Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems
Abstract
1. Introduction
2. Sample Material and Geology
2.1. Ilímaussaq (Southwest Greenland)
2.2. Tamazeght (Morocco)
2.3. Lovozero and Khibina (Russian Federation)
2.4. Norra Kärr (Sweden)
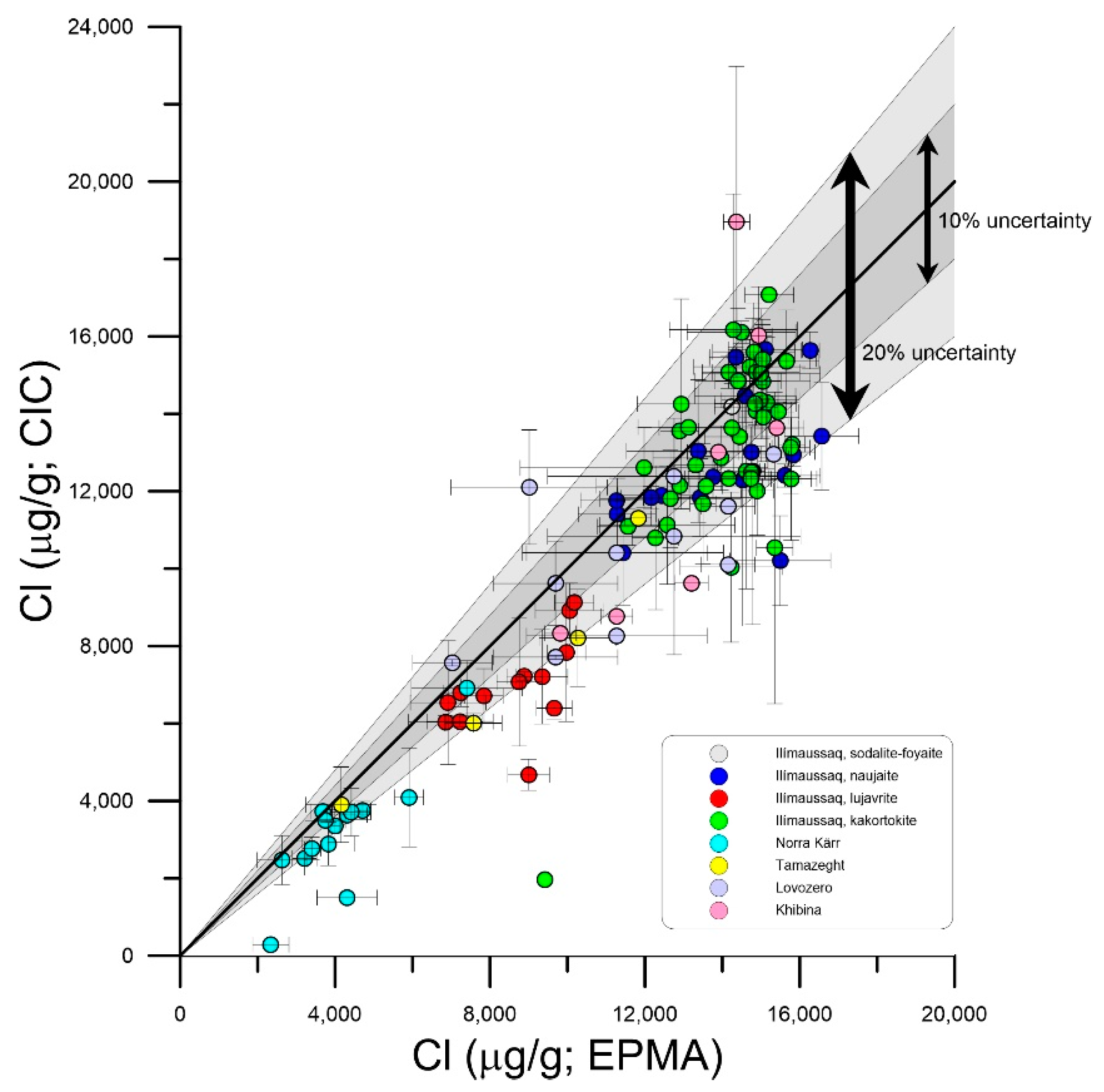
3. Methods
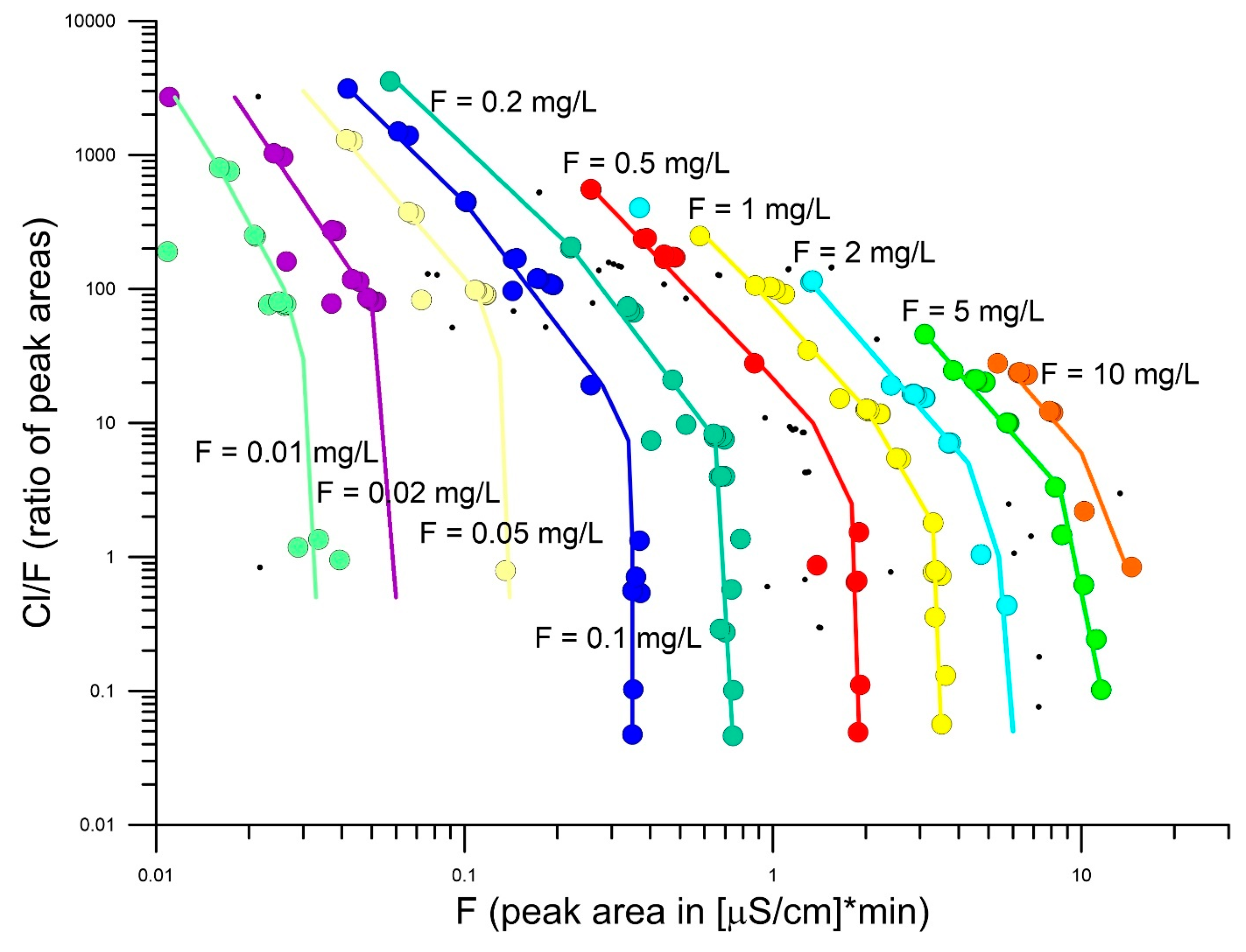
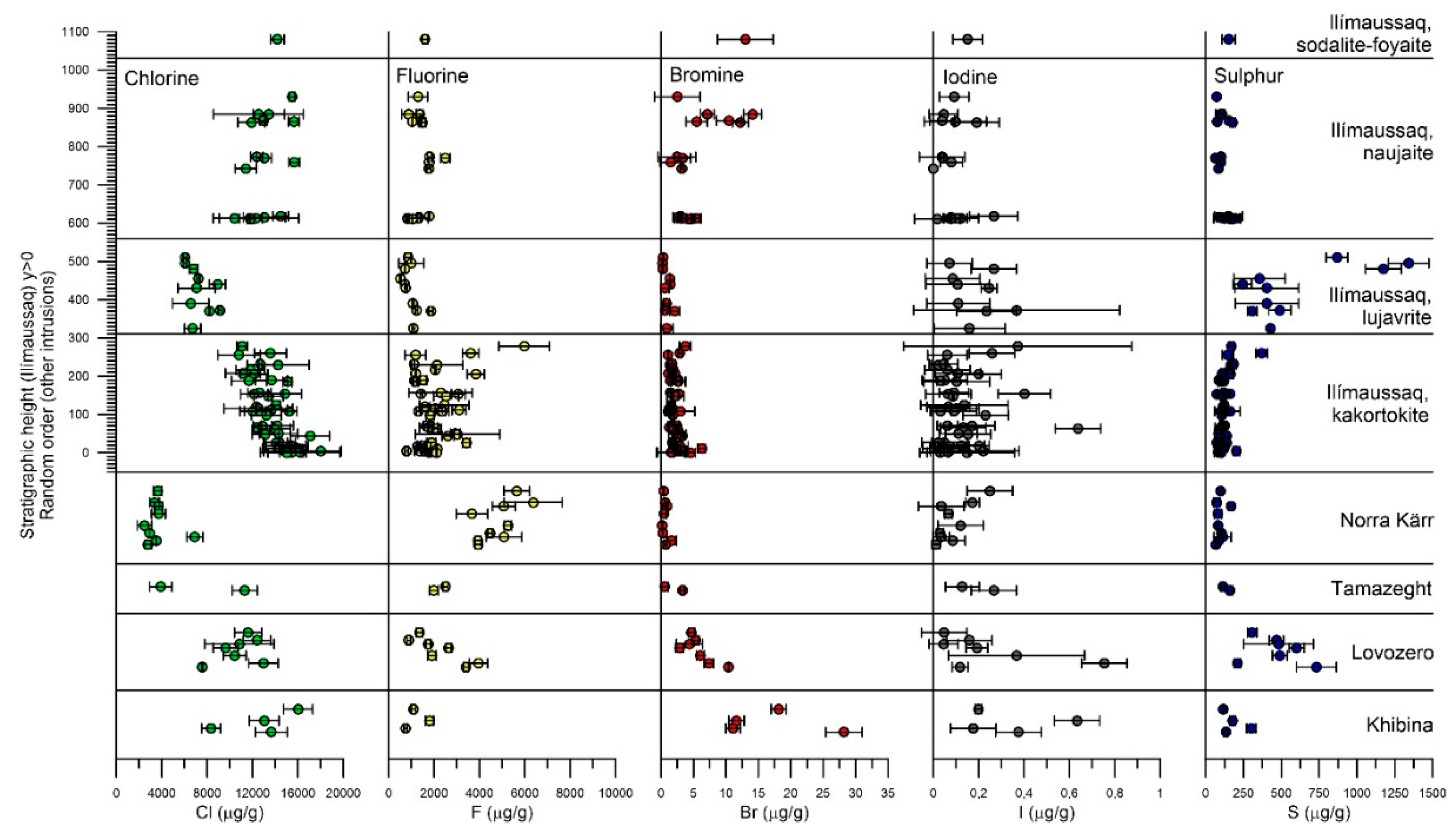
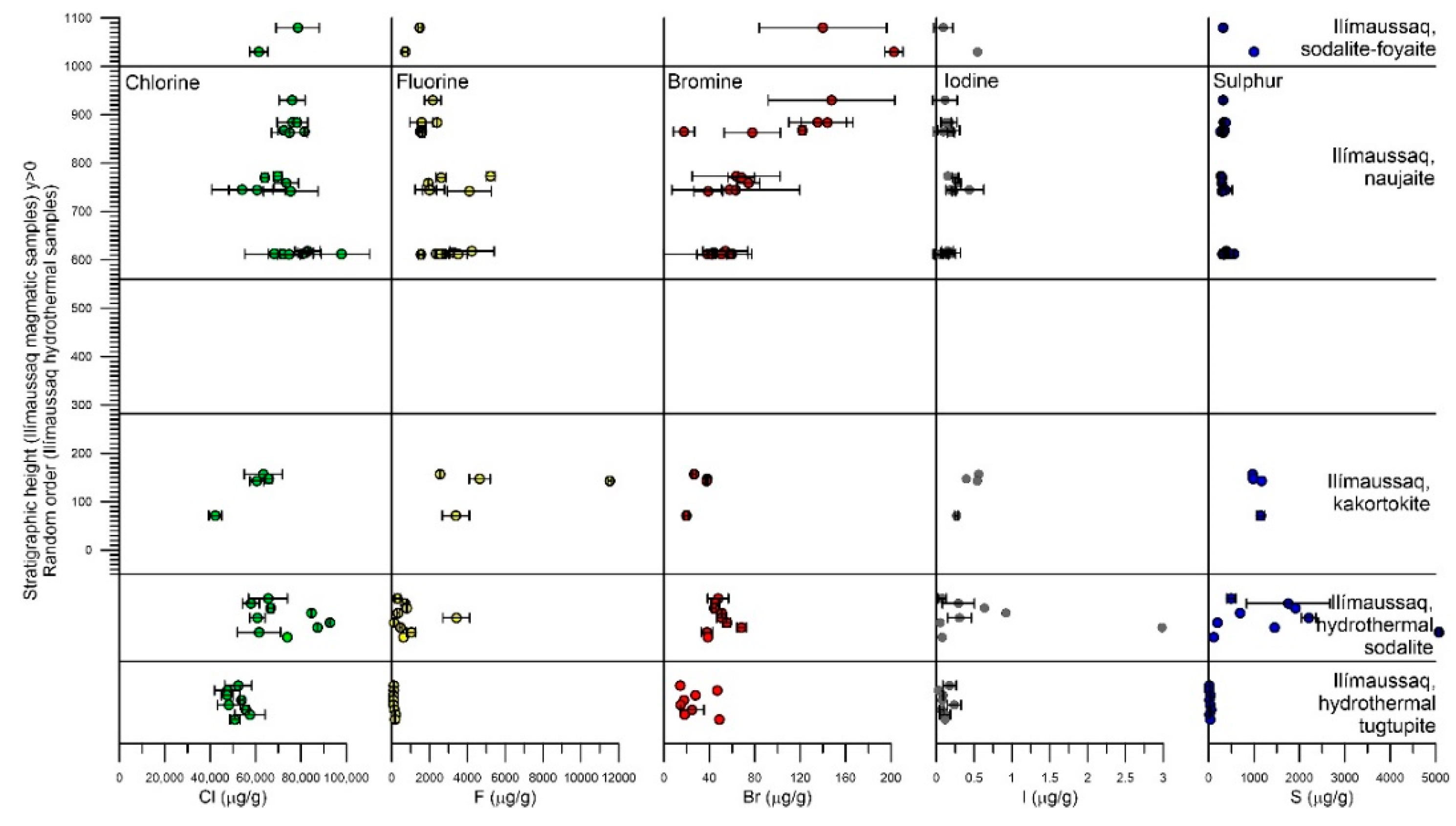
4. Results
4.1. Eudialyte-Group Minerals
4.2. Sodalite
4.3. Tugtupite
5. Discussion
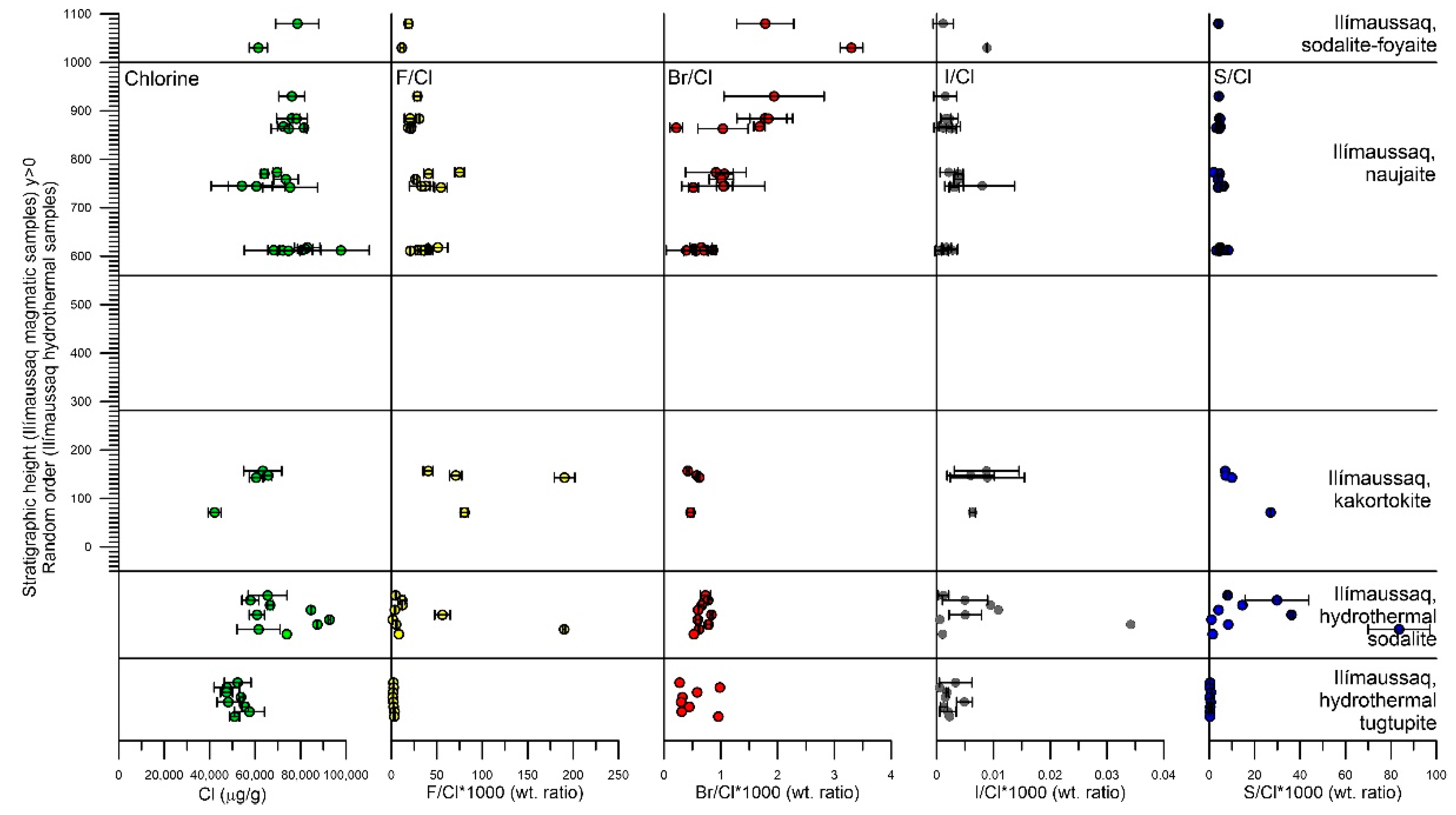
5.1. Halogens in EGM and Sodalite from Ilímaussaq
5.1.1. Eudialyte Group Minerals
5.1.2. Sodalite
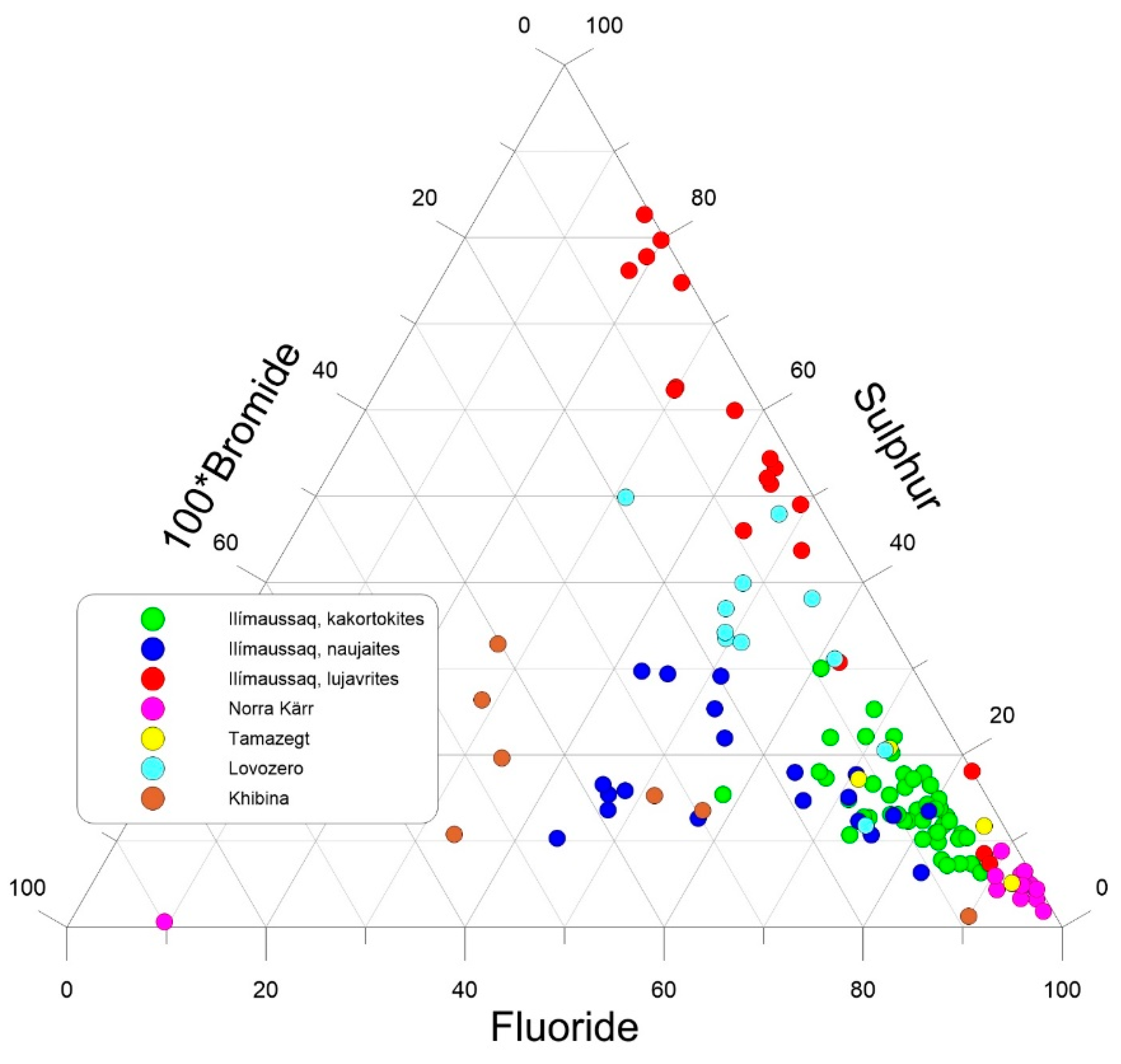
5.2. Halogen and S Partitioning Between EGM and Sodalite
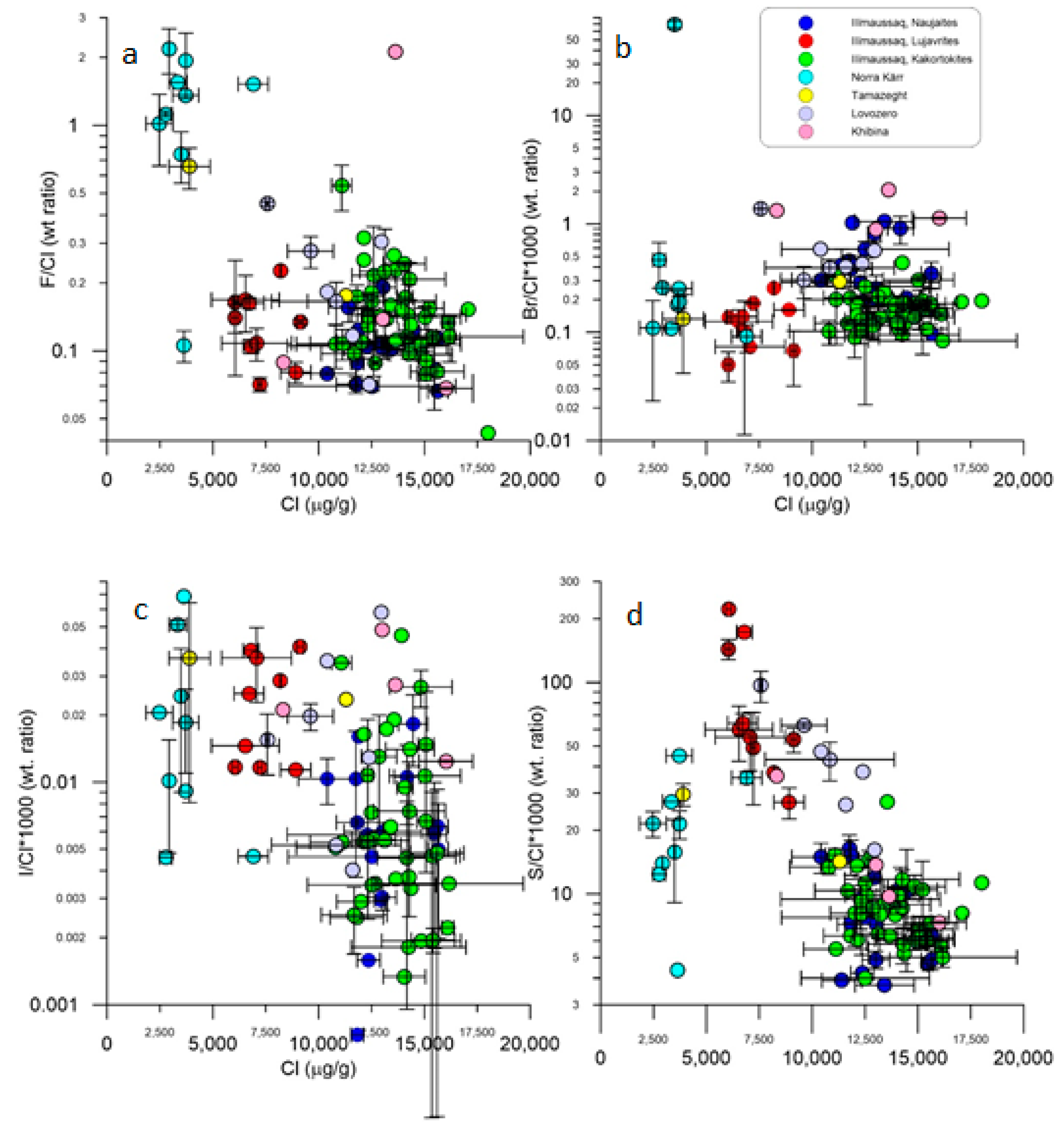
5.3. Halogen and S Systematics of EGM from Different Localities: Clues to Magma Sources?
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aiuppa, A.; Baker, D.R.; Webster, J.D. Halogens in volcanic systems. Chem. Geol. 2009, 263, 1–18. [Google Scholar] [CrossRef]
- Pyle, D.M.; Mather, T.A. Halogens in igneous processes and their fluxes to the atmosphere and oceans from volcanic activity: A review. Chem. Geol. 2009, 263, 110–121. [Google Scholar] [CrossRef]
- Gerlach, T.M. Volcanic sources of tropospheric ozone-depleting trace gases. Geochem. Geophys. Geosyst. 2004, 5, 16. [Google Scholar] [CrossRef]
- Oppenheimer, C.; Bani, P.; Calkins, J.A.; Burton, M.R.; Sawyer, G.M. Rapid FTIR sensing of volcanic gases released by strombolian explosions at Yasur volcano, Vanuatu. Appl. Phys. B 2006, 85, 453–460. [Google Scholar] [CrossRef]
- Theys, N.; Van Rozendael, M.; Dils, B.; Hendrick, F.; de Mazière, M. First satellite detection of volcanic bromine monoxide emission after Kasatochi eruption. Geophys. Res. Lett. 2009, 36, L03809. [Google Scholar] [CrossRef]
- Daniel, J.S.; Solomon, S.; Portmann, R.W.; Garcia, R.R. Stratospheric ozone detruction: The importance of bromine relative to chlorine. J. Geophys. Res. 1999, 104, 23871–23880. [Google Scholar] [CrossRef]
- Bureau, H.; Foy, E.; Raepsaet, C.; Somogyi, A.; Munsch, P.; Simon, G.; Kubsky, S. Bromine cycle in subduction zones through in situ Br monitoring in diamond anvil cells. Geochim. Cosmochim. Acta 2010, 74, 3839–3850. [Google Scholar] [CrossRef]
- Boulyga, S.F.; Heumann, K.G. Direct determination of halogens in powdered geological and environmental samples using isotope dilution laser ablation ICP-MS. Int. J. Mass Spectrom. 2005, 242, 291–296. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Wenzel, T.; Whitehouse, M.; Loose, M.; Zack, T.; Barth, M.; Worgard, L.; Krasz, V.; Eby, G.N.; Stosnach, H.; et al. The volatile inventory (F, Cl, Br, S, C) of magmatic apatite: An integrated analytical approach. Chem. Geol. 2012, 291, 241–255. [Google Scholar] [CrossRef]
- John, T.; Scambelluri, T.; Frische, M.; Barnes, J.D.; Bach, W. Dehydration of subducting serpentinite: Implications for halogen mobility in subduction zones and the deep halogen cycle. Earth Planet. Sci. Lett. 2011, 308, 65–76. [Google Scholar] [CrossRef]
- Cadoux, A.; Iacono-Mariziano, G.; Paonita, A.; Deloule, E.; Aiuppa, A.; Eby, G.N.; Costa, M.; Brusca, L.; Berlo, K.; Geraki, K.; et al. A new set of standards for in–situ measurement of bromine abundances in natural silicate glasses: Application to SR-XRF, LA-ICP-MS and SIMS techniques. Chem. Geol. 2017, 452, 60–70. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Pan, Y.; Feng, R.; Almeev, R.R.; Holtz, F. Electron Probe Microanalysis of Bromine in Minerals and Glasses with Correction for Spectral Interference from Aluminium, and Comparison with Microbeam Synchrotron X-Ray Fluorescence Spectrometry. Geostand. Geoanal. Res. 2017, 41, 449–457. [Google Scholar] [CrossRef]
- Webster, J.D. Partitioning of F between H2O and CO2 fluids and topaz rhyolite melt. Contrib. Mineral. Petrol. 1990, 104, 424–438. [Google Scholar] [CrossRef]
- Webster, J.D.; Halloway, J.R. Partitioning of F and Cl between magmatic hydrothermal fluids and highly evolved granitic magmas. Geol. Soc. Am. Spec. Pap. 1990, 246, 21–34. [Google Scholar]
- Scaillet, B.; Macdonald, R. Fluorite stability in silicic magmas. Contrib. Mineral. Petrol. 2004, 147, 319–329. [Google Scholar] [CrossRef][Green Version]
- Giehl, C.; Marks, M.A.W.; Nowak, M. An experimental study on the influence of fluorine and chlorine on phase relations in peralkaline phonolitic melts. Contrib. Mineral. Petrol. 2014, 167, 977. [Google Scholar] [CrossRef]
- Metrich, N.; Rutherford, M.J. Experimental study of chlorine behaviour in hydrous silicic melts. Geochim. Cosmochim. Acta 1992, 56, 607–616. [Google Scholar] [CrossRef]
- Signorelli, S.; Carroll, M.R. Solubility and fluid-melt partitioning of Cl in hydrous phonolitic melts. Geochim. Cosmochim. Acta 2000, 64, 2851–2862. [Google Scholar] [CrossRef]
- Signorelli, S.; Carroll, M.R. Experimental study of Cl solubility in hydrous alkaline melts: Constraints on the theoretical maximum amount of Cl in trachytic and phonolitic melts. Contrib. Mineral. Petrol. 2002, 143, 209–218. [Google Scholar]
- Bureau, H.; Métrich, N. An experimental study of bromine behaviour in water-saturated silicic melts. Geochim. Cosmochim. Acta 2003, 67, 1689–1697. [Google Scholar] [CrossRef]
- Bureau, H.; Keppler, H.; Métrich, N. Volcanic degassing of bromine and iodine: Experimental fluid/melt partitioning data and applications to stratospheric chemistry. Earth Planet. Sci. Lett. 2000, 183, 51–60. [Google Scholar] [CrossRef]
- Carroll, M.R.; Webster, J.D. Solubilities of sulfur, noble gases, nitrogen, chlorine and fluorine in magmas. Rev. Miner. 1994, 30, 231–279. [Google Scholar]
- Villemant, B.; Boudon, G. H2O and halogen (F, Cl, Br) behaviour during shallow agma degassing processes. Earth Planet. Sci. Lett. 1999, 168, 271–286. [Google Scholar] [CrossRef]
- Wang, L.-X.; Marks, M.A.W.; Keller, J.; Markl, G. Halogen variations in alkaline rocks from the Upper Rhine Graben (SW Germany): Insights into F, Cl and Br behavior during magmatic processes. Chem. Geol. 2014, 380, 133–144. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Dong, P. Halogen-Element (F, Cl and Br) Behaviour in Apatites, Scapolite, and Sodalite: An Experimental Investigation with Field Applications. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2005. [Google Scholar]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. Rev. Mineral. Geochem. 2002, 48, 255–292. [Google Scholar] [CrossRef]
- Zhang, C.; Holtz, F.; Ma, C.; Wolff, P.E.; Li, X. Tracing the evolution and distribution of F and Cl in plutonic systems from volatile-bearing minerals: A case study from the Liujiawa pluton (Dabie Orogen, China). Contrib. Mineral. Petrol. 2012, 164, 859–879. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.A.W.; Wenzel, T.; Zack, T.; Siebel, W.; Altherr, R.; Markl, G. The distribution of halogens (F, Cl, Br) in granitoid rocks. Chem. Geol. 2014, 374–375, 92–109. [Google Scholar] [CrossRef]
- Zhu, C.; Sverjenski, D.A. Partitioning of F-Cl-OH between minerals and hydrothermal fluids. Geochim. Cosmochim. Acta 1991, 55, 1837–1858. [Google Scholar] [CrossRef]
- Ionov, D.A.; Griffin, W.L.; O’Reilly, S.Y. Volatile-bearing minerals and lithophile trace elements in the upper mantle. Chem. Geol. 1997, 141, 153–184. [Google Scholar] [CrossRef]
- Kendrick, M.A. High precision Cl, Br and I determinations in mineral standards using the noble gas method. Chem. Geol. 2012, 292, 116–126. [Google Scholar] [CrossRef]
- Krumrei, T.V.; Pernicka, E.; Kaliwoda, M.; Markl, G. Volatiles in a peralkaline system: Abiogenic hydrocarbons and F-Cl-Br systematics in the naujaite of the Ilimaussaq intrusion, South Greenland. Lithos 2007, 95, 298–314. [Google Scholar] [CrossRef]
- O´Reilly, S.Y.; Griffin, W.L. Apatite in the mantle: Implications for metasomatic processes and high heat production in Phanerozoic mantle. Lithos 2000, 53, 217–232. [Google Scholar] [CrossRef]
- Kusebauch, C.; John, T.; Whitehouse, M.J.; Engvik, A.K. Apatite as probe for the halogen composition of metamorphic fluids (Bamble Sector, SE Norway). Contrib. Mineral. Petrol. 2015, 170, 34. [Google Scholar] [CrossRef]
- Kusebauch, C.; John, T.; Barnes, J.D.; Klügel, A.; Austrheim, H.O. Halogen Element and Stable Chlorine Isotope Fractionation Caused by Fluid–Rock Interaction (Bamble Sector, SE Norway). J. Petrol. 2015, 56, 299–324. [Google Scholar] [CrossRef]
- Teiber, H.; Marks, M.A.W.; Arzamastsev, A.A.; Wenzel, T.; Markl, G. Compositional variation in apatite from various host rocks: Clues with regards to source composition and crystallization conditions. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem. 2015, 192, 151–167. [Google Scholar] [CrossRef]
- Wang, L.-X.; Marks, M.A.W.; Wenzel, T.; von der Handt, A.; Keller, J.; Teiber, H.; Markl, G. Apatites from the Kaiserstuhl Volcanic Complex, Germany: New constraints on the relationship between carbonatite and associated silicate rocks. Eur. J. Mineral. 2014, 26, 397–414. [Google Scholar] [CrossRef]
- Wang, L.-X.; Marks, M.A.W.; Wenzel, T.; Markl, G. Halogen-bearing minerals from the Tamazeght complex (Morocco): Constraints on halogen distribution and evolution in alkaline to peralkaline magmatic systems. Can. Mineral. 2016, 54, 1347–1368. [Google Scholar] [CrossRef]
- Bailey, J.C.; Gwozdz, R.; Rose-Hansen, J.; Sørensen, H. Geochemical overview of the Ilimaussaq alkaline complex, South Greenland. GEUS Bull. 2001, 190, 35–53. [Google Scholar] [CrossRef]
- Smith, M.; Moore, K.; Kavecsánszki, D.; Finch, A.; Kynicky, J.; Wall, F. From mantle to critical zone: A review of large and giant sized deposits of the rare earth elements. Geosci. Front. 2016, 7, 315–334. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Khomyakov, A. Mineralogy of Hyperagpaitic Alkaline Rocks; Oxford Science Publications; Oxford University Press: Oxford, UK, 1995; 222p. [Google Scholar]
- Sørensen, H. The agpaitic rocks—An overview. Mineral. Mag. 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Andersen, T.; Erambert, M.; Larsen, A.O.; Selbekk, R.S. Petrology of nepheline syenite pegmatites in the Oslo rift, Norway: Zirconium silicate mineral assemblages as indicators of alkalinity and volatile fugacity in mildly agpaitic magma. J. Petrol. 2010, 51, 2303–2325. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Hettmann, K.; Schilling, J.; Frost, B.R.; Markl, G. The mineralogical diversity of alkaline igneous rocks: Critical factors for the transition from miaskitic to agpaitic phase assemblages. J. Petrol. 2010, 52, 439–455. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Lindhuber, M.J.; Marks, M.A.W.; Bons, P.D.; Wenzel, T.; Markl, G. Crystal mat-formation as an igneous layering-forming process: Textural and geochemical evidence from the ‘lower layered’ nepheline syenite sequence of the Ilimaussaq complex, South Greenland. Lithos 2015, 224–225, 295–309. [Google Scholar] [CrossRef]
- Ratschbacher, B.C.; Marks, M.A.W.; Bons, P.D.; Wenzel, T.; Markl, G. Emplacement and geochemical evolution of highly evolved syenites investigated by a combined structural and geochemical field study: The lujavrites of the Ilímaussaq complex, SW Greenland. Lithos 2015, 231, 62–76. [Google Scholar] [CrossRef]
- Stormer, J.C.; Carmichael, I. Villiaumite and the occurrence of fluoride minerals in igneous rocks. Am. Mineral. 1970, 55, 126–134. [Google Scholar]
- Sharp, Z.D.; Helffrich, G.R.; Bohlen, S.R.; Essene, E.J. The stability of sodalite in the system NaAlSiO4-NaCl. Geochim. Cosmochim. Acta 1989, 53, 1943–1954. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Lazutkina, L.N.; Romanchev, B.P. The origin of eudialyte mineralization (translated from Russian). Geokhimiya 1982, 10, 1415–1432. [Google Scholar]
- Bailey, J.C. Geochemistry of boron in the Ilimaussaq alkaline complex, South Greenland. Lithos 2006, 91, 319–330. [Google Scholar] [CrossRef]
- Zahoransky, T.; Friis, H.; Marks, M.A.W. Luminescence and tenebrescence of natural sodalites: A chemical and structural study. Phys. Chem. Mineral. 2016, 43, 459–480. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, P. Bromine in scapolite-group minerals and sodalite; XRF microprobe analysis, exchange experiments, and applications to skarn deposits. Can. Mineral. 2003, 41, 529–540. [Google Scholar] [CrossRef][Green Version]
- Ryabchikov, I.D.; Kogarko, L.N. Oxygen fugacity in the apatite-bearing intrusion of the Khibina complex. Geochem. Int. 2009, 47, 1157–1169. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Glaznev, V.N.; Raevsky, A.B.; Arzamastseva, L.V. Morphology and internal structure of the Kola Alkaline intrusions, NE Fennoscandian Shield: 3D density modelling and geological implications. J. Asian Earth Sci. 2001, 18, 213–228. [Google Scholar] [CrossRef]
- Atanasova, P.; Marks, M.A.W.; Heining, T.; Krause, J.; Gutzmer, J.; Markl, G. Distinguishing magmatic and metamorphic processes in peralkaline rocks of the Norra Kärr complex (Southern Sweden) using textural and compositional variations of clinopyroxene and eudialyte-group minerals. J. Petrol. 2017, 58, 361–384. [Google Scholar] [CrossRef]
- Kramm, U.; Kogarko, L.N. Nd and Sr isotope signatures of the Khibina and Lovozero agpaitic centres, Kola Alkaline province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Schilling, J.; Wu, F.-Y.; McCammon, C.; Wenzel, T.; Marks, M.A.W.; Pfaff, K.; Jacob, D.E.; Markl, G. The compositional variability of eudialyte-group minerals. Mineral. Mag. 2011, 75, 87–115. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. The Ilímaussaq alkaline complex, South Greenland. In Layered Intrusions; Charlier, B., Namur, O., Latypov, R., Tegner, C., Eds.; Springer Geology; Springer: Dordrecht, The Netherlands, 2015; pp. 649–691. [Google Scholar]
- Larsen, L.M.; Sørensen, H. The Ilimaussaq intrusion-progressive crystallization and formation of layering in an agpaitic magma. In Alkaline Igneous Rocks; Fitton, J.G., Upton, B.G.J., Eds.; Geological Society Special Publications; Blackwell Scientific Publications: London, UK, 1987; Volume 30, pp. 473–488. [Google Scholar]
- Markl, G.; Marks, M.; Schwinn, G.; Sommer, H. Phase equilibrium constraints on intensive crystallization parameters of the Ilimaussaq Complex, South Greenland. J. Petrol. 2001, 42, 2231–2258. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Rudnick, R.; Vennemann, T.; McCammon, C.; Markl, G. Arrested kinetic Li isotope fractionation at the margin of the Ilimaussaq complex, South Greenland: Evidence for open-system processes during final cooling of peralkaline igneous rocks. Chem. Geol. 2007, 246, 207–230. [Google Scholar] [CrossRef]
- Schönberg, R.; Marks, M.A.W.; Schuessler, J.A.; von Blanckenburg, F.; Markl, G. Fe isotope systematics of coexisting amphibole and pyroxene in the alkaline igneous rock suite of the Ilímaussaq Complex, South Greenland. Chem. Geol. 2009, 258, 65–77. [Google Scholar] [CrossRef]
- Hettmann, K.; Marks, M.A.W.; Kreissig, K.; Zack, T.; Wenzel, T.; Rehkämper, M.; Jacob, D.E.; Markl, G. The geochemistry of Tl and its isotopes during magmatic and hydrothermal processes: The peralkaline Ilimaussaq complex, southwest Greenland. Chem. Geol. 2014, 366, 1–13. [Google Scholar] [CrossRef]
- Kchit, A. Le Plutonisme Alcalin du Tamazeght (Haut Atlas de Midelt, Maroc). Ph.D. Thesis, University Toulouse, Toulouse, France, 1990; 224p. [Google Scholar]
- Marks, M.A.W.; Schilling, J.; Coulson, I.M.; Wenzel, T.; Markl, G. The alkaline-peralkaline Tamazeght complex, High Atlas Mountains, Morocco: Mineral chemistry and petrological constraints for derivation from a compositionally heterogeneous mantle source. J. Petrol. 2008, 49, 1097–1131. [Google Scholar] [CrossRef]
- Marks, M.; Coulson, I.M.; Schilling, J.; Jacob, D.E.; Schmitt, A.K.; Markl, G. The effect of titanite and other HFSE-rich mineral (Ti-bearing andradite, zircon, eudialyte) fractionation on the geochemical evolution of silicate melts. Chem. Geol. 2008, 257, 153–172. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Neukirchen, F.; Vennemann, T.; Markl, G. Textural, chemical, and isotopic effects of late-magmatic carbonatitic fluids in the carbonatite-syenite Tamazeght complex, High Atlas Mountains, Morocco. Mineral. Petrol. 2009, 97, 23–42. [Google Scholar] [CrossRef]
- Allah, R.K.; Fontan, F.; Kadar, M.; Monchoux, P.; Sørensen, H. Reactions between agpaitic nepheline syenitic melts and sedimentary carbonate rocks, exemplified by the Tamazeght complex, Morocco. Geochem. Int. 1998, 36, 569–581. [Google Scholar]
- Schilling, J.; Marks, M.; Wenzel, T.; Markl, G. Reconstruction of magmatic to sub-solidus processes in an agpaitic system using eudialyte textures and composition: A case study from Tamazeght, Morocco. Can. Mineral. 2009, 47, 351–365. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Yakovenchuk, V.; Pakhomovsky, Y.; Ivanyuk, G. The Khibina and Lovozero alkaline massifs: Geology and unique mineralization. In Proceedings of the 33 International Geological Congress (IGC) Excursion No 47, Oslo, Norway, 6–14 August 2008; 58p. [Google Scholar]
- Zaitsev, A.N.; Wall, F.; Le Bas, M.J. REE-Sr-Ba minerals from the Khibina carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Arzamastseva, L.V.; Zhirova, A.M.; Glaznev, V.N. Model of formation of the Khibiny-Lovozero ore-bearing volcanic-plutonic complex. Geol. Ore Depos. 2013, 55, 341–356. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Yakovenchuk, V.N. Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 581. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Magnetite compositions and oxygen fugacity of the Khibina magmatic system. Lithos 2006, 91, 35–45. [Google Scholar] [CrossRef]
- Sjöqvist, A.S.; Cornell, D.H.; Andersen, T.; Erambert, M.; Ek, M.; Leijd, M. Three Compositional Varieties of Rare-Earth Element Ore: Eudialyte-Group Minerals from the Norra Kärr Alkaline Complex, Southern Sweden. Minerals 2013, 3, 94–120. [Google Scholar] [CrossRef]
- Stevensen, F.J. Chemical state of the nitrogen in rocks. Geochim. Cosmochim. Acta 1962, 26, 797–809. [Google Scholar] [CrossRef]
- Scholten, S.O. The Distribution of Nitrogen Isotopes in Sediments. Ph.D. Thesis, Utrecht University Repository, Utrecht, The Netherlands, 1991. [Google Scholar]
- Holloway, J.A.M.; Dahlgren, R.A. Nitrogen in rock: Occurrences and biogeochemical implications. Glob. Biogeochem. Cycles 2002, 16, 65. [Google Scholar] [CrossRef]
- Walter, A. Bestimmung von Iod in Gesteinsmehl nach Verbrennungsaufschluß mit Metrohm Combustion; Application Work AW IC DE8-0898-042015; Metrohm: Filderstadt, Germany, 2016. [Google Scholar]
- Hagen, M. Igneous Layering: Mineralogic and Petrologic Analysis of Slightly-Layered Kakortokites (SLK’s) and Lower Layered Kakortokites (LLK’s) of the Ilimaussaq Intrusion, Southwest Greenland. Master’s Thesis, University of Tübingen, Tübingen, Germany, 2019. [Google Scholar]
- Mundel, F. Igneous Layering in the Lowermost Units of the Eudialyte-Bearing Nepheline Syenitic Kakortokites, Ilímaussaq Alkaline Complex, South Greenland. Master’s Thesis, Eberhard-Karls-Universität Tübingen, Tübingen, Germany, 2019. [Google Scholar]
- Rastsvetaeva, R.K. Structural mineralogy of the eudialyte group: A review. Crystallogr. Rep. 2007, 52, 47–64. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Pekov, I.V.; Varlamov, D.A.; Aksenov, S.M. New Data on the Isomorphism in Eudialyte-Group Minerals. VI: Crystal Structure of the First Member Containing Sulfide Anion with Isomorphic Substitution Cl−–S2−. Crystallogr. Rep. 2020, 65, 215–222. [Google Scholar] [CrossRef]
- Sørensen, H.; Danø, M.; Petersen, O.V. On the mineralogy and paragenesis of Tugtupite, Na8Al2Be2Si8O24(Cl,S)2, from the Ilímaussaq alkaline intrusion, South Greenland. GEUS Bull. 1971, 95, 38. [Google Scholar]
- Danø, M. The crystal structure of tugtupite—A new mineral, Na8Al2Be2Si8O24(Cl,S)2. Acta Crystallogr. 1966, 20, 812–816. [Google Scholar] [CrossRef]
- Gaft, M.; Panczer, G.; Nagli, L.; Yeates, H. Laser-induced time-resolved luminescence of tugtupite, sodalite and hackmanite. Phys. Chem. Mineral. 2009, 36, 127–141. [Google Scholar] [CrossRef]
- Finch, A.A.; Friis, H.; Maghrabi, M. Defects in sodalite-group minerals determined from X-ray-induced luminescence. Phys. Chem. Mineral. 2016, 43, 481–491. [Google Scholar] [CrossRef]
- Povarennykh, A.S.; Platonov, A.N.; Tarashchan, A.N.; Belichenko, V.B. The colour and luminescence of Tugtupite (beryllosodalite) from Ilímaussaq, South Greenland. GEUS Bull. 1971, 95, 12. [Google Scholar]
- Kendrick, M.A.; Jackson, M.G.; Hauri, E.H.; Phillips, D. The halogen (F, Cl, Br, I) and H2O systematics of Samoan lavas: Assimilated-seawater, EM2 and high-3He/4He components. Earth Planet. Sci. Lett. 2015, 410, 197–209. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Honda, M.; Vanko, D.A. Halogens and noble gases in Mathematician Ridge meta-gabbros, NE Pacific: Implications for oceanic hydrothermal root zones and global volatile cycles. Contrib. Mineral. Petrol. 2015, 170, 43. [Google Scholar] [CrossRef]
- Epp, T.; Marks, M.A.W.; Ludwig, T.; Kendrick, M.A.; Eby, N.; Neidhardt, H.; Oelmann, Y.; Markl, G. Crystallographic and fluid compositional effects on the halogen (Cl, F, Br, I) incorporation in pyromorphite-group minerals. Am. Mineral. 2019, 104, 1673–1688. [Google Scholar] [CrossRef]
- Borst, A.M.; Friis, H.; Nielsen, T.F.D.; Waight, T.E. Bulk and mush melt evolution in agpaitic intrusions: Insights from compositional zoning in eudialyte, Ilímaussaq complex, South Greenland. J. Petrol. 2018, 59, 589–612. [Google Scholar] [CrossRef]
- Pfaff, K.; Krumrei, T.; Marks, M.; Wenzel, T.; Rudolf, T.; Markl, G. Chemical and physical evolution of the ‘lower layered sequence’ from the nepheline syenitic Ilímaussaq intrusion, South Greenland: Implications for the origin of magmatic layering in peralkaline felsic liquids. Lithos 2008, 106, 280–296. [Google Scholar] [CrossRef]
- Babiel, R.; Marks, M.A.W.; Neumann, U.; Markl, G. Sulfides in alkaline and peralkaline rocks: Textural appearance and compositional variations. Neues Jahrb. Mineral. Abh. J. Mineral. Geochem 2018, 195, 155–175. [Google Scholar] [CrossRef]
- Graser, G.; Potter, J.; Köhler, J.; Markl, G. Isotope, major, minor and trace element geochemistry of late-magmatic fluids in the peralkaline Ilímaussaq intrusion, South Greenland. Lithos 2008, 106, 207–221. [Google Scholar] [CrossRef]
- Hettmann, K.; Wenzel, T.; Marks, M.; Markl, G. The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals. Am. Mineral. 2012, 97, 1653–1661. [Google Scholar] [CrossRef]
- Fleet, M.E.; Liu, X.; Harmer, S.L.; Nesbitt, H.W. Chemical state of sulfur in natural and synthetic lazurite by S K-edge xanes and X-ray photoelectron spectroscopy. Can. Mineral. 2005, 43, 1589–1603. [Google Scholar] [CrossRef]
- Hassan, I. Transmission electron microscopy and differential thermal studies of lazurite polymorphs. Am. Mineral. 2000, 85, 1383–1389. [Google Scholar] [CrossRef]
- Wulff-Pedersen, E.; Neumann, E.-R.; Burke, E.A.J.; Vannucci, R.; Bottazzi, P.; Ottolini, L.; Gjonnes, J.; Hansen, V. Origin and structural character of hauyne(ss) in spinel dunite xenoliths from La Palma, Canary Islands. Am. Mineral. 2000, 85, 1397–1405. [Google Scholar] [CrossRef]
- Hassan, I.; Grundy, H.D. The crystal structures of sodalite-group minerals. Acta Crystallogr. B Struct. Sci. 1984, 40, 6–13. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Mazzi, F.; Tadini, C. The crystal structure of eudialyte. Tschermaks Mineral. Petrogr. Mitt. 1971, 16, 105–127. [Google Scholar] [CrossRef]
- Johnsen, O.; Grice, J.D.; Gault, R.A. Kentbrooksite from the Kangerdlugssuaq intrusion, East Greenland, a new Mn-REE-Nb-F endmember in a series within the eudialyte group: Description and crystal structure. Eur. J. Mineral. 1998, 10, 207–219. [Google Scholar] [CrossRef]
- Johnsen, O.; Grice, J.D. The crystal chemistry of the eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Sjöqvist, A.S.; Zack, T.; Honn, D.K.; Baxter, E.F. Modification of a rare-earth element deposit by low-temperature partial melting during metamorphic overprinting: Norra Kärr alkaline complex, southern Sweden. Chem. Geol. 2020, 545, 119640. [Google Scholar] [CrossRef]

| Mineral Name | Formula |
|---|---|
| Fluorite | CaF2 |
| Villiaumite | NaF |
| Rinkite | (Ca3REE)Na(NaCa)Ti(Si2O7)2(OF)F2 |
| Wöhlerite | Na2Ca4Zr(Nb,Ti)(Si2O7)2(O,F)4 |
| Natrophosphate | Na7(PO4)2F.19H2O |
| Vuonnemite | Na6Na2Nb2Na3Ti(Si2O7)2(PO4)2O2(OF) |
| Kentbrooksite * | Na15Ca6Mn3Zr3NbSi(Si24O73)(O,OH,H2O)3(F,Cl)2 |
| Eudialyte * | Na15Ca6Fe3Zr3Si2(Si24O73)(O,OH,H2O)3(Cl,F,OH)2 |
| Sodalite | Na8Al6Si6O24Cl2 |
| Tugtupite | Na4BeAlSi4O12Cl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggenkamp, H.G.M.; Marks, M.A.W.; Atanasova, P.; Wenzel, T.; Markl, G. Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems. Minerals 2020, 10, 995. https://doi.org/10.3390/min10110995
Eggenkamp HGM, Marks MAW, Atanasova P, Wenzel T, Markl G. Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems. Minerals. 2020; 10(11):995. https://doi.org/10.3390/min10110995
Chicago/Turabian StyleEggenkamp, Hans G.M., Michael A.W. Marks, Petya Atanasova, Thomas Wenzel, and Gregor Markl. 2020. "Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems" Minerals 10, no. 11: 995. https://doi.org/10.3390/min10110995
APA StyleEggenkamp, H. G. M., Marks, M. A. W., Atanasova, P., Wenzel, T., & Markl, G. (2020). Changes in Halogen (F, Cl, Br, and I) and S Ratios in Rock-Forming Minerals as Monitors for Magmatic Differentiation, Volatile-Loss, and Hydrothermal Overprint: The Case for Peralkaline Systems. Minerals, 10(11), 995. https://doi.org/10.3390/min10110995






