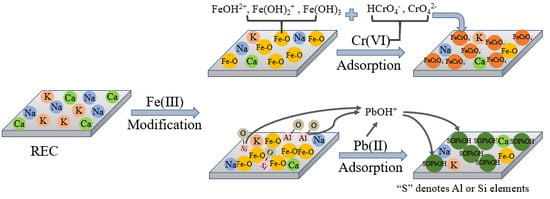
Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Methods
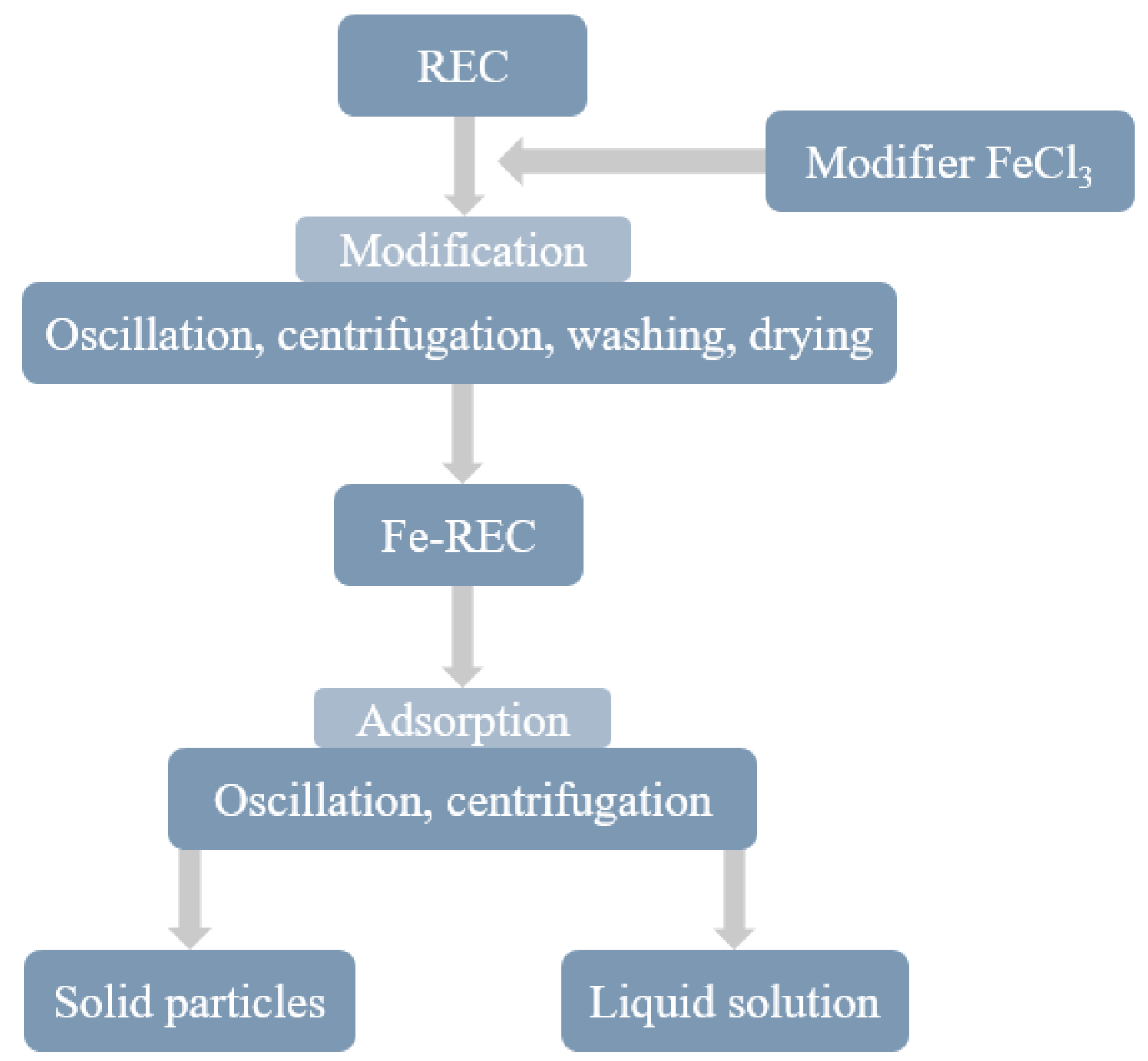
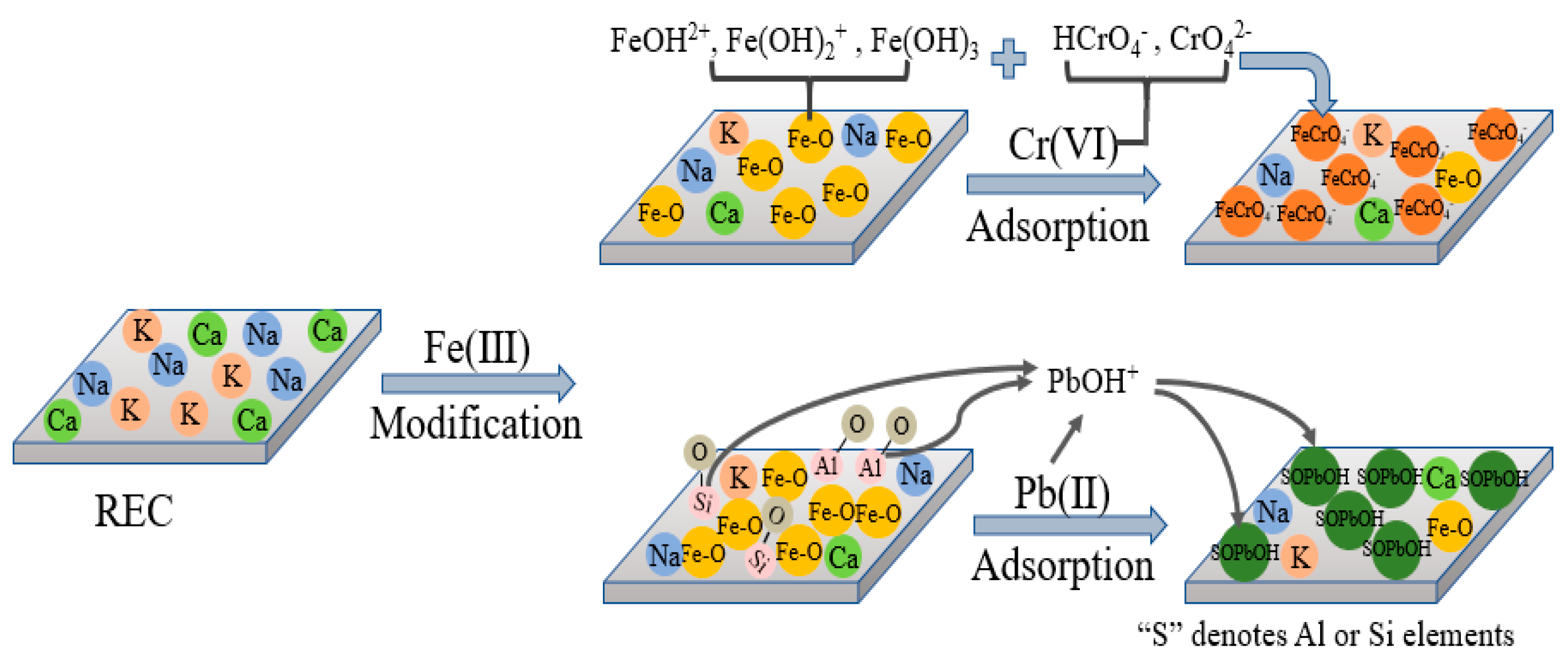
2.2.1. Rectorite Modification
2.2.2. Heavy Metal Ions Adsorption Test
2.2.3. Zeta Potential Test
2.2.4. XPS Analysis
2.2.5. Description of Solution Chemistry Calculations
3. Results and Discussion
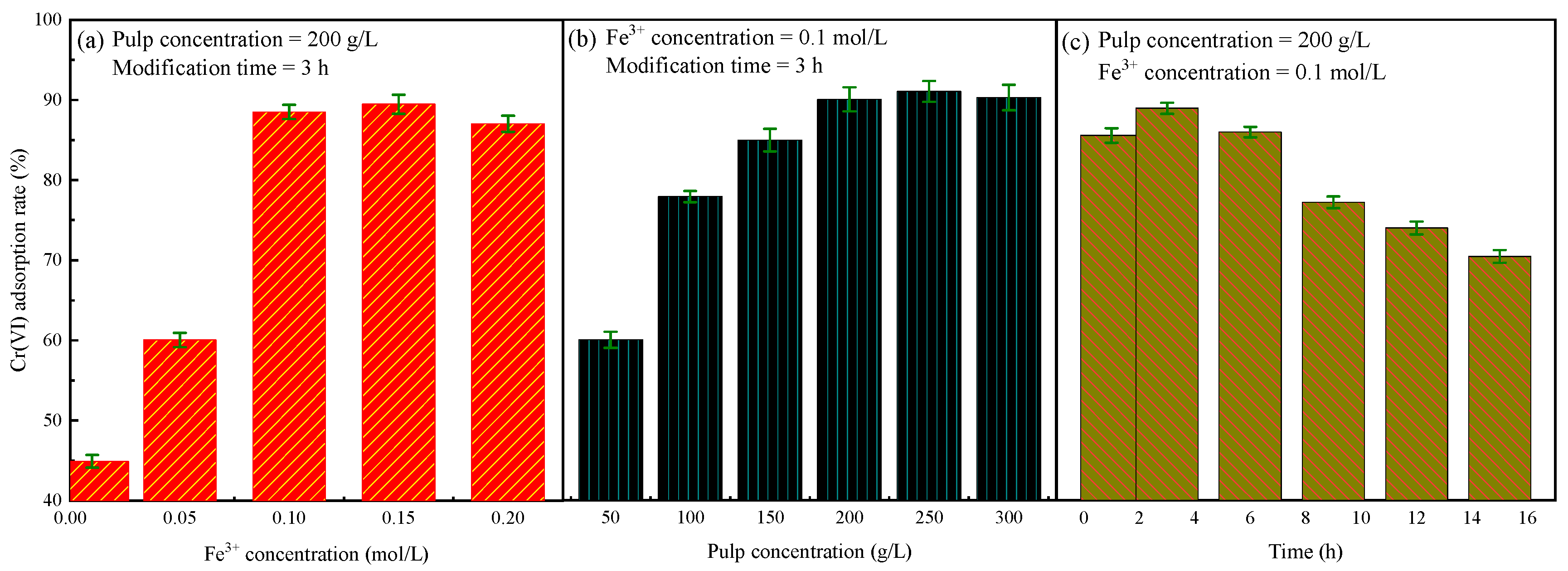
3.1. Modification of Rectorite
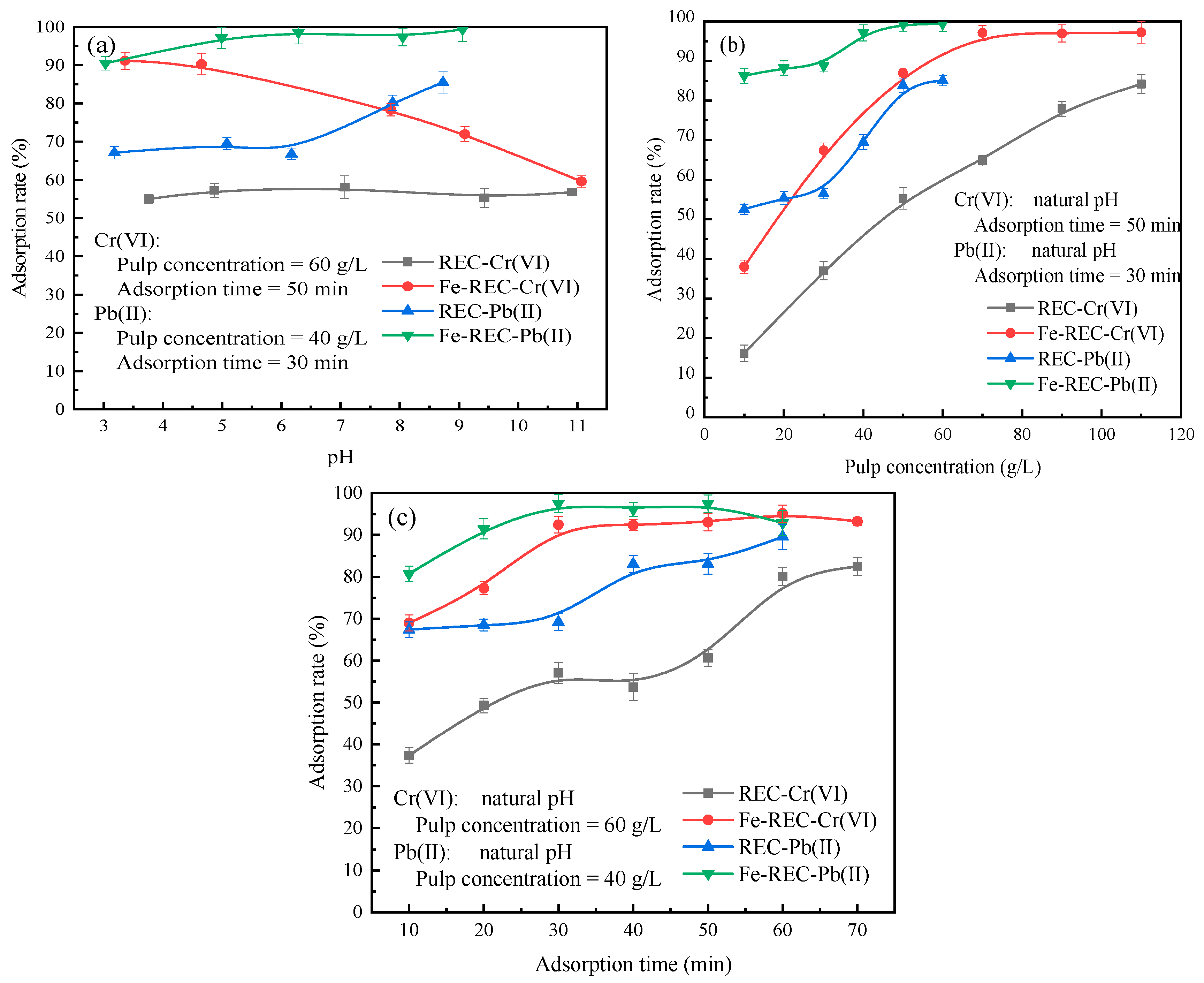
3.2. Adsorption Studies
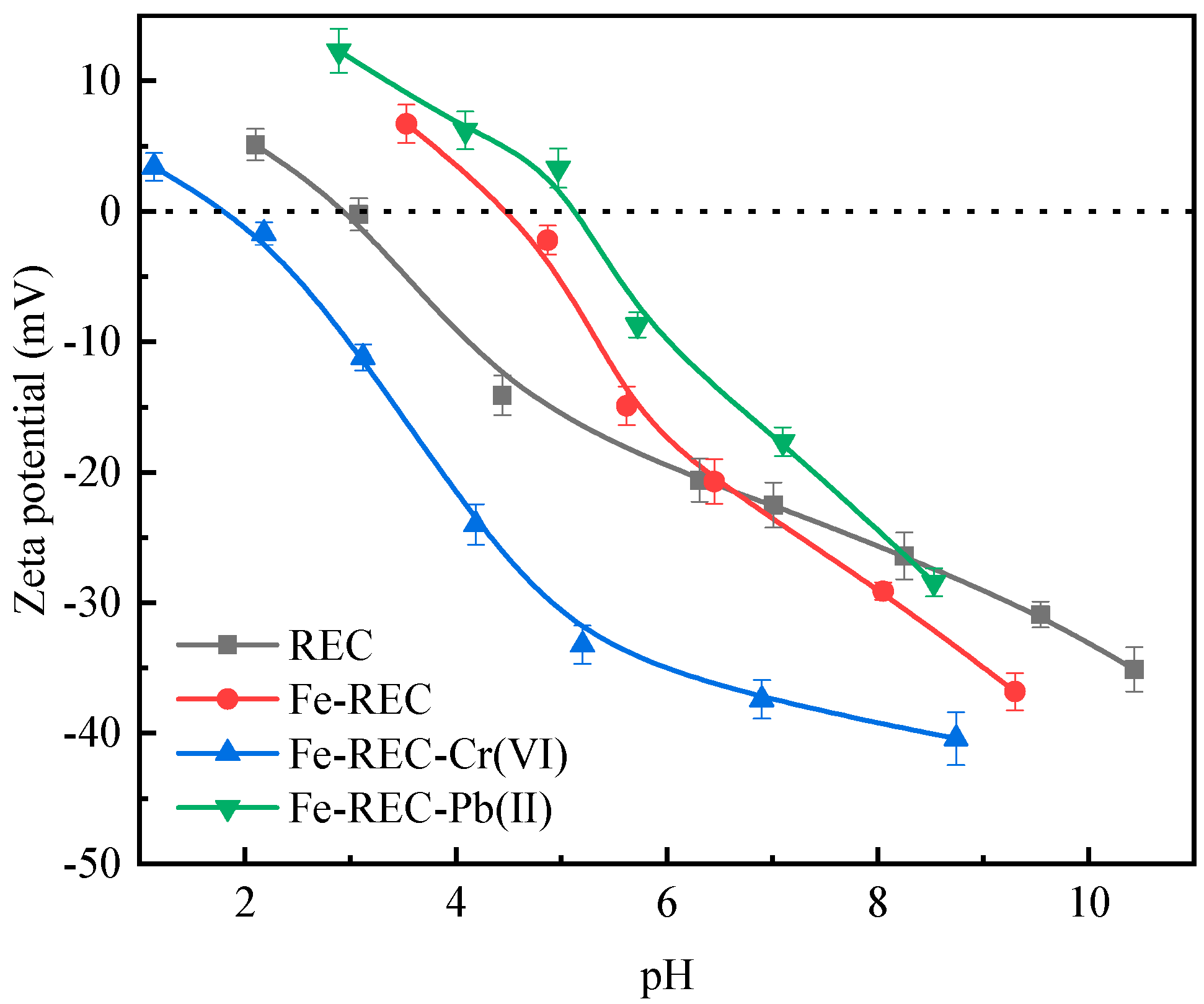
3.3. Zeta Potential Analysis
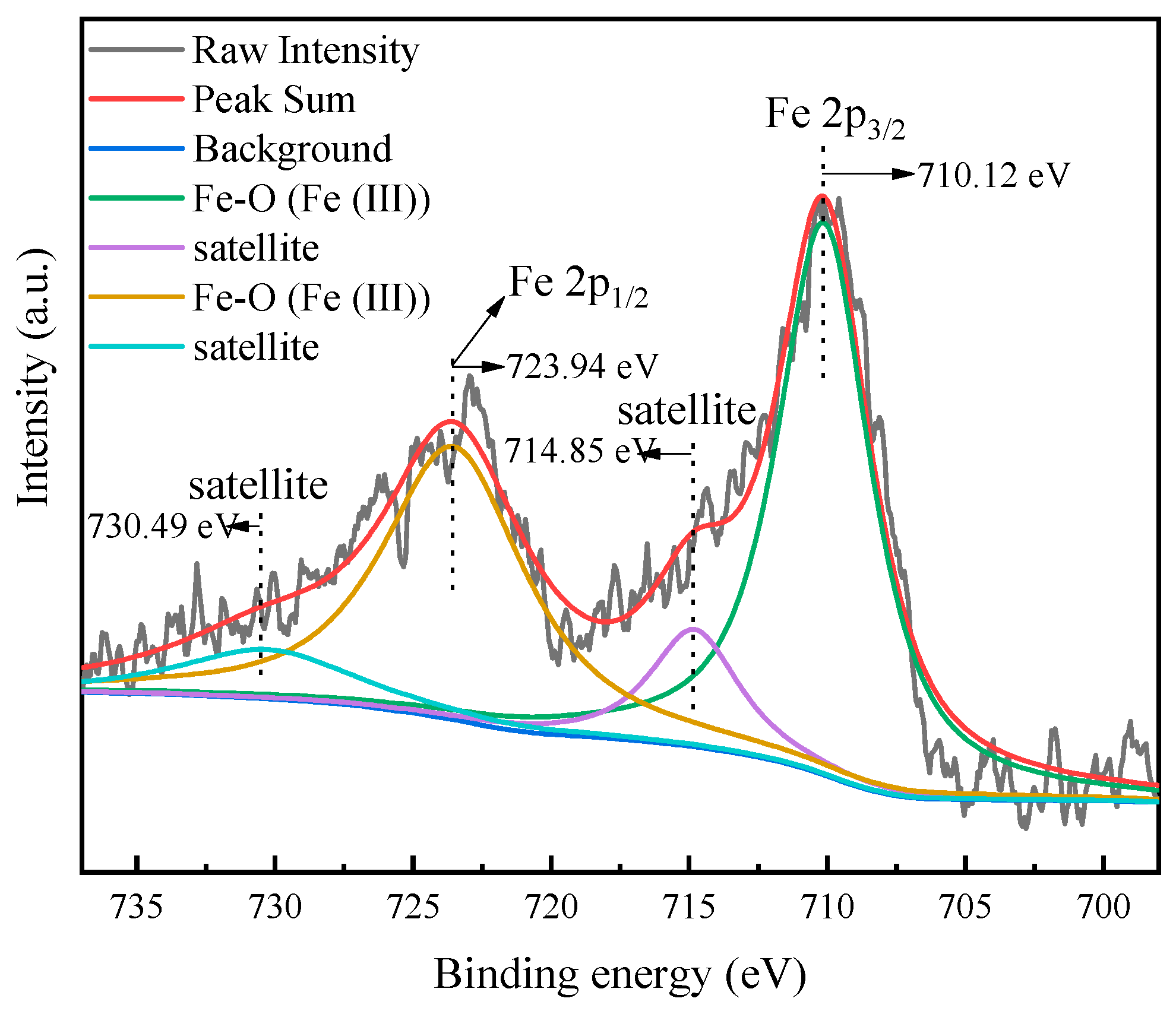
3.4. XPS Analysis
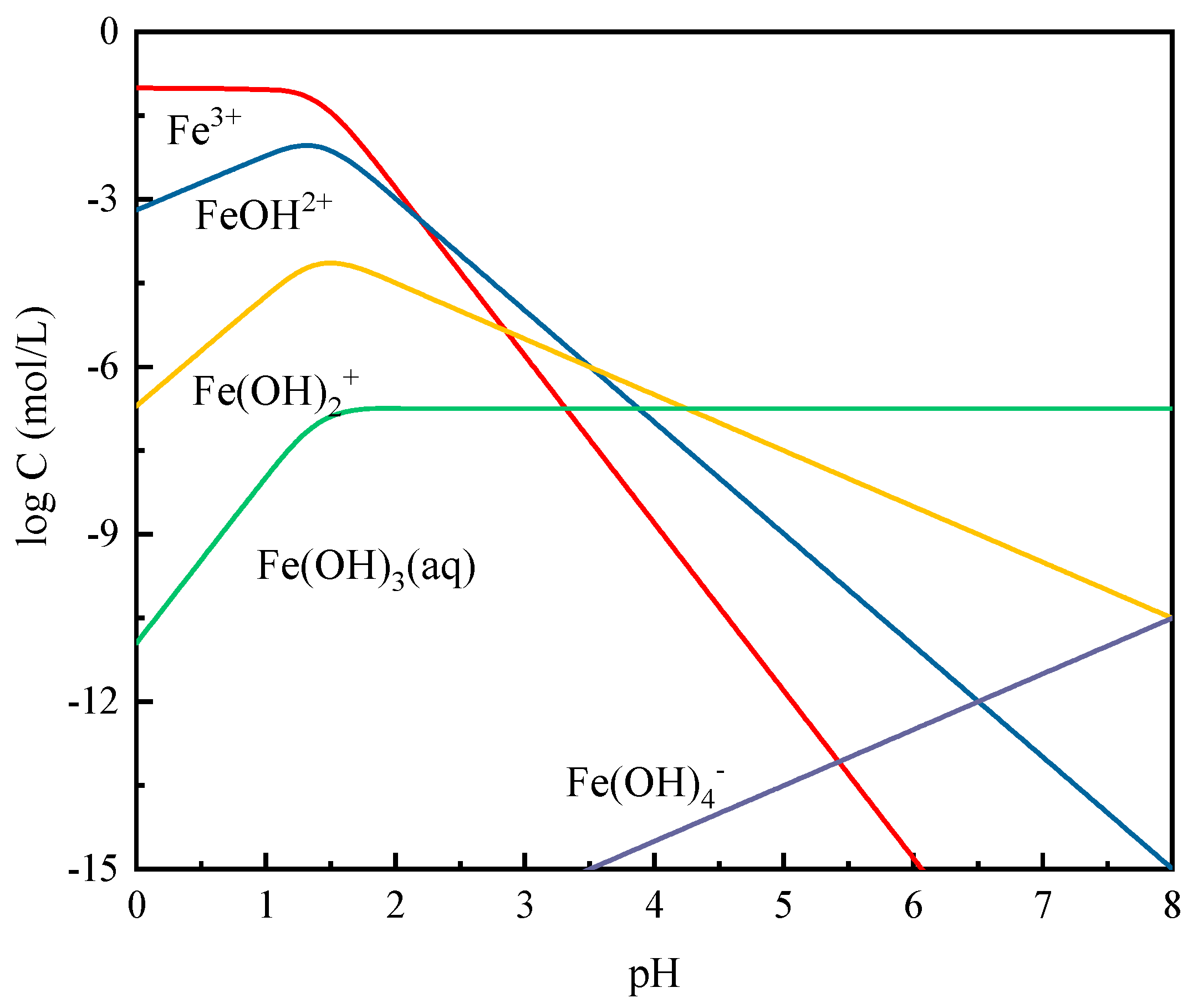
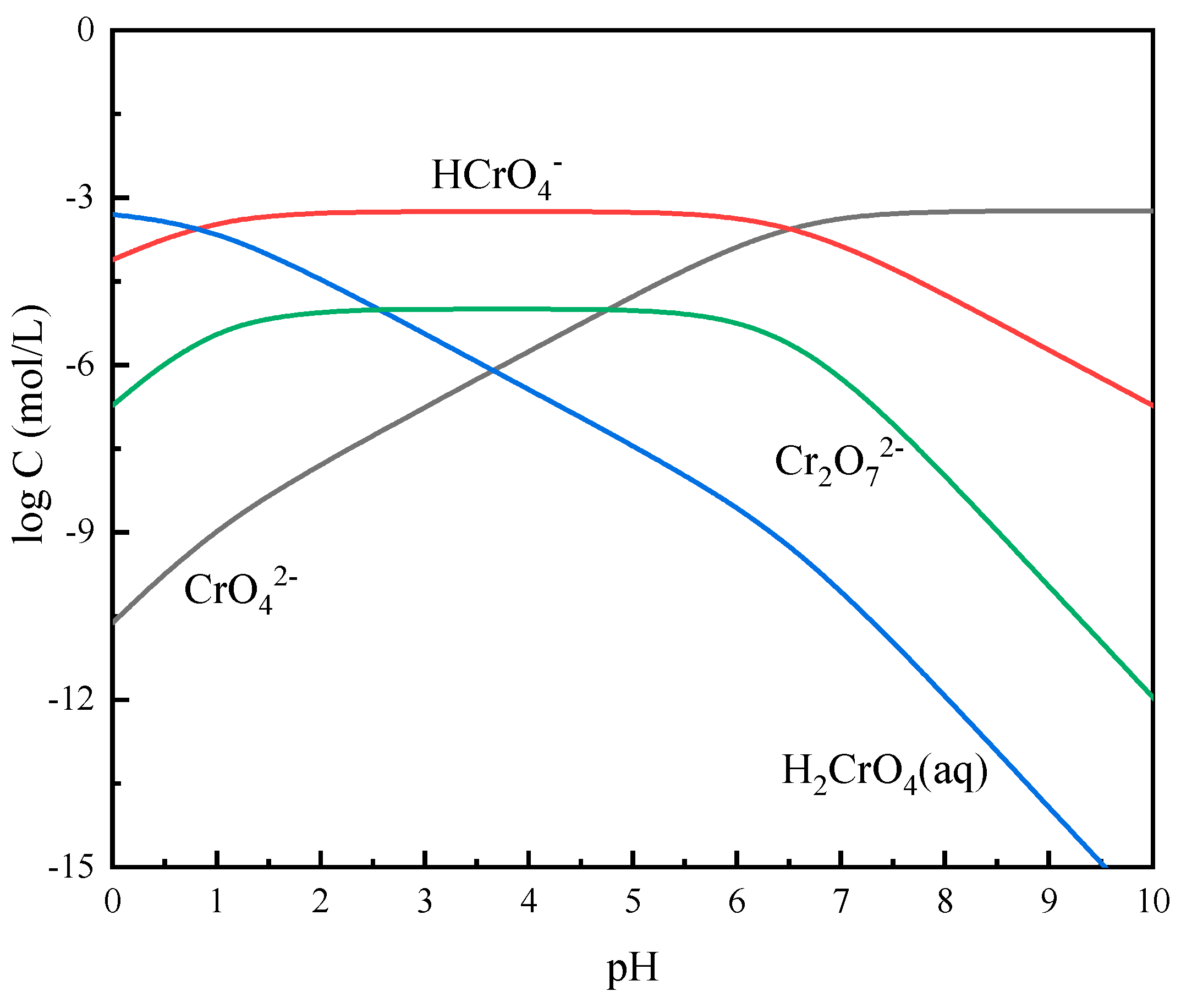
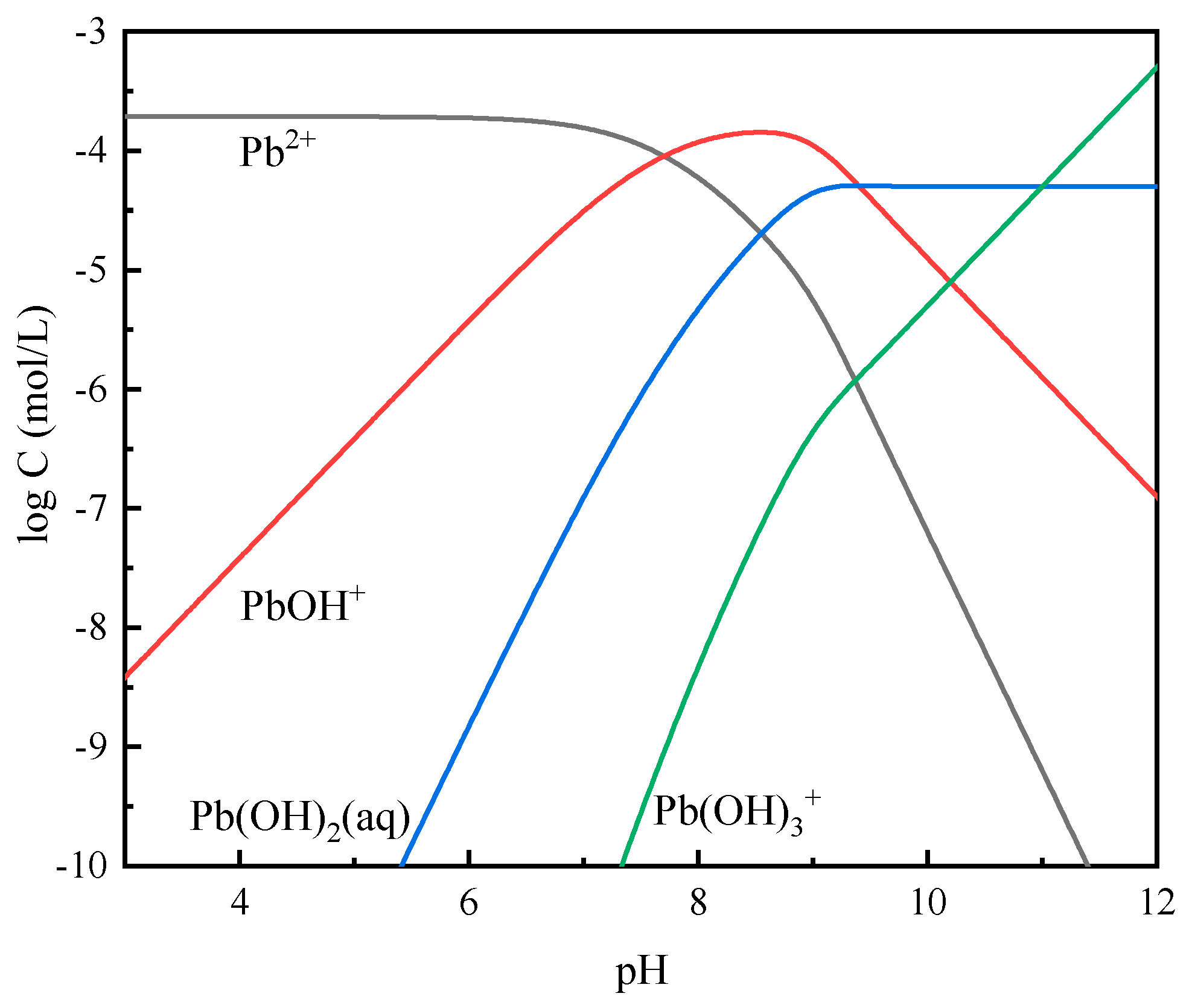
3.5. Solution Chemistry Calculations
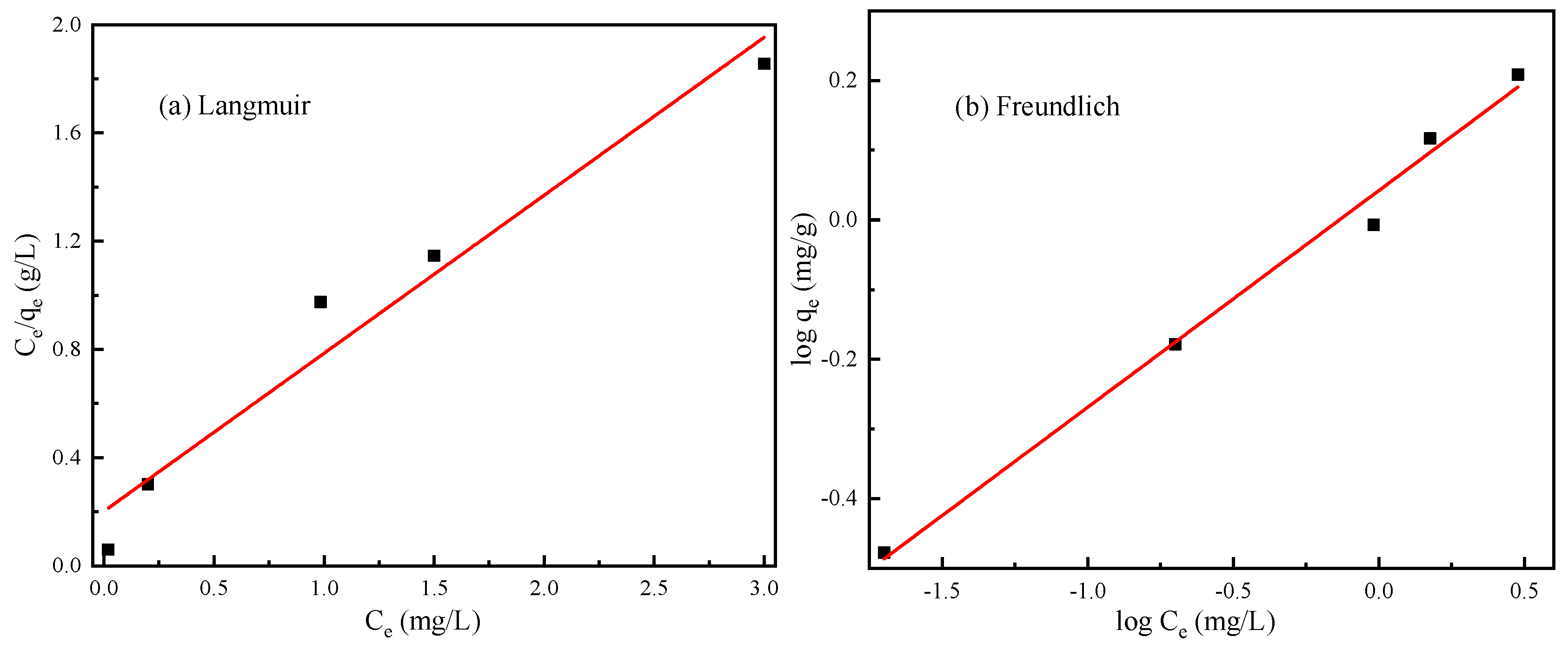
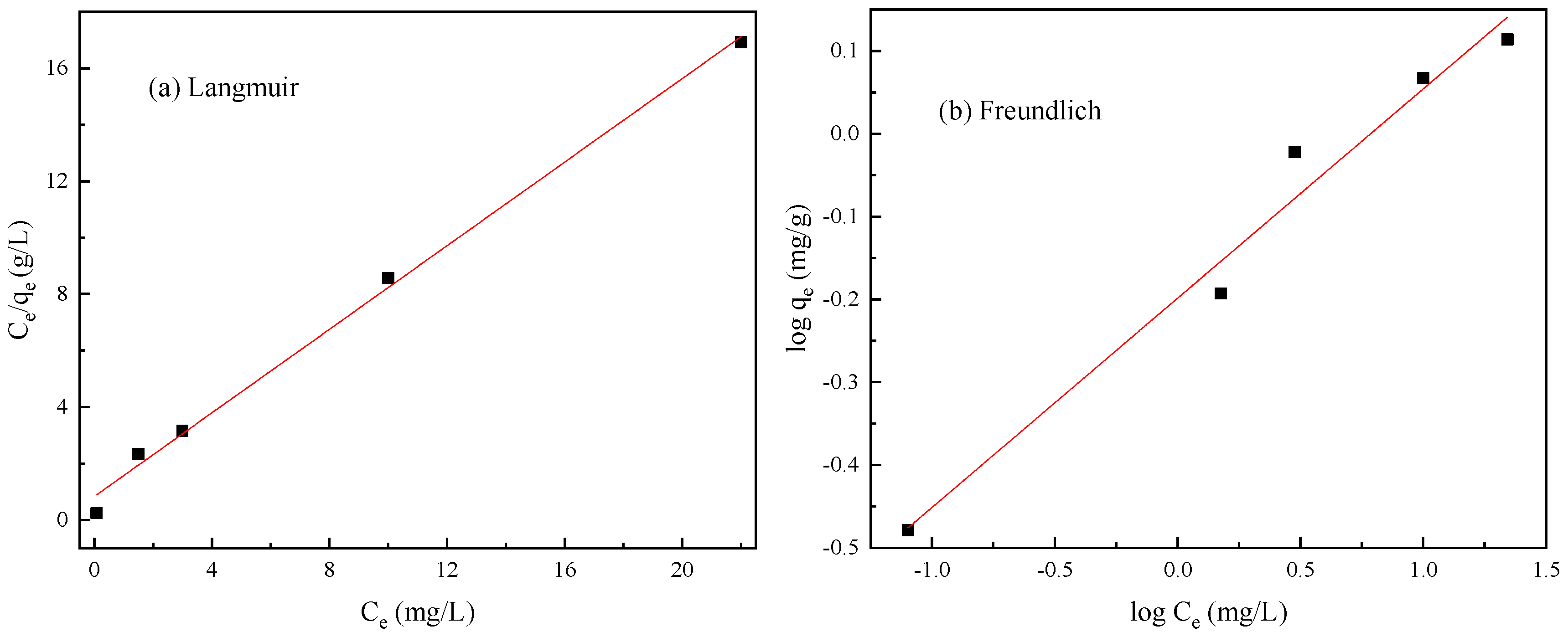
3.6. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Z.; Li, Y.; Guo, M.; Xu, F.; Wang, P.; Du, Y.; Na, P. One-pot synthesis of Mn-doped TiO2 grown on graphene and the mechanism for removal of Cr(VI) and Cr(III). J. Hazard. Mater. 2016, 310, 188–198. [Google Scholar] [CrossRef]
- Song, W.; Shi, T.; Yang, D.; Ye, J.; Zhou, Y.; Feng, Y. Pretreatment effects on the sorption of Cr(VI) onto surfactant-modified zeolite: Mechanism analysis. J. Environ. Manag. 2015, 162, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.; Choi, U.S.; Yun, S.T.; Choi, J.W.; Park, J.A.; Lee, S.H. Evaluation of amine-functionalized acrylic ion exchange fiber for chromium (VI) removal using flow-through experiments modeling and real wastewater. J. Ind. Eng. Chem. 2018, 66, 187–195. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and chromium from aqueous solutions using red mud—An aluminium industry waste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamala, C.T.; Chary, N.S.; Sastry, A.R.K.; Rao, T.N.; Vairamani, M. Removal of lead from aqueous solutions using an immobilized biomaterial derived from a plant biomass. J. Hazard. Mater. 2004, 108, 111–117. [Google Scholar]
- Taka, A.L.; Fosso-Kankeu, E.; Pillay, K.; Mbianda, X.Y. Removal of cobalt and lead ions from wastewater samples using an insoluble nanosponge biopolymer composite: Adsorption isotherm, kinetic, thermodynamic, and regeneration studies. Environ. Sci. Pollut. Res. 2018, 25, 21752–21767. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Simultaneous removal of Pb(II) and Cr(III) by magnetite nanoparticles using various synthesis conditions. J. Ind. Eng. Chem. 2014, 20, 3543–3549. [Google Scholar] [CrossRef]
- Eskandari, E.; Kosari, M.; Davood Abadi Farahani, M.H.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Izidoro, J.D.C.; Fungaro, D.A.; Abbott, J.E.; Wang, S. Synthesis of zeolites X and A from fly ashes for cadmium and zinc removal from aqueous solutions in single and binary ion systems. Fuel 2013, 103, 827–834. [Google Scholar] [CrossRef]
- Guan, B.; Ni, W.; Wu, Z.; Lai, Y. Removal of Mn(II) and Zn(II) ions from flue gas desulfurization wastewater with water-soluble chitosan. Sep. Purif. Technol. 2009, 65, 269–274. [Google Scholar] [CrossRef]
- Shin, K.Y.; Hong, J.Y.; Jang, J. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater. 2011, 190, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, X.; Liang, G.; Yan, Y.; Wang, S. Adsorption behavior of Cr(VI) on organic-modified rectorite. Chem. Eng. J. 2008, 138, 187–193. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Dong, Y.; Zhang, Z. Impact of environmental conditions on the sorption behavior of Co(II) on Na-rectorite studied by batch experiments. Chem. Eng. J. 2011, 289, 851–859. [Google Scholar] [CrossRef]
- Huang, J.; Ming, Y.A.; Du, Y.; Wang, Y.; Wang, C.E. Study on the effect of the three-dimensional electrode in degradation of methylene blue by lithium modified rectorite. J. Anal. Methods Chem. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Mei, H.; Yu, S.; Tan, X.; Wang, S.; Chen, C.; Li, J. Evaluation of the influence of environmental conditions on the removal of Pb(II) from wastewater by ca-rectorite. Sep. Sci. Technol. 2015, 50, 2257–2266. [Google Scholar] [CrossRef]
- Tu, H.; Huang, M.; Yi, Y.; Li, Z.; Zhan, Y.; Chen, J.; Wu, Y.; Shi, X.; Deng, H.; Du, Y. Chitosan-rectorite nanospheres immobilized on polystyrene fibrous mats via alternate electrospinning /electrospraying techniques for copper ions adsorption. Appl. Surf. Sci. 2017, 426, 545–553. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Y.; Zhang, Q.; Guo, X.; Peng, Y.; Xiao, H.; Chen, X.; Luo, J. Adsorption of Cd(II), Cu(II) and Ni(II) ions by cross-linking chitosan/rectorite nano-hybrid composite microspheres. Carbohydr. Polym. 2015, 130, 333–343. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, L.; Zhang, Q.; Yuan, K.; Xiao, H.; Peng, Y.; Chen, X.; Luo, J. Synthesis and adsorption behavior of magnetic microspheres based on chitosan/organic rectorite for low-concentration heavy metal removal. J. Alloy. Compd. 2015, 647, 892–905. [Google Scholar] [CrossRef]
- Hong, H.; Jiang, W.T.; Zhang, X.; Tie, L.; Li, Z. Adsorption of Cr(VI) on STAC-modified rectorite. Appl. Clay Sci. 2008, 42, 292–299. [Google Scholar] [CrossRef]
- Ying, Z.; Shao, Z.; Chen, C.; Hu, J.; Chen, H. Effect of environmental conditions on the adsorption behavior of Sr(II) by Na-rectorite. Appl. Clay Sci. 2014, 87, 1–6. [Google Scholar]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and reduction of chromium(VI) from aqueous solution using polypyrrole/calcium rectorite composite adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Tu, H.; Yu, Y.; Chen, J.; Shi, X.; Zhou, J.; Deng, H.; Du, Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: Chitosan and cellulose. Polym. Chem. 2017, 8, 2913–2921. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y. Flotation Solution Chemistry; Hunan Science and Technology Press: Changsha, China, 1988; pp. 132–138. [Google Scholar]
- Wang, X.; Bo, L.; Ren, J.; Liu, C.; Wang, X.; Wu, J.; Sun, R. Preparation and characterization of new quaternized carboxymethyl chitosan/rectorite nanocomposite. Compos. Sci. Technol. 2010, 70, 1161–1167. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Sun, W.; Zeng, X.; Fang, S.; Han, H.; Hong, K.; Hu, Y. Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. Selective depressive effect of sodium fluorosilicate on calcite during scheelite flotation. Miner. Eng. 2019, 131, 262–271. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Pan, C.; Han, H.; Li, W.; Zhang, C.; Wei, S.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]
- Peng, J.; Moszner, F.; Rechmann, J.; Vogel, D.; Palm, M.; Rohwerder, M. Influence of Al content and pre-oxidation on the aqueous corrosion resistance of binary Fe-Al alloys in sulphuric acid. Corros. Sci. 2019, 149, 123–132. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Use of citric acid and Fe(III) mixture as depressant in calcite flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123579. [Google Scholar] [CrossRef]
- Weerasooriya, R.; Tobschall, H.J. Mechanistic modeling of chromate adsorption onto goethite. Colloids Surf. A Physicochem. Eng. Asp. 2000, 162, 167–175. [Google Scholar] [CrossRef]
- Senanayake, G. A review of effects of silver, lead, sulfide and carbonaceous matter on gold cyanidation and mechanistic interpretation. Hydrometallurgy 2008, 90, 46–73. [Google Scholar] [CrossRef]
- Deng, Y.; Stjernström, M.; Banwart, S. Accumulation and remobilization of aqueous chromium(VI) at iron oxide surfaces: Application of a thin-film continuous flow-through reactor. J. Contam. Hydrol. 1996, 21, 141–151. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. Modelling the sorption of Mn(II), Co(II), Ni(II), Zn(II), Cd(II), Eu(III), Am(III), Sn(IV), Th(IV), Np(V) and U(VI) on montmorillonite: Linear free energy relationships and estimates of surface binding constants for some selected heavy metals and actinides. Geochim. Cosmochim. Acta 2005, 69, 875–892. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef]
- Laatikainen, M.; Lindström, M. Determination of adsorption isotherms with quartz crystal microbalance in liquid phase. J. Colloid Interface Sci. 1988, 125, 610–614. [Google Scholar] [CrossRef]
- Demirbas, A.; Sari, A.; Isildak, O. Adsorption thermodynamics of stearic acid onto bentonite. J. Hazard. Mater. 2006, 135, 226–231. [Google Scholar] [CrossRef]
- Tahir, S.S.; Naseem, R. Removal of Cr(III) from tannery wastewater by adsorption onto bentonite clay. Sep. Purif. Technol. 2007, 53, 312–321. [Google Scholar] [CrossRef]
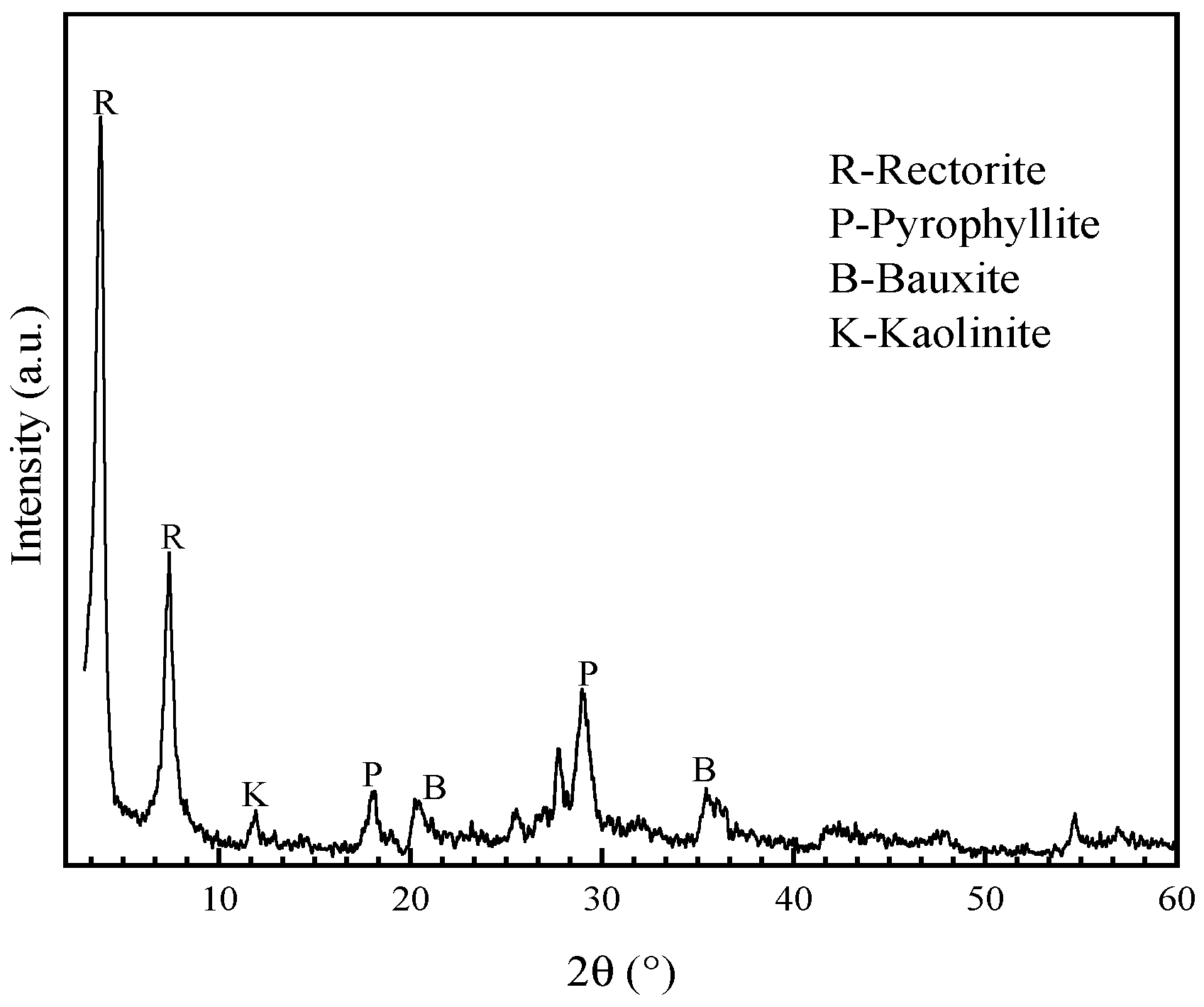

| Sample | Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|
| Rectorite | 36.53 | 47.41 | 0.4 | 4.34 | 5.44 | 0.31 | 0.71 | 1.23 | 3.94 |
| Samples | Element (atomic %) | ||||||
|---|---|---|---|---|---|---|---|
| C 1s | O 1s | Al 2p | Si 2p | Fe 2p | Cr 2p | Pb 4f | |
| REC | 9.15 | 61.89 | 14.22 | 14.75 | - | - | - |
| Fe-REC | 11.16 | 61.75 | 12.45 | 13.26 | 1.38 | - | - |
| Fe-REC-Cr(VI) | 9.48 | 60.66 | 13.23 | 14.43 | 1.26 | 0.94 | - |
| Fe-REC-Pb(II) | 11.35 | 61.03 | 12.48 | 13.96 | 1.15 | - | 1.09 |
| Samples | Binding Energy (eV) | ||||
|---|---|---|---|---|---|
| C 1s | O 1s | Al 2p | Si 2p | Fe 2p | |
| REC | 284.76 | 531.52 | 74.8 | 102.99 | - |
| Fe-REC | 284.83 | 531.89 | 74.8 | 102.92 | 711.82 |
| Shift values | 0.07 | 0.37 | 0 | −0.08 | - |
| Fe-REC-Cr(VI) | 284.82 | 531.02 | 74.82 | 103.07 | 710.59 |
| Shift values | −0.01 | −0.87 | 0.02 | 0.15 | −1.23 |
| Fe-REC-Pb(II) | 284.81 | 530.74 | 75.61 | 103.25 | 710.04 |
| Shift values | −0.02 | −1.15 | 0.81 | 0.33 | −1.78 |
| Ions | Reactions | Reaction Constants |
|---|---|---|
| Fe(III) | (14) | |
| (15) | ||
| (16) | ||
| (17) | ||
| Cr(VI) | (18) | |
| (19) | ||
| (20) | ||
| Pb(II) | (21) | |
| (22) | ||
| (23) |
| Metals Ions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/g) | R2 | KF (mg/g) | n(g/L) | R2 | |
| Cr(VI) | 1.7128 | 2.8927 | 0.9618 | 1.1019 | 3.2146 | 0.9908 |
| Pb(II) | 1.3509 | 0.8914 | 0.9959 | 0.6330 | 3.9568 | 0.9763 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Jiang, H.; Li, X.; Khoso, S.A.; Xiang, G.; Han, W. Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater. Minerals 2020, 10, 176. https://doi.org/10.3390/min10020176
Gao Y, Jiang H, Li X, Khoso SA, Xiang G, Han W. Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater. Minerals. 2020; 10(2):176. https://doi.org/10.3390/min10020176
Chicago/Turabian StyleGao, Ya, Hao Jiang, Xianyuan Li, Sultan Ahmed Khoso, Guoyuan Xiang, and Wenping Han. 2020. "Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater" Minerals 10, no. 2: 176. https://doi.org/10.3390/min10020176
APA StyleGao, Y., Jiang, H., Li, X., Khoso, S. A., Xiang, G., & Han, W. (2020). Different Insights into Silicate Rectorite Modification and Its Role in Removal of Heavy Metal Ions from Wastewater. Minerals, 10(2), 176. https://doi.org/10.3390/min10020176