Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. FTIR Spectroscopy
2.2.1. Experiment
2.2.2. Spectra Decomposition and Curve-Fitting
3. Results and Discussion
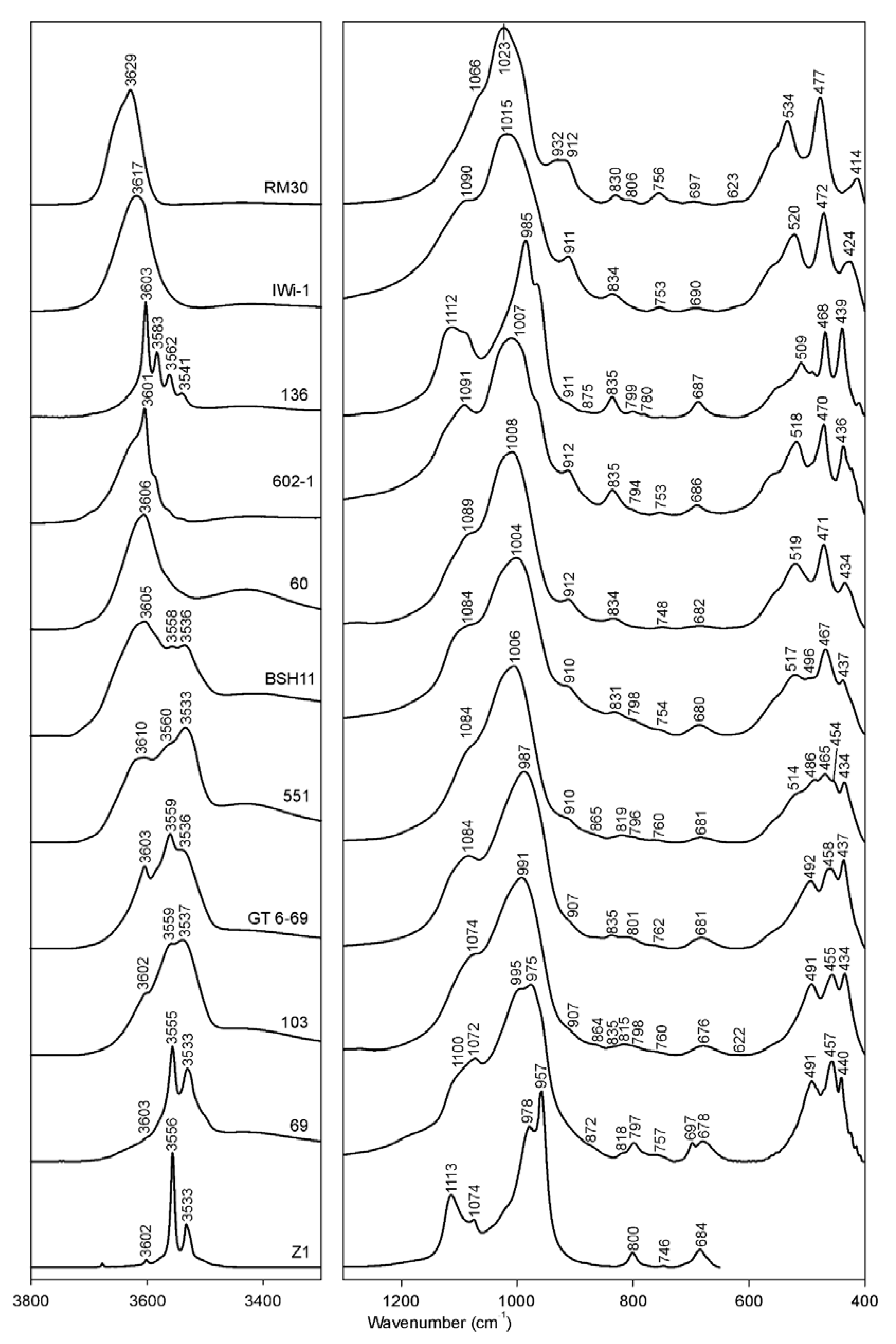
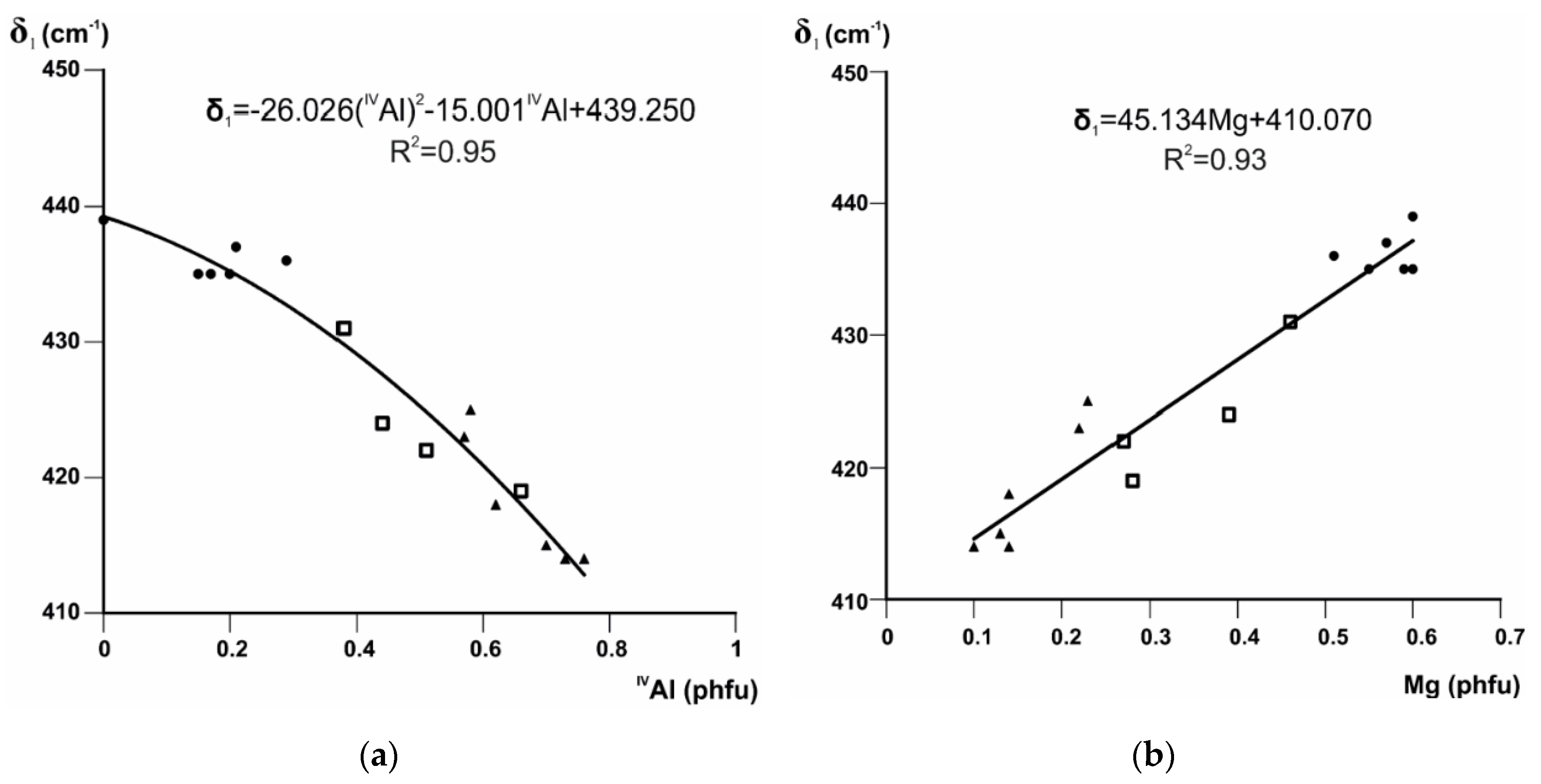
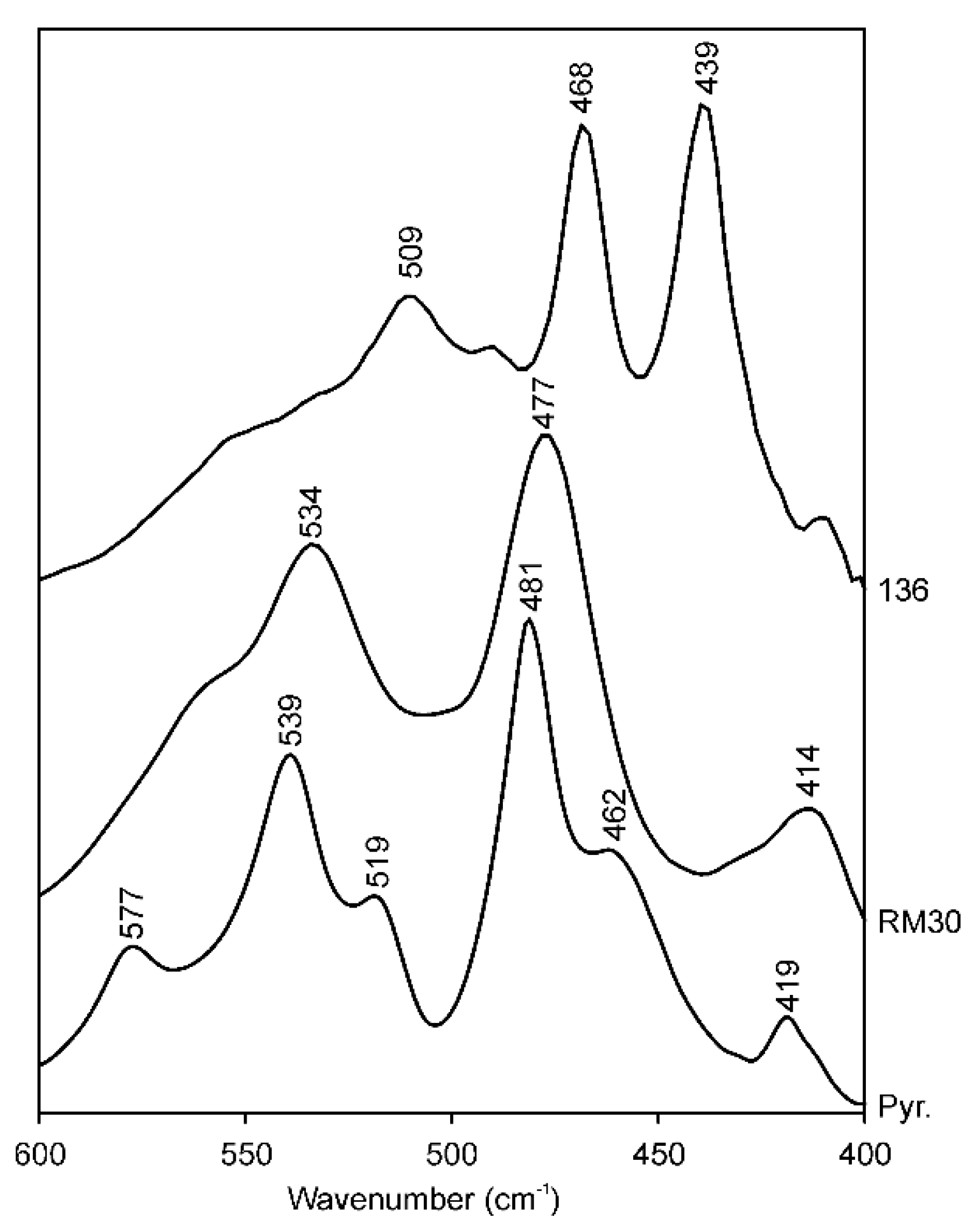
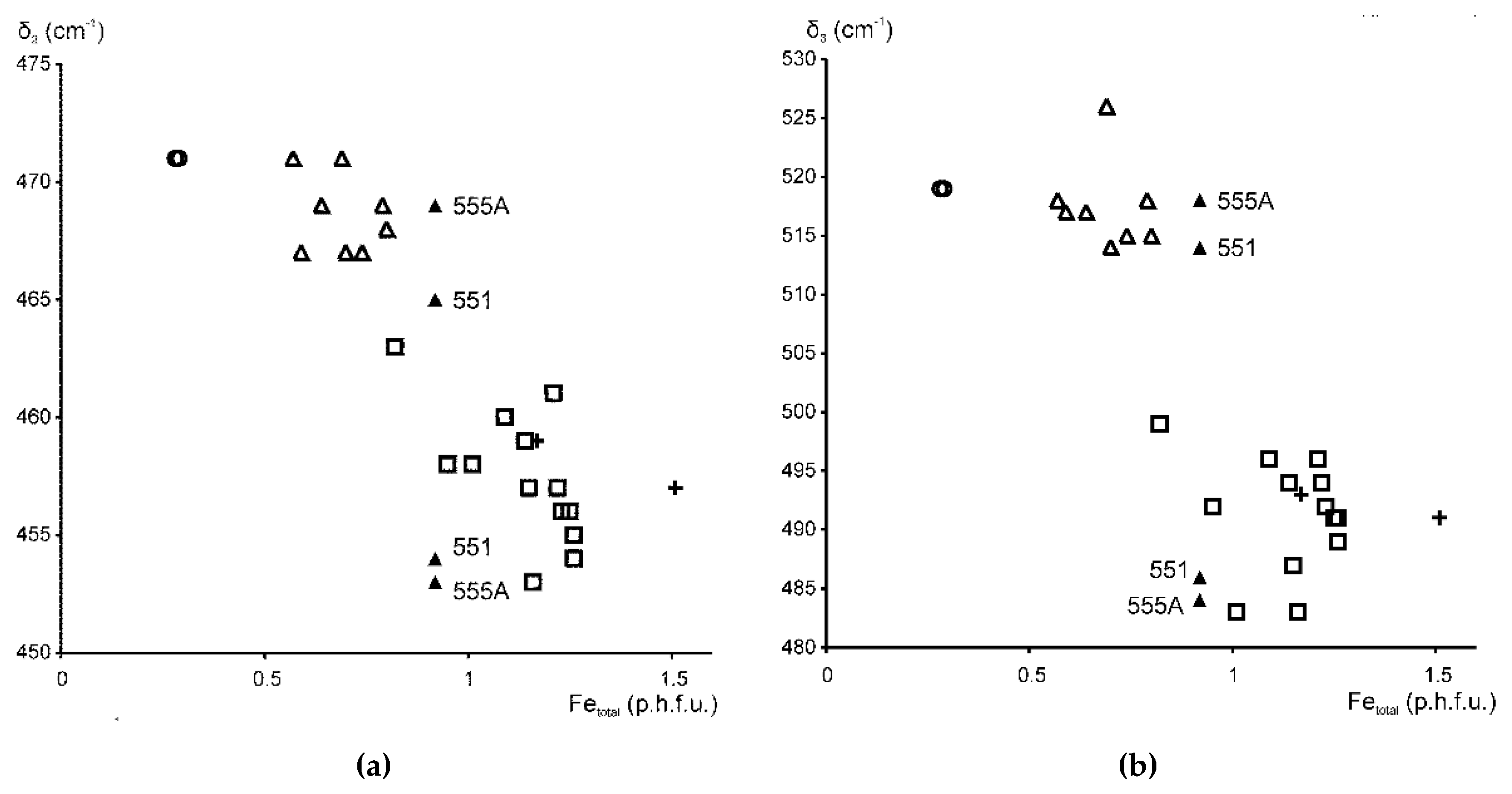
3.1. Si–O Bending Vibration Modes
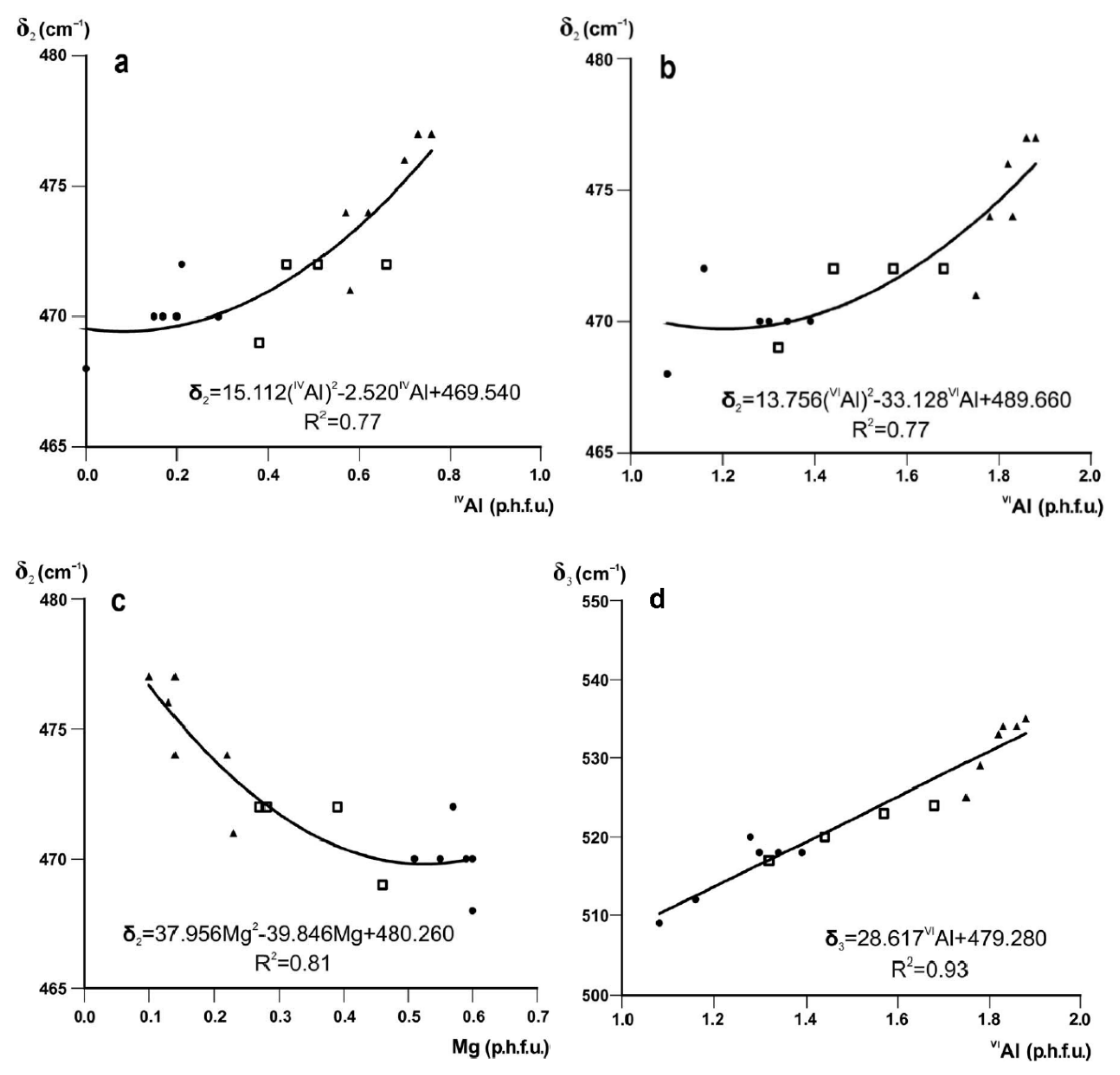
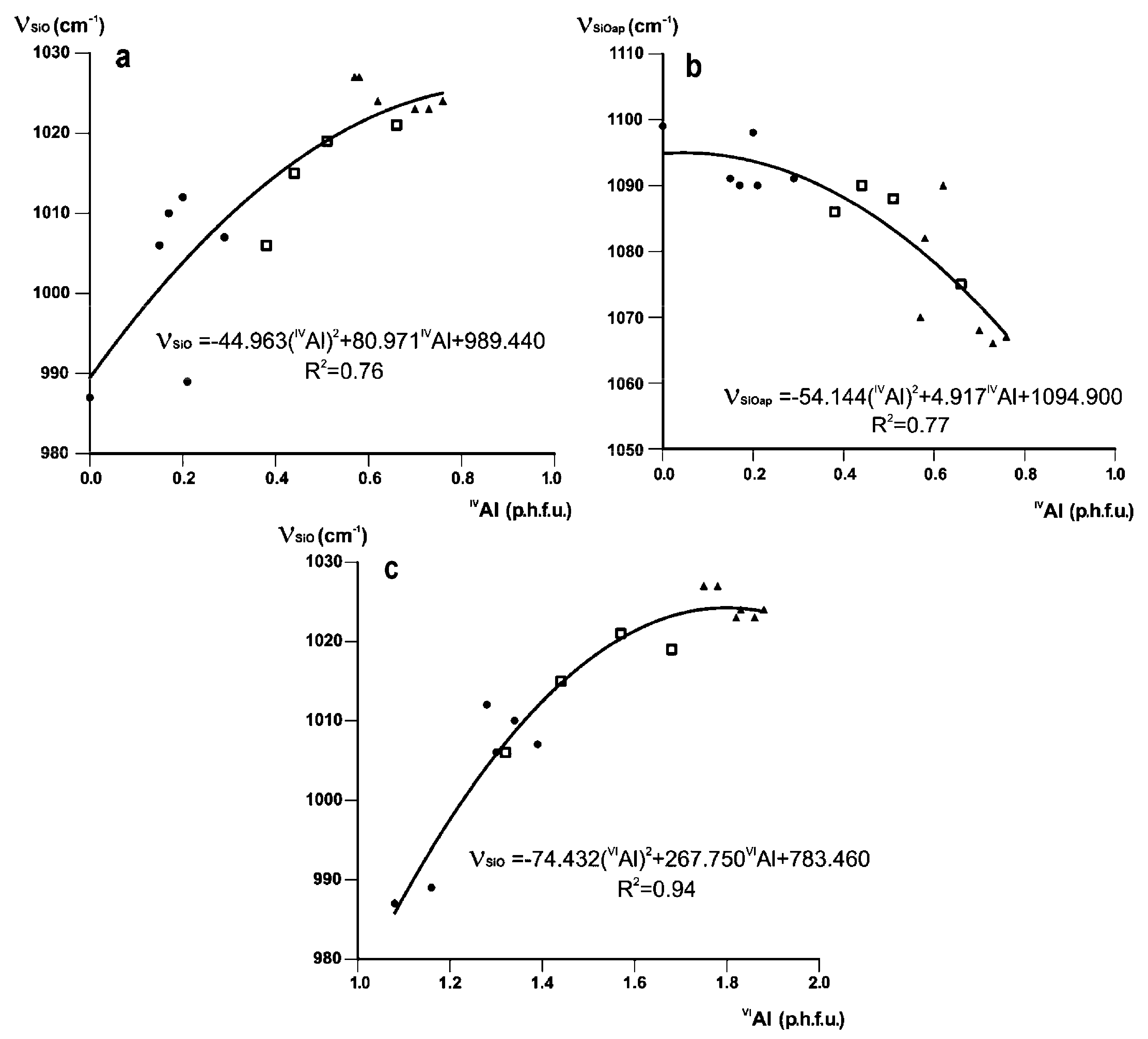
3.2. Si–O Stretching Vibration Modes
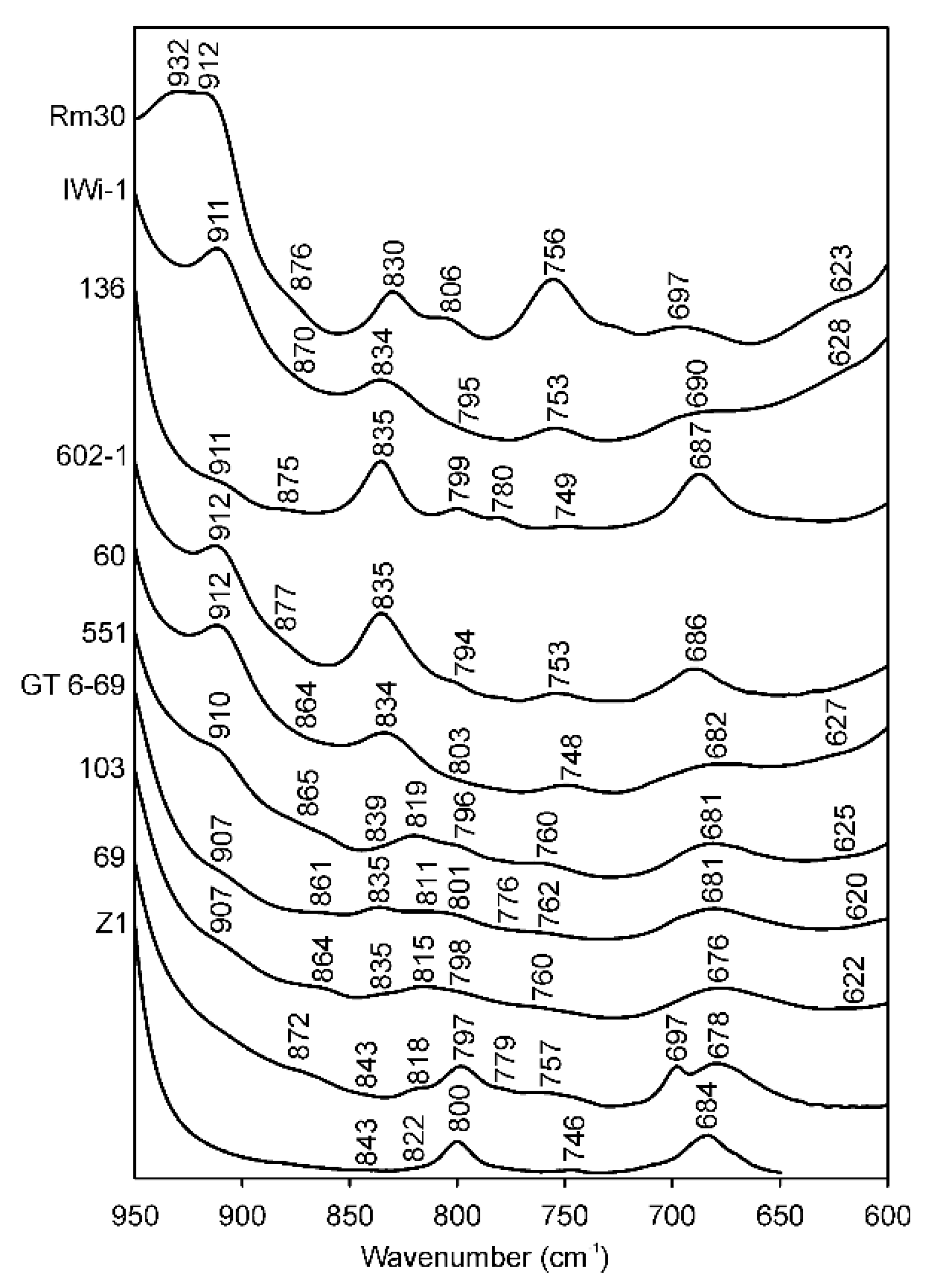
3.3. The 950–600 cm−1 Region
3.4. The OH Stretching Vibration Modes
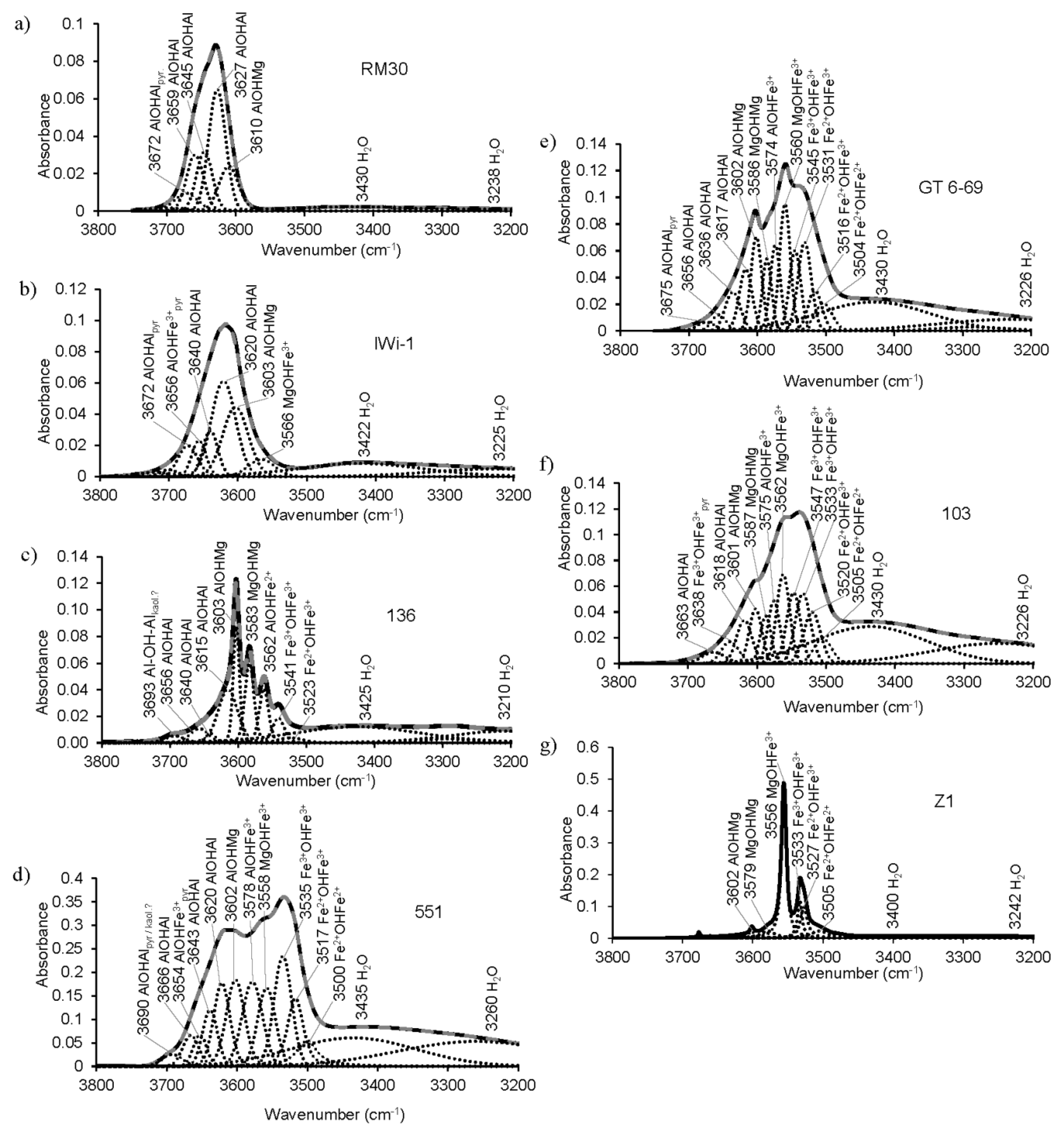
3.4.1. Methodology
3.4.2. Profiles and Positions of OH-Stretching Bands as a Function of Cation Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Środoń, J.; Eberl, D.D. Illite. In Micas: Reviews in Mineralogy; Bailey, S.W., Ed.; Mineralogical Society of America: Washington, DC, USA, 1984; Volume 13, pp. 495–544. [Google Scholar]
- Drits, V.A.; Kossovskaya, A.G. Clay Minerals: Micas and Chlorites; Nauka: Moscow, Russia, 1991; p. 175. (In Russian) [Google Scholar]
- Brigatti, M.F.; Guggenheim, S. Mica crystal chemistry and the influence of pressure, temperature and solid solution on atomistic models. In Micas: Crystal Chemistry and Metamorphic Petrology. Reviews in Mineralogy and Geochemistry; Mottana, A., Sassi, F.E., Thompson, J.B., Jr., Guggenheim, S., Eds.; Mineralogical Society of America: Washington, DC, USA; Accademia Nazionale dei Lincei: Roma, Italy, 2002; Volume 46, Chapter 1; pp. 1–97. [Google Scholar]
- Drits, V.A.; McCarty, D.K.; Zviagina, B.B. Crystal-chemical factors responsible for the distribution of octahedral cations over trans- and cis-sites in dioctahedral 2:1 layer silicates. Clays Clay Miner. 2006, 54, 131–153. [Google Scholar] [CrossRef]
- Drits, V.A.; Zviagina, B.B.; McCarty, D.K.; Salyn, A.L. Factors responsible for crystal-chemical variations in the solid solutions from illite to aluminoceladonite and from glauconite to celadonite. Am. Miner. 2010, 95, 348–361. [Google Scholar] [CrossRef]
- Wilson, M.D. Rock-Forming Minerals, Volume 3C, Sheet Silicates: Clay Minerals; The Geological Society: London, UK, 2013. [Google Scholar]
- Zviagina, B.B.; Drits, V.A.; Środoń, J.; McCarty, D.K.; Dorzhieva, O.V. The illite–aluminoceladonite series: Distinguishing features and identification criteria from X-ray diffraction and infrared spectroscopy data. Clays Clay Miner. 2015, 63, 378–394. [Google Scholar] [CrossRef]
- Zviagina, B.B.; Drits, V.A.; Sakharov, B.A.; Ivanovskaya, T.A.; Dorzhieva, O.V. Crystal-chemical regularities and identification criteria in Fe-bearing, K-dioctahedral 1M micas from X-ray diffraction and infrared spectroscopy data. Clays Clay Miner. 2017, 65, 234–251. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Ivanovskaya, T.A.; Pokrovskaya, E.V. Crystal-chemical microheterogeneity of Precambrian globular dioctahedral mica minerals. Lithol. Miner. Resour. 2013, 48, 503–528. [Google Scholar] [CrossRef]
- Farmer, C.V.; Russell, J.D. The infra-red spectra of layer silicates. Spectrochim. Acta 1964, 20, 1140–1173. [Google Scholar] [CrossRef]
- Farmer, V.C. The characterization of adsorption bonds in clays by infrared spectroscopy. Soil Sci. 1971, 112, 62–68. [Google Scholar] [CrossRef]
- Farmer, V.C. The layer silicates. In Infrared Spectra of Minerals, Monograph 4; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; Chapter 15; pp. 331–363. [Google Scholar]
- Slonimskaya, M.V.; Besson, G.; Dainyak, L.G.; Tchoubar, C.; Drits, V.A. Interpretation of the IR spectra of celadonites and glauconites in the region of OH-stretching frequencies. Clay Miner. 1986, 21, 377–388. [Google Scholar] [CrossRef]
- Velde, B. Infrared spectra of synthetic micas in the series muscovite-MgAl celadonite. Am. Miner. 1978, 63, 343–349. [Google Scholar]
- Velde, B. Clay Minerals: A Physico-Chemical Explanation of Their Occurrence. Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 40. [Google Scholar]
- Russell, J.D.; Fraser, A.R. Infrared methods. In Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Wilson, M.J., Ed.; Chapman & Hall: London, UK, 1994; pp. 11–67. [Google Scholar]
- Besson, G.; Drits, V.A. Refined relationships between chemical composition of dioctahedral fine-dispersed mica minerals and their infrared spectra in the OH stretching region. Part I. Identification of the OH stretching bands. Clays Clay Miner. 1997, 45, 158–169. [Google Scholar] [CrossRef]
- Besson, G.; Drits, V.A. Refined relationship between chemical composition of dioctahedral fine-dispersed mica minerals and their infrared spectra in the OH stretching region. Part II. The main factors affecting OH vibration and quantitative analysis. Clays Clay Miner. 1997, 45, 170–183. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L.; Hickey, L. Infrared absorption and emission study of synthetic mica-montmorillonite in comparison to rectorite, beidellite and paragonite. J. Mater. Sci. Lett. 1999, 18, 1921–1923. [Google Scholar] [CrossRef]
- Beran, A. Infrared spectroscopy of micas. In Micas: Crystal Chemistry and Metamorphic Petrology, Reviews in Mineralogy and Geochemistry; Mottana, A., Sassi, F.P., Thomson, J.B., Jr., Guggenheim, S., Eds.; Mineralogical Society of America: Washington, DC, USA; Accademia Nazionale dei Lincei: Roma, Italy, 2002; Volume 46, pp. 351–369. [Google Scholar]
- Post, J.L.; Borer, L. Physical properties of selected illites, beidellites and mixed-layer illite–beidellites from southwestern Idaho, and their infrared spectra. Appl. Clay Sci. 2002, 22, 77–91. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Weiszburg, T.; Tóth, E.; Beran, A. Celadonite, the 10-Å green clay mineral of the manganese carbonate ore, Úrkút, Hungary. Acta Miner. Petrogr. 2004, 45, 65–80. [Google Scholar]
- Heller-Kallai, L.; Lapides, I. Dehydroxylation of muscovite: Study of quenched samples. Phys. Chem. Miner. 2015, 42, 835–845. [Google Scholar] [CrossRef]
- Singh, M.; Singh, L. Vibrational spectroscopic study of muscovite and biotite layered phyllosilicates. Indian J. Pure Appl. Phys. 2016, 54, 116–122. [Google Scholar]
- Madejová, J.; Gates, W.P.; Petit, S. IR spectra of clay minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W.P., Klopprogge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 5; Volume 8, pp. 107–149. [Google Scholar]
- LeGras, M.; Laukamp, C.; Lau, I.; Mason, P. NVCL Spectral Reference Library—Phyllosilicates Part 2: Micas; CSIRO: Canberra, Australia, 2018.
- Mathian, M.; Hebert, B.; Baron, F.; Petit, S.; Lescuyer, J.-L.; Furic, R.; Beaufort, D. Identifying the phyllosilicate minerals of hypogene ore deposits in lateritic saprolites using the near-IR spectroscopy second derivative methodology. J. Geochem. Explor. 2018, 186, 298–314. [Google Scholar] [CrossRef]
- Ross, P.-S.; Bourke, A.; Schnitzler, N.; Conly, A. Exploration Vectors from Near Infrared Spectrometry near the McLeod Volcanogenic Massive Sulfide Deposit, Matagami District, Québec. Econ. Geol. 2019, 114, 613–638. [Google Scholar] [CrossRef]
- Shishelova, T.I.; Lipovchenko, E.L.; Shulga, V.V. Mica Dehydroxylation Mechanism. J. Appl. Spectr. 2019, 86, 817–821. [Google Scholar] [CrossRef]
- Grandjean, G.; Briottet, X.; Adeline, K.; Bourguignon, A.; Hohmann, A. Clay Minerals Mapping from Imaging Spectroscopy. In Geospatial Analyses of Earth Observation (EO) Data; Pepe, A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Della Ventura, G.; Hawthorne, F.C.; Iezzi, G. Synthesis and solid solution in “rubidium richterite”, Rb(NaCa)Mg5Si8O22(OH,F)2. Phys. Chem. Miner. 2019, 46, 759–770. [Google Scholar] [CrossRef]
- Yang, M.; Han, L.; Xu, Y.; Ke, H.; Zhou, N.; Dong, H.; Liu, S.; Qiao, G. Near Infrared Spectroscopic Study of Trioctahedral Chlorites and Its Remote Sensing Application. Open Geosci. 2019, 11, 815–828. [Google Scholar] [CrossRef]
- Eberl, D.D.; Środoń, J.; Lee, M.; Nadeau, P.H.; Northrop, H.R. Sericite from the Silverton Caldera, Colorado: Correlation among structure, composition, origin, and particle thickness. Am. Miner. 1987, 72, 914–934. [Google Scholar]
- Viczián, I. Hungarian investigations on the “Zempleni” illite. Clays Clay Miner. 1997, 45, 114–115. [Google Scholar] [CrossRef]
- Środoń, J.; Zeelmaekers, E.; Derkowski, A. The charge of component layers of illite-smectite in bentonites and the nature of end-member illite. Clays Clay Miner. 2009, 57, 649–671. [Google Scholar] [CrossRef]
- Środoń, J.; Clauer, N.; Huff, W.; Dudek, T.; Banaś, M. K-Ar dating of the Lower Palaeozoic K-bentonites from the Baltic Basin and the Baltic Shield: Implications for the role of temperature and time in the illitization of smectite. Clay Miner. 2009, 44, 361–387. [Google Scholar] [CrossRef]
- Grathoff, G.H.; Moore, D.M.; Kluessendorf, J.; Mikulic, D.G. The Waukesha illite, a Silurian Residuum from Karstification, Proposed as a Candidate for the Source Clay Repository. Program with abstracts. In Proceedings of the 32nd Annual Clay Minerals Society Meeting, Baltimore, MD, USA, 3–8 June 1995; p. 54. [Google Scholar]
- Ivanovskaya, T.A.; Tsipursky, S.I.; Yakovleva, O.V. Mineralogy of globular glauconites from Vendian and Riphean of the Ural and Siberia. Litologia i Poleznye Iskopaemye 1989, 3, 83–99. (In Russian) [Google Scholar]
- Seifert, F. X-ray powder data for Mg-A1-celadonite (leucophyllite) from Barcza, Poland. Contrib. Miner. Petrol. 1968, 19, 93–96. [Google Scholar] [CrossRef]
- Kardymowicz, I. O seladonicie z Barczy w Górach Swietokrzyskich. Geol. Quart. 1960, 4, 609–618. (In Polish) [Google Scholar]
- Sokolova, T.N.; Drits, V.A.; Sokolova, A.L.; Stepanov, S.S. Structural and mineralogical characteristics and conditions of formation of leucophyllite from salt-bearing deposits of Inder. Litologia i Poleznye Iskopaemye 1976, 6, 80–95. (In Russian) [Google Scholar]
- Środoń, J.; Paszkowski, M.; Drygant, D.; Anczkiewicz, A.; Banas, M. Thermal history of Lower Paleozoic rocks on the Peri-Tornquist margin of the East European Craton (Podolia, Ukraine) inferred from combined XRD, K-Ar, and AFT data. Clays Clay Miner. 2013, 61, 107–132. [Google Scholar] [CrossRef]
- Petrova, V.V.; Amarjargal, P. Zeolites of Mongolia; Nauka: Moscow, Russia, 1996; p. 150. (In Russian) [Google Scholar]
- Ivanovskaya, T.A.; Kats, A.G.; Florova, Z.B.; Tsipursky, S.I.; Yakovleva, O.V. Structure and lithomineralogical peculiarities of the Basal Lower Riphean in the Olenek Uplift (Osorkhayata Formation), Stratigrafiya. Geologicheskaya Korrelyatsiya 1993, 1, 84–92. (In Russian) [Google Scholar]
- Ivanovskaya, T.A. Globular glauconite-illite layer silicates in Middle Riphean Debengdinsk Formation of the Olenek Uplift. Lithol. Miner. Resour. 1994, 29, 595–605. [Google Scholar]
- Zaitseva, T.S.; Gorokhov, I.M.; Ivanovskaya, T.A.; Semikhatov, M.A.; Kuznetsov, A.B.; Mel’nikov, N.N.; Arakelyants, M.M.; Yakovleva, O.V. Mössbauer characteristics, mineralogy and isotopic age (Rb–Sr, K–Ar) of Upper Riphean glauconites from the Uk Formation, the southern Urals. Stratigr. Geol. Correl. 2008, 14, 227–247. [Google Scholar] [CrossRef]
- Ivanovskaya, T.A.; Zviagina, B.B.; Sakharov, B.A.; Zaitseva, T.S.; Pokrovskaya, E.V.; Dorzhieva, O.V. Globular layer silicates of the glauconite–illite composition in Upper Proterozoic and Lower Cambrian rocks. Lithol. Miner. Resour. 2015, 50, 452–477. [Google Scholar] [CrossRef]
- Ivanovskaya, T.A.; Tsipursky, S.I. First find of globular glauconite in the Lower Riphean, Anabar Uplift. Litologia i Poleznye Iskopaemye 1990, 25, 110–121. (In Russian) [Google Scholar]
- Nikolaeva, I.V. Minerals of the Glauconite Group in Sedimentary Formations; Nauka: Novosibirsk, Russia, 1977; p. 319. (In Russian) [Google Scholar]
- Drits, V.A.; Kameneva MYu Sakharov, B.A.; Dainyak, L.G.; Smoliar, B.B.; Bookin, A.S.; Salyn, A.L. Problems in Determination of the Actual Crystal Structure of Glauconites and Related Phyllosilicates; Nauka: Novosibirsk, Russia, 1993; p. 198. (In Russian) [Google Scholar]
- Thompson, J.R.; Hower, J. The mineralogy of glauconite. Clays Clay Miner. 1975, 23, 289–300. [Google Scholar] [CrossRef]
- Murav’ev, V.I. Mineral Parageneses of Glauconiee-Siliceous Formations; Nauka: Moscow, Russia, 1983; p. 208. (In Russian) [Google Scholar]
- Kimbara, K.; Shimoda, S. A ferric celadonite in amygdales of dolerite at Taiheizan, Akita prefecture, Japan. Appl. Clay Sci. 1973, 4, 143–150. [Google Scholar]
- Lazarenko, E.K.; Pavlishin, V.I. Relationship of celadonite and svitalskite. In Mineralogy of Sedimentary Rocks; Naukova Dumka: Kiev, Ukraine, 1976. (In Russian) [Google Scholar]
- Malkova, K.M. On the celadonite of Pobuzhye. In Collected Papers on Mineralogy. Lvov Geol. Soc. 1956, 10, 305–318. (In Russian) [Google Scholar]
- Strens, R.G., Jr. The comon chain, ribbon and ring silicates. In Infrared Spectra of Minerals, Monograph 4; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; Chapter 14; pp. 305–330. [Google Scholar]
- Chryssikos, G.D.; Gates, W.P. Spectral Manipulation and Introduction to Multivariate Analysis. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W.P., Klopprogge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, Chapter 4; pp. 64–106. [Google Scholar]
- Madejová, J.; Komadel, P.; Číčel, B. Infrared study of octahedral site populations in smectites. Clay Miner. 1994, 29, 319–326. [Google Scholar] [CrossRef]
- Cuadros, J.; Sainz-Diaz, C.I.; Ramirez, R.; Hernandez-Laguna, A. Analysis of Fe segregation in the octahedral sheet of bentonitic illite-smectite by means of FTIR, 27Al MAS NMR and reverse Monte-Carlo simulations. Am. J. Sci. 1999, 299, 289–308. [Google Scholar] [CrossRef]
- Zviagina, B.B.; McCarty, D.K.; Środoń, J.; Drits, V.A. Interpretation of infrared spectra of dioctahedral smectites in the region of OH-stretching vibrations. Clays Clay Miner. 2004, 52, 399–410. [Google Scholar] [CrossRef]
- Vantelon, D.; Pelletier, M.; Michot, L.J.; Barres, O.; Thomas, F. Fe, Mg and Al distribution in the octahedral sheet of montmorillonites. An infrared study in the OH-bending region. Clay Miner. 2001, 36, 369–379. [Google Scholar] [CrossRef]
- Fialips, C.I.; Huo, D.; Yan, L.; Wu, J.; Stucki, J.W. Effect of Fe oxidation state on the IR spectra of Garfield nontronite. Am. Miner. 2002, 87, 630–641. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the Clay Minerals Society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Beran, A.; Voll, D.; Schneider, H. IR spectroscopy as a tool for the characterization of ceramic precursor phases. In Spectroscopic Methods in Mineralogy, EMU Notes in Mineralogy; Beran, A., Libowitzky, E., Eds.; Eötvös University Press: Budapest, Hungary, 2004; Volume 6, pp. 189–226. [Google Scholar]
- Rothbauer Von, R. Untersuchung eines 2M1-muskovits mit neutronenstrahlen. Neues Jahrbuch für Mineralogie Monatshefte 1971, 1971, 143–154. [Google Scholar]
- Ivaldi, G.; Ferraris, G.; Curetti, N.; Compagnoni, R. Coexisting 3T and 2M1 polytypes in a phengite from Cima Pal (Val Savenca, western Alps): Chemical and polytypic zoning and structural characterization. Eur. J. Miner. 2001, 13, 1025–1034. [Google Scholar] [CrossRef]
- Smyth, J.R.; Jacobsen, S.D.; Swope, R.J.; Angel, R.J.; Arlt, T.; Domanik, K.; Holloway, J.R. Crystal structures and compressibilities of synthetic 2M1 and 3T phengite micas. Eur. J. Miner. 2000, 12, 955–963. [Google Scholar] [CrossRef]
- Lee, J.H.; Guggenheim, S. Single crystal X-ray refinement of pyrophyllite-1Tc. Am. Miner. 1981, 66, 350–357. [Google Scholar]
- Zviagina, B.B.; Drits, V.A. Structural regularities in 2M1 dioctahedral micas: The structure modeling approach. Am. Miner. 2012, 97, 1939–1954. [Google Scholar] [CrossRef]
- Goodman, B.A.; Russell, J.D.; Fraser, A.R. A Mössbauer and I.R. spectroscopy study of the structure of nontronite. Clays Clay Miner. 1976, 24, 53–59. [Google Scholar] [CrossRef]
- Gates, W.P. Infrared spectroscopy and the chemistry of dioctahedral smectites. In The Application of Vibrational Spectroscopy to Clay Minerals and Layered Double Hydroxides, CMS Workshop Lectures; Klopproge, J.T., Ed.; The Clay Mineral Society: Aurora, CO, USA, 2005; pp. 125–168. [Google Scholar]
- Gates, W.P. Cation mass—Valence sum (CM-VS) approach to assigning OH-bending bands in dioctahedral smectites. Clays Clay Miner. 2008, 56, 10–22. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Information available from infrared spectra of the fine fraction of bentonites. In The Application of Vibrational Spectroscopy to Clay Minerals and Layered Double Hydroxides, CMS Workshop Lectures, Klopproge, J.T., Ed.; The Clay Mineral Society: Aurora, CO, USA, 2005; pp. 65–98. [Google Scholar]
- Martinez-Alonso, S.; Rustad, J.R.; Goetz, A.F.H. Ab initio quantum mechanical modelling of infrared vibrational frequencies of the OH group in dioctahedral phyllosilicates. Part 1: Methods, results and comparison to experimental data. Am. Miner. 2002, 87, 1215–1223. [Google Scholar] [CrossRef]
- Moenke, H.H.W. Silica, the three-dimensional silicates, borosilicates, and beryllium silicates. In Infrared Spectra of Minerals, Monograph 4; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; Chapter 16; pp. 365–380. [Google Scholar]
- White, W.B. The carbonate minerals. In Infrared Spectra of Minerals, Monograph 4; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; Chapter 12; pp. 227–284. [Google Scholar]
- Ryskin, Y.I. The vibrations of protons in minerals: Hydroxyl, water, and ammonium. In Infrared Spectra of Minerals, Monograph 4; Farmer, V.C., Ed.; Mineralogical Society: London, UK, 1974; Chapter 9; pp. 137–182. [Google Scholar]
- Chukhrov, F.V.; Gorshkov, A.I.; Rudnitskaya, E.S.; Berezovskaya, V.V.; Sivtsov, A.V. On vernadite. Izvestiya Akademii Nauk SSR, Seriya Geologicheskaya 1978, 6, 5–19. [Google Scholar] [CrossRef]
- Lantenois, S.; Bény, J.-M.; Muller, F.; Champallie, R. Integration of iron in natural and synthetic Al-pyrophyllites: An infrared spectroscopic study. Clay Miner. 2007, 42, 129–143. [Google Scholar] [CrossRef][Green Version]
- Langer, K.; Chatterjee, N.D.; Abraham, K. Infrared studies of some synthetic and natural 2M1 dioctahedral micas. N. Jb. Miner. Abh. 1981, 142, 91–110. [Google Scholar]
- Besson, G.; Drits, V.A.; Dayniak, L.G.; Smoliar, B.B. Analysis of cation distribution in dioctahedral micaceous minerals on the basis of IR spectroscopy data. Clays Clay Miner. 1987, 22, 465–478. [Google Scholar] [CrossRef]
- Dainyak, L.G.; Drits, V.A.; Zviagina, B.B.; Lindgreen, H. Cation redistribution in the octahedral sheet during diagenesis of illite-smectites from Jurassic and Cambrian oil source rock shales. Am. Miner. 2006, 91, 589–603. [Google Scholar] [CrossRef]
- Dainyak, L.G.; Rusakov, V.S.; Sukhorukov, I.A.; Zviagina, B.B.; Drits, V.A. An improved model for the interpretation of Mössbauer spectra of dioctahedral 2:1 trans-vacant Fe-rich micas: Refinement of parameters. Eur. J. Miner. 2009, 21, 995–1008. [Google Scholar] [CrossRef]
| # | Sample Name | Mineral Variety | Origin, Location, and Reference |
|---|---|---|---|
| 1 | RM30 | illite, (Mg, Fe)-poor | Miocene hydrothermal, Silverton Caldera, San Juan Mountains., Colorado [34] |
| 2 | SG4 | illite, (Mg, Fe)-poor | Miocene hydrothermally altered volcanics, Silverton Caldera, San Juan Mountains., Colorado [34] |
| 3 | RM35C | illite, (Mg, Fe)-poor | Miocene hydrothermally altered volcanics, Silverton Caldera, San Juan Mountains., Colorado [34] |
| 4 | Fuzz-5 | illite-smectite, (Ws 1 = 16%) | Miocene hydrothermally altered volcanics, Fuzzeradvany, Hungary [35,36] |
| 5 | Fuzz-6 | illite-smectite, (Ws 1 = 16%) | Miocene hydrothermally altered volcanics, Fuzzeradvany, Hungary [35,36] |
| 6 | Zempleni | illite-smectite, (Ws 1 = 13%) | Miocene hydrothermally altered volcanics, Fuzzeradvany, Hungary [35,36] |
| 7 | L-2A-1 | illite, Mg-rich | Triassic sandstone, Poland [1] |
| 8 | Swe-151 | illite-smectite, (Ws 1 = 15%) | Ordovician K-bentonite, Röstånga, Sweden [37] |
| 9 | IWi-1 | illite, Mg-rich | Waukesha illite, Silurian, Wisconsin, USA [38] |
| 10 | 60 | illite, Mg-rich, Fe-bearing | Silty shales, Upper Riphean, South Urals, Russia [9,39] |
| 11 | 60/3 | illite, Mg-rich, Fe-bearing | Clayey siltstones, Upper Riphean, South Urals, Russia [5,39] |
| 12 | 602-1 | aluminoceladonite | Altered tuff in the Leeds Creek member of Twin Creek formation, Wyoming [5] |
| 13 | Z2 | aluminoceladonite | Devonian bentonite, Barcza, Poland [40,41] |
| 14 | 99-3/5 | aluminoceladonite | Vitric tuff in saliferous strata, Inder, Russia [42] |
| 15 | Mal-4 | aluminoceladonite | Silurian K-bentonite, Podolia, Ukraine [43] |
| 16 | Mal-6 | aluminoceladonite | Silurian K-bentonite, Podolia, Ukraine [43] |
| 17 | 136 | aluminoceladonite | Upper-Jurassic conglomerates, Tushleg, Mongolia [44] |
| 18 | 560/3 | Fe-illite | Sandstones, Lower Riphean, Olenek Uplift, northern Siberia, Russia [45] |
| 19 | 553/1 | Fe-illite | Siltstones, Middle Riphean, Olenek Uplift, northern Siberia, Russia [46] |
| 20 | 2076A | Fe-illite | Sandstones, Lower Riphean, Olenek Uplift, northern Siberia, Russia [45] |
| 21 | BSH11 | Fe-illite | Siltstones, Upper Riphean, South Urals, Russia [47,48] |
| 22 | BSH12 | Fe-illite | Siltstones, Upper Riphean, South Urals, Russia [47,48] |
| 23 | KUL1 | Fe-illite | Siltstones, Upper Riphean, South Urals, Russia [47,48] |
| 24 | 400/3 | Al-glauconite | Gravelly sandstones, Lower Riphean, Anabar Uplift, northern Siberia, Russia [9,49] |
| 25 | KUL2 | Al-glauconite | Siltstones, Upper Riphean, South Urals, Russia [47,48] |
| 26 | 551 | Al-glauconite | Gravelly sandstones, Middle Riphean, Olenek Uplift, northern Siberia, Russia [9,46] |
| 27 | 555A | Al-glauconite | Gravelly sandstones, Middle Riphean, Olenek Uplift, northern Siberia, Russia [46] |
| 28 | 402/1 | glauconite | Silty shale, Lower Riphean, Anabar Uplift, Russia [9,49] |
| 29 | 103 | glauconite | Glauconitites, Lower Ordovician, Parila, Estonia [8] |
| 30 | 37/71 | glauconite | Glauconitites, Lower Ordovician, Vergale, Latvia [8] |
| 31 | 37/71A | glauconite | Siltstones, Middle Cambrian, Vergale, Latvia [8] |
| 32 | PILT | glauconite | Ordovician sandstone, Latvia [5,50,51] |
| 33 | 68-69 | glauconite | Leningrad region, Russia [5,50,51] |
| 34 | G294 | glauconite | Ordovician, Sweden [52] |
| 35 | GT6-69 | glauconite | Cretaceous, Bornholm Island [52] |
| 36 | GT8-66 | glauconite | Cambrian, Wyoming [52] |
| 37 | 821-057 | glauconite | Greensand, Birmingham, New Jersey, Ward’s Natural Science Establishment Inc., Rochester, NY [8] |
| 38 | 541 | glauconite | Lower Cambrian, East Siberia, Russia [48] |
| 39 | 79/73 | glauconite | Zeolite-free sandstones, the Dnieper river, Ukraine [53] |
| 40 | 372/70 | glauconite | Zeolite-free sands, the Tyk-Butak river, Kazakhstan [53] |
| 41 | TAIH | celadonite | Taiheizan, Akita Prefecture, Japan [5,54] |
| 42 | 69 | celadonite | Krivoi Rog mining district, Russia [5,55] |
| 43 | Z1 | celadonite | Russia [5,56] |
| # | Sample | Cation Composition | KAl2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tetrahedra | Octahedra | Interlayer | |||||||||||
| Si | IVAl | VIAl | Fe3+ | Fe2+ | Mg | Σoct 1 | K | Ca | Na | NH4 | |||
| 1 | RM30 | 3.27 | 0.73 | 1.86 | 0 | 0 | 0.14 | 2.00 | 0.80 | 0.0 | 0.01 | n.d. | 1.00 |
| 2 | SG4 | 3.24 | 0.76 | 1.88 | 0.02 | n.d. | 0.1 | 2.00 | 0.79 | 0.01 | 0.05 | n.d. | 0.99 |
| 3 | RM35C | 3.30 | 0.70 | 1.82 | 0.05 | n.d. | 0.13 | 2.00 | 0.73 | 0.04 | 0.02 | n.d. | 0.97 |
| 4 | Fuzz-5 | 3.38 | 0.62 | 1.83 | 0.01 | n.d. | 0.14 | 1.98 | 0.68 | 0.06 | 0 | 0.01 | 0.99 |
| 5 | Fuzz-6 | 3.42 | 0.58 | 1.75 | 0.02 | n.d. | 0.23 | 2.00 | 0.70 | 0.05 | 0 | 0.01 | 0.99 |
| 6 | Zempleni | 3.43 | 0.57 | 1.78 | 0.01 | n.d. | 0.22 | 2.01 | 0.66 | 0.03 | 0.03 | n.d. | 0.99 |
| 7 | L-2A-1 | 3.45 | 0.55 | 1.57 | 0.13 | n.d. | 0.28 | 1.98 | 0.78 | 0.02 | 0.06 | n.d. | 0.92 |
| 8 | Swe-151 | 3.49 | 0.51 | 1.68 | 0.04 | n.d. | 0.27 | 1.99 | 0.58 | 0.06 | 0.01 | 0.06 | 0.99 |
| 9 | IWi-1 | 3.56 | 0.44 | 1.44 | 0.14 | n.d. | 0.39 | 1.97 | 0.72 | 0 | 0.16 | n.d. | 0.91 |
| 10 | 60 | 3.63 | 0.37 | 1.32 | 0.13 | 0.16 | 0.46 | 2.07 | 0.77 | 0 | 0.01 | n.d. | 0.91 |
| 11 | 60/3 | 3.65 | 0.35 | 1.38 | 0.13 | 0.15 | 0.40 | 2.06 | 0.75 | 0.02 | 0.01 | n.d. | 0.91 |
| 12 | 602-1 | 3.71 | 0.29 | 1.39 | 0.04 | 0.09 | 0.51 | 2.03 | 0.75 | 0.01 | 0.03 | n.d. | 0.97 |
| 13 | Z2 | 3.81 | 0.19 | 1.19 | 0.06 | 0.17 | 0.57 | 1.99 | 0.86 | 0.04 | 0 | n.d. | 0.95 |
| 14 | 99-3/5 | 3.78 | 0.22 | 1.28 | 0.11 | 0.06 | 0.59 | 2.04 | 0.65 | 0.05 | 0.01 | n.d. | 0.92 |
| 15 | Mal-4 | 3.84 | 0.16 | 1.33 | 0.09 | n.d. | 0.55 | 1.97 | 0.65 | 0.05 | 0.01 | 0.05 | 0.93 |
| 16 | Mal-6 | 3.86 | 0.14 | 1.29 | 0.09 | n.d. | 0.60 | 1.98 | 0.67 | 0.04 | 0.01 | 0.05 | 0.93 |
| 17 | 136 | 4.00 | 0 | 1.08 | 0.13 | 0.19 | 0.60 | 2.00 | 0.78 | 0 | 0.01 | n.d. | 0.89 |
| 18 | 560/3 | 3.62 | 0.38 | 1.12 | 0.37 | 0.20 | 0.32 | 2.01 | 0.80 | 0 | 0.05 | n.d. | 0.75 |
| 19 | 553/1 | 3.57 | 0.43 | 1.13 | 0.50 | 0.19 | 0.19 | 2.01 | 0.69 | 0.04 | 0.02 | n.d. | 0.69 |
| 20 | 2076A | 3.68 | 0.32 | 1.03 | 0.55 | 0.09 | 0.34 | 2.01 | 0.69 | 0.01 | 0.01 | n.d. | 0.65 |
| 21 | BSH11 | 3.71 | 0.29 | 1.06 | 0.36 | 0.23 | 0.39 | 2.04 | 0.74 | 0.02 | 0 | n.d. | 0.75 |
| 22 | BSH12 | 3.73 | 0.27 | 0.88 | 0.37 | 0.37 | 0.47 | 2.09 | 0.78 | 0.02 | 0 | n.d. | 0.70 |
| 23 | KUL1 | 3.71 | 0.29 | 0.99 | 0.43 | 0.27 | 0.37 | 2.06 | 0.78 | 0.01 | 0 | n.d. | 0.70 |
| 24 | 400/3 | 3.63 | 0.37 | 0.91 | 0.60 | 0.20 | 0.34 | 2.05 | 0.76 | 0.01 | 0.02 | n.a | 0.60 |
| 25 | KUL2 | 3.72 | 0.28 | 0.86 | 0.54 | 0.25 | 0.39 | 2.04 | 0.76 | 0.02 | 0 | n.d. | 0.61 |
| 26 | 551 | 3.67 | 0.33 | 0.85 | 0.77 | 0.15 | 0.22 | 1.99 | 0.77 | 0.01 | 0.02 | n.d. | 0.52 |
| 27 | 555A | 3.63 | 0.37 | 0.77 | 0.76 | 0.16 | 0.34 | 2.03 | 0.73 | 0.01 | 0.02 | n.d. | 0.50 |
| 28 | 402/1 | 3.80 | 0.20 | 0.52 | 0.78 | 0.28 | 0.47 | 2.05 | 0.74 | 0 | 0.04 | n.d. | 0.40 |
| 29 | 103 | 3.67 | 0.33 | 0.37 | 1.15 | 0.11 | 0.40 | 2.03 | 0.74 | 0 | 0 | n.d. | 0.24 |
| 30 | 37/71 | 3.57 | 0.43 | 0.38 | 1.15 | 0.10 | 0.45 | 2.08 | 0.72 | 0.01 | 0.02 | n.d. | 0.25 |
| 31 | 37/71A | 3.56 | 0.44 | 0.64 | 0.96 | 0.19 | 0.28 | 2.07 | 0.68 | 0 | 0.01 | n.d. | 0.40 |
| 32 | PILT | 3.73 | 0.27 | 0.44 | 0.92 | 0.22 | 0.47 | 2.05 | 0.77 | 0 | 0.03 | n.d. | 0.32 |
| 33 | 68-69 | 3.73 | 0.27 | 0.43 | 0.92 | 0.30 | 0.39 | 2.04 | 0.80 | 0.01 | 0.03 | n.d. | 0.32 |
| 34 | G294 | 3.72 | 0.28 | 0.74 | 0.82 | n.d. | 0.40 | 1.96 | 0.67 | 0.06 | 0.02 | n.d. | 0.47 |
| 35 | GT6-69 | 3.78 | 0.22 | 0.57 | 0.95 | n.d. | 0.47 | 1.99 | 0.69 | 0.01 | 0 | n.d. | 0.38 |
| 36 | GT8-66 | 3.60 | 0.40 | 0.37 | 1.26 | n.d. | 0.32 | 1.95 | 0.72 | 0 | 0.12 | n.d. | 0.23 |
| 37 | 821-057 | 3.74 | 0.26 | 0.33 | 1.23 | n.d. | 0.41 | 1.97 | 0.68 | 0.03 | 0.01 | n.d. | 0.22 |
| 38 | 541 | 3.53 | 0.47 | 0.68 | 0.72 | 0.29 | 0.40 | 2.09 | 0.78 | 0.06 | 0.01 | n.d. | 0.49 |
| 39 | 79/73 | 3.65 | 0.35 | 0.37 | 1.16 | 0.05 | 0.44 | 2.02 | 0.67 | 0.06 | 0.01 | n.d. | 0.27 |
| 49 | 372/70 | 3.68 | 0.32 | 0.51 | 1.01 | 0.08 | 0.40 | 2.00 | 0.65 | 0.06 | 0.02 | n.d. | 0.27 |
| 41 | TAIH | 3.72 | 0.28 | 0.16 | 1.07 | 0.10 | 0.71 | 2.04 | 0.82 | 0 | 0.18 | n.d. | 0.13 |
| 42 | 69 | 3.94 | 0.06 | 0.06 | 1.15 | 0.36 | 0.41 | 1.98 | 0.83 | 0.03 | 0.01 | n.d. | 0.05 |
| 43 | Z1 | 3.96 | 0.04 | 0.05 | 0.96 | 0.26 | 0.73 | 2.00 | 0.89 | 0 | 0.07 | n.d. | 0.05 |
| # | Sample | Si–O Bend | Si–O–Si Bend | Si–O–Si Bend (Additional) | Si–O–Fe Bend | Si–O–Al Bend | Si–O Stretch | Si–Oap Stretch | OH-Stretch, Visually Discernable Absorption Maxima | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RM30 | 414 | 477 | - | - | 534 | 1023 | 1066sh | - | - | - | 3629 |
| 2 | SG4 | 414 | 477 | - | - | 535 | 1024 | 1067sh | - | - | - | 3627 |
| 3 | RM35C | 415 | 476 | - | - | 533 | 1023 | 1068sh | - | - | - | 3629 |
| 4 | Fuzz-5 | 418 | 474 | - | - | 534 | 1024 | 1090 | - | - | - | 3630 |
| 5 | Fuzz-6 | 425 | 471 | - | - | 525 | 1027 | 1082sh | - | - | - | 3625 |
| 6 | Zempleni | 423 | 474 | - | - | 529 | 1027 | 1070sh | - | - | - | 3624 |
| 7 | L-2A-1 | 419 | 472 | - | - | 523 | 1021 | 1075 | - | - | - | 3618 |
| 8 | Swe-151 | 422 | 472 | - | - | 524 | 1019 | 1088sh | - | - | - | 3626 |
| 9 | IWi-1 | 424 | 472 | - | - | 520 | 1015 | 1090 | - | - | - | 3617 |
| 10 | 60 | 434 | 471 | - | - | 519 | 1008 | 1089 | - | - | - | 3606 |
| 11 | 60/3 | 435 | 470 | - | - | 519 | 1010 | 1084 | - | - | - | 3607 |
| 12 | 602-1 | 436 | 470 | - | - | 518 | 1007 | 1091 | - | - | - | 3601 |
| 13 | Z2 | 437 | 472 | - | - | 512 | 989 | 1090 | 3538 | 3559 | 3583 | 3603 |
| 14 | 99-3/5 | 435 | 470 | - | - | 520 | 1012 | 1098 | - | - | - | 3605 |
| 15 | Mal-4 | 435 | 470 | - | - | 518 | 1010 | 1090 | - | - | - | 3606 |
| 16 | Mal-6 | 435 | 470 | - | - | 518 | 1006 | 1091 | - | - | - | 3603 |
| 17 | 136 | 439 | 468 | - | - | 509 | 985 | 1112 | 3541 | 3562 | 3583 | 3603 |
| 18 | 560/3 | 436 | 471 | - | - | 518 | 1008 | 1084 | 3528 | - | - | 3612 |
| 19 | 553/1 | 434 | 471 | 451sh | - | 526 | 1017 | 1083sh | 3534 | - | - | 3624 |
| 20 | 2076 | 437 | 469 | 453 | 486sh | 517 | 1000 | 1085 | 3530 | 3554 | - | 3607 |
| 21 | BSh11 | 437 | 467 | 450sh | 496* | 517 | 1004 | 1084 | 3536 | 3558 | - | 3605 |
| 22 | BSh12 | 437 | 467 | - | - | 515 | 1007 | 1084 | 3534 | 3555sh | 3582sh | 3602 |
| 23 | KUL1 | 438 | 467 | 454sh | 490 | 514 | 998 | 1087 | 3533 | 3554 | 3584 | 3602 |
| 24 | 400/3 | 438 | 468 | 455sh | 484sh | 515sh | 994 | 1079 | 3532 | 3555sh | - | 3603 |
| 25 | KUL2 | 438 | 469 | 454sh | 484sh | 518 | 1012 | 1081sh | 3533 | 3555sh | - | 3602, 3618sh |
| 26 | 551 | 434 | 465 | 454 | 486sh | 514sh | 1006 | 1084sh | 3533 | 3560sh | - | 3610 |
| 27 | 555A | 437 | 469 | 455 | 486sh | 518sh | 1002 | 1080sh | 3532 | 3560sh | - | 3609 |
| 28 | 402/1 | 438 | 453 | 467 | 483sh | - | 990 | 1082 | 3533 | 3555sh | - | 3603* |
| 29 | 103 | 434 | 455 | - | 491 | - | 991 | 1074 | 3537 | 3559 | - | 3602sh |
| 30 | 37/71 | 434 | 456 | - | 491 | - | 995 | 1072 | 3535 | 3561 | - | 3603sh |
| 31 | 37/71A | 435 | 457 | - | 487 | - | 1001 | 1079sh | 3538 | - | 3581sh | 3603sh |
| 32 | PILT | 439 | 459 | - | 494 | - | 1010 | 1081sh | - | 3550 | - | |
| 33 | 68/69 | 437 | 457 | - | 494 | - | 1013 | 1082sh | 3550 (3530 + 3542 + 3567) (2nd drv) | - | ||
| 34 | G294 | 435 | 463 | - | 499sh | - | 995 | 1082 | 3534 | 3558 | 3604 | |
| 35 | GT6-69 | 437 | 458 | - | 492 | - | 987 | 1084 | 3536 | 3559 | 3583sh | 3603 |
| 36 | GT8-66 | 434 | 454 | - | 489 | - | 992 | 1075 | 3536 | 3564sh | 3604sh | |
| 37 | 821-057 | 438 | 456 | - | 492 | - | 992 | 1078 | 3538 | 3568 | 3602 | |
| 38 | 541 | 438 | 458 | - | 483 | 526sh | 1000 | 1072 | 3533 | 3558 | - | 3621 |
| 39 | 79/73 | 439 | 461 | - | 496 | - | 1016 | 1099sh | 3553 (3537 + 3558) (2nd drv) | - | 3601sh | |
| 40 | 372/70 | 440 | 460 | - | 496 | - | 1014 | 1101 | 3550 (3538 + 3563) (2nd drv) | - | 3614sh | |
| 41 | TAIH | 439 | 459 | - | 493 | - | 978 | 1090 | 3532 | 3556 | - | 3601 |
| 42 | 69 | 440 | 457 | - | 491 | - | 975, 995sh | 1072, 1100sh | 3533 | 3555sh | - | 3603 (2nd drv) |
| 43 | Z1 | n.d. | n.d. | - | n.d. | - | 957, 978 | 1074, 1113 | 3533 | 3556 | - | 3602 |
| # | Sample | Coupled Al–O and Si–O out-of-Plane of Smectite | Si–O; Fe–O out-of-Plane; Quartz; Carbonates | Al–O–Si in-Plane; δ(Fe2+OHFe3+)? | Al–O–Al; δ(Fe3+OHMg); δ(Fe2+OHFe3+); Silica/Quartz; Goethite | δ(Fe3+OHFe3+) | δ(AlOHMg)/ Al–O out of Plane/ Second δ(Fe3+OHFe3+) | Al–O–Al; δ(AlOHFe3+); Goethite | δ(AlOHAl) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | RM30 | 623sh | 697 | 756 | 806 | - | 830 | 876sh | (912, 932) *** |
| 2 | SG4 | 626sh | 695 | 756 | 804 | - | 831 | 876sh | (917, 933) *** |
| 3 | RM35C | 624sh | 695 | 756 | 803 | - | 826 | 876 sh | (918, 934) *** |
| 4 | Fuzz-5 | 626sh | 694 | 755 | 803sh | - | 826 | 876sh | (913, 932) *** |
| 5 | Fuzz-6 | 618 | 699 | 754 | 799 * | - | 823, 848 | 882sh | 913 |
| 6 | Zempleni | 618 | 695 | 755 | 804 | - | 826 | 882 * | 917 |
| 7 | L-2A-1 | 615sh | 687 | 752 | 801sh | - | 827 | 871 ** | 913 |
| 8 | Swe-151 | 620sh | 693 | 753 | 782, 799 * | - | 832 | 876 | 912 |
| 9 | IWi-1 | 628 | 690sh | 753 | 795 * | - | 834 | 870 | 911 |
| 10 | 60 | 627 ** | 682 | 748 | 803 | - | 834 | 864 | 912 |
| 11 | 60/3 | 635 | 681 | 750 | 790 | - | 831 | 868 | 909 |
| 12 | 602-1 | - | 686 | 753 | 794 * | - | 835 | 877 | 912 |
| 13 | Z2 | - | 686 | 750 | 780, 800 * | - | 835 | 874 | 912sh |
| 14 | 99-3/5 | 623 | 688 | 752 ** | 780, 800 ** | - | 838 | 873 * | 914 |
| 15 | Mal-4 | 625 | 685 | 752 | 802sh | - | 839 | 875sh | 912 |
| 16 | Mal-6 | 626 ** | 686 | 752 | 787 * | - | 836 | 876 * | 913 |
| 17 | 136 | - | 687 | 749 | 780, 799 | - | 835 | 875sh | 911sh |
| 18 | 560/3 | - | 683 | 753 | ~799sh | 829 | 829 | 862, 878 | 915 |
| 19 | 553/1 | 620 | 683 | 753 | ~800sh | 824 | 824 | 870sh | 913sh |
| 20 | 2076A | - | 682 | 754 | 795sh | - | 830 | 864, 878 | 915sh |
| 21 | BSH11 | - | 680 | 754sh | 798sh | - | 831 | 860, 874 | 910 |
| 22 | BSH12 | - | 691 | 751sh | 780, 799 | - | 833 | 877sh | 914sh |
| 23 | KUL1 | 625 | 681 | 752sh | ~797sh | - | 830 | (860, 876) | 910sh |
| 24 | 400/3 | - | 682 | 754sh | 797sh | 823 | 838 | 863sh | 911sh |
| 25 | KUL2 | 625 | 683 | 752sh | 801 | 824 | 824 | 870sh | 912sh |
| 26 | 551 | 625 | 681 | 760sh | 796sh | 819 | 839 | 865sh * | 910sh * |
| 27 | 555A | - | 682 | 759sh | 798sh | 822 | 841 | 865sh * | 911sh * |
| 28 | 402/1 | - | 683 | 797sh | 821sh | 838 | (861, 878) | 907 | |
| 29 | 103 | 622 | 676 | 760sh | 798 | 815 | 835sh | 864sh | 907 |
| 30 | 37/71 | 620 ** | 678 | 764sh | 800 | 812 | 837sh | 865sh | 905sh |
| 31 | 37/71A | 624 | 681 | 753sh | 780, 797 ** | 820 | 834 * | 871sh | 911sh |
| 32 | PILT | 620 ** | 670, 698sh | 760 | 798 | 815 | 836 | 872sh | 907 |
| 33 | 68-69 | - | 677, 696srp | 762sh | 778 **, 796 | 815 | 834 | 863sh | 907 |
| 34 | G294 | - | 683 | 760sh | 804sh | 817* | 836 | 861sh | 911sh |
| 35 | GT6-69 | 620 ** | 681 | 762sh | 776sh, 801 | 811 | 835 | 861sh | 907sh * |
| 36 | GT8-66 | 620 | 680 | 760sh | 776 | 814 | 836 | 864sh | 907sh |
| 37 | 821-057 | - | 676 | 779, 800 | 815sh | 835sh | 866sh | 908 | |
| 38 | 541 | 625 | 684 | 755 | 780, 800 | 812 | 834 | 870sh | 913sh |
| 39 | 79/73 | - | 678, 698srp | 760 | 797 | 817 * | 838 * | 870sh | 907sh |
| 40 | 372/70 | 623 ** | 677, 698srp | 760 | - | 816 | 834 * | 867sh * | 910sh |
| 41 | TAIH | 618 | 681, 695srp | 760 * | 799 | 821 | 840 | (873, 888) * | 908sh |
| 42 | 69 | - | 678, 697srp | 757sh | 779sh, 797 | 818sh | 843 | 872sh | - |
| 43 | Z1 | - | 684 | 746* | 800 | 822 | 843 ** | - | - |
| # | 1 | 9 | 12 | 17 | 26 | 27 | 29 | 31 | 35 | 43 | [17,18] | This work | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | RM30 | IWi-1 | 602-1 | 136 | 551 | 555A | 103 | 3771A | GT6-69 | Z1 | ||||||||||||
| Band | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | A | ν(OH) | ν(OH) |
| Fe2+OHFe2+ | - | - | - | - | - | - | - | - | 3500 | 0.062 | 3505 | 0.087 | 3505 | 0.059 | 3505 | 0.051 | 3504 | 0.055 | 3505 | 0.091 | 3505±2 | 3503±3 |
| Fe2+OHFe3+ | - | - | - | - | - | - | 3523 | 0.032 | 3517 | 0.107 | 3517 | 0.022 | 3520 | 0.111 | 3516 | 0.048 | 3516 | 0.065 | 3527 | 0.151 | 3521±3 | 3521±6 |
| Fe3+OHFe3+ | - | - | - | - | - | - | - | - | 3535 | 0.16 | 3534 | 0.236 | 3533 | 0.138 | 3530 | 0.149 | 3531 | 0.134 | 3533 | 0.119 | 3535±2 | 3533±3 |
| Fe3+OHFe3+ | - | - | - | - | - | - | 3541 | 0.076 | - | - | - | - | 3547 | 0.127 | 3544 | 0.104 | 3545 | 0.105 | - | - | - | 3544±3 |
| MgOHFe2+ | - | - | - | - | 3542 | 0.020 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3543±3 | 3541 |
| AlOHFe2+ | - | - | - | - | 3567 | 0.052 | 3562 | 0.134 | - | - | - | - | - | - | 3560 | 0.071 | - | - | 3559±3 | 3563±3 | ||
| MgOHFe3+ | - | - | 3566 | 0.072 | - | - | - | - | 3558 | 0.114 | 3559 | 0.118 | 3562 | 0.148 | 3560 | 0.069 | 3560 | 0.154 | 3556 | 0.527 | 3559±3 | 3561±5 |
| AlOHFe3+ | - | - | - | - | - | - | - | - | 3578 | 0.14 | 3574 | 0.055 | 3575 | 0.095 | 3576 | 0.088 | 3574 | 0.098 | - | - | 3573±2 | 3576±2 |
| MgOHMg | - | - | - | - | 3586 | 0.099 | 3583 | 0.194 | - | - | 3587 | 0.073 | 3587 | 0.066 | 3587 | 0.051 | 3586 | 0.071 | 3579 | 0.073 | 3583±3 | 3583±4 |
| AlOHMg | 3610 | 0.166 | 3603 | 0.280 | 3604 | 0.247 | 3603 | 0.192 | 3602 | 0.135 | 3605 | 0.12 | 3601 | 0.088 | 3601 | 0.119 | 3602 | 0.116 | 3602 | 0.029 | 3604±3 | 3605±5 |
| AlOHAl | 3627 | 0.393 | 3620 | 0.366 | 3619 | 0.210 | 3615 | 0.276 | 3620 | 0.117 | 3623 | 0.101 | 3618 | 0.086 | 3616 | 0.076 | 3617 | 0.091 | - | - | 3621±4 | 3621±6 |
| AlOHAl | 3645 | 0.181 | 3640 | 0.109 | 3636 | 0.220 | 3640 | 0.027 | 3643 | 0.069 | 3638 | 0.074 | - | - | - | - | 3636 | 0.068 | - | - | 3641±3 | 3641±5 |
| AlOHAl | 3659 | 0.181 | - | - | 3661 | 0.042 | 3656 | 0.070 | 3666 | 0.041 | 3663 | 0.033 | 3663 | 0.034 | 3665 | 0.026 | 3656 | 0.026 | - | - | 3658±3 | 3661±5 |
| Fe3+OHFe3+pyr | - | - | - | - | - | - | - | - | - | - | - | - | 3638 | 0.049 | 3631 | 0.081 | - | - | - | - | 3631±3 | 3634±4 |
| AlOHFe3+pyr | - | - | 3656 | 0.079 | 3651 | 0.061 | - | - | 3654 | 0.035 | 3652 | 0.041 | - | - | 3649 | 0.05 | - | - | - | - | 3652±2 | 3652±4 |
| AlOHAlpyr | 3672 | 0.079 | 3672 | 0.094 | 3672 | 0.050 | - | - | 3690 * | 0.020 | 3680 | 0.028 | - | - | 3679 | 0.017 | 3675 | 0.018 | 3677 | 0.009 | 3675±4 | 3675±5 |
| χ2 | 1.13 | 4.64 | 4.26 | 1.34 | 6.04 | 3.78 | 2.56 | 5.48 | 1.21 | 3.25 | ||||||||||||
| Sample | Al | Mg | Fe2+ | Fe3+ | Fetot * | Σoct ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.a. | FTIR | c.a. | FTIR | c.a. | FTIR | c.a. | FTIR | c.a. | FTIR | c.a. | FTIR | |
| RM30 | 1.86 | 1.83 | 0.14 | 0.17 | - | - | - | - | - | - | 2.00 | 2.00 |
| IWi-1 | 1.44 | 1.50 | 0.39 | 0.35 | - | - | 0.14 | 0.15 | 0.14 | 0.15 | 1.97 | 2.00 |
| 602-1 | 1.39 | 1.40 | 0.51 | 0.47 | 0.09 | 0.07 | 0.04 | 0.06 | 0.13 | 0.13 | 2.03 | 2.00 |
| 136 | 1.08 | 1.07 | 0.60 | 0.58 | 0.19 | 0.17 | 0.13 | 0.18 | 0.32 | 0.35 | 2.00 | 2.00 |
| 551 | 0.85 | 0.80 | 0.22 | 0.25 | 0.15 | 0.23 | 0.77 | 0.72 | 0.92 | 0.95 | 1.99 | 2.00 |
| 555A | 0.76 | 0.70 | 0.34 | 0.39 | 0.16 | 0.20 | 0.76 | 0.72 | 0.92 | 0.92 | 2.03 | 2.00 |
| 103 | 0.37 | 0.42 | 0.40 | 0.37 | 0.11 | 0.23 | 1.15 | 0.98 | 1.26 | 1.21 | 2.03 | 2.00 |
| 3771A | 0.64 | 0.56 | 0.28 | 0.29 | 0.19 | 0.22 | 0.96 | 0.92 | 1.15 | 1.14 | 2.07 | 2.00 |
| GT6-69 | 0.57 | 0.62 | 0.47 | 0.41 | n.d. | 0.18 | 0.95 | 0.79 | 0.95 | 0.97 | 1.99 | 2.00 |
| Z1 | 0.05 | 0.05 | 0.73 | 0.70 | 0.26 | 0.33 | 0.96 | 0.92 | 1.22 | 1.25 | 2.00 | 2.00 |
| Si–O Bend (δ1, cm−1) | Si–O–Si Bend (δ2, cm−1) | Si–O–Al Bend (δ3, cm−1) | Si–O–Fe Bend (δ3, cm–1) | Si–O Stretch (cm–1) | Si–Oapical Stretch (cm–1) | OH Stretch | |
|---|---|---|---|---|---|---|---|
| Fe-poor, K-dioctahedral micas (Fetot ≤ 0.3 cations p.h.f.u) | |||||||
| (Mg, Fe)-poor illites | 414–425 | 471–477 | 525–535 | - | 1023–1027 | ~1070–1080 (shoulder) | Broad band, maximum at ~3625–3630 cm−1 |
| Mg-rich illites | 419–435 | ~470 | 519–525 | - | 1008–1021 | 1075–1090 | Broad band, maximum at ~3605–3620 cm−1 |
| Aluminoceladonites | 435–439 | ~470 | 509–520 | - | 985–1012 | 1090–1112 | Sharp bands at ~3600 cm−1 (AlOHMg, strongest); 3583 cm−1 (MgOHMg); ~3560 cm−1 (AlOHFe2+ or MgOHFe3+); ~3540 cm−1 (FeOHFe); OR broad band with a sharp maximum at ~3600 cm−1 |
| Fe-bearing, K-dioctahedral micas (Fetot≥0.3 cations p.h.f.u) | |||||||
| Fe-bearing Mg-rich illites (KAl ~ 0.9) | 434–440 | ~470 | ~520 | - | ~1010 | 1084–1089 | Broad band, maximum at 3600–3610 cm−1 |
| Fe illites (0.65≤ KAl ≤0.75) Al-glauconites (0.5< KAl ≤0.6) | 467–471; 450–455 (* or sh) | 514–526 | 484–496 | 987–1017 | 1072–1101 (band or shoulder) | Two bands, 3528–3534 (“Fe”) and 3600–3610 cm−1 (“Al, Mg”). Similar intensity or “Al, Mg” band stronger | |
| Al-glauconites (KAl ~0.5) | 465–469; 454–455 | 514–518 (sh) | 486 (sh) | “Fe” band stronger | |||
| Glauconites (KAl <0.5) | 453–463 | - | 483–499 | Bands at 3533–3538 cm−1 and ~3550–3560 cm−1, variable resolution and relative intensity | |||
| Celadonites (KAl <0.2) | (957), 975–978 (sharp) | 1090 or 1072–1074 (sharp), 1100–1113 (sharp band or shoulder) | Sharp, well-resolved bands at 3556 cm−1 (MgOHFe3+) and 3532–3533 cm−1 (Fe3+ OHFe3+) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zviagina, B.B.; Drits, V.A.; Dorzhieva, O.V. Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data. Minerals 2020, 10, 153. https://doi.org/10.3390/min10020153
Zviagina BB, Drits VA, Dorzhieva OV. Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data. Minerals. 2020; 10(2):153. https://doi.org/10.3390/min10020153
Chicago/Turabian StyleZviagina, Bella B., Victor A. Drits, and Olga V. Dorzhieva. 2020. "Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data" Minerals 10, no. 2: 153. https://doi.org/10.3390/min10020153
APA StyleZviagina, B. B., Drits, V. A., & Dorzhieva, O. V. (2020). Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data. Minerals, 10(2), 153. https://doi.org/10.3390/min10020153




