Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia)
Abstract
1. Introduction
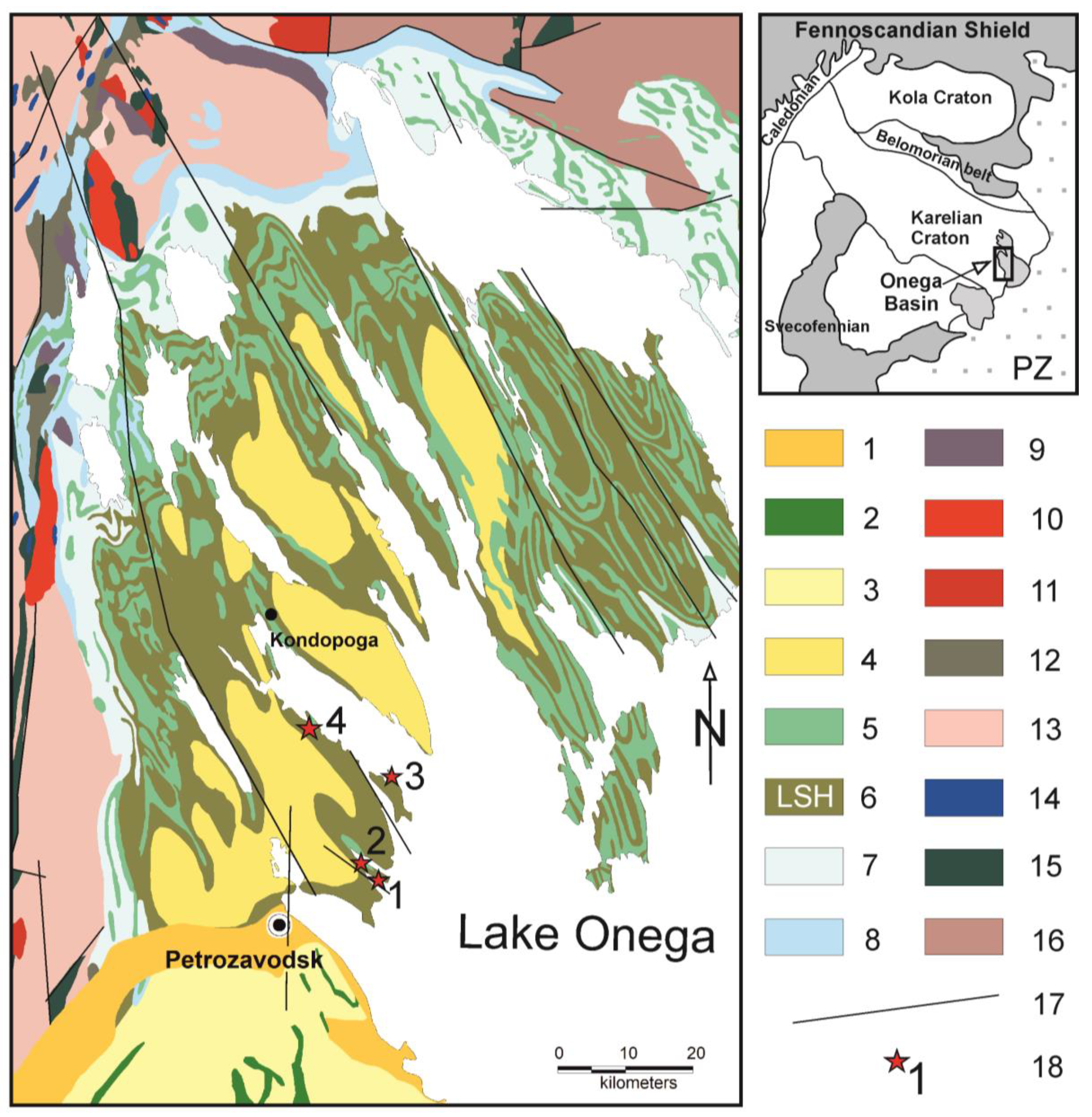
2. Geological Setting
- (1)
- (2)
- Suisari Formation (SF) has a total thickness up to 1 km and is represented by mafic lava flows interbedded with tuffs, hyaloclastites, tuffites. The subvolcanic complex comprises dolerite and peridotite dykes and sills. A depositional age of the SF by the Sm/Nd isotopic data is 1975 ± 24 Ma [28].
3. Agate Occurrences
- Pinguba. Shore outcrops of the northern part of the Pinguba bay of Lake Onega. Here, pillow porphyric basalt lava flows attributed to the upper part of the SF were examined. The lava outcrops cover an area along the water’s edge for 1 km at a width of 5–10 m. The pillows have rounded, elongated shapes, shell-like cleavage, and thin chill zones (up to 1 cm), their size ranges from 0.3 × 0.6 to 1.5 × 2.5 m. There are many gas vesicles (1–2 mm in size) filled by chlorite-carbonate-quartz in lavas. Agates form prominent nodules between pillows (Figure 2a) or fill gas cavities in pillow cores. Most agates are cone-shaped; lens-shaped bodies and veinlets are less frequent. The size of such agates ranges from 3–5 to 50 cm across. Agates also occur as rounded fragments on the shore. The frequency of agates occurrence is 3–5 per 10 m2 of the lava flow surface.
- Yalguba Ridge. The upper part of the SF section reaches 60 m in depth and is formed by massive and pillow Pl-Cpx porphyric picrobasalts. The section is well exposed in the Yalguba area of Lake Onega. The pillowed and massive lava flow interbed with tuffs with thickness up to 1–3 m. The pillows have compressed shapes, thin chill zones (up to 1 cm), their size ranges from 0.3 × 0.4 to 4 × 5 m. Some pillows have a well-developed sagging tail. Agates are present in the inter-pillow space of lava flows as large (up to 50–60 cm) segregations and veinlets varying in shape (Figure 2b). In some cases, agate mineralization is exposed in the central gas vesicles in the pillow cores. On the lava flow surface, the frequency of agates occurrence is 5–7 per 10 m2.
- Suisari Island. In the northeastern part of Lake Onega Island, numerous agates are observed in the Pl-porphyric picrobasalt lava flows. The stratigraphic attribution of volcanic rocks to ZF or SF on Suisari Island is unclear. The pillows are lightly deformed and have chill zones, their diameter ranges from 0.5 to 4 m. Agates fill cavities in the inter-pillow space of basalt flows, as well as in pillow cores (Figure 2e). These are found on the shore and underwater outcrops and can have a size of up to 50 cm. The frequency of agate occurrence is 4–6 per 10 m2 of the lava flow surface. After storms, agates join the beach pebbles.
- Tulguba. The studied area was by Lake Onega within an open-pit mine. The middle part of the SF section of the picrobasalt lava shows flows with thickness about 100–120 m. The lavas contain small (from 5 to 30 cm) and large (up to 2.5 m) pillows with chill zones (from 0.5 to 2 cm). Individual pillows have amygdales in the top flow (Figure 2f). Some pillows have flow banding—pahoehoe. The inter-pillow space is composed of a mixture of agglutinate tuff and terrigenous material. Lenses of agglomerate tuffs (with single large volcanic bombs) overlie on the lava top flows. Most of the agates are composed of quartz and calcite. Agates are present in the inter-pillow space, in the pillow cores and tuffs as large (up to 40 cm) segregations. The frequency of agates occurrence is up to 12 per 10 m2 of the lava flow surface.
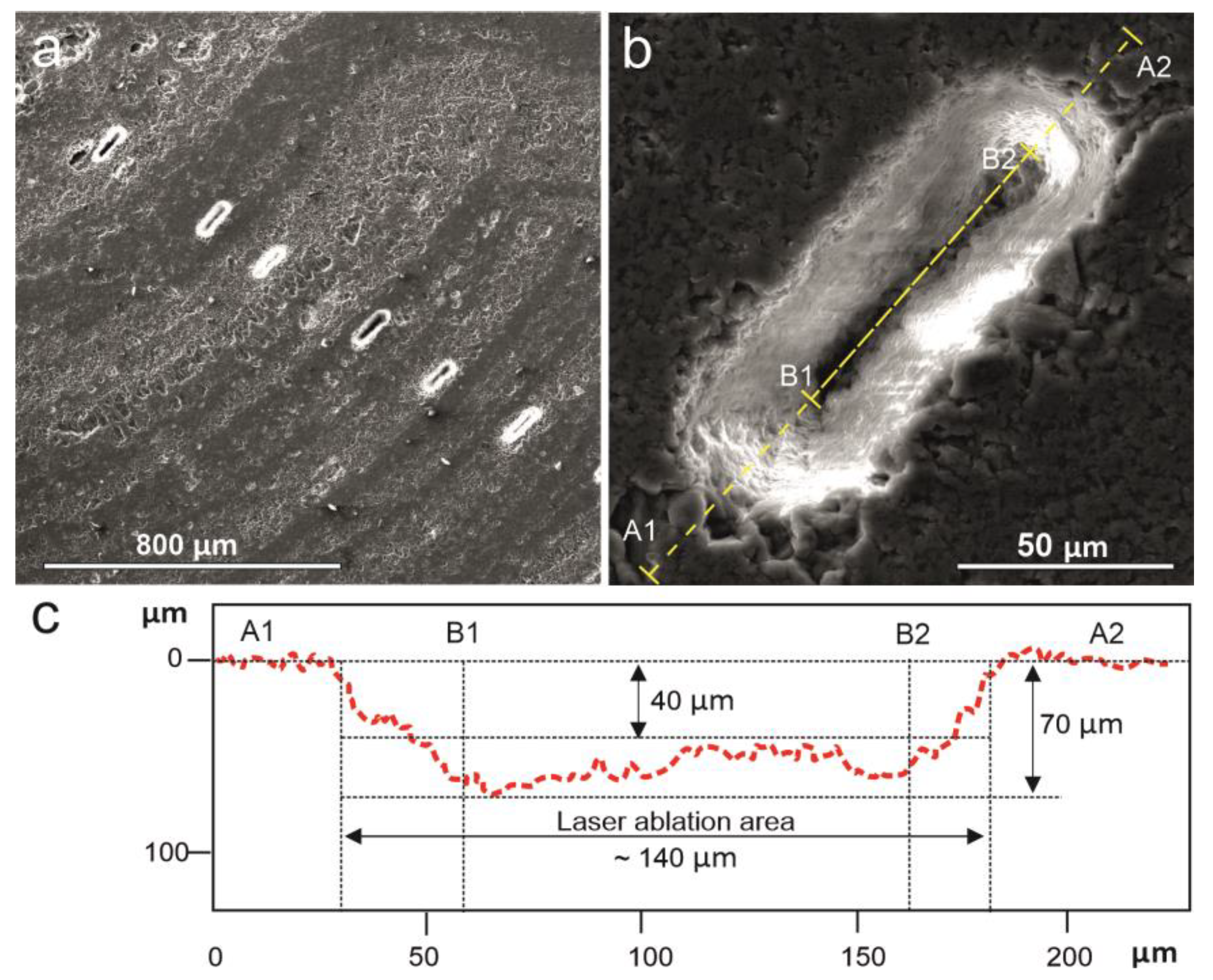
4. Methods
5. Results and Discussion
5.1. Host Rock Characteristic
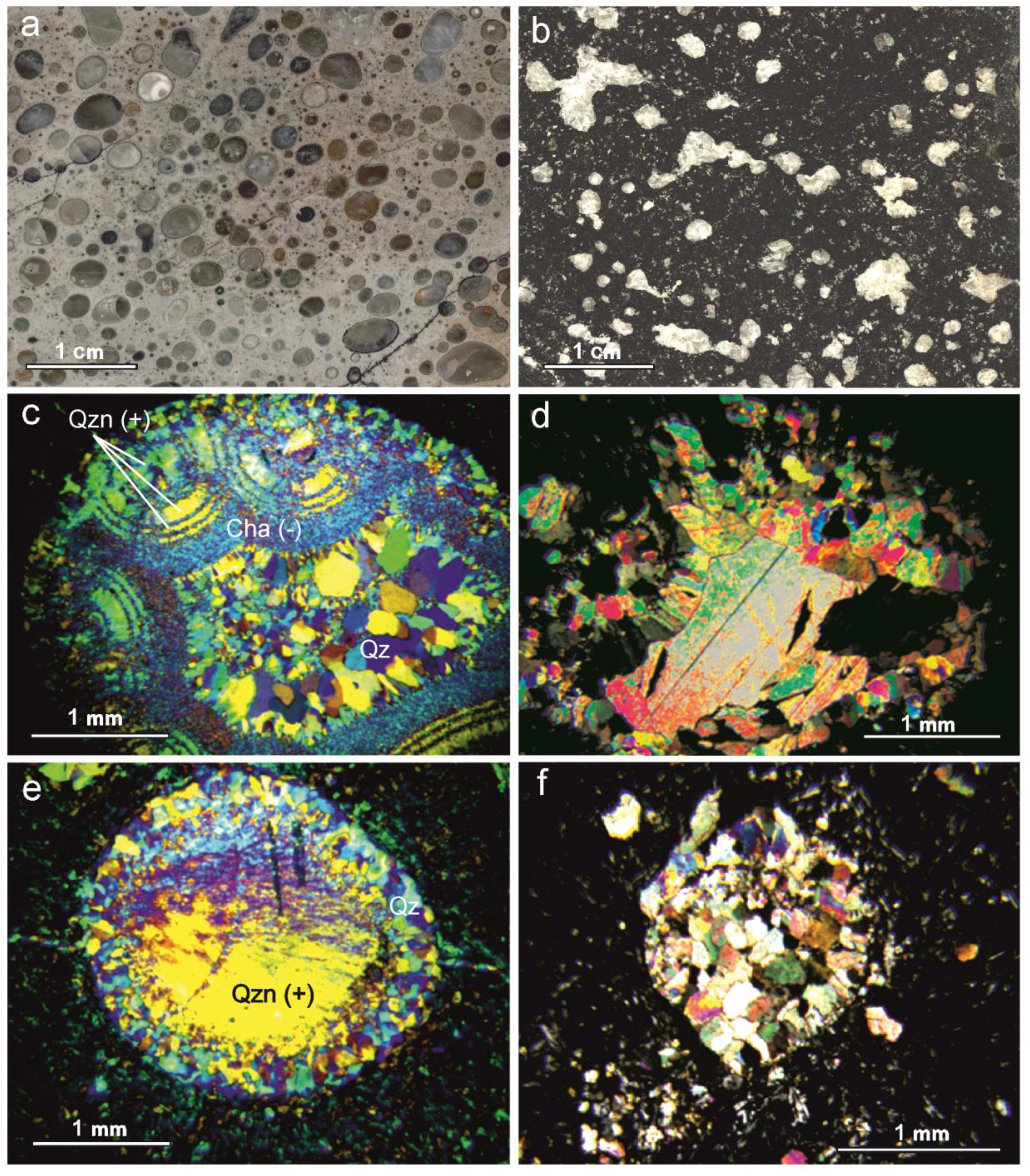
5.2. Morphology, Coloration, and Anatomy of Agates
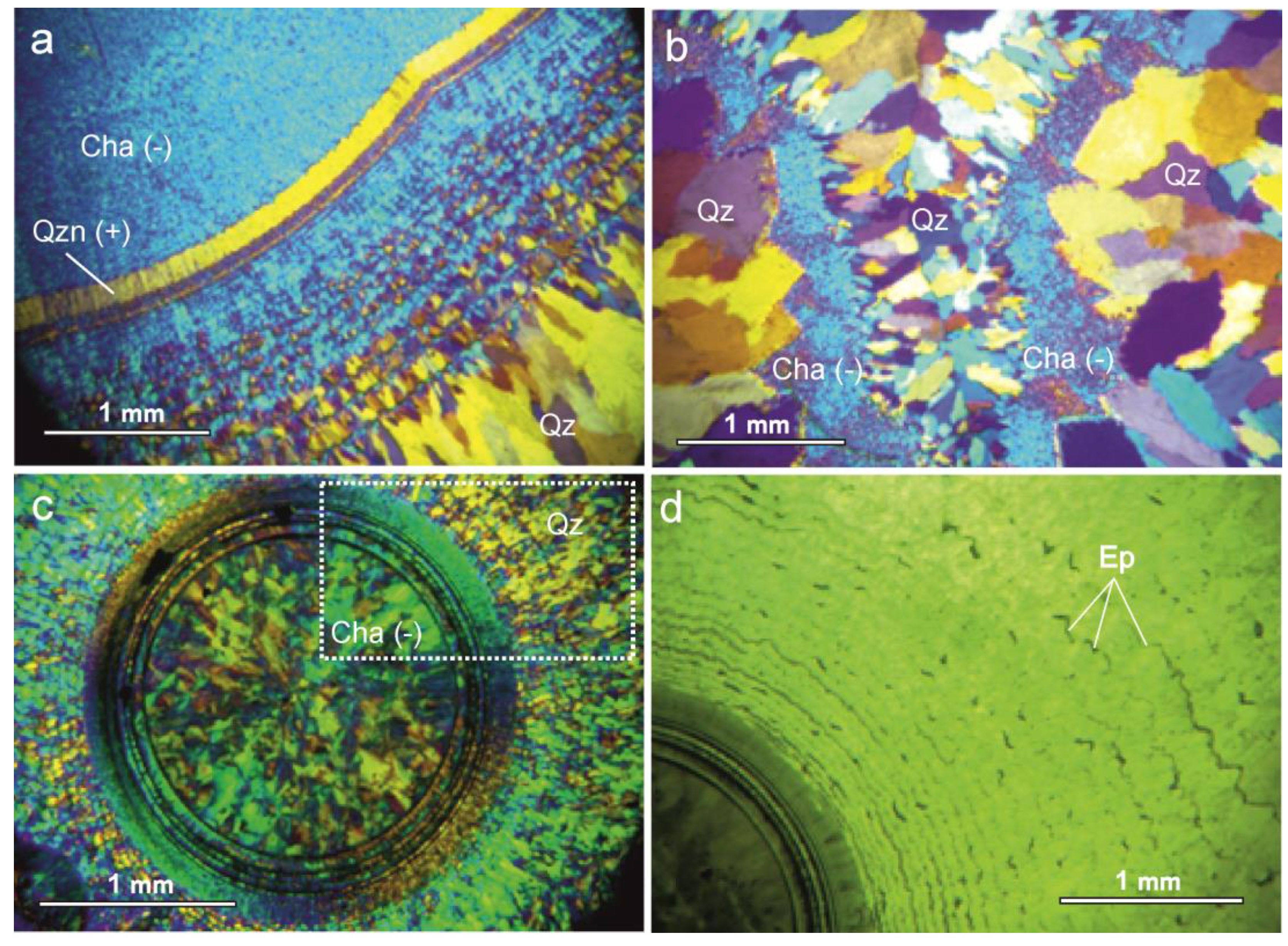
5.3. Optical Microscopy
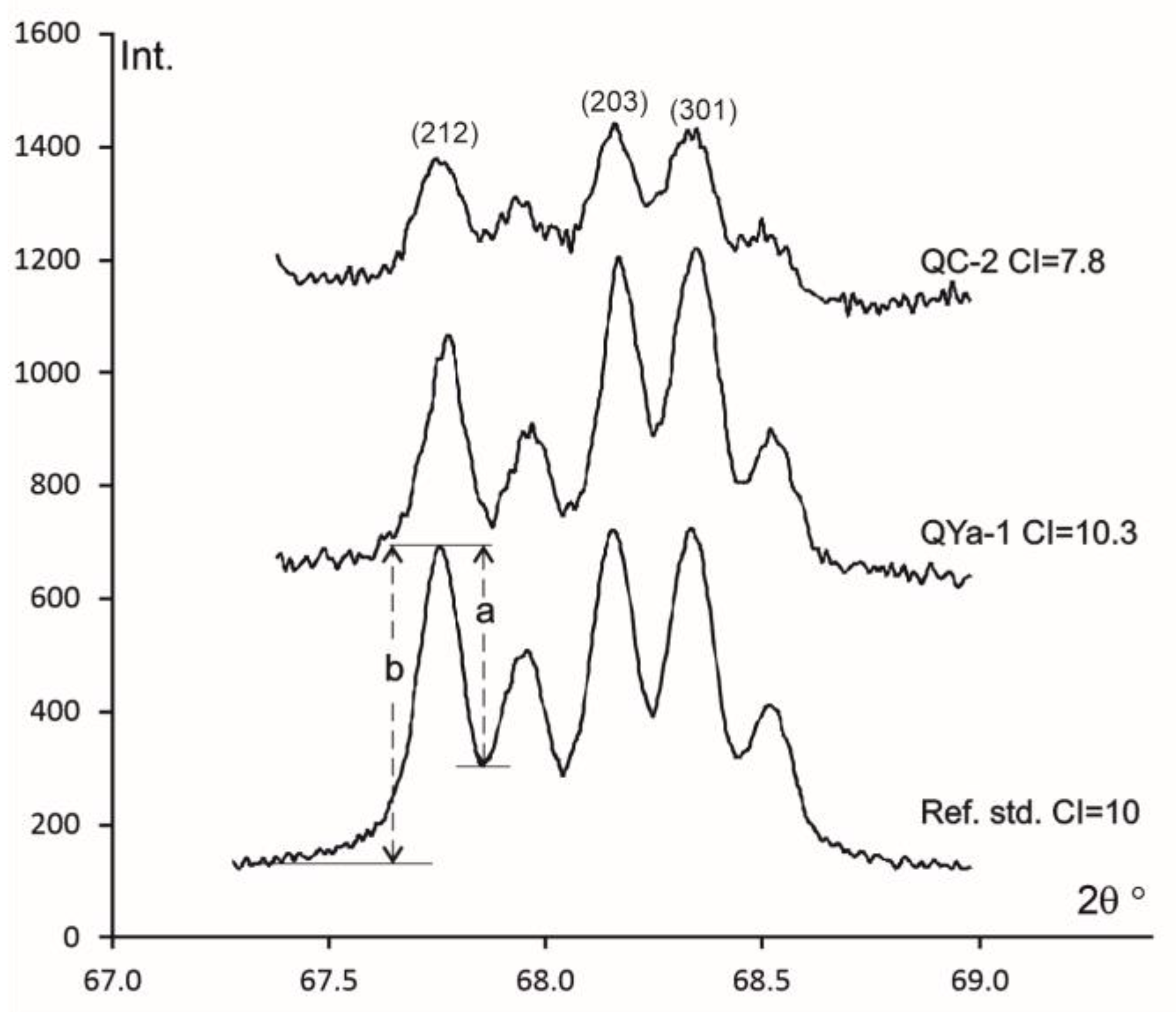
5.4. X-Ray Powder Diffraction
5.5. SEM and Microprobe Investigation
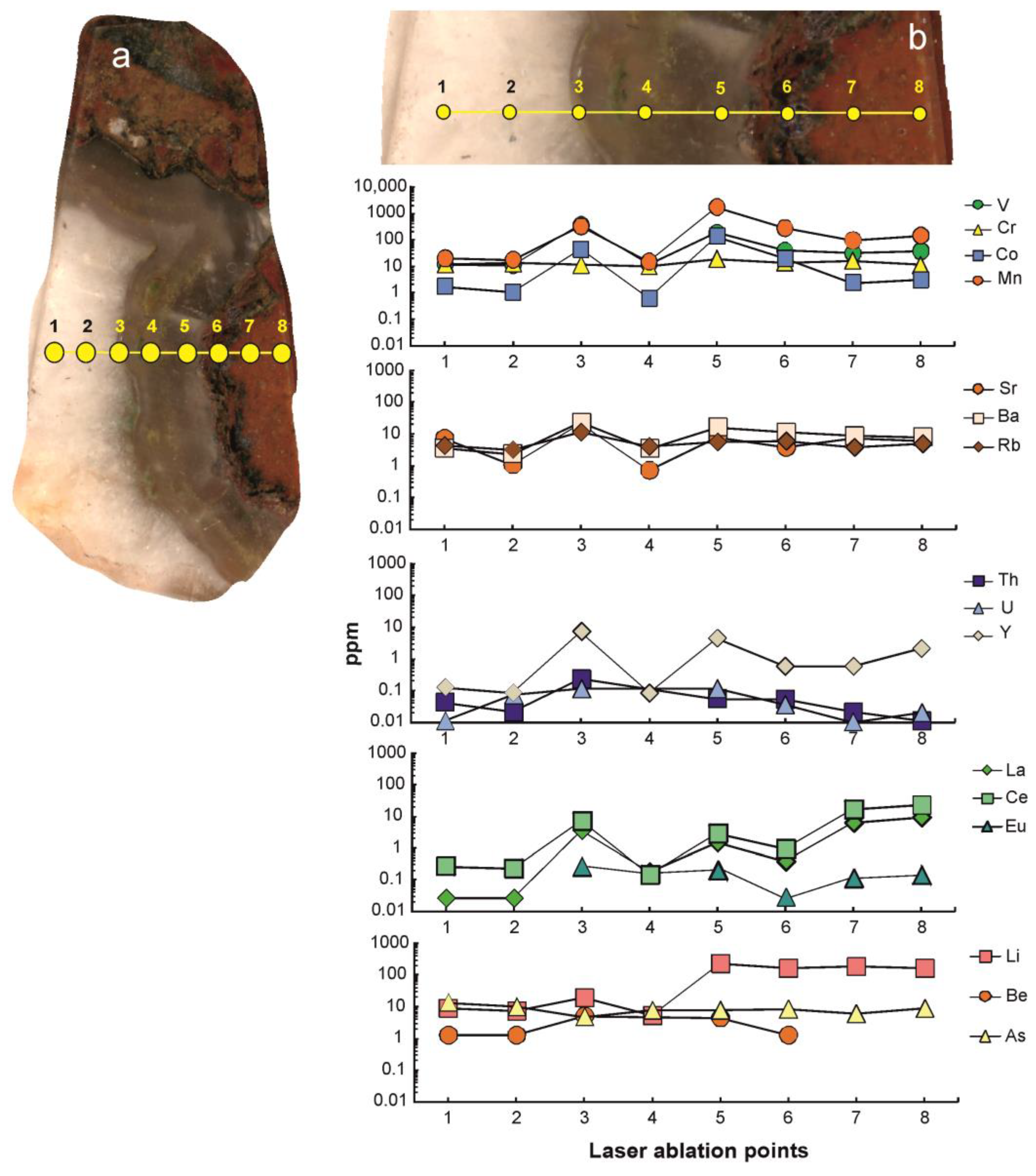
5.6. Geochemistry Investigation
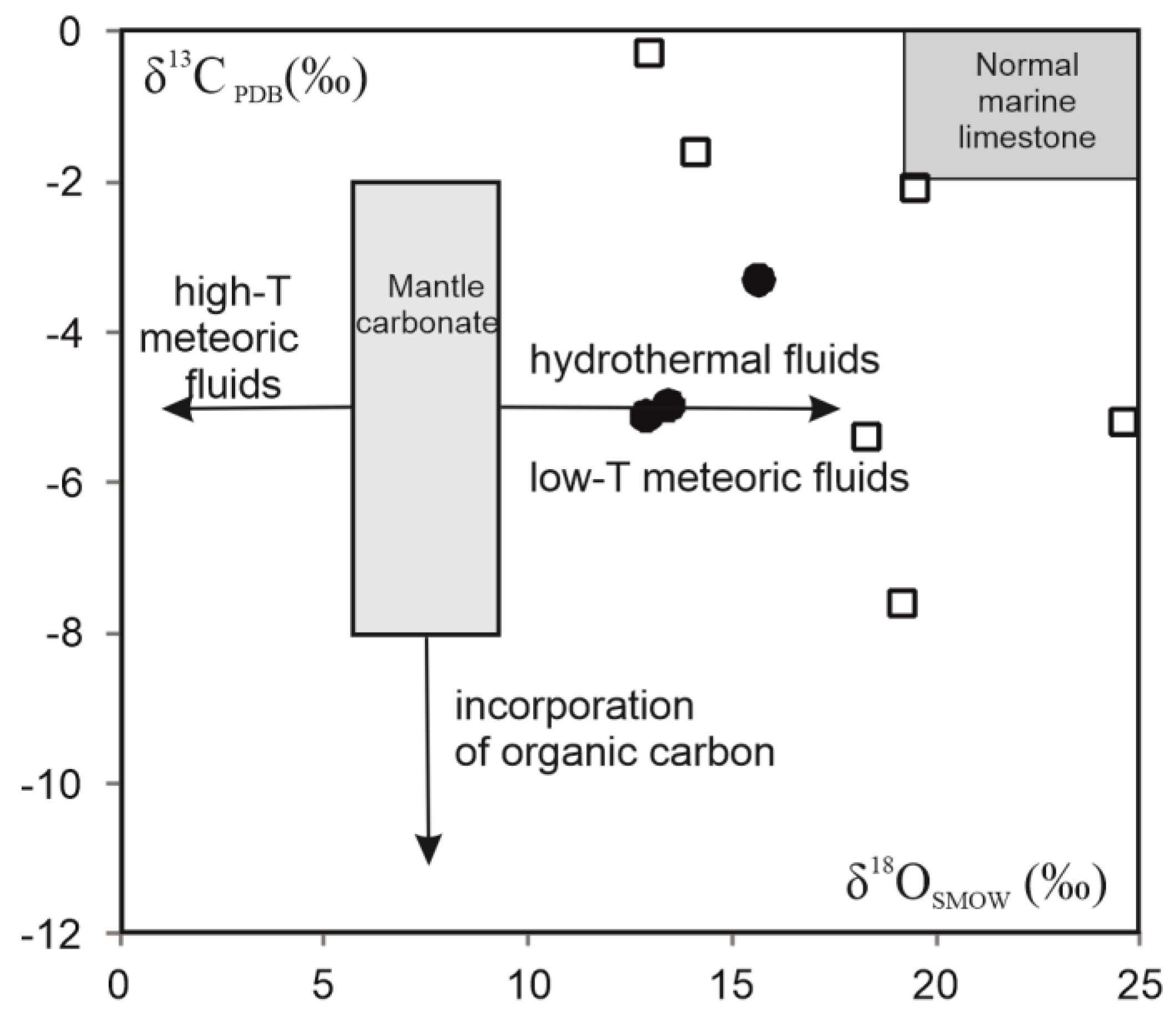
5.7. C-O Isotopic Composition
6. Conclusions
- (1)
- The present study describes the key occurrences of agate mineralization in volcanic rocks of the LSH within the Onega Basin, Karelian Craton, Southeast Fennoscandia, Russia (2100−1920 Ma). Agates occur in the inter-pillow lava space, fills gas vesicles, and tectonic cracks of volcanics.
- (2)
- Investigations showed that this mineralization is represented by silicates, oxides and hydroxides, carbonates, phosphates, sulfides, and sulfates. Among the silica minerals in agates only chalcedony, quartz, and quartzine are found. The parameters of the quartz structure according to the X-ray diffraction data (well-develops reflections (212), (203), (301), large crystallite sizes (Cs 710–1050 Å) and CI (7.8–10.3) provide evidence for multi-stage silica minerals recrystallization due to a metamorphic (thermal) effect.
- (3)
- The elevated concentrations of certain elements (V, Co, Mn, Ba, Y, La, Ce, Eu, Li) in chalcedonic layers of banded agates in comparison with macro crystalline quartz areas were confirmed. The concentrations of Cr, As, Be, and Rb are only slightly varying. The observed decrease of trace element concentration in banded agates from the agate host rock to core is probably reflecting a chemical purification process during crystallization.
- (4)
- C−O isotope characteristic of agate-associated calcite reflects a primary magmatic origin with the influence of hydrothermal or low-thermal meteoric fluids.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moxon, T. Agate Microstructure and Possible Origin; Terra Publications: Doncaster, UK, 1996; p. 106. [Google Scholar]
- Moxon, T.; Palyanova, G. Agate Genesis: A Continuing Enigma. Minerals 2020, 10, 953. [Google Scholar] [CrossRef]
- Saunders, J. A Oxygen-isotope zonation of agates from Karoo volcanic of the Skeleton Coast, Namibia: Discussion and Reply. Am. Mineral. 1990, 75, 1205–1206. [Google Scholar]
- Fallick, A.E.; Jocelyn, J.; Donelly, T.; Guy, M.; Behan, C. Origin of agates in the volcanic rocks of Scotland. Nature 1985, 313, 672–674. [Google Scholar] [CrossRef]
- Godovikov, A.A.; Ripinen, O.I.; Motorin, S.G. Agates; Nedra: Moscow, Russia, 1987; p. 368. (In Russian) [Google Scholar]
- Kigai, I.N. The genesis of agates and amethyst geodes. Can. Mineral. 2019, 57, 867–883. [Google Scholar] [CrossRef]
- Liesegang, R.E. Die Achate; T. Verlag von Theodor Steinkopff: Dresden, Germany; Leipzig, Germany, 1915; p. 122. [Google Scholar]
- Landmesser, M. Mobility by metastability: Silica transport and accumulation at low temperatures. Chem. Erde 1995, 55, 149–176. [Google Scholar]
- Goncharov, V.I.; Gorodinsky, M.E.; Pavlov, G.F.; Savva, N.E.; Fadeev, A.P.; Vartanov, V.V.; Gunchenko, E.V. Chalcedony of North-East of the USSR; Science: Moscow, Russia, 1987; p. 192. (In Russian) [Google Scholar]
- Nacken, R. Über die Nachbildung von Chalcedon–Mandeln. Natur Folk 1948, 78, 2–8. [Google Scholar]
- Spiridonov, E.M.; Ladygin, V.M.; Frolova, Y.V. When and how the vesicular lavas are transformed into mandelstones, agate-bearing ones included. In Proceedings of the Conference «Paleovulcanology, Vulcano-Sedimentary Lithogenesis, Hydrothermal Metamorphism and Ore Formation in the Precambrian», Petrozavodsk, Russia, 20–25 August 2001; Svetov, A.P., Ed.; IG KarRC RAS: Petrozavodsk, Russia, 2001; pp. 123–124. (In Russian). [Google Scholar]
- Pilipenko, P.P. Zur Frage der Achat genese. Bul. Soc. Nat. Mosc. 1934, 12, 279–299, (In German and Russian). [Google Scholar]
- Mockel, R.; Götze, J.; Sergeev, S.A.; Kapitonov, I.N.; Adamskaya, E.V.; Goltsin, N.A.; Vennemann, T. Trace-Element Analysis by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): A Case Study for Agates from Nowy Kościoł, Poland. J. Sib. Fed. Univ. Eng. Technol. 2009, 2, 123–138. [Google Scholar]
- Florke, O.W.; Kohler-Herbertz, B.; Langer, K.; Tonges, I. Water in microcrystalline quartz of volcanic origin: Agates. Contrib. Mineral. Petrol. 1982, 80, 324–333. [Google Scholar] [CrossRef]
- Harris, C. Oxygen-isotope zonation of agates from Karoo volcanics of the Skeleton Coast. Namibia Am. Mineral. 1989, 74, 476–481. [Google Scholar]
- Wang, Y.; Merino, E. Self-organizational origin of agates: Banding, fiber twisting, composition, and dynamic crystallization model. Geochim. Cosmochim. Acta 1990, 54, 1627–1638. [Google Scholar] [CrossRef]
- Heaney, P.J. A Proposed Mechanism for the Growth of Chalcedony. Contrib. Mineral. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Götze, J.; Tichomirowa, M.; Fuchs, H.; Pilot, J.; Sharp, Z.D. Geochemistry of agates: A trace element and stable isotope study. Chem. Geol. 2001, 175, 523–541. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Vennemann, T.; Muller, A. Origin and geochemistry of agates in Permian volcanic rocks of the Sub-Erzgebirge basin, Saxony (Germany). Chem. Geol. 2016, 428, 77–91. [Google Scholar] [CrossRef]
- Moxon, T.; Nelson, D.R.; Zhang, M. Agate recrystallization: Evidence from samples found in Archaean and Proterozoic host rocks, Western Australia. Aust. J. Earth Sci. 2006, 53, 235–248. [Google Scholar] [CrossRef]
- Moxon, T.; Reed, S.J.B.; Zhang, M. Metamorphic effects on agate found near the Shap granite, Cumbria: As demonstrated by petrography, X-ray diffraction spectroscopic methods. Miner. Mag. 2007, 71, 461–476. [Google Scholar] [CrossRef]
- Moxon, T.; Carpenter, M.A. Crystallite growth kinetics in nanocrystalline quartz (agate and chalcedony). Miner. Mag. 2009, 73, 551–568. [Google Scholar] [CrossRef]
- Moxon, T.; Petrone, C.M.; Reed, S.J.B. Characterization and genesis of horizontal banding in Brazilian agate: An X-ray diffraction, thermogravimetric and microprobe study. Miner. Mag. 2013, 77, 227–248. [Google Scholar] [CrossRef]
- Zenz, J. Achate; Bode-Verlag GmbH: Salzhemmendorf, Germany, 2005; p. 656. (In German) [Google Scholar]
- Melezhik, V.A.; Medvedev, P.V.; Svetov, S.A. The Onega basin. In Reading the Archive of Earth’s Oxygenation; Melezhik, V.A., Prave, A.R., Fallick, A.E., Kump, L.R., Strauss, H., Lepland, A., Hanski, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 387–490. [Google Scholar]
- Timofeev, V.M. Chalcedony of Sujsar Island. Proc. Soc. St. Petersburg Nat. 1912, 35, 157–174. (In Russian) [Google Scholar]
- Puchtel, I.S.; Arndt, N.T.; Hofmann, A.W.; Haase, K.M.; Kröner, A.; Kulikov, V.S.; Kulikova, V.V.; Garbe-Schönberg, C.D.; Nemchin, A.A. Petrology of mafic lavas within the Onega plateau, central Karelia: Evidence for 2.0 Ga plume-related continental crustal growth in the Baltic Shield. Contrib. Mineral. Petrol. 1998, 130, 134–153. [Google Scholar] [CrossRef]
- Kulikov, V.S.; Kulikova, V.V.; Lavrov, B.S.; Pisarevskii, S.A.; Pukhtel, I.S.; Sokolov, S.Y. The Paleoroterozoic Suisarian Picrite–Basalt Complex in Karelia: Key Section and Petrology; KNTs RAN: Petrozavodsk, Russia, 1999; p. 96. (In Russian) [Google Scholar]
- Glushanin, L.V.; Sharov, N.V.; Shchiptsov, V.V. Paleoproterozoic Onega Structure: Geology, Tectonics, Structure, and Metallogeny; Karelian Research Centre, RAS: Petrozavodsk, Russia, 2011; p. 431. (In Russian) [Google Scholar]
- Glebovitskii, V.A.; Bushmin, S.A.; Belyatsky, B.V.; Bogomolov, E.S.; Borozdin, A.P.; Savva, E.V.; Lebedeva, Y.M. RB-SR age of metasomatism and ore formation in the low-temperature shear zones of the Fenno-Karelian Craton, Baltic Shield. Petrology 2014, 22, 184–204. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Putintzeva, E.V.; Lavrov, O.B.; Ladygin, V.M. Kronstedtite, pumpelliite, prehnite and lennilenapeite in the metaagates and metabasalts of the early Proterozoic trap formation in the northern Onega region. In Proceedings of the Conference «Lomonosov Readings», Moscow, Russia, 17–27 April 2017; Moscow State University: Moscow, Russia. Available online: https://conf.msu.ru/file/event/4305/eid4305_attach_b0acc3e7de2cd859225469534617a6272d70ce50.pdf (accessed on 8 December 2020). (In Russian).
- Spiridonov, E.; Ladygin, V.; Frolova, Y.; Lavrov, O.; Putintseva, E.; Semikolennykh, E.; Sokolov, V.; Chernov, M. Agates in the low-grade metamorphic volcanic rocks: “bearing”, “existence”, “extinction”. In Book of Abstracts of the International Conference on Clay Science and Technology EUROCLAY 2019, Paris, France, 1–5 July 2019; Sorbonne University: Paris, France, 2019; p. 563. [Google Scholar]
- Kulikov, V.S.; Svetov, S.A.; Slabunov, A.I.; Kulikova, V.V.; Polin, A.K.; Golubev, A.I.; Gorkovets, V.Y.; Ivashchenko, V.I.; Gogolev, M.A. Geological map of Southeastern Fennoscandia (scale 1:750,000): A new approach to map compilation. Trans. KarRC RAS 2017, 2, 3–41. (In Russian) [Google Scholar] [CrossRef]
- Slabunov, A.I.; Lobach-Zhuchenko, S.B.; Bibikova, E.V.; Sorjonen-Ward, P.; Balagansky, V.V.; Volodichev, O.I.; Shchipansky, A.A.; Svetov, S.A.; Chekulaev, V.P.; Arestova, N.A.; et al. The Archaean nucleus of the Fennoscandian (Baltic) Shield. In European Lithosphere Dynamics; GEE, D.G., Stephenson, R.A., Eds.; Memoirs, no. 32; Geological Society: London, UK, 2006; pp. 627–644. [Google Scholar]
- Sokolov, V.A.; Galdobina, L.P. The Ludicovi—A new stratigraphic subdivision of the lower Proterozoic in Karelia. Trans. USSR Acad. Sci. 1982, 267, 187–190. (In Russian) [Google Scholar]
- Narkisova, V.V. Petrology and geochemistry of igneous rocks in the OPB section. In Paleoproterozoic Onega Structure: Geology, Tectonics, Structure, and Metallogeny; Glushanin, L.V., Sharov, N.V., Shchiptsov, V.V., Eds.; KarRC of RAS: Petrozavodsk, Russia, 2011; pp. 195–208. (In Russian) [Google Scholar]
- Martin, A.P.; Prave, A.R.; Condon, D.J.; Lepland, A.; Fallick, A.E.; Romashkin, A.E.; Medvedev, P.V.; Rychanchik, D.V. Multiple Palaeoproterozoic carbon burial episodes and excursions. Earth Planet. Sci. Lett. 2015, 424, 226–236. [Google Scholar] [CrossRef]
- Svetov, S.A.; Chazhengina, S.J. Geological Phenomenon of Yalguba Ridge Variolite from F. Yu. Levinson-Lessing’s Time until Today: Mineralogical and Geochemical Aspects. Geol. Ore Depos. 2018, 60, 547–558. [Google Scholar] [CrossRef]
- Gudin, A.N.; Dubinina, E.O.; Nosova, A.A. Petrogenesis of Variolitic Lavas of the Onega Structure, Central Karelia. Petrology 2012, 20, 255–270. [Google Scholar] [CrossRef]
- Svetov, S.A.; Chazhengina, S.Y.; Stepanova, A.V. Geochemistry and texture of clinopyroxene phenocrysts from Paleoproterozoic picrobasalts, Onega Basin, Fennoscandian Shield: Records of magma mixing processes. Minerals 2020, 10, 434. [Google Scholar] [CrossRef]
- Bibikova, E.V.; Kirnozova, T.I.; Lazarev, Y.I.; Makarov, V.A.; Nikolaev, A.A. U–Pb isotopic age of the Karelian Vepsian. Trans. USSR Acad. Sci. 1990, 310, 189–191. [Google Scholar]
- Lokhov, K.I.; Goltsin, N.A.; Kapitonov, I.N.; Prasolov, E.M.; Polekhovsky, Y.S.; Bogomolov, E.S.; Akhmedov, A.M.; Sergeyev, S.A. Isotopic dating of Zaonega Formation rocks subjected to stepwise alteration in the Khmelozerskaya syncline. In Paleoproterozoic Onega Structure: Geology, Tectonics, Structure, and Metallogeny; Glushanin, L.V., Sharov, N.V., Shchiptsov, V.V., Eds.; KarRC of RAS: Petrozavodsk, Russia, 2011; pp. 297–314. (In Russian) [Google Scholar]
- Svetov, S.A.; Stepanova, A.V.; Chazhengina, S.Y.; Svetova, E.N.; Rybnikova, Z.P.; Mikhailova, A.I.; Paramonov, A.S.; Utitsyna, V.L.; Ekhova, M.V.; Kolodey, B.S. Precision geochemical (ICP–MS, LA–ICP–MS) analysis of rock and mineral composition: The method and accuracy estimation in the case study of Early Precambrian mafic complexes. Tr. KarRC RAS 2015, 7, 54–73. [Google Scholar] [CrossRef]
- Moxon, T.; Rios, S. Moganite and water content as a function of age in agate: An XRD and 1497 thermogravimetric study. Eur. J. Miner. 2004, 4, 693–706. [Google Scholar]
- Murata, J.; Norman, M.B. An index of crystallinity for quartz. Am. J. Sci. 1976, 276, 1120–1130. [Google Scholar] [CrossRef]
- Kuznetsov, S.K.; Svetova, E.N.; Shanina, S.N.; Filippov, V.N. Minor Elements in Quartz from Hydrothermal-Metamorphic Veins in the Nether Polar Ural Province. Geochemistry 2012, 50, 911–925. [Google Scholar] [CrossRef]
- Svetova, E.N.; Svetov, S.A. Agates from Paleoproterozoic volcanic rocks of the Onega Structure, Central Karelia. Geol. Ore Depos. 2020, 62. In Press. [Google Scholar] [CrossRef]
- Faure, G. Principles of Isotope Geology, 2nd ed.; Wiley: New York, NY, USA, 1986; p. 589. [Google Scholar]
- Giuliani, A.; Phillips, D.; Kamenetsky, V.S.; Fiorentini, M.L.; Farquhar, J.; Kendrick, M.A. Stable isotope (C, O, S) compositions of volatile-rich minerals in kimberlites: A review. Chem. Geol. 2014, 374–375, 61–83. [Google Scholar] [CrossRef]
- Valley, J.W. Stable isotope geochemistry of metamorphic rocks. Rev. Mineral. Geochem. 1986, 16, 445–489. [Google Scholar]



| Sample | SiO2 | Al2O3 | FeOtot | Na2O | CaO | K2O | MgO | MnO | TiO2 | P2O5 | S | LOI | Σ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pinguba | |||||||||||||
| BP-1 * | 57.73 | 12.32 | 9.05 | 3.97 | 3.33 | 0.39 | 7.34 | 0.10 | 1.55 | 0.15 | 0.08 | 3.87 | 99.88 |
| BP-2 * | 53.87 | 13.69 | 9.04 | 3.94 | 3.82 | 0.80 | 7.96 | 0.10 | 1.49 | 0.15 | 0.07 | 5.10 | 100.03 |
| BP-3 * | 49.88 | 15.01 | 11.22 | 4.16 | 2.62 | 0.34 | 8.75 | 0.12 | 1.64 | 0.20 | 0.07 | 5.36 | 99.37 |
| BP-4 | 50.48 | 13.92 | 9.85 | 4.37 | 4.00 | 0.55 | 9.12 | 0.11 | 1.61 | 0.15 | 0.08 | 5.85 | 100.09 |
| BP-5 | 49.09 | 14.33 | 10.62 | 4.39 | 3.22 | 0.28 | 9.49 | 0.10 | 1.65 | 0.17 | 0.07 | 6.33 | 99.74 |
| Yalguba Ridge | |||||||||||||
| BYa-1 ** | 39.32 | 9.30 | 7.47 | 0.23 | 16.01 | 3.62 | 7.81 | 0.15 | 1.11 | 0.10 | 0.07 | 14.34 | 99.53 |
| BYa-2 | 45.91 | 11.49 | 14.47 | 0.13 | 9.58 | 0.56 | 10.82 | 0.21 | 1.50 | 0.14 | 0.08 | 4.96 | 99.85 |
| Suisari Island | |||||||||||||
| BC-1 | 54.78 | 12.27 | 8.45 | 4.59 | 7.43 | 0.34 | 8.14 | 0.13 | 1.54 | 0.17 | 0.08 | 1.93 | 99.85 |
| BC-2 | 61.08 | 13.26 | 8.30 | 3.66 | 2.27 | 1.26 | 4.89 | 0.08 | 1.73 | 0.23 | 0.07 | 2.95 | 99.78 |
| Tulguba | |||||||||||||
| S20-19 | 47.61 | 13.19 | 12.31 | 1.91 | 8.00 | 1.95 | 9.97 | 0.14 | 2.10 | 0.18 | 0.00 | 2.38 | 99.74 |
| S20-21 | 48.48 | 12.44 | 11.81 | 2.53 | 8.78 | 0.62 | 10.13 | 0.17 | 2.06 | 0.19 | 0.00 | 2.22 | 99.43 |
| Occurrences | Main Minerals | Accessory Minerals |
|---|---|---|
| Pinguba | quartz | calcite, chlorite, epidote, hematite, goethite, pyrite |
| Yalguba Ridge | calcite, quartz | chlorite, epidote, phengite, prehnite, apatite, titanite, leucoxene, albite, covellite, pentlandite, bornite, chalcopyrite, pyrite, hematite |
| Suisari island | quartz | calcite, chlorite, epidote, K-feldspar, titanite, barite, carbonaceous phases |
| Tulguba | calcite, quartz | chlorite, epidote, mica, titanite, chalcopyrite, chalcocite, bornite, hematite, magnetite |
| Sample | a ± Δa, Å | c ± Δc, Å | V, Å3 | Cs, Å | CI | |
|---|---|---|---|---|---|---|
| Pinguba | ||||||
| A4x1 | Wall-lining chalcedony | 4.9131 ± 0.0001 | 5.4048 ± 0.0002 | 112.98 | 1047 | 8.5 |
| A9x | Quartz from agate core | 4.9133 ± 0.0001 | 5.4051 ± 0.0001 | 113.00 | 922 | 9.1 |
| A10x | Chalcedony from the outer layer of agate | 4.9137 ± 0.0001 | 5.4054 ± 0.0001 | 113.02 | 922 | 8.7 |
| A10k | Quartz from agate core | 4.9137 ± 0.0001 | 5.4054 ± 0.0001 | 113.02 | 934 | 9.1 |
| A11x | Wall-lining chalcedony | 4.9137 ± 0.0001 | 5.4052 ± 0.0001 | 113.02 | 848 | 8.7 |
| Yalguba Ridge | ||||||
| QYa-1 | Quartz from agate core | 4.9133 ± 0.0001 | 5.4050 ± 0.0002 | 113.00 | 908 | 10.3 |
| QYa-2 | Quartz from agate core | 4.9136 ± 0.0001 | 5.4051 ± 0.0002 | 113.02 | 817 | 10.2 |
| Suisari island | ||||||
| QC-1 | Quartz from agate core | 4.9134 ± 0.0001 | 5.4050 ± 0.0002 | 113.00 | 709 | 9.7 |
| QC-2 | Chalcedony from the outer layer of agate | 4.9136 ± 0.0001 | 5.4050 ± 0.0002 | 113.01 | 763 | 7.8 |
| Tulguba | ||||||
| QT-2 | Quartz from agate core | 4.9137 ± 0.0001 | 5.4050 ± 0.0001 | 113.02 | 801 | 10.1 |
| QT-3 | Quartz from agate core | 4.9137 ± 0.0001 | 5.4051 ± 0.0001 | 113.02 | 749 | 10.2 |
| Ref. std. | Quartz crystal | 4.9133 ± 0.0001 | 5.4052 ± 0.0001 | 113.00 | 935 | 10 |
| Sample | a ± Δa, Å | c ± Δc, Å | V, Å3 | |
|---|---|---|---|---|
| Tulguba | ||||
| CT-1 | Colorless crystals | 4.9851 ± 0.0003 | 17.050 ± 0.002 | 366.9 |
| CT-2 | Smoky crystals | 4.9872 ± 0.0002 | 17.057 ± 0.002 | 367.4 |
| CT-4 | Smoky crystals | 4.9865 ± 0.0004 | 17.057 ± 0.002 | 367.3 |
| CT-5 | Smoky crystals | 4.9876 ± 0.0004 | 17.056 ± 0.002 | 367.4 |
| CSh-2 | Rose crystals | 4.9865 ± 0.0004 | 17.057 ± 0.004 | 367.3 |
| Yalguba Ridge | ||||
| CYa-1 | Colorless crystals | 4.9867 ± 0.0004 | 17.041 ± 0.002 | 367.0 |
| CYa-3 | Smoky crystals | 4.9907 ± 0.0004 | 17.065 ± 0.002 | 368.1 |
| CYa-6 | Rose crystals | 4.9810 ± 0.0004 | 17.028 ± 0.002 | 365.9 |
| CYa-7 | Colorless crystals | 4.9848 ± 0.0005 | 17.053 ± 0.002 | 367.0 |
| Sample | δ13C PDB(‰) | δ18O SMOW(‰) |
|---|---|---|
| CYa-1 | −3.31 | 15.67 |
| CYa-2 | −4.98 | 13.46 |
| CYa-3 | −5.12 | 12.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetova, E.N.; Svetov, S.A. Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia). Minerals 2020, 10, 1106. https://doi.org/10.3390/min10121106
Svetova EN, Svetov SA. Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia). Minerals. 2020; 10(12):1106. https://doi.org/10.3390/min10121106
Chicago/Turabian StyleSvetova, Evgeniya N., and Sergei A. Svetov. 2020. "Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia)" Minerals 10, no. 12: 1106. https://doi.org/10.3390/min10121106
APA StyleSvetova, E. N., & Svetov, S. A. (2020). Mineralogy and Geochemistry of Agates from Paleoproterozoic Volcanic Rocks of the Karelian Craton, Southeast Fennoscandia (Russia). Minerals, 10(12), 1106. https://doi.org/10.3390/min10121106






