Colloidal ZnCO3 as a Powerful Depressant of Arsenopyrite in Weakly Alkaline Pulp and the Interaction Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Flotation Tests
2.2.1. Micro Flotation
2.2.2. Bench Scale Flotation
2.3. Electrokinetic Potential Tests
2.4. Adsorption Measurements
2.5. SEM Observation and EDS Detection
3. Results and Discussion
3.1. Flotation
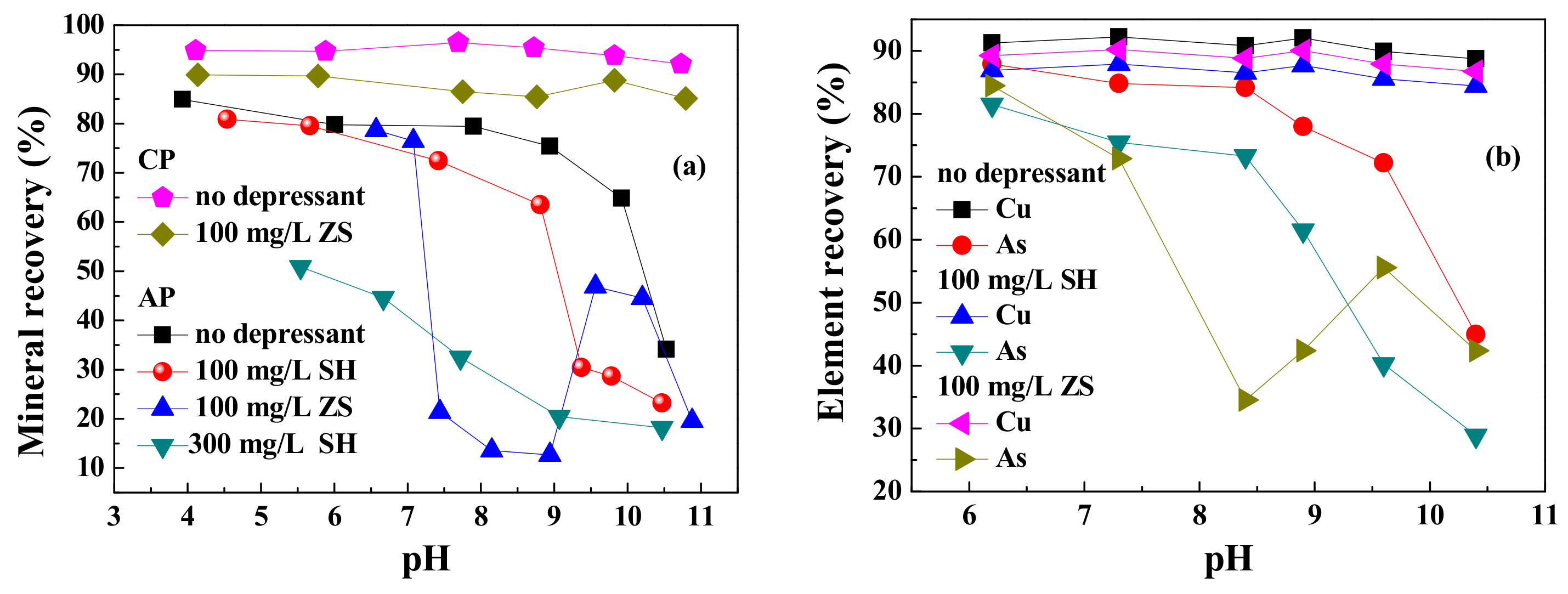
3.1.1. Micro Flotation
3.1.2. Bench Scale Flotation
3.2. Electrokinetic Potential
3.3. Adsorption Amounts of SIBX
3.4. SEM and EDS Results
3.5. Suggested Adsorption Model
4. Conclusions
- (1)
- The depression effect of ZnSO4 on arsenopyrite was superior to the traditional depressant sodium humate in Na2CO3 and SIBX solution. The greatest recovery difference between chalcopyrite and arsenopyrite was achieved in the weakly alkaline pH range 7.5–9.0 with ZnSO4 as the depressant.
- (2)
- For real ore flotation, ZnSO4 also exhibited better depression performance on the arsenopyrite minerals with Na2CO3 as the pH regulator, and Cu roughing concentrate with 8.5%/0.2% Cu/As grades and 84%/39% Cu/As recoveries was obtained through the open circuit flow with one rougher and one scavenger. The As recovery in concentrate decreased 20 percentage points.
- (3)
- With the addition of ZnSO4 into the pH 7.5–9.0 Na2CO3 and SIBX solution, the adsorption of SIBX onto arsenopyrite surface was inhibited, resulting in the higher electrokinetic potentials of arsenopyrite and low adsorbed amount of SIBX. This depression effect of ZnSO4 was attributed to the formation of colloidal ZnCO3 on the arsenopyrite surface.
Author Contributions
Funding
Conflicts of Interest
References
- Angino, E.E.; Magnuson, L.M.; Waugh, T.C.; Galle, O.K.; Bredfeldt, J. Arsenic in detergents: Possible danger and pollution hazard. Science 1970, 168, 389–390. [Google Scholar] [CrossRef] [PubMed]
- Filippou, D.; St-Germain, P.; Grammatikopoulos, T. Recovery of metal values from copper—Arsenic minerals and other related resources. Miner. Process. Extr. Metall. Rev. 2007, 28, 247–298. [Google Scholar] [CrossRef]
- Xiong, D.; Hu, Y.; He, Z.; Zhan, X. Advances in research on Organic depressor’s depressing of arsenopyrite in sulfide flotation. Min. Metall. Eng. 2004, 24, 42–44. (In Chinese) [Google Scholar]
- David, D.M.; Quast, K.B. Arsenic depression in the flotation of Broken Hill lead concentrates. In Proceedings of the Fourth Mill Operators’ Conference, AusIMM, Burnie, Australia, 10–14 March 1991; pp. 103–108. [Google Scholar]
- Matveeva, T.N.; Gromova, N.K.; Lantsova, L.B. Adsorption of tannin-bearing organic reagents on stibnite, arsenopyrite and chalcopyrite in complex gold ore flotation. J. Min. Sci. 2016, 52, 551–558. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, D.; Qin, W. Selective Flotation Separation of Marmatite and Arsenopyrite by a New Organic Depressant. Min. Metall. Eng. 2006, 26, 42–44. (In Chinese) [Google Scholar]
- Lu, J.; Tong, Z.; Yuan, Z.; Li, L. Investigation on flotation separation of chalcopyrite from arsenopyrite with a novel collector: N-Butoxycarbonyl-O-Isobutyl Thiocarbamate. Miner. Eng. 2019, 137, 118–123. [Google Scholar] [CrossRef]
- Ran, J.; Qiu, X.; Hu, Z.; Liu, Q.; Song, B.; Yao, Y. Enhance flotation separation of arsenopyrite and pyrite by low-temperature oxygen plasma surface modification. Appl. Surf. Sci. 2019, 480, 1136–1146. [Google Scholar] [CrossRef]
- Tapley, B.; Yan, D. The selective flotation of arsenopyrite from pyrite. Miner. Eng. 2003, 16, 1217–1220. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.; Bradshaw, D. A review of copper–arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 36, 179–186. [Google Scholar] [CrossRef]
- Ma, X.; Bruckard, W.J. Rejection of arsenic minerals in sulfide flotation—A literature review. Int. J. Miner. Process. 2009, 93, 89–94. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Sirkeci, A.A. The flotation separation of pyrite from arsenopyrite using hexyl thioethylamine as collector. Int. J. Miner. Process. 2000, 60, 263–276. [Google Scholar] [CrossRef]
- Herkenhoff, E.C. Separation of Pyrite from Arsenopyrite. U.S. Patent 2,342, 277, 22 February 1994. [Google Scholar]
- Dai, Z.; Garritsen, J.; Wells, P.F.; Xu, M. Arsenic rejection in the flotation of Garson Ni–Cu ore. In Proceedings of the Centenary of Flotation Symposium, Brisbane, Australia, 5–9 June 2005; pp. 939–946. [Google Scholar]
- Chanturiya, V.A.; Ivanova, T.A.; Lunin, V.D. New reagent for flotation separation of pyrite and arsenopyrite. Tsvet. Met. 2001, 4, 22–27. [Google Scholar]
- Wang, H.; Wei, Z.; Zeng, M.; Peng, R.; Xue, C. Research progress on the mechanism of sphalerite inhibitors in the separation of copper and zinc minerals. Conserv. Util. Miner. Resour. 2019, 39, 124–130. (In Chinese) [Google Scholar]
- He, X.; Luo, C.; Zheng, S. Study on mineral processing technology for a rich Ag-bearing Pb-Zn ore. J. Guangdong Non-Ferr. Met. 2008, 4, 297–299. (In Chinese) [Google Scholar]
- Zeng, K. Study on Flotation Behavior and Separation of Sulfur-Arsenic Minerals. Ph.D. Thesis, Central South University, Changsha, China, 2010. (In Chinese). [Google Scholar]
- Feng, B.; Feng, Q.; Lu, Y. A novel method to limit the detrimental effect of serpentine on the flotation of pentlandite. Int. J. Miner. Process. 2012, 114, 11–13. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.; Bu, Y.; Wang, C.; Wang, L.; Sun, W.; Hu, Y. Oxidative Depression of Arsenopyrite by Using Calcium Hypochlorite and Sodium Humate. Minerals 2018, 8, 463. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Ma, L.; Qin, W.; Jiao, F. Depression mechanism of the zinc sulfate and sodium carbonate combined inhibitor on talc. Colloids Surf. A Physicochem. Eng. Asp. 2016, 501, 92–97. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- He, J.; Liu, C.; Yao, Y. Flotation intensification of the coal slime using a new compound collector and the interaction mechanism between the reagent and coal surface. Powder Technol. 2018, 325, 333–339. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Wang, Z.; Yan, W.; Zhou, X.; Xu, L.; Wang, C. A novel depressant for selective flotation separation of pyrite and pyrophyllite. Appl. Surf. Sci. 2019, 487, 9–16. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Fang, S.; Wang, Z.; Xu, Y.; Zhang, Z. Effects of ultrasonic pre-treatment on the flotation of ilmenite and collector adsorption. Miner. Eng. 2019, 137, 124–132. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Z.; Xiao, J.; Wang, H.; Deng, B.; Zhang, Y.; Chen, C. Novel Selective Depressant of Titanaugite and Implication for Ilmenite Flotation. Minerals 2019, 9, 703. [Google Scholar] [CrossRef]
- Jones, M.H.; Woodcock, J.T. Spectrophotometric determination of flotation collectors and organic reagents in ore treatment process liquors and effluents with an atomic absorption spectrometer. Anal. Chim. Acta 1976, 87, 463–471. [Google Scholar] [CrossRef]
- Xia, W.; Zhou, C.; Peng, Y. Enhancing flotation cleaning of intruded coal dry-ground with heavy oil. J. Clean. Prod. 2017, 161, 591–597. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, Y.; Xu, L.H.; Dai, B.; Xiao, J.H.; Fu, K. Selective chalcopyrite flotation from pyrite with glycerine-xanthate as depressant. Miner. Eng. 2015, 74, 86–90. [Google Scholar] [CrossRef]
- Multani, R.S.; Williams, H.; Johnson, B.; Li, R.; Water, K.E. The effect of superstructure on the zeta potential, xanthate adsorption, and flotation response of pyrrhotite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 108–116. [Google Scholar] [CrossRef]
- Valdivieso, A.L.; López, A.S.; Escamilla, C.O.; Fuerstenau, M.C. Flotation and depression control of arsenopyrite through pH and pulp redox potential using xanthate as the collector. Int. J. Miner. Process. 2006, 81, 27–34. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Little, L.; Mainza, A.N.; Becker, M.; Wiese, J. Fine grinding: How mill type affects particle shape characteristics and mineral liberation. Miner. Eng. 2017, 111, 148–157. [Google Scholar] [CrossRef]
- Patra, A.; Taner, H.A.; Bordes, R.; Holmberg, K.; Larsson, A. Selective flotation of calcium minerals using double-headed collectors. J. Dispers. Sci. Technol. 2019, 40, 1205–1216. [Google Scholar] [CrossRef]
- McFadzean, B.; Marozva, T.; Wiese, J. Flotation frother mixtures: Decoupling the sub-processes of froth stability, froth recovery and entrainment. Miner. Eng. 2016, 85, 72–79. [Google Scholar] [CrossRef]
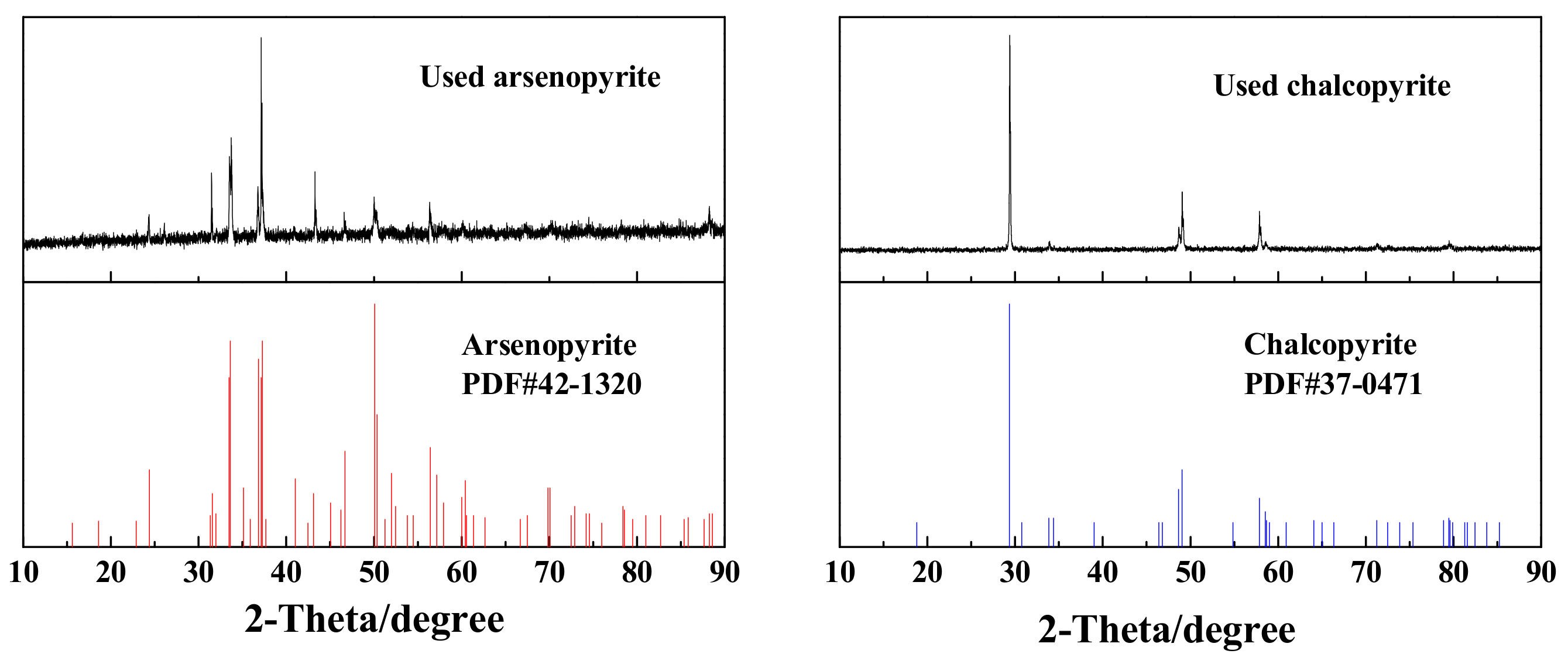







| Elements | Cu | As | S | WO3 | Pb | Zn | CaO | Al2O3 | MgO | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 0.36 | 0.02 | 2.58 | 0.039 | 0.0053 | 0.031 | 18.146 | 5.721 | 4.682 | 61.410 |
| Minerals | Chalcopyrite | Covellite | Malachite | Arsenopyrite | Pyrite | Quartz | Feldspar | Biotite |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 1.165 | 0.064 | 0.015 | 0.042 | 0.0064 | 42.343 | 28.650 | 6.214 |
| Depressant Type* | Products | Ratio/wt. % | Cu Grade/% | Cu Recovery/% | As Grade/% | As Recovery/% |
|---|---|---|---|---|---|---|
| SH, 350/150 g/t (Roughing/Scavenging) | C | 4.08 | 7.301 | 82.74 | 0.284 | 57.89 |
| T | 95.92 | 0.0651 | 17.26 | 0.0088 | 42.11 | |
| F | 100.00 | 0.36 | 100.00 | 0.02 | 100.00 | |
| ZS, 200/100 g/t (Roughing/Scavenging) | C | 3.58 | 8.467 | 84.19 | 0.218 | 38.79 |
| T | 96.42 | 0.059 | 15.81 | 0.0127 | 61.21 | |
| F | 100.00 | 0.36 | 100.00 | 0.02 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Ming, P.; Xie, Z.; Li, F.; Xing, Q.; Wang, Z. Colloidal ZnCO3 as a Powerful Depressant of Arsenopyrite in Weakly Alkaline Pulp and the Interaction Mechanism. Minerals 2020, 10, 315. https://doi.org/10.3390/min10040315
Guan Y, Ming P, Xie Z, Li F, Xing Q, Wang Z. Colloidal ZnCO3 as a Powerful Depressant of Arsenopyrite in Weakly Alkaline Pulp and the Interaction Mechanism. Minerals. 2020; 10(4):315. https://doi.org/10.3390/min10040315
Chicago/Turabian StyleGuan, Youguo, Pingtian Ming, Zhuohong Xie, Fei Li, Qingqing Xing, and Zhen Wang. 2020. "Colloidal ZnCO3 as a Powerful Depressant of Arsenopyrite in Weakly Alkaline Pulp and the Interaction Mechanism" Minerals 10, no. 4: 315. https://doi.org/10.3390/min10040315
APA StyleGuan, Y., Ming, P., Xie, Z., Li, F., Xing, Q., & Wang, Z. (2020). Colloidal ZnCO3 as a Powerful Depressant of Arsenopyrite in Weakly Alkaline Pulp and the Interaction Mechanism. Minerals, 10(4), 315. https://doi.org/10.3390/min10040315





