Past Hydrological Conditions in a Fluvial Valley: Records from C-O Isotope Signatures of Holocene Sediments in the Loire River (France)
Abstract
1. Introduction
2. Materials and Methods
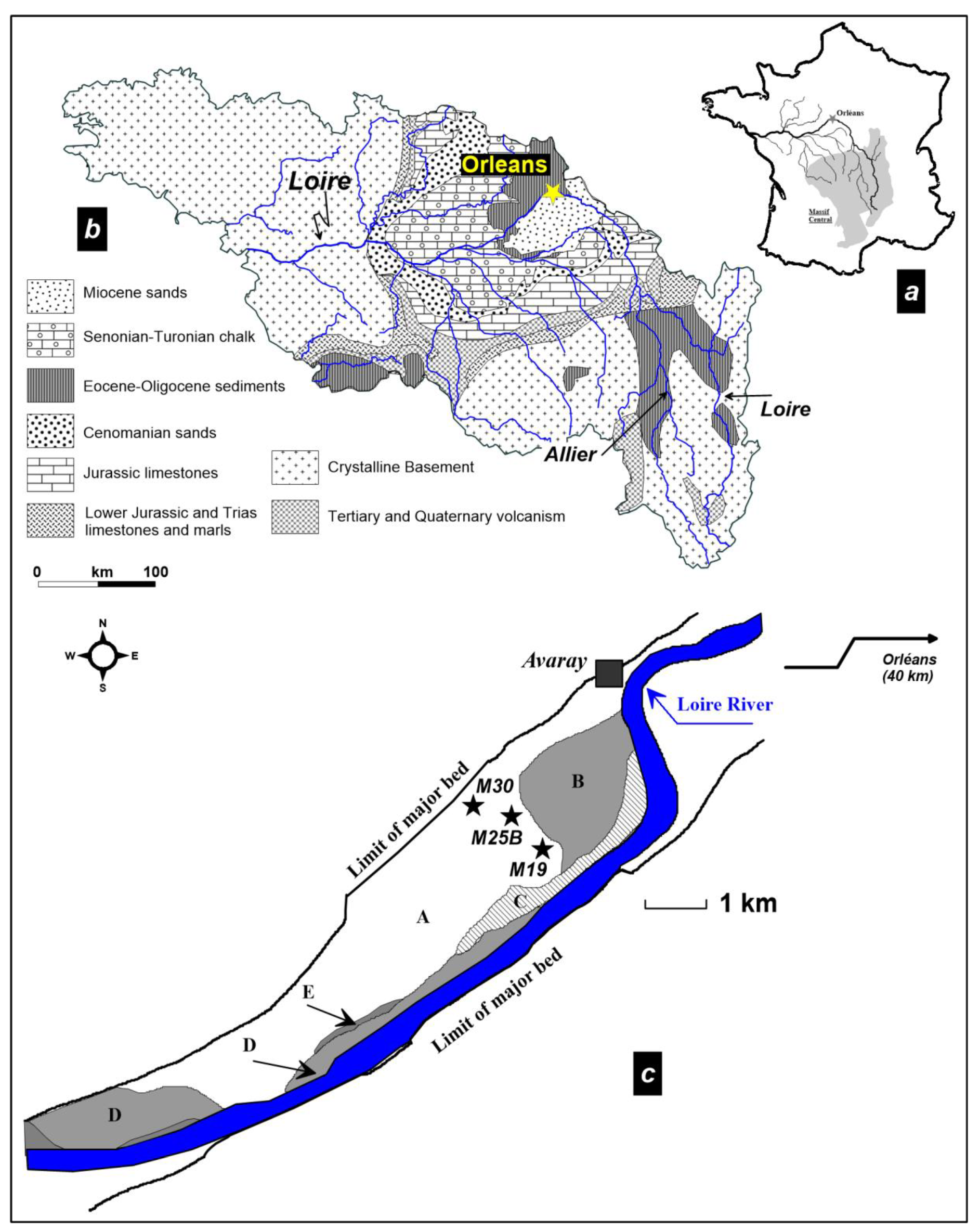
2.1. Site Description
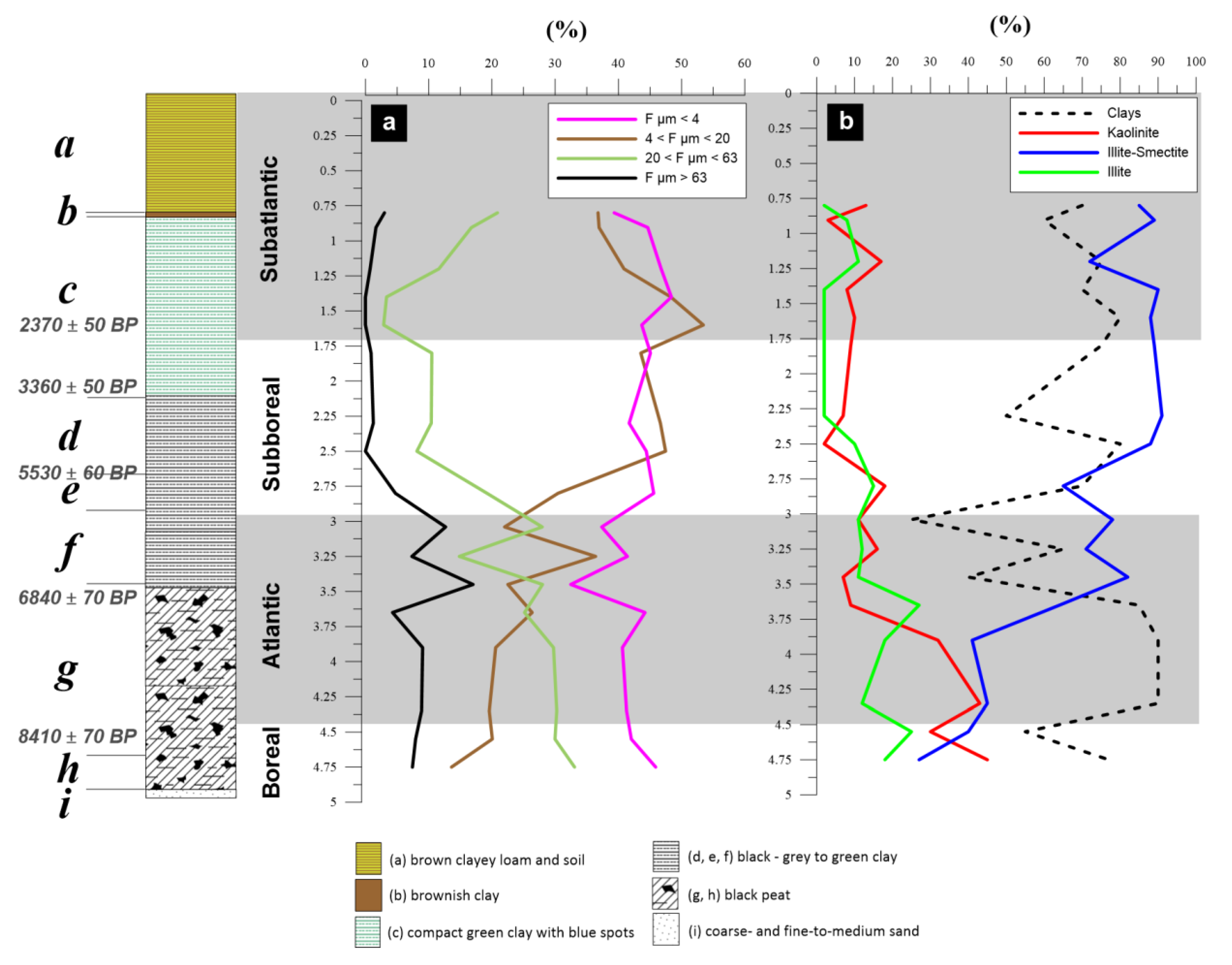
- (i)
- lower part (interval 6.3–4.9 m) of the core consists of coarse- and fine-to-medium grained micaceous sand;
- (h)
- 4.9–4.7 m: very fine black peat, the base is dated 8410 ± 70 a BP;
- (g)
- 4.7–3.5 m: black peat, becoming brownish towards the top, containing ligneous debris and gastropods (6840 ± 70 a BP at 3.58 m);
- (f)
- 3.5–2.9 m: black clay, rich in well-preserved gastropods, with ligneous debris dated as Atlantic;
- (e)
- 2.9–2.7 m: black clay with sparse gastropods and wood fragments (5350 ± 60 a BP at 2.77 m, end of Atlantic);
- (d)
- 2.7–2.2 m: grey to green clay, sparse shelly debris and very rare vegetal debris (Atlantic);
- (c)
- 2.2–1.0 m: compact green clay with blue spots and sparse gastropod debris (3360 ± 50 a BP at 2.16 m depth; Subboreal 2370 ± 50 a BP at 1.65 m depth, Subatlantic);
- (b)
- 1.0–0.9 m: brownish clay grading into loamy clay;
- (a)
- 0.9–0 m: brown clayey loam and soil.
2.2. Sample Pretreatment and Methods
3. Results and Discussion
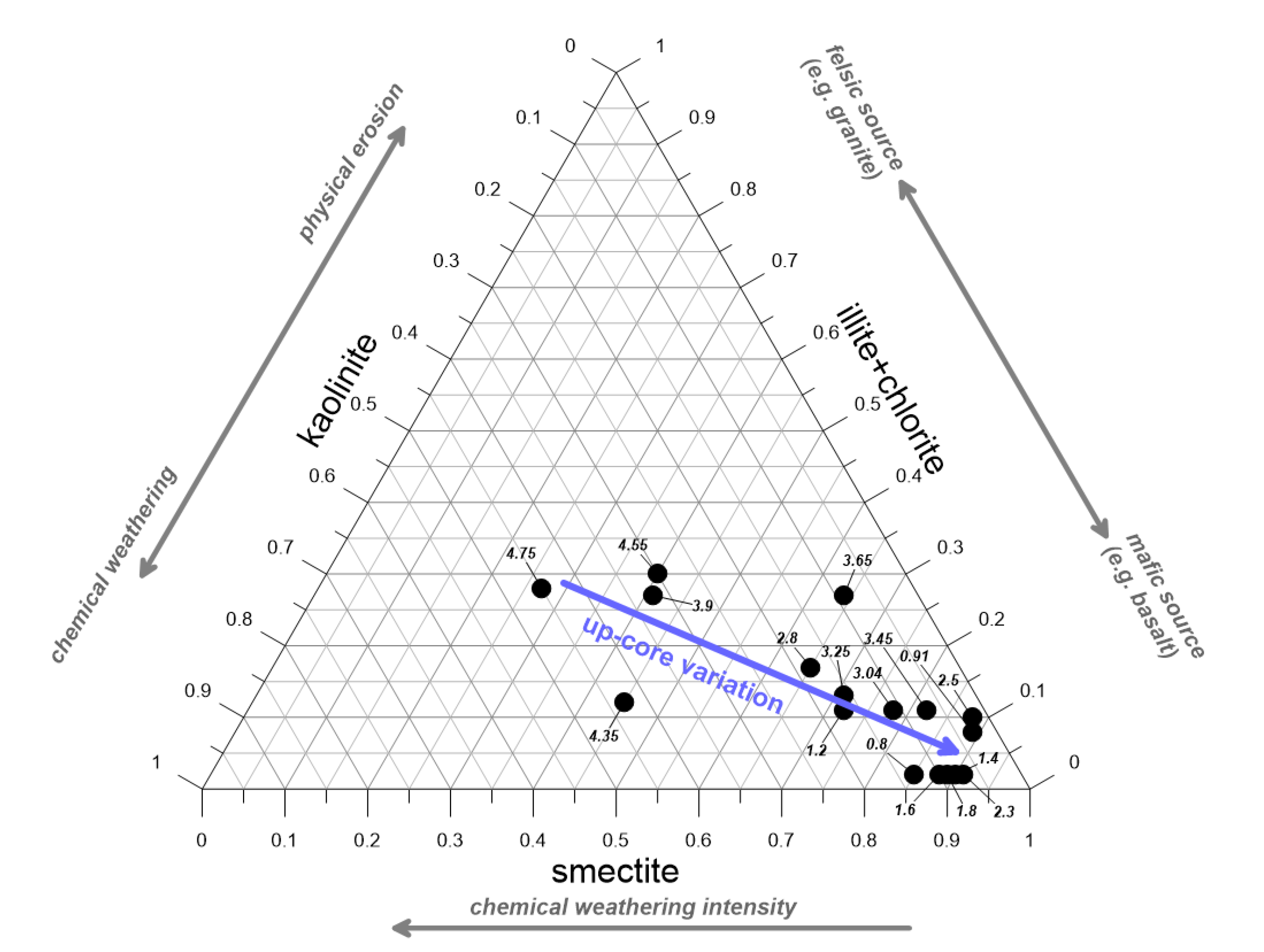
3.1. Clay Mineralogical Changes in the Loire River Reflecting Weathering Intensity
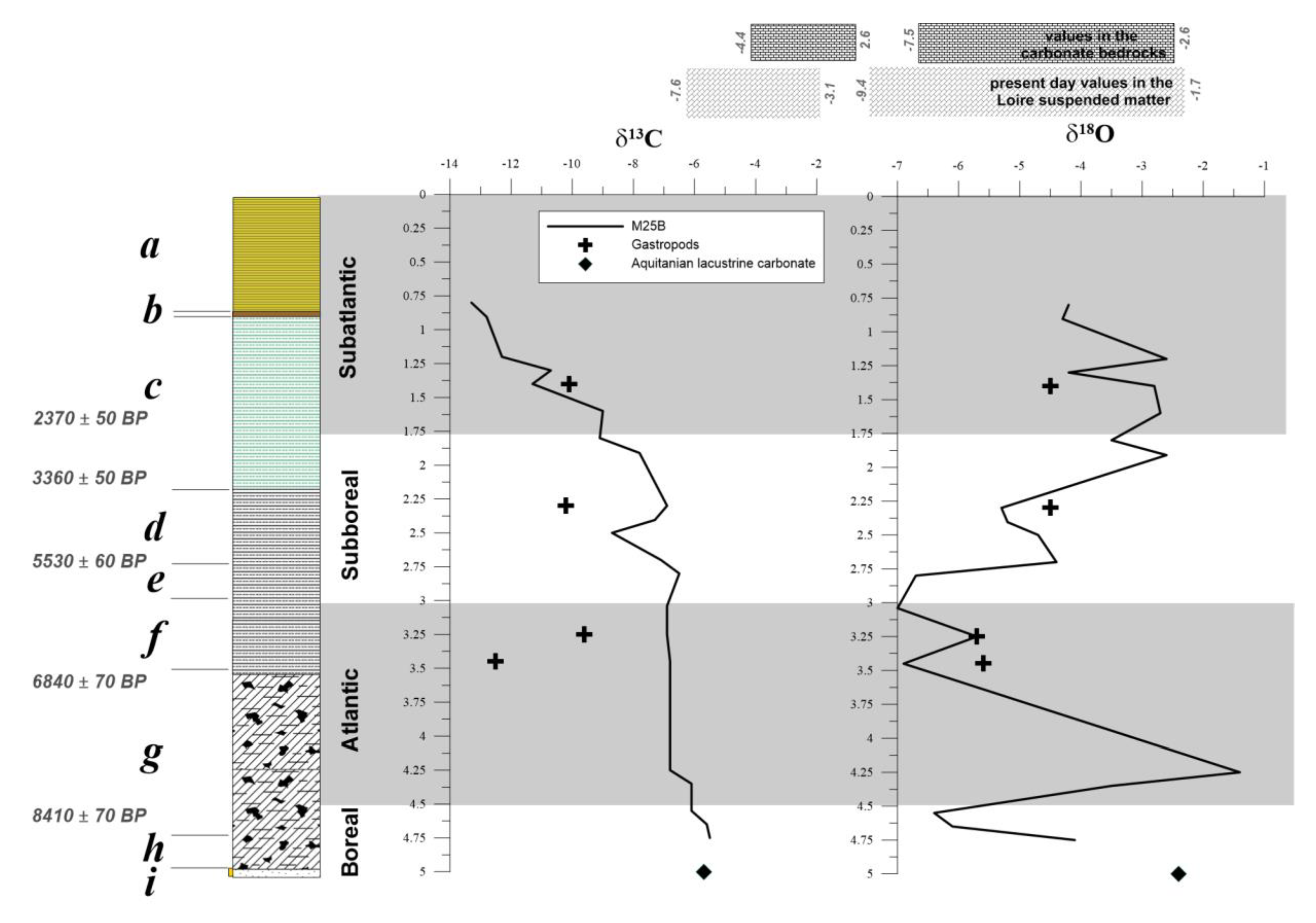
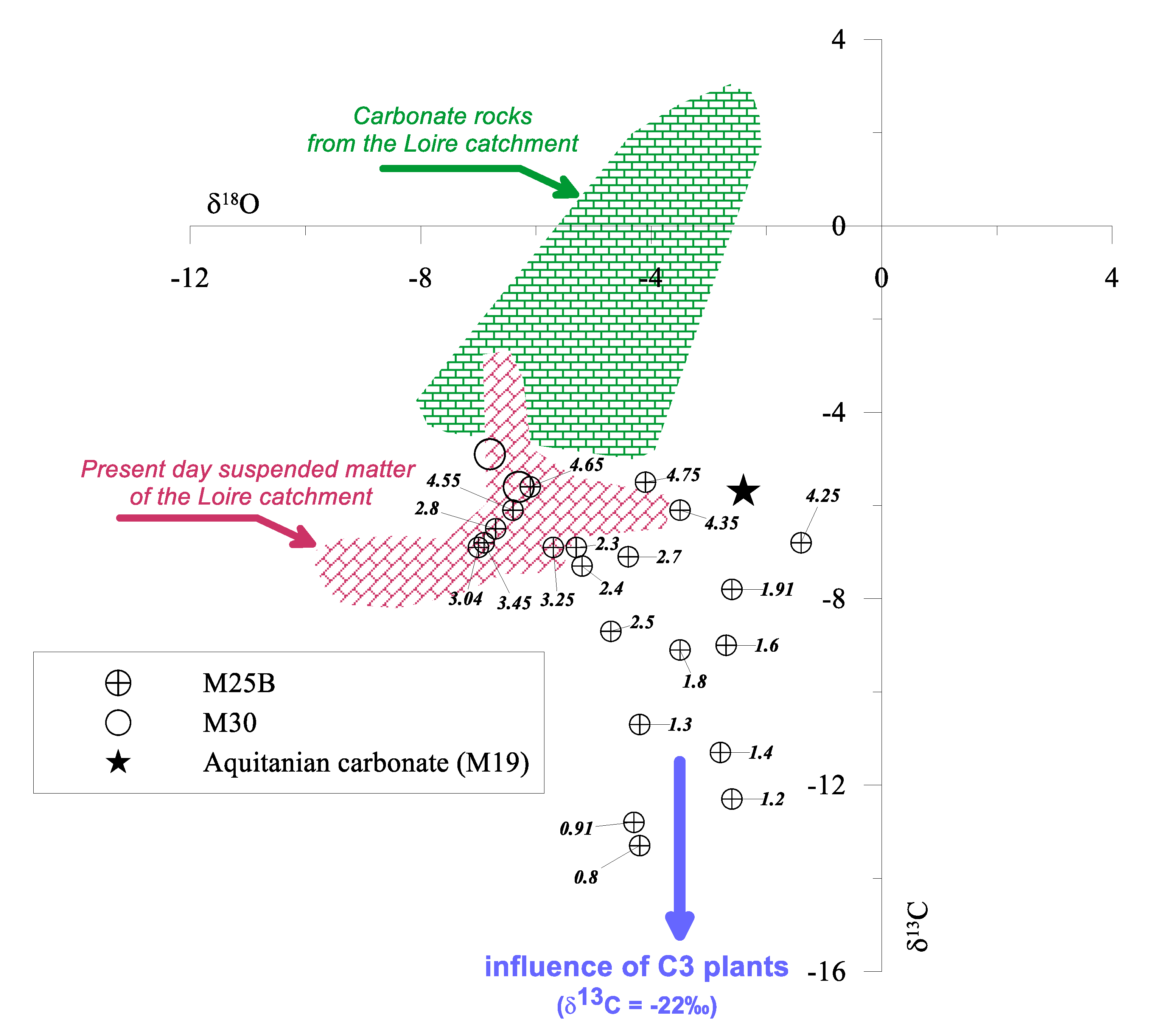
3.2. Origin of Carbonate in the Flood Sediments
4. Conclusions
- (1)
- The kaolinite vs. illite/chlorite ratio indicates the importance of chemical weathering vs. physical erosion that affected the sediments, with smectite content as weathering intensity proxy. Their variations might reflect a less-intense chemical weathering and a weak physical erosion in the case of the younger sediments, and a more mafic (basalt) than felsic source for the clay-mineral origin, likely related to the increase in the soil cultivation in the mid to late Holocene and a higher contribution of volcanic material to the alluvial deposits [14].
- (2)
- Variations of carbon and oxygen isotopes ratios over the last 10,000 years reveal a wide range. The Atlantic climatic optimum is reflected by a positive δ18O shift. The opposite trends for δ13C and δ18O values observed in the Subboreal and the Subatlantic variations is ascribed to a progressive closure of the meander and the installation of vegetation in a non-permanent wetland. The closure is consistent with the decrease in particle size and reflects the progressive disconnection of the meander with its bed infill, changes in the C origin, and possible difference in oxygen availability affecting the degradation processes of organic matter.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meade, R.H. Movement and storage of sediment in river systems. In Physical and Chemical Weathering in Geochemical Cycles; Lerman, A., Meybeck, M., Eds.; Kluwer Academic Pub: Dordrecht, The Netherlands, 1988; pp. 165–180. [Google Scholar]
- Runge, J. Holocene landscape history and palaeohydrology evidenced by stable carbon isotope (δ13C) analysis of alluvial sediments in the Mbari valley (5°N/23°E), Central African Republic. Catena 2002, 48, 67–87. [Google Scholar] [CrossRef]
- May, D.W. Properties of a 5500-year-old flood-plain in the Loup River Basin, Nebraska. Geomorphology 2003, 56, 243–254. [Google Scholar] [CrossRef]
- Dhivert, E.; Grosbois, C.; Rodrigues, S.; Desmet, M. Influence of fluvial environments on sediment archiving processes and temporal pollutant dynamics (Upper Loire River, France). Sci. Tot. Environ. 2015, 505, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Meade, R.H.; Yuzyk, T.R.; Day, T.J. Movement and storage of sediment in rivers of the United States and Canada. In The Geology of North America, Surface Water Hydrology; Geological Society of America: Boulder, CO, USA, 1990; pp. 255–280. [Google Scholar]
- Gaillardet, J.; Dupré, B.; Allègre, C.J. Geochemistry of large river suspended sediments: Silicate weathering or recycling tracer? Geochim. Cosmochim. Acta 1999, 63, 4037–4051. [Google Scholar] [CrossRef]
- Benito, G.; Sopena, A.; Sanchez-Moya, Y.; Machado, M.J.; Pérez-Gonzalez, A. Palaeoflood record of the Tagus River (Central Spain) during the Late Pleistocene and Holocene. Quart. Sci. Rev. 2003, 22, 1737–1756. [Google Scholar] [CrossRef]
- Négrel, P.; Grosbois, C. Changes in chemical and 87Sr/86Sr signature distribution patterns of suspended matter and bed sediments in the upper Loire River basin (France). Chem. Geol. 1999, 156, 231–249. [Google Scholar] [CrossRef]
- Négrel, P.; Grosbois, C.; Kloppmann, W. The labile fraction of suspended matter in the Loire River (France): Multi-element chemistry and isotopic (Rb-Sr and C-O) systematics. Chem. Geol. 2000, 166, 271–285. [Google Scholar] [CrossRef]
- Grosbois, C.; Négrel Ph Grimaud, D.; Fouillac, C. An overview of dissolved and suspended matter fluxes in the Loire River basin: Natural and anthropogenic inputs. Aquat. Geochem. 2001, 7, 81–105. [Google Scholar] [CrossRef]
- Négrel, P.; Roy, S. Investigating the sources of the labile fraction in sediments from silicate-drained rocks using trace elements, and strontium and lead isotopes. Sci. Total Environ. 2002, 298, 163–182. [Google Scholar] [CrossRef]
- Garcin, M.; Giot, D.; Farjanel, G.; Gourry, J.C.; Kloppmann, W.; Négrel, P. Géométrie et âge des alluvions du lit majeur de la Loire moyenne, exemple du val d’Avaray (Loir-et-Cher, France). Comptes Rendus Acad. Sci. 1999, 329, 405–412. [Google Scholar] [CrossRef]
- Négrel, P.; Kloppmann, W.; Garcin, M.; Giot, D. Strontium isotopic record of signatures of Holocene fluvial sediments in the Loire valley, France. Hydrol. Earth Syst. Sci. 2002, 6, 849–858. [Google Scholar] [CrossRef]
- Négrel, P.; Kloppmann, W.; Garcin, M.; Giot, D. Lead isotope signatures of Holocene fluvial sediments from the Loire River valley. Appl. Geochem. 2004, 19, 957–972. [Google Scholar] [CrossRef]
- Fontes, J.C.; Gasse, F.; Gibert, E. Holocene environmental changes in Lake Bangong basin (Western Tibet). Part 1: Chronology and stable isotopes of carbonates of a Holocene lacustrine core. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 120, 25–47. [Google Scholar] [CrossRef]
- Makhnach, N.; Zemitskaja, V.; Kolosov, I.; Simakova, G. Stable oxygen and carbon isotopes in Late Glacial-Holocene freshwater carbonates from Belarus and their palaeoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 209, 73–102. [Google Scholar] [CrossRef]
- Garzanti, E.; Padoan, M.; Setti, M.; López-Galindo, A.; Villa, I.M. Provenance versus weathering control on the composition of tropical river mud (southern Africa). Chem. Geol. 2014, 366, 61–74. [Google Scholar] [CrossRef]
- Mayer, B.; Schwark, L. A 15,000-year stable isotope record from sediments of lake Steisslingen, Southwest Germany. Chem. Geol. 1999, 161, 315–337. [Google Scholar] [CrossRef]
- Lojen, S.; Dolenec, T.; Vokal, B.; Cukrov, N.; Mihelcic, G.; Papesch, W. C and O stable isotope variability in recent freshwater carbonates (River Krha, Croatia). Sedimentology 2004, 51, 361–375. [Google Scholar] [CrossRef]
- Schulte, P.; van Geldern, R.; Freitag, H.; Karim, A.; Négrel Ph Petelet-Giraud, E.; Probst, A.; Probst, J.L.; Telmer, K.; Veizer, J.; Barth, J.A.C. Applications of stable water and carbon isotopes in watershed research: Weathering, carbon cycling, and water balances. Earth Sci. Rev. 2011, 109, 20–31. [Google Scholar] [CrossRef]
- Parrish, J.T.; Hyland, E.G.; Chan, M.A.; Hasiotis, S.T. Stable and clumped isotopes in desert carbonate spring and lake deposits reveal palaeohydrology: A case study of the Lower Jurassic Navajo Sandstone, south-western USA. Sedimentology 2019, 66, 32–52. [Google Scholar] [CrossRef]
- Reis, A.; Erhardt, A.M.; McGlue, M.M.; Waite, L. Evaluating the effects of diagenesis on the δ13C and δ18O compositions of carbonates in a mud-rich depositional environment: A case study from the Midland Basin, USA. Chem. Geol. 2019, 524, 196–212. [Google Scholar] [CrossRef]
- Kämpf, L.; Plessen, B.; Lauterbach, S.; Nantke, C.; Meyer, H.; Chapligin, B.; Brauer, A. Stable oxygen and carbon isotopes of carbonates in lake sediments as a paleoflood proxy. Geology 2019, 48, 3–7. [Google Scholar] [CrossRef]
- Benjelloun, Y.; Carlut, J.; Hélie, J.F.; Chazot, G.; Le Callonnec, L. Geochemical study of carbonate concretions from the aqueduct of Nîmes (southern France): A climatic record for the first centuries AD? Sci. Rep. 2019, 9, 5209. [Google Scholar] [CrossRef] [PubMed]
- Garcin, M.; Giot, D.; Farjanel, G. Radiocarbon dating and palynology of alluvial deposits in the Loire flood plain (Val d’Avaray, Loir et Cher, France). Quaternaire 2001, 12, 69–88. [Google Scholar] [CrossRef]
- Wilson, M. The origin and formation of clay minerals in soils: Past, present and future perspectives. Clay Miner. 1999, 34, 7–25. [Google Scholar] [CrossRef]
- Négrel, P.; Sadeghi, M.; Ladenberger, A.; Reimann, C.; Birke, M. Geochemical fingerprinting and source discrimination of agricultural soils at continental scale. Chem. Geol. 2015, 396, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Hantoro, W.S.; Sathiamurthy, E.; Colin, C.; Zhao, Y.; Li, J. Climatic and tectonic controls on chemical weathering in tropical Southeast Asia (Malay Peninsula, Borneo, and Sumatra). Chem. Geol. 2012, 291, 1–12. [Google Scholar] [CrossRef]
- Singer, A. The paleoclimatic interpretation of clay minerals in soils and weathering profiles. Earth Sci. Rev. 1980, 15, 303–326. [Google Scholar] [CrossRef]
- Chamley, H. Clay Sedimentology; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1989; 623p. [Google Scholar]
- Hu, B.; Li, J.; Cui, R.; Wei, H.; Zhao, J.; Li, G.; Fang, X.; Ding, X.; Zou, L.; Bai, F. Clay mineralogy of the riverine sediments of Hainan Island, South China Sea: Implications for weathering and provenance. J. Asian Earth Sci. 2014, 96, 84–92. [Google Scholar] [CrossRef]
- Shanahan, T.M.; Pigati, J.S.; Dettman, D.L.; Quade, J. Isotopic variability in the aragonite shells of freshwater gastropods living in springs with nearly constant temperature and isotopic composition. Geochim. Cosmochim. Acta 2005, 69, 3949–3966. [Google Scholar] [CrossRef]
- Li, H.C.; Ku, T.L. δ13C-δ18O covariance as a paleohydrological indicator for closed-basin lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 133, 69–80. [Google Scholar] [CrossRef]
- Rowland, J.C.; Lepper, K.; Dietrich, W.E.; Wilson, C.J.; Sheldon, R. Tie channel sedimentation rates, oxbow formation age and channel migration rate from optically stimulated luminescence (OSL) analysis of floodplain deposits. Earth Sur. Proc. Landf. 2005, 30, 1161–1179. [Google Scholar] [CrossRef]
- Hammarlund, D.; Björck, S.; Buchardt, B.; Israelson, C.; Thomsen, C. Rapid hydrological changes during the Holocene revealed by stable isotope records of lacustrine carbonates from Lake Igelsjön, southern Sweden. Quart. Sci. Rev. 2003, 22, 353–370. [Google Scholar] [CrossRef]
| Sample | Z | Age | Quartz | Feldspaths | Calcite | Phyllosilicates | Smectite-Illite | Illite | Kaolinite | Chlorite | Illite-Chlorite |
|---|---|---|---|---|---|---|---|---|---|---|---|
| m | a BP | % | % | % | % | % | % | % | % | % | |
| M25B/A | 0.80 | 1137 | 15 | 15 | 70 | 85 | 2 | 13 | 2 | ||
| M25B/B | 0.91 | 1309 | 15 | 25 | 60 | 89 | 8 | 3 | 8 | ||
| M25B/C | 1.20 | 1791 | 10 | 10 | 5 | 75 | 72 | 11 | 17 | 11 | |
| M25B/D | 1.40 | 2117 | 10 | 15 | 5 | 70 | 90 | 2 | 8 | 2 | |
| M25B/E | 1.60 | 2444 | 10 | 5 | 5 | 80 | 88 | 2 | 10 | 2 | |
| M25B/F | 1.80 | 2771 | 12 | 10 | 3 | 75 | 89 | 2 | 9 | 2 | |
| M25B/G | 2.30 | 3587 | 5 | 10 | 35 | 50 | 91 | 2 | 7 | 2 | |
| M25B/H | 2.50 | 3914 | 5 | 5 | 10 | 80 | 88 | 10 | 2 | 10 | |
| M25B/I | 2.80 | 4404 | 5 | 5 | 20 | 70 | 65 | 15 | 18 | 2 | 17 |
| M25B/J | 3.04 | 4796 | 5 | 30 | 40 | 25 | 78 | 11 | 11 | 11 | |
| M25B/K | 3.25 | 5139 | 15 | 10 | 10 | 65 | 71 | 12 | 16 | 1 | 13 |
| M25B/L | 3.45 | 5466 | 5 | 15 | 40 | 40 | 82 | 11 | 7 | 11 | |
| M25B/M | 3.65 | 6831 | 5 | 10 | 85 | 64 | 27 | 9 | 27 | ||
| M25B/N | 3.90 | 7202 | 5 | 5 | 90 | 41 | 18 | 32 | 9 | 27 | |
| M25B/O | 4.35 | 7870 | 5 | 5 | 90 | 45 | 12 | 43 | 0.1 | 12 | |
| M25B/P | 4.55 | 8166 | 3 | 2 | 40 | 55 | 40 | 25 | 30 | 5 | 30 |
| M25B/Q | 4.75 | 8463 | 3 | 5 | 15 | 77 | 27 | 18 | 45 | 10 | 28 |
| Sample | Z | age | δ18O | δ13C |
|---|---|---|---|---|
| m | a BP | ‰ vs. V-PDB | ‰ vs. V-PDB | |
| M25B core | ||||
| M25B/A | 0.80 | 1137 | −4.2 | −13.3 |
| M25B/B | 0.91 | 1309 | −4.3 | −12.8 |
| M25B/C | 1.20 | 1791 | −2.6 | −12.3 |
| M25B/Ca | 1.30 | 1954 | −4.2 | −10.7 |
| M25B/D | 1.40 | 2117 | −2.8 | −11.3 |
| M25B/E | 1.60 | 2444 | −2.7 | −9 |
| M25B/F | 1.80 | 2771 | −3.5 | −9.1 |
| M25B/Fa | 1.91 | 2950 | −2.6 | −7.8 |
| M25B/G | 2.30 | 3587 | −5.3 | −6.9 |
| M25B/Ga | 2.41 | 3759 | −5.2 | −7.3 |
| M25B/H | 2.50 | 3914 | −4.7 | −8.7 |
| M25B/Ha | 2.70 | 4241 | −4.4 | −7.1 |
| M25B/I | 2.80 | 4404 | −6.7 | −6.5 |
| M25B/J | 3.04 | 4796 | −7 | −6.9 |
| M25B/K | 3.25 | 5139 | −5.7 | −6.9 |
| M25B/L | 3.45 | 5466 | −6.9 | −6.8 |
| M25B/Na | 4.25 | 7721 | −1.4 | −6.8 |
| M25B/O | 4.35 | 7870 | −3.5 | −6.1 |
| M25B/P | 4.55 | 8166 | −6.4 | −6.1 |
| M25B/Pa | 4.65 | 8315 | −6.1 | −5.6 |
| M25B/Q | 4.75 | 8463 | −4.1 | −5.5 |
| M30B core | ||||
| M30A | 2.43 | 11,460 | −6.3 | −5.6 |
| M30B | 2.18 | - | −6.8 | −4.9 |
| Aquitanian carbonate | ||||
| M19/5 | 5.00 | - | −2.4 | −5.7 |
| gastropod shells | ||||
| M25B/Ds | 1.40 | - | −4.5 | −10.1 |
| M25B/Gs | 2.30 | - | −4.5 | −10.2 |
| M25B/Ks | 3.25 | - | −5.7 | −9.6 |
| M25B/Ls | 3.45 | - | −5.6 | −12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Négrel, P.; Kloppmann, W. Past Hydrological Conditions in a Fluvial Valley: Records from C-O Isotope Signatures of Holocene Sediments in the Loire River (France). Minerals 2020, 10, 400. https://doi.org/10.3390/min10050400
Négrel P, Kloppmann W. Past Hydrological Conditions in a Fluvial Valley: Records from C-O Isotope Signatures of Holocene Sediments in the Loire River (France). Minerals. 2020; 10(5):400. https://doi.org/10.3390/min10050400
Chicago/Turabian StyleNégrel, Philippe, and Wolfram Kloppmann. 2020. "Past Hydrological Conditions in a Fluvial Valley: Records from C-O Isotope Signatures of Holocene Sediments in the Loire River (France)" Minerals 10, no. 5: 400. https://doi.org/10.3390/min10050400
APA StyleNégrel, P., & Kloppmann, W. (2020). Past Hydrological Conditions in a Fluvial Valley: Records from C-O Isotope Signatures of Holocene Sediments in the Loire River (France). Minerals, 10(5), 400. https://doi.org/10.3390/min10050400






