Experimental Study on Preparation of Ferropericlase by Oxalate Coprecipitation
Abstract
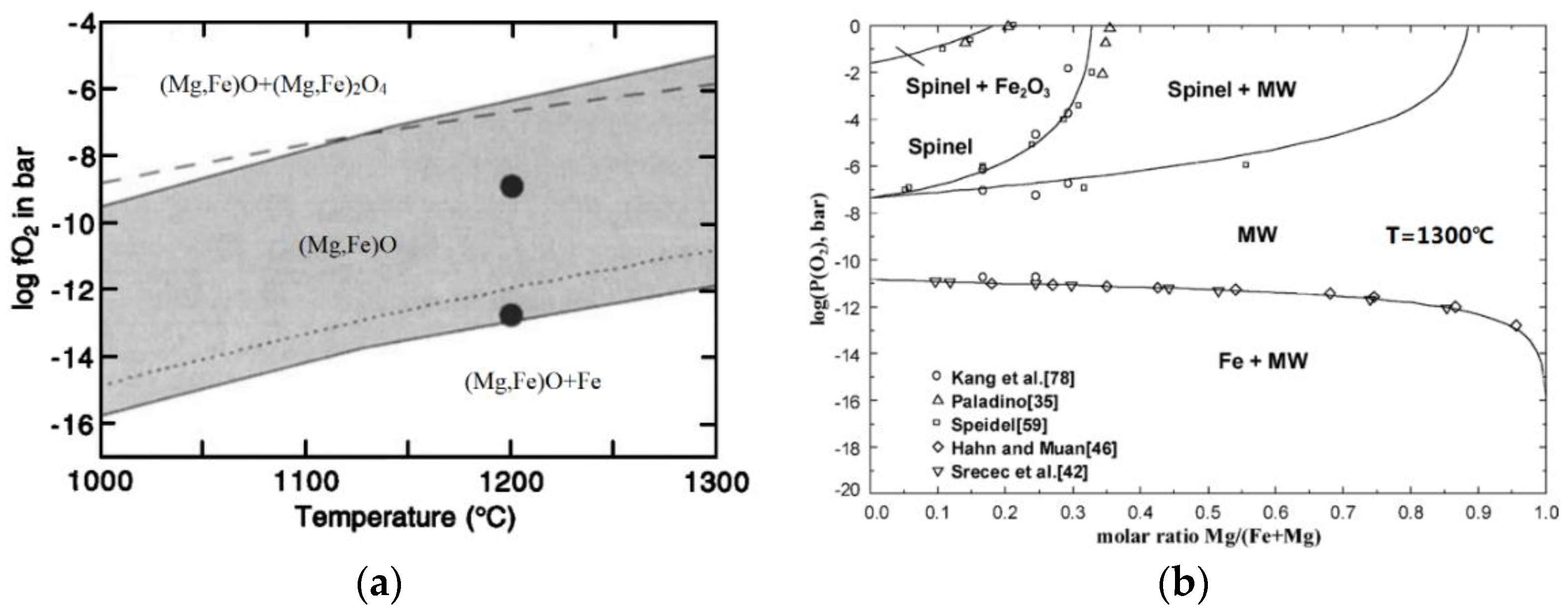
1. Introduction
2. Materials and Methods
3. Results
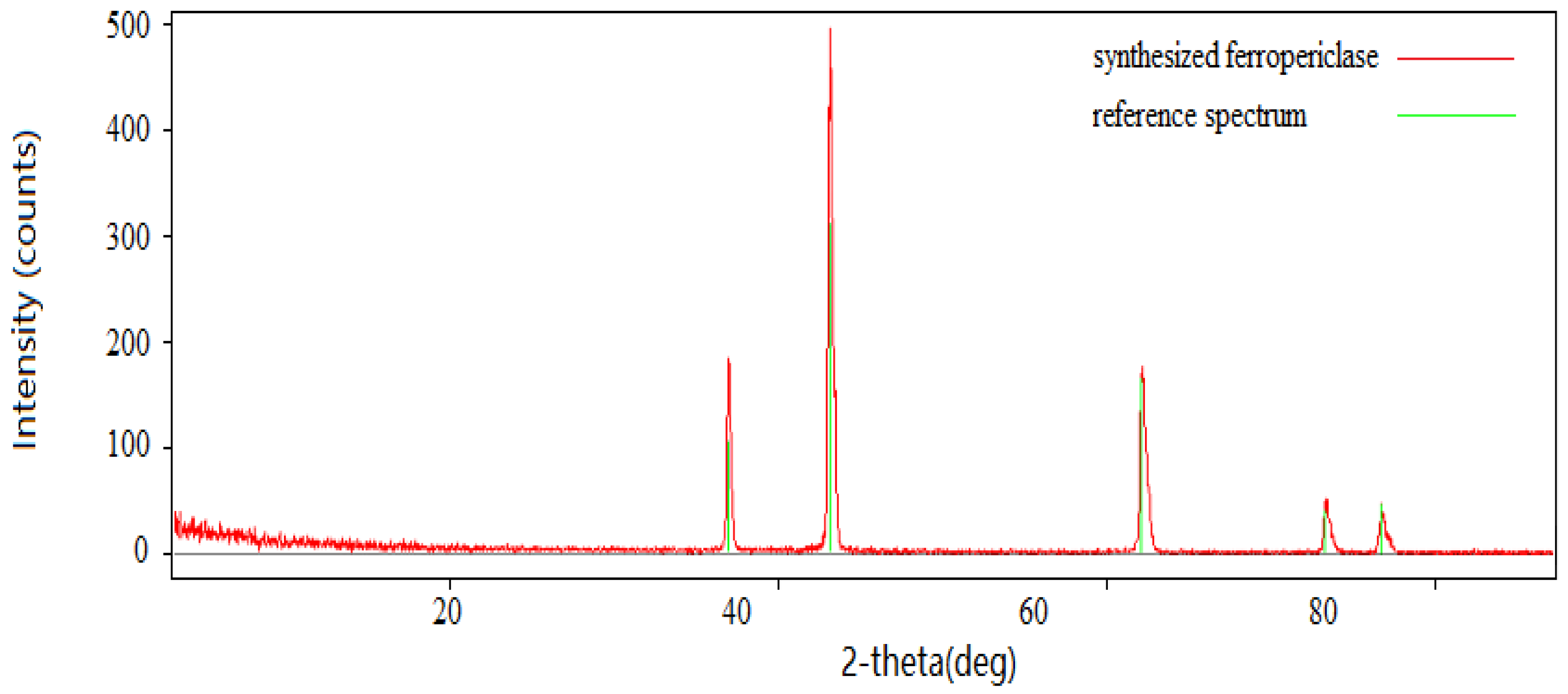
3.1. XRD Result
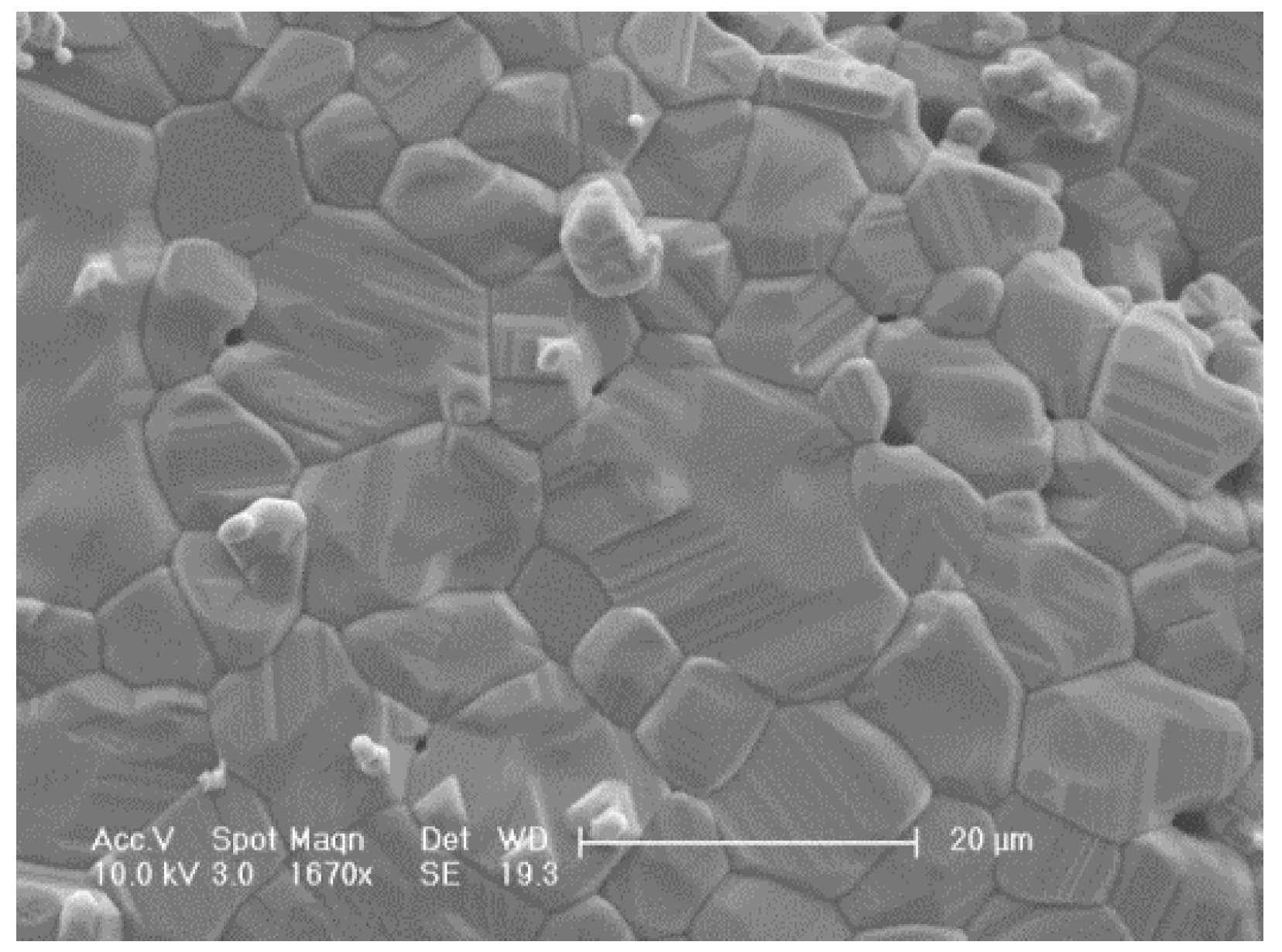
3.2. SEM Result
3.3. EDS Result
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manghnani, M.H. High Pressure Research in Mineral Physics; American Geophysical Union: Washington, DC, USA, 1987. [Google Scholar]
- Ito, E.; Takahashi, E. Ultrahigh-pressure phase transformations and the constitution of the deep mantle. In High-Pressure Research in Mineral Physics; American Geophysical Union: Washington, DC, USA, 1987; pp. 221–229. [Google Scholar]
- Irifune, T.; Ringwood, A.E. Phase transformations in primitive MORB and pyrolite compositions to 25 GPa and some geophysical implications. High Press. Res. Miner. Phys. 1987, 39, 235–246. [Google Scholar]
- Irifune, T. Absence of an aluminous phase in the upper part of the Earth’s lower mantle. Nature 1994, 370, 131. [Google Scholar] [CrossRef]
- Murakami, M.; Hirose, K.; Sata, N.; Ohishi, Y. Post-perovskite phase transition and mineral chemistry in the pyrolitic lowermost mantle. Geophys. Res. Lett. 2005, 32, L03304. [Google Scholar] [CrossRef]
- Dubrovinsky, L.S.; Dubrovinskaia, N.A.; Saxena, S.K.; Annersten, H.; Hålenius, E.; Harryson, H.; Tutti, F.; Rekhi, S.; Le Bihan, T. Stability of ferropericlase in the lower mantle. Science 2000, 289, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.; Li, B.; Liebermann, R.C. Elasticity of ferropericlase at high pressure and temperature: Implications in earth’s interior. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2004. [Google Scholar]
- Dubrovinsky, L.; Dubrovinskaia, N.; Kantor, I.; McCammon, C.; Crichton, W.; Urusov, V. Decomposition of ferropericlase (Mg0.80Fe0.20) O at high pressures and temperatures. J. Alloy. Compd. 2005, 390, 41–45. [Google Scholar] [CrossRef]
- Demouchy, S.; Mackwell, S.J.; Kohlstedt, D.L. Effect of hydrogen on Mg–Fe interdiffusion in ferro-periclase. In AGU Fall Meeting Abstracts; American Geophysical Union: Washington, DC, USA, 2005. [Google Scholar]
- Lin, J.F.; Jacobsen, S.D.; Sturhahn, W.; Jackson, J.M.; Zhao, J.; Yoo, C.S. Sound velocities of ferropericlase in the Earth’s lower mantle. Geophys. Res. Lett. 2006, 33, L22304. [Google Scholar] [CrossRef]
- Skorikov, N.A.; Shorikov, A.O.; Skornyakov, S.L.; Korotin, M.A.; Anisimov, V.I. Mechanism of magnetic moment collapse under pressure in ferropericlase. J. Phys. Condens. Matter 2015, 27, 275501. [Google Scholar] [CrossRef]
- Deng, J.; Lee, K.K.M. Viscosity jump in the lower mantle inferred from melting curves of ferropericlase. Nat. Commun. 2017, 8, 1997. [Google Scholar] [CrossRef]
- Davies, C.J.; Pozzo, M.; Gubbins, D.; Alfè, D. Partitioning of oxygen between ferropericlase and earth’s liquid core. Geophys. Res. Lett. 2018, 45, 6042–6050. [Google Scholar] [CrossRef]
- McCammon, C.; Peyronneau, J.; Poirier, J.P. Low ferric iron content of (Mg,Fe)O at high pressures and temperatures. Geophys. Res. Lett. 1998, 25, 1589–1592. [Google Scholar] [CrossRef]
- Kung, J.; Li, B.; Weidner, D.J.; Zhang, J.; Liebermann, R.C. Elasticity of (Mg0.83,Fe0.17)O ferropericlase at high pressure: Ultrasonic measurements in conjunction with X-radiation techniques. Earth Planet. Sci. Lett. 2002, 203, 557–566. [Google Scholar] [CrossRef]
- Holzapfel, C.; Rubie, D.C.; Mackwell, S.; Frost, D.J. Effect of pressure on Fe-Mg interdiffusion in (FexMg1−x)O, ferropericlase. Phys. Earth Planet. Inter. 2003, 139, 21–34. [Google Scholar] [CrossRef]
- Lin, J.F.; Struzhkin, V.V.; Jacobsen, S.D.; Hu, M.Y.; Chow, P.; Kung, J.; Liu, H.; Mao, H.; Hemley, R.J. Spin transition of iron in magnesiowüstite in the Earth’s lower mantle. Nature 2005, 436, 377. [Google Scholar] [CrossRef] [PubMed]
- Keppler, H.; Kantor, I.; Dubrovinsky, L.S. Optical absorption spectra of ferropericlase to 84 GPa. Am. Mineral. 2007, 92, 433–436. [Google Scholar] [CrossRef]
- Heidelbach, F.; Terry, M.P.; Bystricky, M.; Holzapfel, C.; McCammon, C. A simultaneous deformation and diffusion experiment: Quantifying the role of deformation in enhancing metamorphic reactions. Earth Planet. Sci. Lett. 2009, 278, 386–394. [Google Scholar] [CrossRef]
- Narygina, O.; Mattesini, M.; Kantor, I.; Pascarelli, S.; Wu, X.; Aquilanti, G.; McCammon, C.; Dubrovinsky, L. High-pressure experimental and computational XANES studies of (Mg,Fe)(Si,Al)O3 perovskite and (Mg,Fe)O ferropericlase as in the Earth’s lower mantle. Phys. Rev. B 2009, 79, 174115. [Google Scholar] [CrossRef]
- Otsuka, K.; Karato, S. The influence of ferric iron and hydrogen on Fe–Mg interdiffusion in ferropericlase ((Mg,Fe)O) in the lower mantle. Phys. Chem. Miner. 2015, 42, 261–273. [Google Scholar] [CrossRef]
- Ohta, K.; Yagi, T.; Hirose, K.; Ohishi, Y. Thermal conductivity of ferropericlase in the Earth’s lower mantle. Earth Planet. Sci. Lett. 2017, 465, 29–37. [Google Scholar] [CrossRef]
- Fu, S.; Yang, J.; Zhang, Y.; Liu, J.; Greenberg, E.; Prakapenka, V.B.; Okuchi, T.; Lin, J.-F. Melting behavior of the lower-mantle ferropericlase across the spin crossover: Implication for the ultra-low velocity zones at the lowermost mantle. Earth Planet. Sci. Lett. 2018, 503, 1–9. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloy. Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Sinmyo, R.; Hirose, K.; Nishio-Hamane, D.; Seto, Y.; Fujino, K.; Sata, N.; Ohishi, Y. Partitioning of iron between perovskite/postperovskite and ferropericlase in the lower mantle. J. Geophys. Res. Solid Earth 2008, 113, B11204. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Chen, H.I.; Chang, H.Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, P.; Lu, C.H. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J. 2008, 144, 509–513. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Niihara, K. Synthesis of nanoscaled yttrium aluminum garnet powder by the co-precipitation method. Mater. Sci. Eng. A 2000, 288, 1–4. [Google Scholar] [CrossRef]
- Jal, P.K.; Sudarshan, M.; Saha, A.; Patel, S.; Mishra, B.K. Synthesis and characterization of nanosilica prepared by precipitation method. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 173–178. [Google Scholar] [CrossRef]
- Parida, K.M.; Pradhan, A.C.; Das, J.; Sahu, N. Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method. Mater. Chem. Phys. 2009, 113, 244–248. [Google Scholar] [CrossRef]
- Supothina, S.; Seeharaj, P.; Yoriya, S.; Sriyudthsak, M. Synthesis of tungsten oxide nanoparticles by acid precipitation method. Ceram. Int. 2007, 33, 931–936. [Google Scholar] [CrossRef]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Ataie, A.; Heshmati-Manesh, S. Synthesis of ultra-fine particles of strontium hexaferrite by a modified co-precipitation method. J. Eur. Ceram. Soc. 2001, 21, 1951–1955. [Google Scholar] [CrossRef]
- Valenzuela, R.; Fuentes, M.C.; Parra, C.; Baeza, J.; Duran, N.; Sharma, S.K.; Knobel, M.; Freer, J. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. J. Alloy. Compd. 2009, 488, 227–231. [Google Scholar] [CrossRef]
- Shami, M.Y.; Awan, M.S.; Anis-ur-Rehman, M. Phase pure synthesis of BiFeO3 nanopowders using diverse precursor via co-precipitation method. J. Alloy. Compd. 2011, 509, 10139–10144. [Google Scholar] [CrossRef]
- Nejati-Moghadam, L.; Esmaeili Bafghi-Karimabad, A.; Salavati-Niasari, M.; Safardoust, H. Synthesis and characterization of SnO2 nanostructures prepared by a facile precipitation method. J. Nanostruct. 2015, 5, 47–53. [Google Scholar]
- Spiridigliozzi, L.; Dell’Agli, G.; Biesuz, M.; Sglavo, V.M.; Pansini, M. Effect of the precipitating agent on the synthesis and sintering behavior of 20 mol Sm-doped ceria. Adv. Mater. Sci. Eng. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, R.; Yu, Z.; Shan, L.; Dong, L.; Zhang, X. The magnetic properties of permanent strontium ferrite doped with rare-earth by chemical co-precipitation method. Ferroelectrics 2018, 529, 120–127. [Google Scholar] [CrossRef]
- Rezaee, O.; Chenari, H.M.; Ghodsi, F.E. Precipitation synthesis of tungsten oxide nanoparticles: X-ray line broadening analysis and photocatalytic efficiency study. J. Sol-Gel Sci. Technol. 2016, 80, 109–118. [Google Scholar] [CrossRef]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Critical thermodynamic evaluation and optimization of the Fe-Mg-O system. J. Phys. Chem. Solids 2004, 65, 1683–1695. [Google Scholar] [CrossRef]


| Title | MgCl2·6H2O | FeSO4 | H2C2O4 | Na2CO3 |
|---|---|---|---|---|
| The coefficient | 0.8 | 0.2 | 1 | 1 |
| n (mol) | 0.016 | 0.004 | 0.022 | 0.022 |
| M (g/mol) | 203.3 | 151.9 | 126 | 106 |
| m (g) | 3.2528 | 0.6076 | 2.772 | 2.332 |
| Formula | Mass% | Mol% | Cation | Sigma | Net | K Ratio | Line |
|---|---|---|---|---|---|---|---|
| O | K | ||||||
| Na | Nd | Nd | K | ||||
| MgO | 69.84 | 80.60 | 19.22 | 0.35 | 592,191 | 2.4425994 | K |
| Al | Nd | Nd | K | ||||
| SiO2 | 0.13 | 0.10 | 0.02 | 0.13 | 718 | 0.0036947 | K |
| P | Nd | Nd | K | ||||
| S | Nd | Nd | K | ||||
| Cl | 0.07 | 0.10 | 0.00 | 0.05 | 611 | 0.0049666 | K |
| K | Nd | Nd | K | ||||
| CaO | 0.25 | 0.21 | 0.05 | 0.12 | 950 | 0.0138662 | K |
| TiO2 | 0.46 | 0.27 | 0.06 | 0.18 | 846 | 0.0183841 | K |
| Cr2O3 | 0.60 | 0.18 | 0.09 | 0.22 | 769 | 0.0278279 | K |
| Mn | Nd | Nd | K | ||||
| FeO | 28.65 | 18.55 | 4.42 | 0.71 | 18,907 | 1.4436706 | K |
| Ni | Nd | Nd | K | ||||
| Total | 100.00 | 100.00 | 23.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Sun, T.; Zhao, Y.-H. Experimental Study on Preparation of Ferropericlase by Oxalate Coprecipitation. Minerals 2020, 10, 179. https://doi.org/10.3390/min10020179
Xiao Y, Sun T, Zhao Y-H. Experimental Study on Preparation of Ferropericlase by Oxalate Coprecipitation. Minerals. 2020; 10(2):179. https://doi.org/10.3390/min10020179
Chicago/Turabian StyleXiao, Yanjun, Tong Sun, and Yong-Hong Zhao. 2020. "Experimental Study on Preparation of Ferropericlase by Oxalate Coprecipitation" Minerals 10, no. 2: 179. https://doi.org/10.3390/min10020179
APA StyleXiao, Y., Sun, T., & Zhao, Y.-H. (2020). Experimental Study on Preparation of Ferropericlase by Oxalate Coprecipitation. Minerals, 10(2), 179. https://doi.org/10.3390/min10020179




