Molybdenum Isotope Constraints on the Origin of Vanadium Hyper-Enrichments in Ediacaran–Phanerozoic Marine Mudrocks
Abstract
1. Introduction
2. Background Information
2.1. Vanadium
2.2. Molybdenum
3. Materials and Methods
- Iron speciation: FePY/FeHR > 0.7 (must also record FeHR/FeT > 0.38 for anoxia), where FeHR (highly reactive Fe) is the sum of Fe pools, which can react with H2S in the water or sediment column during deposition or early diagenesis (i.e., FePY + Feox + Fecarb + Femag) [4].
- Total organic carbon content: TOC > 1 weight percent (wt%).
- Molybdenum concentration: [Mo] > 100 μg/g [1].
4. Results
4.1. Mo Isotope Composition of Sediments with and without Vanadium Hyper Enrichments
4.2. Vanadium Concentration and Molybdenum Isotope Covariation
4.2.1. Sediments with Negative V-δ98Mo Trends
4.2.2. Sediments within a Positive V-δ98Mo Trend
4.2.3. Organic-Rich Mudrocks without V Hyper-Enrichments
5. Discussion
5.1. Universal Redox Interpretation from V Hyper Enrichments?
5.2. Influence of Fe-Mn Particulate Shuttles on V Enrichments
5.3. V Hyper-Enrichments in Euxinic Semi-Restricted Basins
5.4. Ancient Localities without V Hyper-Enrichments
5.5. Why Are V Hyper-Enrichments Absent in Modern Euxinic Sediments?
6. Conclusions
- Fe-Mn particulate shuttling providing excess adsorbed V to euxinic bottom waters facilitating removal into sediments when redoxclines deepen towards the sediment–water interface;
- Water column stratification in moderately semi-restricted, highly productive basins with rapid water renewal from the open ocean is hypothesized to produce V hyper-enrichments under strongly euxinic conditions;
- In a similar basin configuration to that of mechanism (2), weakly euxinic sediments may also obtain V hyper-enrichments, however, an association with organic matter and slow clastic sedimentation may amplify relative V concentrations leading to hyper-enrichments.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Scott, C.; Lyons, T.W. Contrasting molybdenum cycling and isotopic properties in euxinic versus non-euxinic sediments and sedimentary rocks: Refining the paleoproxies. Chem. Geol. 2012, 324–325, 19–27. [Google Scholar] [CrossRef]
- Crusius, J.; Calvert, S.; Pedersen, T.; Sage, D. Rhenium and molybdenum enrichments in sediments as indicators of oxic, suboxic and sulfidic conditions of deposition. Earth Planet. Sci. Lett. 1996, 145, 65–78. [Google Scholar] [CrossRef]
- Lyons, T.W.; Severmann, S. A critical look at iron paleoredox proxies: New insights from modern euxinic marine basins. Geochim. Cosmochim. Acta 2006, 70, 5698–5722. [Google Scholar] [CrossRef]
- Raiswell, R.; Hardisty, D.S.; Lyons, T.W.; Canfield, D.E.; Owens, J.D.; Planavsky, N.J.; Poulton, S.W.; Reinhard, C.T. The iron paleoredox proxies: A guide to the pitfalls, problems and proper practice. Am. J. Sci. 2018, 318, 491–526. [Google Scholar] [CrossRef]
- Turgeon, S.; Brumsack, H.-J. Anoxic vs dysoxic events reflected in sediment geochemistry during the Cenomanian–Turonian Boundary Event (Cretaceous) in the Umbria–Marche Basin of central Italy. Chem. Geol. 2006, 234, 321–339. [Google Scholar] [CrossRef]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Kendall, B.; Dahl, T.W.; Anbar, A.D. Good Golly, Why Moly? The Stable Isotope Geochemistry of Molybdenum. Rev. Min. Geochem. 2017, 82, 683–732. [Google Scholar] [CrossRef]
- Andersen, M.B.; Stirling, C.H.; Weyer, S. Uranium Isotope Fractionation. Rev. Min. Geochem. 2017, 82, 799–850. [Google Scholar] [CrossRef]
- Scott, C.; Slack, J.F.; Kelley, K.D. The hyper-enrichment of V and Zn in black shales of the Late Devonian-Early Mississippian Bakken Formation (USA). Chem. Geol. 2017, 452, 24–33. [Google Scholar] [CrossRef]
- McLennan, S.M. Relationship between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosys. 2001, 2, 24. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Hoboken, NJ, USA, 1985. [Google Scholar]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef]
- Emerson, S.R.; Huested, S.S. Ocean anoxia and the concentrations of molybdenum and vanadium in seawater. Mar. Chem. 1991, 34, 177–196. [Google Scholar] [CrossRef]
- Whitfield, M.; Turner, D.R. Water-rock partition coefficients and the composition of seawater and river water. Nature 1979, 278, 132–137. [Google Scholar] [CrossRef]
- Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere—speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
- Tyson, R.V.; Pearson, T.H. Modern and ancient continental shelf anoxia: An overview. In Modern and Ancient Shelf Anoxia, Geological Society Special Publication 58; Geological Society London: London, UK, 1991; pp. 1–26. [Google Scholar]
- Wanty, R.B.; Goldhaber, M.B. Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochim. Cosmochim. Acta 1992, 56, 1471–1483. [Google Scholar] [CrossRef]
- Trefry, J.H.; Metz, S. Role of hydrothermal precipitates in the geochemical cycling of vanadium. Nature 1989, 342, 531–533. [Google Scholar] [CrossRef]
- Wehrli, B.; Stumm, W. Vanadyl in natural waters: Adsorption and hydrolysis promote oxygenation. Geochim. Cosmochim. Acta 1989, 53, 69–77. [Google Scholar] [CrossRef]
- Breit, G.N.; Wanty, R.B. Vanadium accumulation in carbonaceous rocks: A review of geochemical controls during deposition and diagenesis. Chem. Geol. 1991, 91, 83–97. [Google Scholar] [CrossRef]
- Algeo, T.J.; Li, C. Redox classification and calibration of redox thresholds in sedimentary systems. Geochim. Cosmochim. Acta 2020, 287, 8–26. [Google Scholar] [CrossRef]
- Schwertmann, U.; Pfab, G. Structural vanadium in sythnetic goethite. Geochim. Cosmochim. Acta 1994, 58, 4349–4352. [Google Scholar] [CrossRef]
- Schwertmann, U.; Pfab, G. Structural vanadium and chromium in lateritic iron oxides: Genetic implications. Geochim. Cosmochim. Acta 1996, 60, 4279–4283. [Google Scholar] [CrossRef]
- Algeo, T.J.; Maynard, J.B. Trace-element behaviour and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems. Chem. Geol. 2004, 206, 289–318. [Google Scholar] [CrossRef]
- Ostrander, C.M.; Sahoo, S.K.; Kendall, B.; Jiang, G.; Planavsky, N.J.; Lyons, T.W.; Nielsen, S.G.; Owens, J.D.; Gordon, G.W.; Romaniello, S.J.; et al. Multiple negative molybdenum isotope excursions in the Doushantuo Formation (South China) fingerprint complex redox-related processes in the Ediacaran Nanhua Basin. Geochim. Cosmochim. Acta 2019, 261, 191–209. [Google Scholar] [CrossRef]
- Kendall, B.; Wang, J.; Zheng, W.; Romaniello, S.J.; Over, D.J.; Bennett, Y.; Xing, L.; Kunert, A.; Boyes, C.; Liu, J. Inverse correlation between the molybdenum and uranium isotope compositions of Upper Devonian black shales caused by changes in local depositional conditions rather than global ocean redox variations. Geochim. Cosmochim. Acta 2020, 287, 141–164. [Google Scholar] [CrossRef]
- Erickson, B.E.; Helz, G.R. Molybdenum(VI) speciation in sulfidic waters: Stability and lability of thiomolybdates. Geochim. Cosmochim. Acta 2000, 64, 1149–1158. [Google Scholar] [CrossRef]
- Vorlicek, T.P.; Kahn, M.D.; Kasuya, Y.; Helz, G.R. Capture of molybdenum in pyrite-forming sediments: Role of ligand-induced reduction by polysulfides. Geochim. Cosmochim. Acta 2004, 68, 547–556. [Google Scholar] [CrossRef]
- Dahl, T.W.; Chappaz, A.; Fitts, J.P.; Lyons, T.W. Molybdenum reduction in a sulfidic lake: Evidence from X-ray absorption fine-structure spectroscopy and implications for the Mo paleoproxy. Geochim. Cosmochim. Acta 2013, 103, 213–231. [Google Scholar] [CrossRef]
- King, E.K.; Perakis, S.S.; Pett-Ridge, J.C. Molybdenum isotope fractionation during adsorption to organic matter. Geochim. Cosmochim. Acta 2018, 222, 584–598. [Google Scholar] [CrossRef]
- Nägler, T.F.; Anbar, A.D.; Archer, C.; Goldberg, T.; Gordon, G.W.; Greber, N.D.; Siebert, C.; Sohrin, Y.; Vance, D. Proposal for an international molybdenum isotope measurement standard and data representation. Geostand. Geoanalytical Res. 2014, 38, 149–151. [Google Scholar] [CrossRef]
- Siebert, C.; Nägler, T.F.; von Blanckenburg, F.; Kramers, J.D. Molybdenum isotope records as a potential new proxy for paleoceanography. Earth Planet. Sci. Lett. 2003, 211, 159–171. [Google Scholar] [CrossRef]
- Poulson Brucker, R.L.; McManus, J.; Severmann, S.; Berelson, W.M. Molybdenum behaviour during early diagenesis: Insights from Mo isotopes. Geochem. Geophys. Geosys. 2009, 10, 25. [Google Scholar] [CrossRef]
- Goldberg, T.; Archer, C.; Vance, D.; Poulton, S.W. Mo isotope fractionation during adsorption to Fe (oxyhydr)oxides. Geochim. Cosmochim. Acta 2009, 73, 6502–6516. [Google Scholar] [CrossRef]
- Tossell, J.A. Calculating the partitioning of the isotopes of Mo between oxidic and sulfidic species in aqueous solution. Geochim. Cosmochim. Acta 2005, 69, 2981–2993. [Google Scholar] [CrossRef]
- Goldberg, T.; Gordon, G.; Izon, G.; Archer, C.; Pearce, C.R.; McManus, J.; Anbar, A.D.; Rehkämper, M. Resolution of inter-laboratory discrepancies in Mo isotope data: An intercalibration. J. Anal. At. Spectrom. 2013, 28, 724–735. [Google Scholar] [CrossRef]
- Archer, C.; Vance, D. The isotopic signature of the global riverine molybdenum flux and anoxia in the ancient oceans. Nature Geosci. 2008, 1, 597–600. [Google Scholar] [CrossRef]
- King, E.K.; Pett-Ridge, J.C. Reassessing the dissolved molybdenum isotopic composition of ocean inputs: The effect of chemical weathering and groundwater. Geology 2018, 46, 955–958. [Google Scholar] [CrossRef]
- Neely, R.A.; Gislason, S.R.; Ólafsson, M.; McCoy-West, A.J.; Pearce, C.R.; Burton, K.W. Molybdenum isotope behaviour in groundwaters and terrestrial hydrothermal systems, Iceland. Earth Planet. Sci. Lett. 2018, 486, 108–118. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Planavsky, N.J.; Kendall, B.; Wang, X.; Shi, X.; Scott, C.; Anbar, A.D.; Lyons, T.W.; Jiang, G. Ocean oxygenation in the wake of the Marinoan glaciation. Nature 2012, 489, 546–549. [Google Scholar] [CrossRef]
- Cheng, M.; Li, C.; Zhou, L.; Feng, L.; Algeo, T.J.; Zhang, F.; Romaniello, S.; Jin, C.; Ling, H.; Jiang, S. Transient deep-water oxygenation in the early Cambrian Nanhua Basin, South China. Geochim. Cosmochim. Acta 2017, 210, 42–58. [Google Scholar] [CrossRef]
- Cheng, M.; Li, C.; Zhou, L.; Algeo, T.J.; Zhang, F.; Romaniello, S.; Jin, C.-S.; Lei, L.-D.; Feng, L.-J.; Jiang, S.-Y. Marine Mo biogeochemistry in the context of dynamically euxinic mid-depth waters: A case study of the lower Cambrian Niutitang shales, South China. Geochim. Cosmochim. Acta 2016, 183, 79–93. [Google Scholar] [CrossRef]
- Xu, L.; Lehmann, B.; Mao, J.; Nägler, T.F.; Neubert, N.; Böttcher, M.E.; Escher, P. Mo isotope and trace element patterns of Lower Cambrian black shales in South China: Multi-proxy constraints on the paleoenvironment. Chem. Geol. 2012, 318–319, 45–59. [Google Scholar] [CrossRef]
- Westermann, S.; Vance, D.; Cameron, V.; Archer, C.; Robinson, S.A. Heterogeneous oxygenation states in the Atlantic and Tethys oceans during Oceanic Anoxic Event 2. Earth Planet Sci. Lett. 2014, 404, 178–189. [Google Scholar] [CrossRef]
- Kurzweil, F.; Drost, K.; Pašava, J.; Wille, M.; Taubald, H.; Schoeckle, D.; Schoenberg, R. Coupled sulfur, iron and molybdenum isotope data from black shales of the Teplá-Barrandian unit argue against deep ocean oxygenation during the Ediacaran. Geochim. Cosmochim. Acta 2015, 171, 121–142. [Google Scholar] [CrossRef]
- Schröder, S.; Grotzinger, J.P. Evidence for anoxia at the Ediacaran-Cambrian boundary: The record of redox-sensitive trace metals and rare earth elements in Oman. J. Geol. Soc. 2007, 164, 175–187. [Google Scholar] [CrossRef]
- Wille, M.; Nägler, T.F.; Lehmann, B.; Schröder, S.; Kramers, J.D. Hydrogen sulphide release to surface waters at the Precambrian/Cambrian boundary. Nature 2008, 453, 767–769. [Google Scholar] [CrossRef]
- Lu, X.; Kendall, B.; Stein, H.J.; Li, C.; Hannah, J.L.; Gordon, G.W.; Ebbestad, J.O.R. Marine redox conditions during deposition of Late Ordovician and Early Silurian organic-rich mudrocks in the Siljan ring district, central Sweden. Chem. Geol. 2017, 457, 75–94. [Google Scholar] [CrossRef]
- Zhou, L.; Wignall, P.B.; Su, J.; Feng, Q.; Xie, S.; Zhao, L.; Huang, J. U/Mo ratios and δ98/85Mo as local and global redox proxies during mass extinction events. Chem. Geol. 2012, 324–325, 99–107. [Google Scholar] [CrossRef]
- Goldberg, T.; Poulton, S.W.; Wagner, T.; Kolonic, S.F.; Rehkämper, M. Molybdenum drawdown during Cretaceous Oceanic Anoxic Event 2. Earth Planet Sci. Lett. 2016, 440, 81–91. [Google Scholar] [CrossRef]
- Scheiderich, K.; Zerkle, A.L.; Helz, G.R.; Farquhar, J.; Walker, R.J. Molybdenum and sulphur isotope ratios of early Pleistocene sapropels of ODP Hole 160-969D. PANGAEA 2010. [Google Scholar] [CrossRef]
- Gordon, G.W.; Lyons, T.W.; Arnold, G.L.; Roe, J.; Sageman, B.B.; Anbar, A.D. When do black shales tell molybdenum isotope tales? Geology 2009, 37, 535–538. [Google Scholar] [CrossRef]
- Ruvalcaba Baroni, I.; Coe, A.L.; Harding, S.M.; Papadomanolaki, N.M.; van Helmond, N.A.G.M.; van de Schootbrugge, B.; Cohen, A.S.; Slomp, C.P. Biogeochemical redox proxies in sediments from Dotternhausen during the Toarcian (Early Jurassic). PANGAEA 2018. [Google Scholar] [CrossRef]
- Dickson, A.J.; Gill, B.C.; Ruhl, M.; Jenkyns, H.C.; Porcelli, D.; Idiz, E.; Lyons, T.W.; van den Boorn, S.H.J.M. Molybdenum-isotope chemostratigraphy and paleoceanography of the Toarcian Oceanic Anoxic Event (Early Jurassic). Paleocean. Paleoclim. 2017, 32, 813–829. [Google Scholar] [CrossRef]
- Pearce, C.R.; Cohen, A.S.; Coe, A.L.; Burton, K.W. Molybdenum isotope evidence for global ocean anoxia coupled with perturbations to the carbon cycle during the Early Jurassic. Geology 2008, 36, 231–234. [Google Scholar] [CrossRef]
- Ruvalcaba Baroni, I.; Coe, A.L.; Harding, S.M.; Papadomanolaki, N.M.; van Helmond, N.A.G.M.; van de Schootbrugge, B.; Cohen, A.S.; Slomp, C.P. Biogeochemical redox proxies in sediments from Yorkshire during the Toarcian (Early Jurassic). PANGAEA 2018. [Google Scholar] [CrossRef]
- Pearce, C.R.; Coe, A.L.; Cohen, A.S. Seawater redox variations during the deposition of the Kimmeridge Clay Formation, United Kingdom (Upper Jurassic): Evidence from molybdenum isotopes and trace metal ratios. Paleocean. Paleoclim. 2010, 25, 15. [Google Scholar] [CrossRef]
- Andersen, M.B.; Matthews, A.; Vance, D.; Bar-Matthews, M.; Archer, C.; de Souza, G.F. A 10-fold decline in the deep Eastern Mediterranean thermohaline overturning circulation during the last interglacial period. Earth Planet Sci. Lett. 2018, 503, 58–67. [Google Scholar] [CrossRef]
- Dahl, T.W.; Hammarlund, E.U.; Anbar, A.D.; Bond, D.P.G.; Gill, B.C.; Gordon, G.W.; Knoll, A.H.; Nielsen, A.T.; Schovsbo, N.H.; Canfield, D.E. Devonian Rise in atmospheric oxygen correlated to the radiations of terrestrial plants and large predatory fish. Proc. Natl. Acad. Sci. USA 2010, 107, 17911–17915. [Google Scholar] [CrossRef]
- Algeo, T.J.; Heckel, P.H. The Late Pennsylvanian midcontinent sea of North America: A review. Palaeogeog. Palaeoclim. Palaeoecol. 2008, 268, 205–221. [Google Scholar] [CrossRef]
- Bura-Nakić, E.; Andersen, M.B.; Archer, C.; de Souza, G.F.; Marguš, M.; Vance, D. Coupled Mo-U abundances and isotopes in a small marine euxinic basin: Constraints on processes in euxinic basins. Geochim. Cosmochim. Acta 2018, 222, 212–229. [Google Scholar] [CrossRef]
- Algeo, T.J.; Lyons, T.W. Mo–total organic carbon covariation in modern anoxic marine environments: Implications for analysis of paleoredox and paleohydrographic conditions. Paleoceanogr. Paleoclimatol. 2006, 21, 23. [Google Scholar] [CrossRef]
- Smith, M.G.; Bustin, R.M. Production and preservation of organic matter during deposition of the Bakken Formation (Late Devonian and Early Mississippian), Williston Basin. Palaeogeog. Palaeoclim. Palaeoecol. 1998, 142, 185–200. [Google Scholar] [CrossRef]
- Reinhard, C.T.; Planavsky, N.J.; Robbins, L.J.; Partin, C.A.; Gill, B.C.; Lalonde, S.V.; Bekker, A.; Konhauser, K.O.; Lyons, T.W. Proterozoic ocean redox and biogeochemical stasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5357–5362. [Google Scholar] [CrossRef]
- Partin, C.A.; Bekker, A.; Planavsky, N.J.; Scott, C.T.; Gill, B.C.; Li, C.; Podkovyrov, V.; Maslov, A.; Konhauser, K.O.; Lalonde, S.V.; et al. Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales. Earth Planet Sci. Lett. 2013, 369–370, 284–293. [Google Scholar] [CrossRef]
- Sheen, A.I.; Kendall, B.; Reinhard, C.T.; Creaser, R.A.; Lyons, T.W.; Bekker, A.; Poulton, S.W.; Anbar, A.D. A model for the oceanic mass balance of rhenium and implications for the extent of Proterozoic ocean anoxia. Geochim. Cosmochim. Acta 2018, 227, 75–95. [Google Scholar] [CrossRef]
- Kunert, A. Reconstruction of Local and Global Marine Paleoredox Conditions for the Northeast-Panthalassan (British Columbia, Canada) Expression of the Toarcian Oceanic Anoxic Event Using a Multi-Proxy Approach. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2020. [Google Scholar]
- Holmden, C.; Creaser, R.A.; Muehlenbachs, K.; Leslie, S.A.; Bergström, S.M. Isotopic evidence for geochemical decoupling between ancient epeiric seas and bordering oceans: Implications for secular curves. Geology 1998, 26, 567–570. [Google Scholar] [CrossRef]
- Shiller, A.M.; Boyle, E.A. Dissolved vanadium in rivers and estuaries. Earth Planet. Sci. Lett. 1987, 86, 214–224. [Google Scholar] [CrossRef]
- Wright, M.T.; Stollenwerk, K.G.; Belitz, K. Assessing the solubility of controls on vanadium in groundwater, northeastern San Joaquin Valley, CA. Appl. Geochem. 2014, 48, 41–52. [Google Scholar] [CrossRef]
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Wright, M.T.; Belitz, K. Factors controlling the regional distribution of vanadium in groundwater. Groundwater 2010, 48, 515–525. [Google Scholar] [CrossRef]

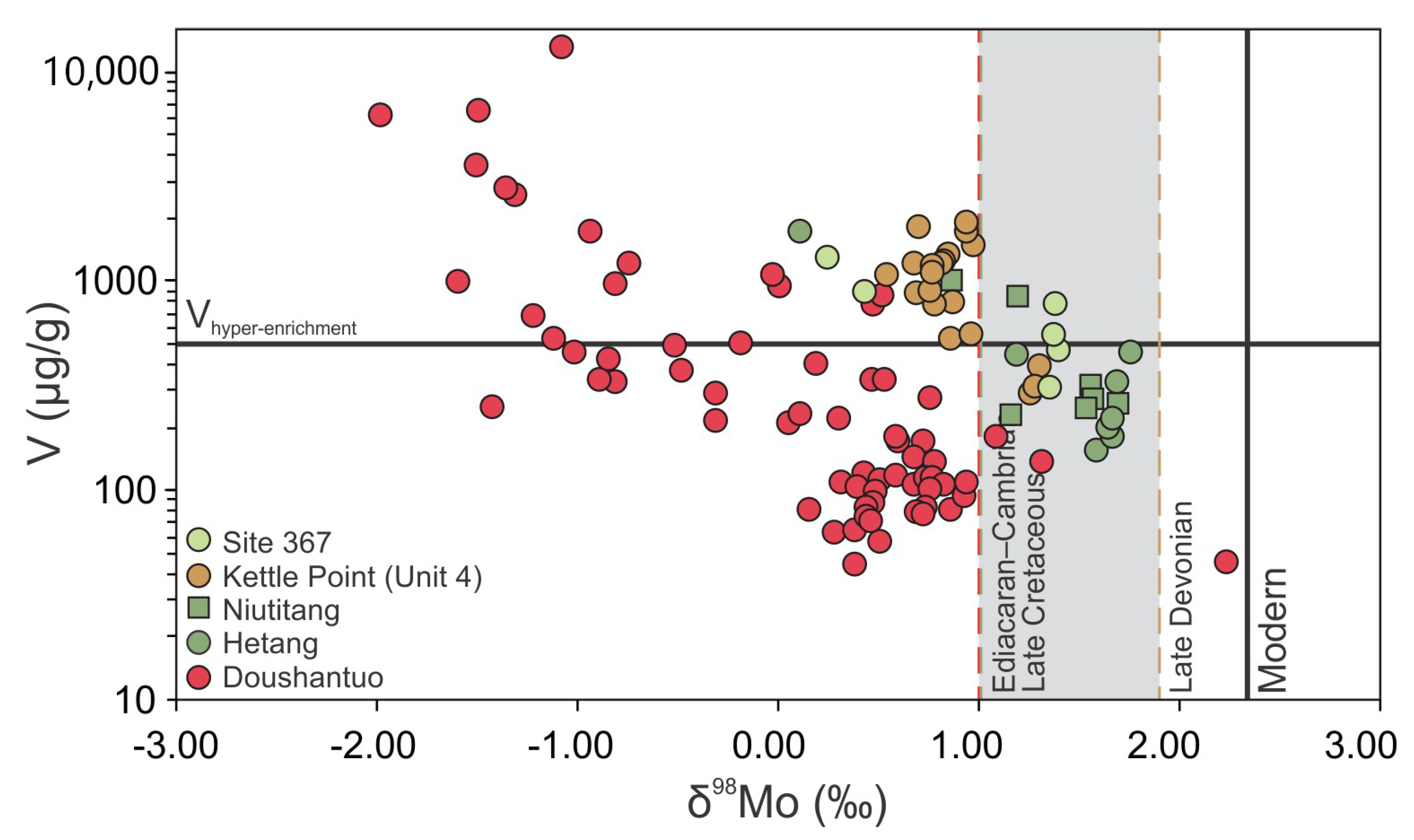
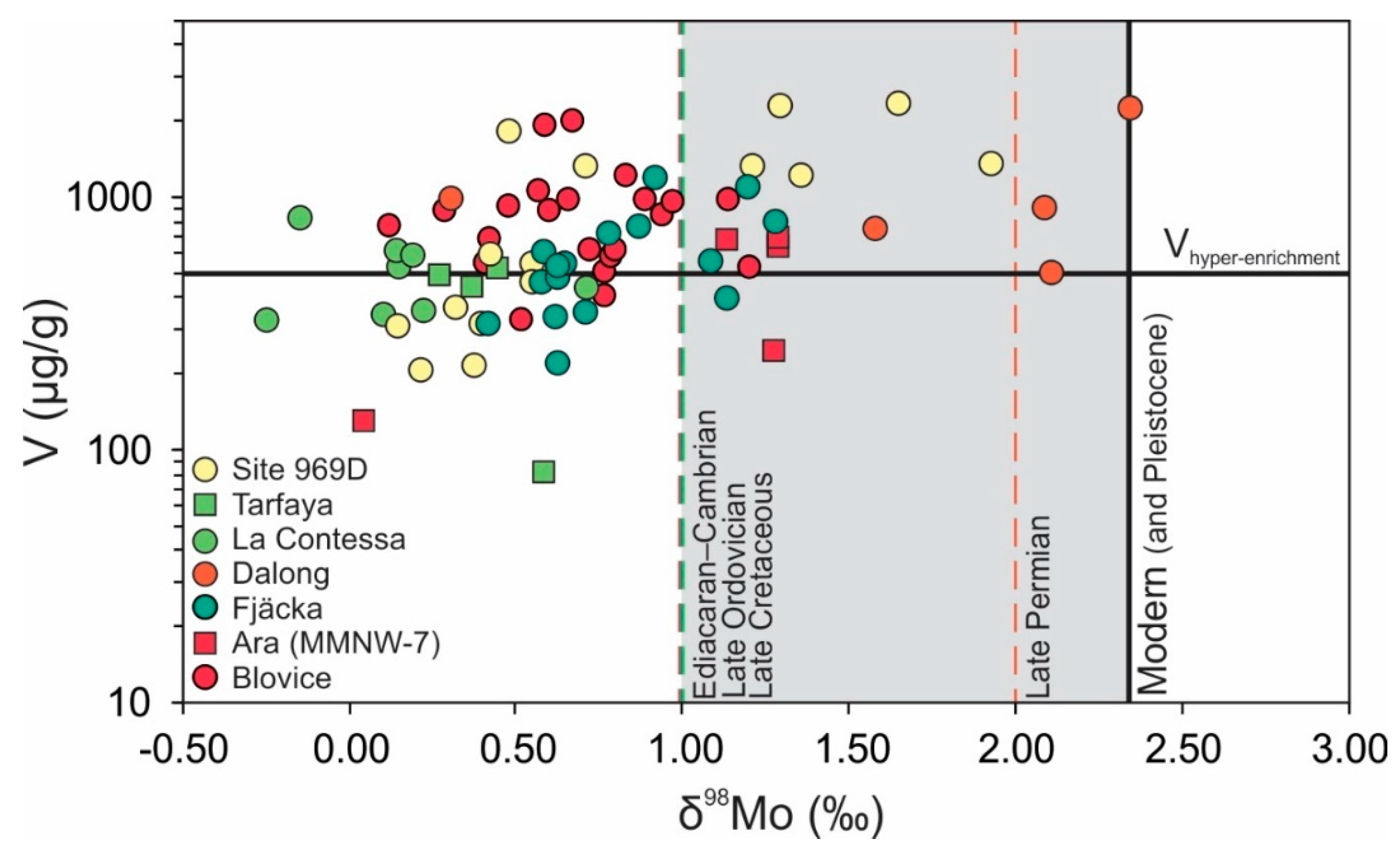
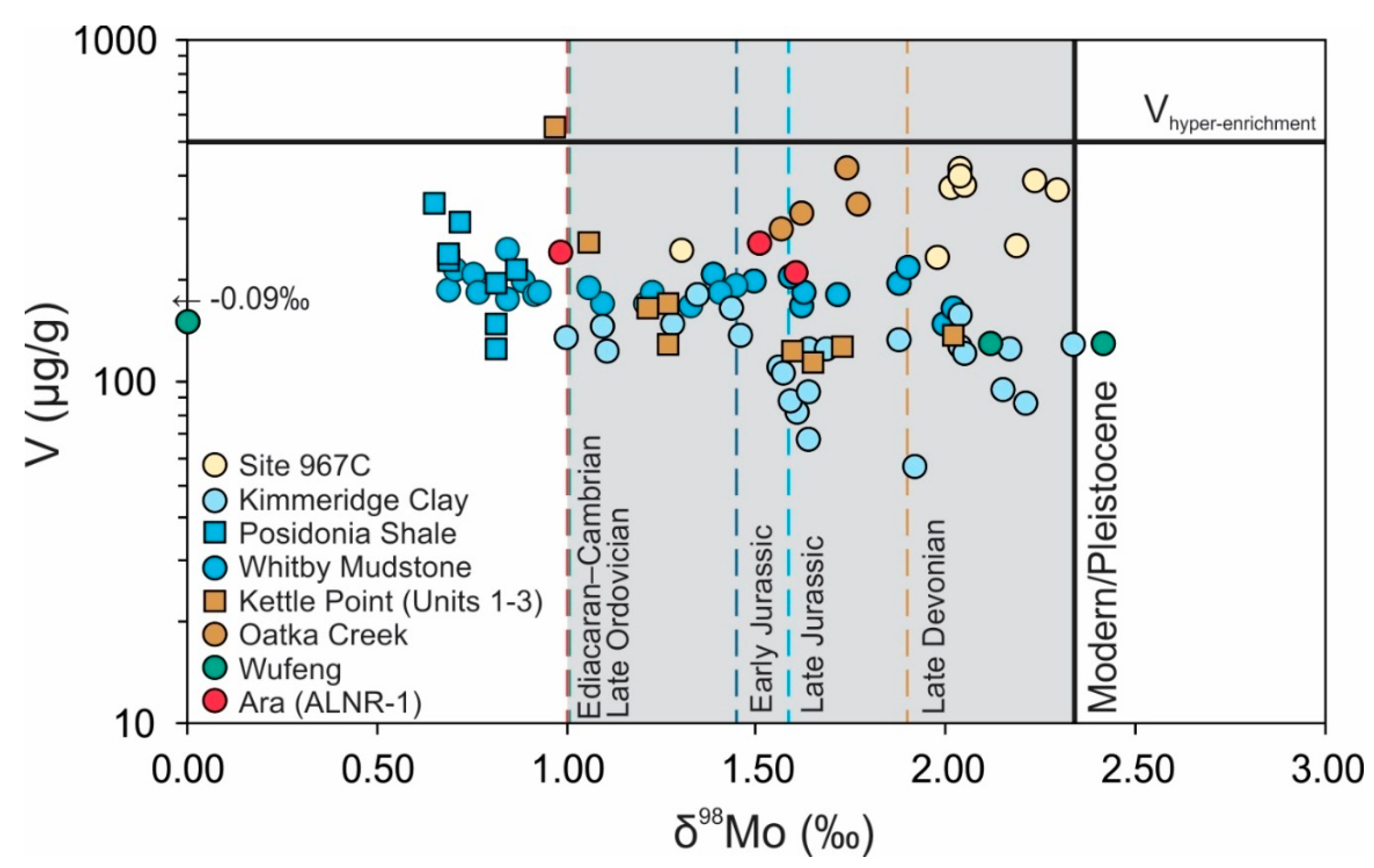
| Geological Unit | Location | Section (n) | Characteristics (in Ref.) | Age (Ma) | V (range) μg/g | δ98Mo (range) ‰ | Ref. |
|---|---|---|---|---|---|---|---|
| Negative V–δ98Mo Trend | 804 (44–15271) | 0.44 (−1.98–2.24) | |||||
| Doushantuo Formation | South China | Rongxi (7) | Mn Particulate Shuttle | 632 | 454 (137–1066) | 0.54 (−0.02–1.32) | [27] |
| Taoying (8) | Mn Particulate Shuttle | 1175 (119–6096) | −0.91 (−1.98–0.78) | ||||
| Wuhe (46) | Mn Particulate Shuttle | 671 (44–15271) | 0.23 (−1.50–2.24) | ||||
| Yuanjia (7) | Mn Particulate Shuttle | 1775 (336–6417) | −0.45 (−1.49–0.54) | ||||
| Hetang Formation | South China | Lantian (8) | Particulate Shuttle | 535–521 | 461 (155–1726) | 1.42 (0.11–1.76) | [43] |
| Niutitang Formation | South China | Yangjiaping (4) | Weak Euxinia, Peripheral Particulate Activity | 529–514 | 592 (228–1005) | 1.20 (0.87–1.49) | [44] |
| Maluhe (2) | 521 | 364 (310–417) | 1.62 (1.55–1.68) | [45] | |||
| Dazhuliushui (1) | 320 | 1.56 | |||||
| Kettle Point Formation | Ontario (Canada) | Chatham Unit 4 (18) | Particulate Shuttle, Brackish, Low Sea Level | 372 | 1044 (288–1912) | 0.88 (0.55–1.31) | [28] |
| OAE2 Black Shale | Cape Verde Basin | DSDP Site 367 (6) | Particulate Shuttle | 716 (466–1300) | 0.83 (0.15–1.15) | [46] | |
| Positive V–δ98Mo Trend | 750 (81–2310) | 0.74 (−0.40–2.35) | |||||
| Blovice Complex | Pilsen (Czechia) | HRM-3 (14) | Low T Hydrothermal | 560–535 | 926 (410–2020) | 0.82 (0.57–1.20) | [47] |
| KA-5 (9) | Low T Hydrothermal | 759 (331–1223) | 0.48 (0.12–0.83) | ||||
| Ara Group | Central Oman | MM NW-7 (5) | Salinity Stratification | 550–540 | 477 (130–689) | 1.01 (0.04–1.29) | [48,49] 1 |
| Fjäcka Shale | Central Sweden | Solberga #1 (6) | Persistent Euxinia | 448 | 457 (219–720) | 0.62 (0.42–0.78) | [50] |
| Stumsnäs #1 (10) | Persistent Euxinia | 658 (347–1175) | 0.91 (0.58–1.28) | ||||
| Dalong Formation | South China | Shangsi (5) | Well-ventilated | 252 | 1069 (496–2227) | 1.69 (0.31–2.35) | [51] |
| Scaglia Bianca Formation | Central Italy | La Contessa (9) | Weak Particulate Shuttle | 531 (310–860) | 0.30 (0.14–0.71) | [46] | |
| OAE2 Black Shale | Moroccan Shelf | Tarfaya S57 (4) | Upwelling Zone | 94 | 381 (81–517) | 0.00 (−0.40–0.63) | [52] |
| Sapropels 35–25 | Mediterranean Basin | ODP Hole 969D (15) | Upwelling Zone, Weak Euxinia | 1.88–1.44 | 975 (206–2310) | 0.78 (0.15–1.93) | [53] |
| No V Hyper-Enrichments | 187 (56–548) | 1.45 (−0.09–2.42) | |||||
| Ara Group | Central Oman | ALNR-1 (3) | Salinity Stratification | 550–540 | 233 (208–253) | 1.37 (0.99–1.61) | [48,49] 1 |
| Wufeng Formation | South China | Wangjiawan (3) | Slow sedimentation | 444 | 133 (127–145) | 1.48 (−0.09–2.42) | [51] |
| Oatka Creek Formation | New York (US) | Akzo #9455 (4) | Persistent Euxinia | 388 | 334 (277–421) | 1.68 (1.57–1.77) | [54] |
| Kettle Point Formation | Ontario (Canada) | Chatham Units 1–3 (9) | Brackish + Marine, High Sea Level (Units 1–2), Epeiric Sea | 372 | 195 (113–548) | 1.42 (0.97–2.02) | [28] |
| Posidonia Shale | South Germany | Dotternhausen (8) | Epeiric Sea | 183 | 221 (124–336) | 0.76 (0.65–0.87) | [55,56] 2 |
| Whitby Mudstone | United Kingdom | Yorkshire Outcrops (27) | Epeiric Sea | 183 | 187 (147–242) | 1.27 (0.69–2.02) | [57,58] 3 |
| Kimmeridge Clay | United Kingdom | Dorset Outcrops (24) | Weak Particulate Shuttle | 155–148 | 119 (56–178) | 1.69 (1.00–2.34) | [59] |
| Sapropel S5 | Mediterranean Basin | ODP Hole 967C (9) | Persistent Euxinic (↑H2S with time), Redox Stratification | 0.13–0.12 | 332 (228–397) | 2.02 (1.31–2.30) | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunert, A.; Clarke, J.; Kendall, B. Molybdenum Isotope Constraints on the Origin of Vanadium Hyper-Enrichments in Ediacaran–Phanerozoic Marine Mudrocks. Minerals 2020, 10, 1075. https://doi.org/10.3390/min10121075
Kunert A, Clarke J, Kendall B. Molybdenum Isotope Constraints on the Origin of Vanadium Hyper-Enrichments in Ediacaran–Phanerozoic Marine Mudrocks. Minerals. 2020; 10(12):1075. https://doi.org/10.3390/min10121075
Chicago/Turabian StyleKunert, Alexandra, Johnathan Clarke, and Brian Kendall. 2020. "Molybdenum Isotope Constraints on the Origin of Vanadium Hyper-Enrichments in Ediacaran–Phanerozoic Marine Mudrocks" Minerals 10, no. 12: 1075. https://doi.org/10.3390/min10121075
APA StyleKunert, A., Clarke, J., & Kendall, B. (2020). Molybdenum Isotope Constraints on the Origin of Vanadium Hyper-Enrichments in Ediacaran–Phanerozoic Marine Mudrocks. Minerals, 10(12), 1075. https://doi.org/10.3390/min10121075




