.5 → As 0.2. Framboidal pyrite accumulates heavy metals, which enter the composition of sulfide geochemical association: Bi, Pb, Cu, Zn, Ag, Cd, Te, Au, and Co, as well as organophilic elements (Cr, V, Ni). It should be noted that some researchers refer to such elements as Cu, Zn, Cd, and Ag, contained in seawater, as biogenic [
45]. Pyrite nodules, which were formed at the later diagenetic stage, accumulate only Sb and As.
Sometimes, the background sedimentary-diagenetic pyrite from black shale formations, which even show no connection with ore deposits, contain high concentrations of the majority of chemical elements [
6]. At the global scale, pyrite from highly metalliferous bituminous deposits, associated, as a rule, with gas and oil-and-gas deposits, is characterized by higher Se content plus the complex of metals (Mo, Zn, Cu, Ni, Cd) [
19]. The data of framboidal pyrite plotted on a Se–(Mo + Zn + Se + Cu + Ni + Cd) diagram lie in the field of pyrite from highly metalliferous black shales associated with oil-gas deposits (see
Figure 3). In the Se/Co–Mo/Co diagram, most of the measurement points of the Bazhenov nodular pyrite occur in the field of pyrite from highly metalliferous deposits, while those of framboidal pyrite occupy the boundary zone between pyrites from highly metalliferous bituminous shales and from common background carbonaceous deposits (see
Figure 3). In general, relationships between median trace element contents in studied framboidal pyrite from the Bazhenov Formation and those in sedimentary-diagenetic pyrite (framboids, small crystals) from world-wide black shales (after [
19] form the following series in descending order (Bazhenov framboids/general framboids): Mo 22 → Au 18 → Zn 13 → Tl 4.4 → Cu 4.3 → Ni 2.7 → Co 2.6 → Ag 2.1 → Sb 1.3 → Te 0.5 → Pb 0.3. Thus, the occurrence of relative high Mo, Zn, and Au contents in framboidal pyrite of the Bazhenov Formation is clearly demonstrated. It is likely that thallium contributes to the incorporation of Au into the Bazhenov framboidal pyrite lattice bound. In terms of the majority of trace elements, with the exception of Mo, the Bazhenov nodular pyrite compares significantly to the global background variety of sedimentary (sedimentary–diagenetic) pyrite (Bazhenov nodules/general sedimentary pyrite): Mo 7.5 → Tl 2.1 → Sb 1.5 → As 1.0 → Au 0.9 → Zn 0.2 → Ag 0.12 → Cu 0.10 → Te 0.07 → Ni 0.06 → Co 0.03 → Pb 0.001. The low contents of many elements are also connected with the high degree of crystallinity of most pyrite nodules from the Bazhenov Formation.
The comparison of framboidal and nodular varieties of pyrite from the Bazhenov Formation with pyrite from major ore-formational types of deposits provides interesting information about their metal bearing capacity. By now, the information on the median trace element contents was published without recognizing pyrite varieties in relation to different ore-formational types of deposits [
19].
Framboidal pyrite from the Bazhenov Formation deposits is characterized by high contents of Mo, Tl, Sb, Zn, Cu and Ag in comparison with pyrite from gold–copper–iron skarn, porphyry copper–gold, and gold-ore orogenic deposits, being different from them in extremely low contents of Te and Co. The introduction of Te into ores of these deposits, as well as into ores of volcanogenic copper-zinc massive sulfide deposits, is associated with high-temperature slightly reduced hydrothermal fluids [
55].
It should be noted that framboidal pyrite from the Bazhenov Formation is characterized by a high Au content compared to that from ore deposits generally, as well as to sedimentary pyrite from black-shale sequences which lack oil, gas and Pb–Zn massive sulfide deposits. In terms of Au concentration, framboidal pyrite from the Bazhenov Formation is close, on average, to that from copper sulfide deposits (
Table 4).
In comparison with pyrite from the major ore-formational types of deposits, nodular pyrite from deposits of the Bazhenov Formation contains higher contents of Mo, As, Sb, and Tl. According to [
11,
40], these elements are characterized by high contents in marine water (
Table 5). Syngenetic pyrite formed within a euxinic water column is typically enriched in As, Mo and Sb but is low in heavy metals, and the geochemical variation reflects changes in seawater composition [
3].
The only exception is abnormally high Tl contents in sedimentary–diagenetic pyrite from stratiform sulfide deposits of black-shale association, for which Tl is considered as a pathfinder indicator [
56]. A significant number of other elements show minimum concentration coefficients (Co, Te, Pb). These are elements that occur in marine water in minimum concentrations [
11,
40].
The difference in concentration factors indicates the geochemical specialization of different ore-formational types of deposits. For example, pyrites from the copper–zinc massive sulfide deposits, which are associated with volcanogenic and sedimentary sequences, are characterized by higher median contents of the majority of trace elements (Zn, Cu, Mo, Pb, Tl, Sb) in comparison with pyrite from porphyry copper–gold, gold–copper–iron skarn and gold-ore orogenic deposits. The extremely low As concentration in pyrites are used to separate gold–copper–iron skarn (median value, 2.2 ppm) and porphyry copper–gold deposits (53.4 ppm) [
3]. Nodular-like pyrite is enriched in As in comparison to framboidal pyrite studied here. This may be caused by As-based metabolisms [
57] in initial microbial mats or existence of marcasite enriched in As. The pyritized microbial mat structures are very well preserved in some pyrite nodules (see
Figure 2d).
The enrichment of Mo is known to occur in many Phanerozoic black shales, especially in those, which were formed under euxinic conditions [
3]. It follows that Mo can be used to identify pyrite from the copper sulfide deposits of the sedimentary association (median value, 23 ppm), as well as background sedimentary–diagenetic pyrite (median value, 28 ppm). On the contrary, pyrite from volcanogenic copper sulfide deposits is characterized in most cases by low median Mo content (about 1 ppm). Pyrite from copper sulfide deposits is commonly enriched in Ag and Pb. This relates to both sedimentary deposits and volcanogenic deposits (median values: Ag 22 ppm and Pb 320 ppm). Pyrite from porphyry copper–gold deposits is Ni-rich (median value, 590 ppm) in comparison with other types of ore deposits. Pyrite from gold–copper–iron skarn formation is enriched in Co (median value, 1735 ppm). The mean Au content in pyrite from most of the studied deposits is close to the detection limits.
The enrichment of organic-rich mudstone with redox-sensitive and biophilic elements is usually connected with three main processes: (1) capture by phytoplankton and bacteria; (2) absorption on organic and detrital particles; (3) accumulation of Ni, Mo, Zn, As, Se, Cu, Pb and Sb in sedimentary–diagenetic pyrite during the diagenesis of iron monosulfides under oxidizing conditions [
15,
18].
Within the framework of development of a genetic model of the behavior of chemical elements under the formation of highly metalliferous pyrite varieties, it is necessary to consider possible sources of metals. The accumulation of abnormally high contents of metals in highly metalliferous bituminous deposits and, correspondingly, in pyrite has been under discussion for a long time [
19]. Some researchers support a hydrothermal origin of metals, including their transfer from distal sources into seawater [
58,
59,
60], while other researchers argue in favor of the model of transferring most metals to seawater under the weathering of the continental crust [
39,
61,
62]. The consideration of Se or Se/Co, as indicators, recording degree of oxygenation of the atmosphere is of particular importance in the second model [
11,
63].
The oil deposits of the Bazhenov Formation are located above a rift system [
64]. It could be inferred from this that the precipitation may be accompanied by hydrothermal activity similar to processes that formed framboidal and nodular pyrite in black shale hosted massive sulfide deposits. However, the direct comparison of these pyrite varieties with the similar types of framboidal and nodular pyrite of Safyanovskoe massive sulfide deposits [
65]; display strong differences. The pyrite of the Bazhenov Formation contains relative higher Cr, V, Ni, Mo, which is related to an oceanic water source. In contrast, the same pyrite varieties located in the black shales of Safyanovskoe VHMS deposits are enriched with heavy metals (Bi, Pb, Ag, Au, Te) which are typical trace elements of massive sulfide ores. Concentration of transitional elements such as Zn and Cu in framboidal pyrite are similar in both deposits (
Table 6). However, the IXPY calculated for framboidal pyrite of Safyanovskoe deposit display very high average values (1.9) and standard deviation (2.1) in comparison to IXPY typical of pyrite from Bazhenov Formation (average value 0.3 with standard deviation 0.14). The cause is the highly variable composition of clastic sulfide turbidite transformed to framboidal pyrite layers resulting in different styles of diagenesis in the Safyanovskoe deposit [
65,
66]. Consequently, the framboidal pyrite formed in black shales of the Bazhenov Formation do not reflect a dramatic impact of typical hydrothermal massive sulfide fluids. The source of Zn and Cu in the Bazhenov Formation could be massive sulfide deposits of the Urals Mountains weathered at the same time rather than some hydrothermal source of these elements.
Many researchers have stated that metals migrate to organic-rich mudstone directly from seawater with a slight dilution of sediment [
67,
68,
69]. In particular, recent studies [
62], supported by isotope data on Cr, Mo and Os, provide firm evidence that metals migrated from seawater due to the oxidation weathering of the continental crust and accumulated in highly metalliferous organic-rich mudstone in the Yangtze Cambrian Basin (South China). In terms of a complex of chemical elements (Mo, Se, Ni, Zn, V and P), the studied black shales resemble both highly metalliferous deposits and “normal black shales” which lack trace element anomalies [
27].
These of trace elements (Мo, Se, Ni, Zn, V, P) are bioessential and they are sources to the oceans primarily though oxidative weathering of the continents and surface runoff. However, these group of trace elements are not not typical of sedimentary–diagenetic pyrite of SEDEX Pb–Zn–Cu stratiform which is usually enriched with Tl, Mn, Zn, Cu, Pb, As and Sb, [
56,
70]. Zinc is the only element common to both systems, but its occurrence is not unambiguous evidence of a hydrothermal origin. Nevertheless, some HMBS samples from the Mecca Quarry shales (IL, USA) and the Lisburne Group (Nothern Alaska, USA) have extremely high contents of Zn, Cd, Ag and/or Tl that may be due to the addition of metals after sedimentation or their hydrothermal migration to seawater from distal sources [
58,
71].
Most researchers believe that organic matter in the highly metalliferous black shales is of biological origin [
72], mainly as phytoplankton detritus, deposited from the photic zone. They have seen an important connection between this process and the enrichment of organic matter with metals. In our opinion, however, they ignore the possibility of an abnormal development of bacterial mats associated with seep activity. The development of gas seepage processes on the bottom of the West Siberian Sea during the Late Jurassic could have taken place in connection with rifting, although it is not yet proven. However, this idea is supported by evidence of the influence of CH
4 seepage on authigenic iron sulfide formation in a range of hypoxic to anoxic conditions in Cretaceous–Paleocene of the eastern part of West Siberian Sea where giant iron-oxide deposits were formed [
49]. In the Bazhenov Formation, abundance of pyritized nodules with relict microbial mat-related laminated structures (MRS) are similar to MRS found in black shales of different ages [
26].
The emergence of such mats, based on methanotrophic chemosynthesis could provide the observed uneven accumulation of organic matter in sediments of the Bazhenov Formation. Moreover, one can assume that there were zones of anomalous development of plankton (radiolarians) above zones of bacterial chemosynthesis, where seawater was also in need of enrichment with nutrient chemical elements. It is not unlikely that the Bazhenov Formation resulted from a combination of fluid seepage and biogenic processes in contrast to the theory of background active erosion of the Ural Mountains. Many papers emphasise that methane seeps and methane oxidized bacteria produce microbial mats [
73]. A possible oxic modern analogue is the coeval seepage of hydrothermal and organic-rich fluids in the Guaymas basin with black smokers, seeps and microbial mats associated with oil redistribution [
74].
The distal hydrothermal genesis of metals in respect of the Bazhenov Formation has not yet been proven. In the Jurassic, after the Triassic calm period, the global events of pyrite formation, resulted in the formation of the Se-rich copper sulfide deposits took place in the Mediterranean, Cordillera and Caribbean pyrite-bearing belts [
75,
76,
77]. However, epochs of sulfide ore formation were usually followed by epochs of formation of bituminous deposits with a span of a few million years between them. As a rule, the residence time of many metals in the ocean are short (<100 years) or long (millennia) [
63]. Therefore, we cannot generalize the influence of all hydrothermal trace elements on geochemical composition of Bazhenov Formation.
Intensive hydrothermal alteration or proximal sulfides have not yet been recognized at the level of the Bazhenov Formation. One can suppose, of course, that there were diffuse fluid seepages on the sea floor, with which the formation of bacterial mats–potential oil sources were associated. The development of sulfide-forming organic-rich seepages, surrounded by biotic assemblages, occurred during the Jurassic in the Middle Russian Sea (Tarkhany locality, Kazan area) [
78]. However, the enrichment of pyrite with metals is not connected with such seepages, possibly due to low temperatures of mineral genesis.
The processes of erosion of the Ural Mountains, enclosing sulfide, iron, molybdenum–copper porphyry, gold, chromite, nickel and other deposits in the Mesozoic, could presumably supply adjacent marine basins with diverse bioessential chemical elements (Cu, Zn, Se, Мo, Cr, Ni, V and others), which are necessary for plankton to flourish with the formation of corresponding bituminous sediments as previously inferred [
79]. Trace element redox-indicators and, first of all, Mo/Co and Se/Co suggest their formation under the conditions of higher oxygen content in the atmosphere [
6,
18,
63]. It is suggested a cyclic distribution of Se and Se/Co in sedimentary–diagenetic pyrite across the Phanerozoic [
38].
According to our data, the Bazhenov Formation of the Volgian Stage of the Upper Jurassic follows this tendency. A surprising combination of anoxic and euxinic conditions of sedimentation corresponding with peaks of oxygen concentration in the atmosphere is considered to be the main reason for the formation of this-type of highly metalliferous deposit. It would seem that the enrichment of sedimentary–diagenetic pyrite in Se and other trace elements is correlated with periods of plate-tectonic collision processes that, on the one hand, contributed to a supply of the sea biota with abundant nutrition [
6] and, on the other hand, provide conditions for CH
4 seep formation favorable for bacterial chemosynthesis and forming of microbial mats as precursors of oil deposits.
Anoxic and euxinic conditions of bottom waters have constrained Co and Mn concentration in pyrite to low levels. These conditions are characterized by high enrichment of seafloor waters in Mo, Zn, Se and V [
80,
81]. The euxinic waters promote capture of Fe
2+ and storage by the precipitation of sulfides, whereas Mn
2+ and Co
2+ are preserved in solution [
11]. It is interesting that in our case framboidal pyrite is enriched in Mn which contradicts this statement.
In a first approximation, the epochs of sulfide ore formation in vast regions were followed by epochs of oil accumulation. This also relates to the change of Devonian sulfide-ore complexes of the Urals followed by oil-bearing complexes of the Domanik Formation [
2]. It is obvious that rift-related volcanogenic settings were followed by accumulation of sedimentary deposits; the change of high-temperature sulfide ore-forming hydrothermal systems to organic-rich seepages in shallow-water seas followed the same tendency.
We assume that, by analogy with the Devonian cycle, there was the Triassic–Jurassic cycle, which included the formation of the North Sosva graben at the early stage. The latter is described in detail in a recent monograph [
64]. Copper massive sulfide deposits have not been discovered so far in the volcanogenic basement of the West Siberian megabasin, but one can assume their discovery will be with rift-related structures in deep-water areas. For example, younger cycles are known on Honshu Island, where the sulfide ore formation in the Miocene was followed by the deposition of oil-bearing sequences [
82].
The subsequent history of the development of the Bazhenov Formation also seems promising. A part of this formation, obviously, entered into the zones of catagenetic and early metamorphic pyrrhotite transformation from pyrite—the process, which leads to the formation of gold deposits [
18,
83]. It makes sense that the Bazhenov bituminous shales, being in the tectonic fault zones, could be a source of formation of gold-ore deposits, since framboidal pyrite contains at least 0.1 ppm Au [
18].
6. Conclusions
Framboidal and nodular pyrite are dominant varieties in the Bazhenov Formation. The large framboidal pyrite sizes and their cluster indicate that most have grown below the seafloor at the dysoxic-anoxic interface within organic-rich sediments, rather than in euxinic seawater columns. The dysoxic–anoxic conditions are supported by moderate values of the syngenetic pyrite index. The abundant barite in the black shales could be explained by excesses of Ba2+ and SO42− formed highly likely due to early diagenetic decay of feldspar and oxidation of H2S-rich seep fluids, respectively.
Some nodule-like pyritized bituminous layers and pyrite nodules with fine-grained laminated pyrite are similar by structure to pyritized microbial mat fragments. The microbial mats may have accumulated due to H2S–CH4 seepage providing massive bioproductivity by bacterial chemosynthesis. Coarse-grained pyrite in the nodules result from crystallization toward the end of early diagenesis.
Framboidal pyrite of the Bazhenov Formation is enriched in redox-sensitive elements such as Mo, V, Au, Cu, Pb, Ag, Ni, Se, and Zn in comparison with the host shales and nodular pyrite. Nodular pyrite has higher concentrations of As and Sb only. The trace element associations are characterized by strong positive correlations that can be interpreted as nano-inclusions of organic matter (Mo, V, Au), sphalerite (Zn, Cd, Hg, Sn, In, Ga, Ge), galena (Pb, Bi, Sb, Te, Ag, Tl) and tennantite (Cu, As, Sb, Bi, Te, Ag, Tl) in the pyrite studied. The substitution of Co, Ni, As and Sb into the pyrite lattice cannot be rejected. Many of the trace elements of the mineral inclusions, except sphalerite assemblage, show an inverse proportional relationship with V, suggesting a prior association with organic matter.
On the global scale, pyrite of the Bazhenov Formation is very similar to pyrite from highly metalliferous bituminous deposits, associated, as a rule, with gas and oil-and-gas deposits, which are characterized by higher Se and the complex of metals (Mo, Zn, Cu, Ni, Cd). In the Bazhenov Formation, the framboidal pyrites with high Mo, Zn, and Au and low Pb are in contrast with sedimentary-diagenetic pyrite (framboids, small crystals) from worldwide black shales barren of oil and gas. In general, the pyrite varieties from the Bazhenov Formation is characterized by high contents of Mo, Tl, Sb, Zn, Cu, Au and Ag in comparison with pyrite from gold–copper–iron skarn, porphyry copper–gold, and gold-ore orogenic deposits, being different from them in the extremely low contents of Co. The pyrite of SEDEX deposits are enriched in Pb, Ag, Co, Cu, Ni, Zn, Te and Tl and depleted in Mo in comparison with pyrite from the Bazhenov Formation. Enrichment with Mo and Sb indicate a higher influence of seawater during the formation of pyrite from the Bazhenov Formation in comparison to other styles of ore deposit presented herein.
The most important criteria for evaluation of hydrothermal versus seawater metal sources come from our study of geochemical differences of the pyrite in VHMS deposits hosted by black shales compared with pyrite from the Bazhenov Formation. The pyrite of the Bazhenov Formation contains relative higher Cr, V, Ni, Mo, which is related to an oceanic water source. In contrast, the same pyrite varieties located in the black shales of Safyanovskoe VHMS deposit are enriched in heavy metals (Bi, Pb, Ag, Au, Te) indicating a variable temperature of initial hydrothermal concentration. Concentrations of transitional elements such as Zn and Cu in framboidal pyrite are similar in both deposit types. The framboidal pyrite formed in black shales of the Bazhenov Formation does not reflect any impact of fluids from a typical hydrothermal massive sulfide-forming system. The source of Zn and Cu in the Bazhenov Formation could be massive sulfide deposits of the Urals Mountains weathered at the same Mesozoic time rather than some hydrothermal source of these elements. It is not improbable that the formation of the Bazhenov Formation has resulted from either a unique combination of the erosion of the Ural Mountains from one side and the simultaneous manifestation of organic-rich gas seep activity in the West Siberian Sea from another direction. It is suggested, that cold CH4 seeps provided bacterial chemosynthesis of organic mats as scavengers of trace elements and became precursors of pyrite and oil deposits. This theory may explain the formation of bituminous deposits unique in reserves of oil, gas and metals.