Function of Interface Deposition of Calcium Sulfate in Pressure Acid Leaching of Black Shale-Hosted Vanadium
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Effect of Sulfate Type and Dosage on Vanadium Leaching
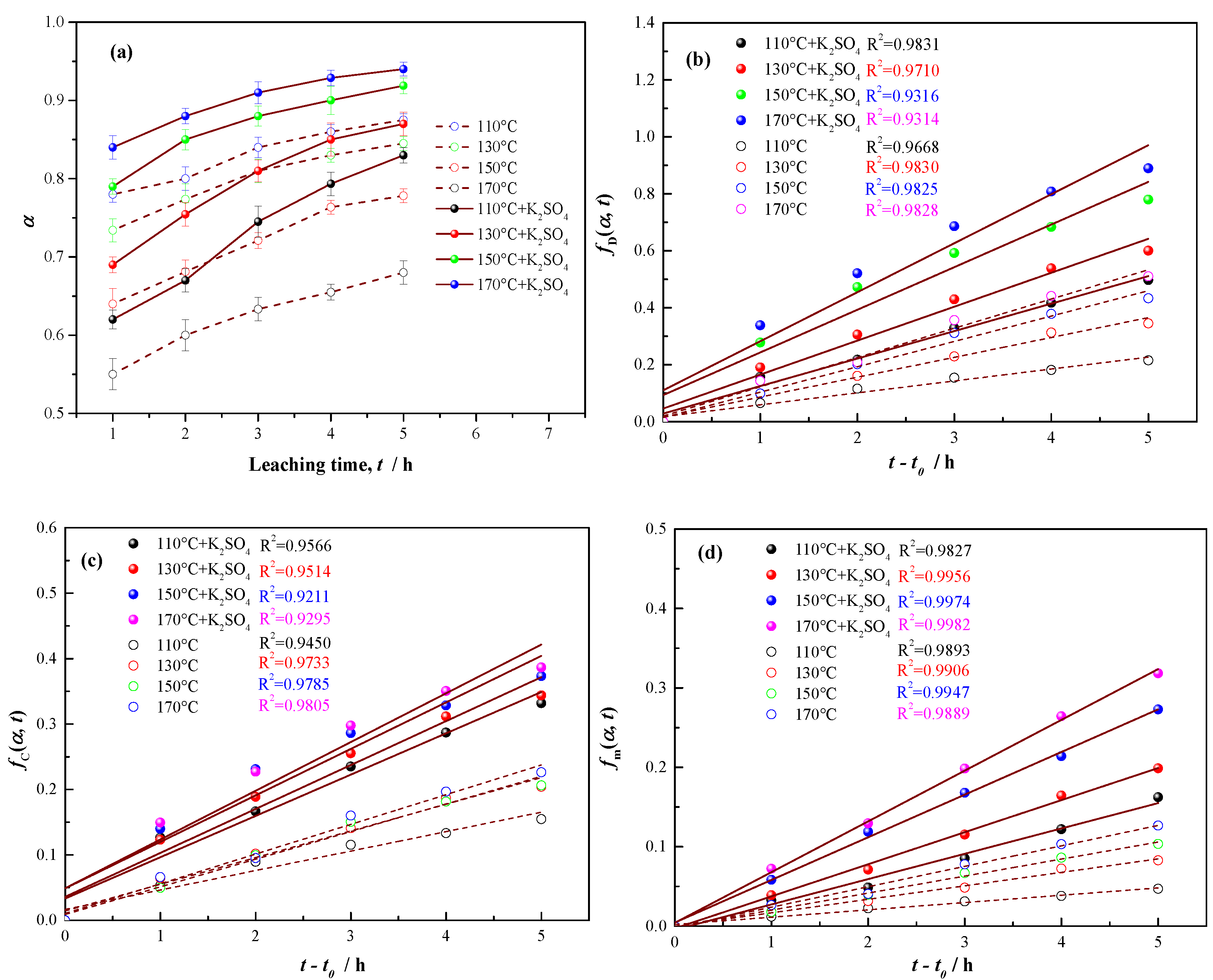
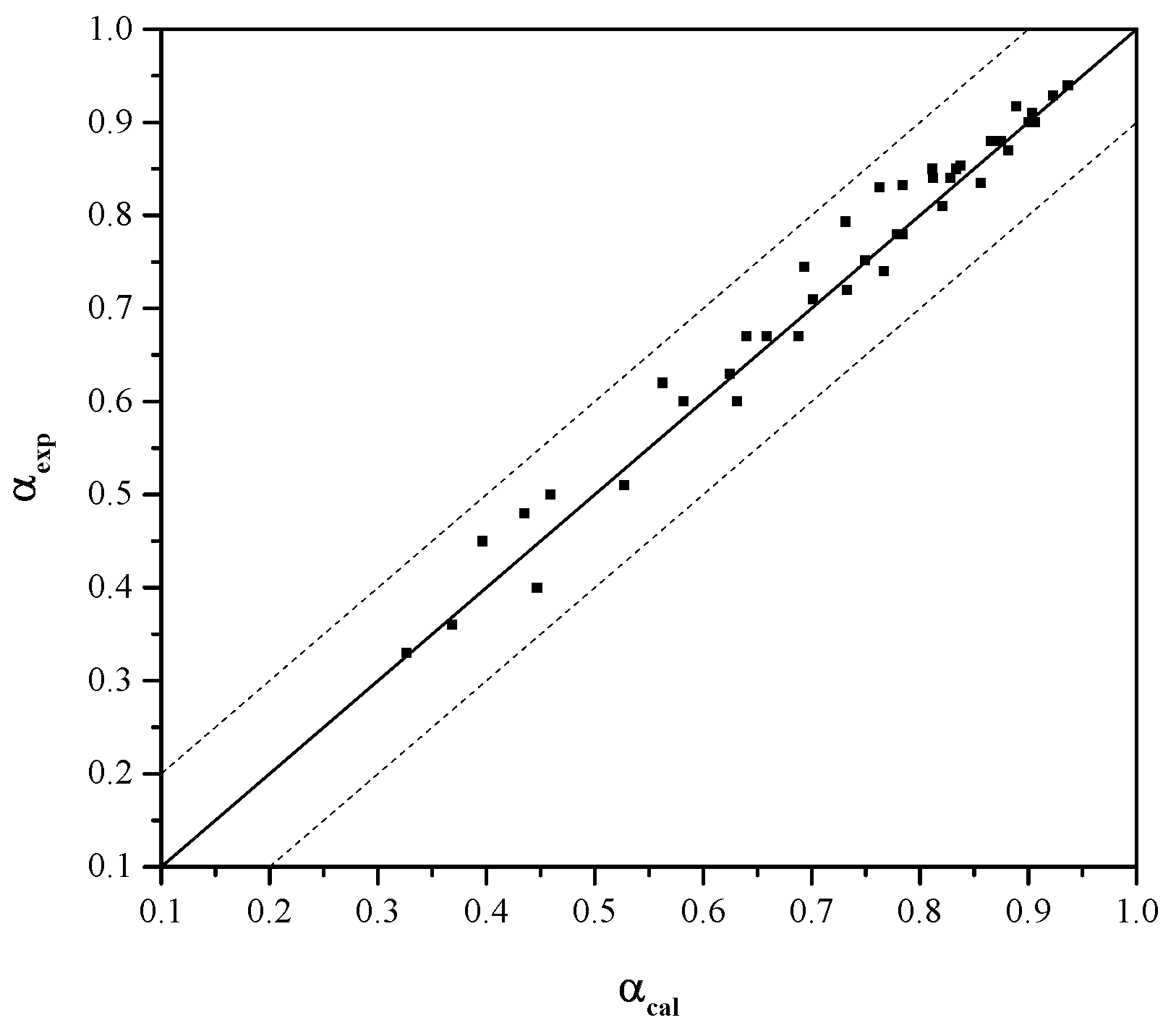
3.2. K2SO4-Assisted Pressure Acid Leaching Kinetics
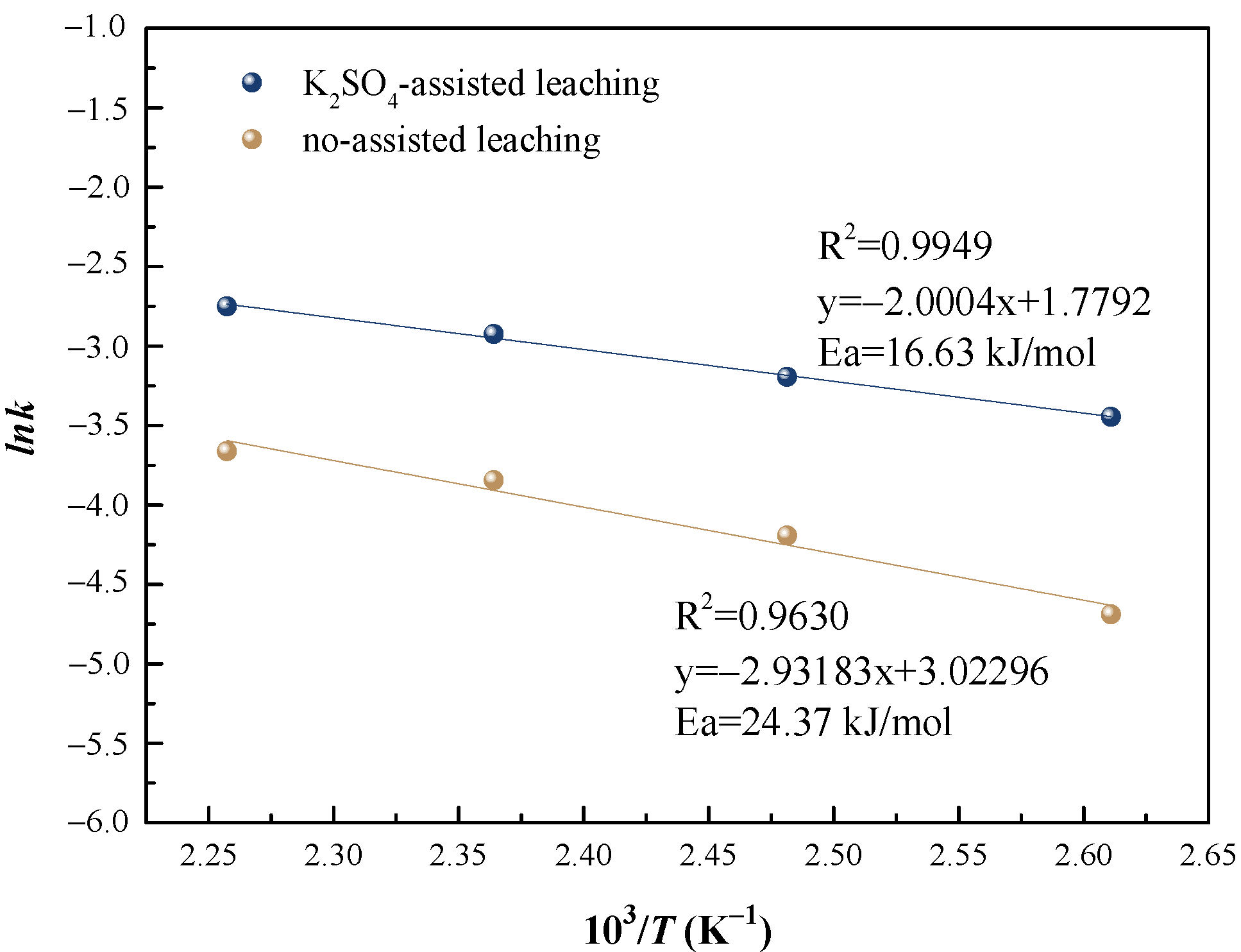
3.2.1. Apparent Activation Energy of Vanadium Leaching
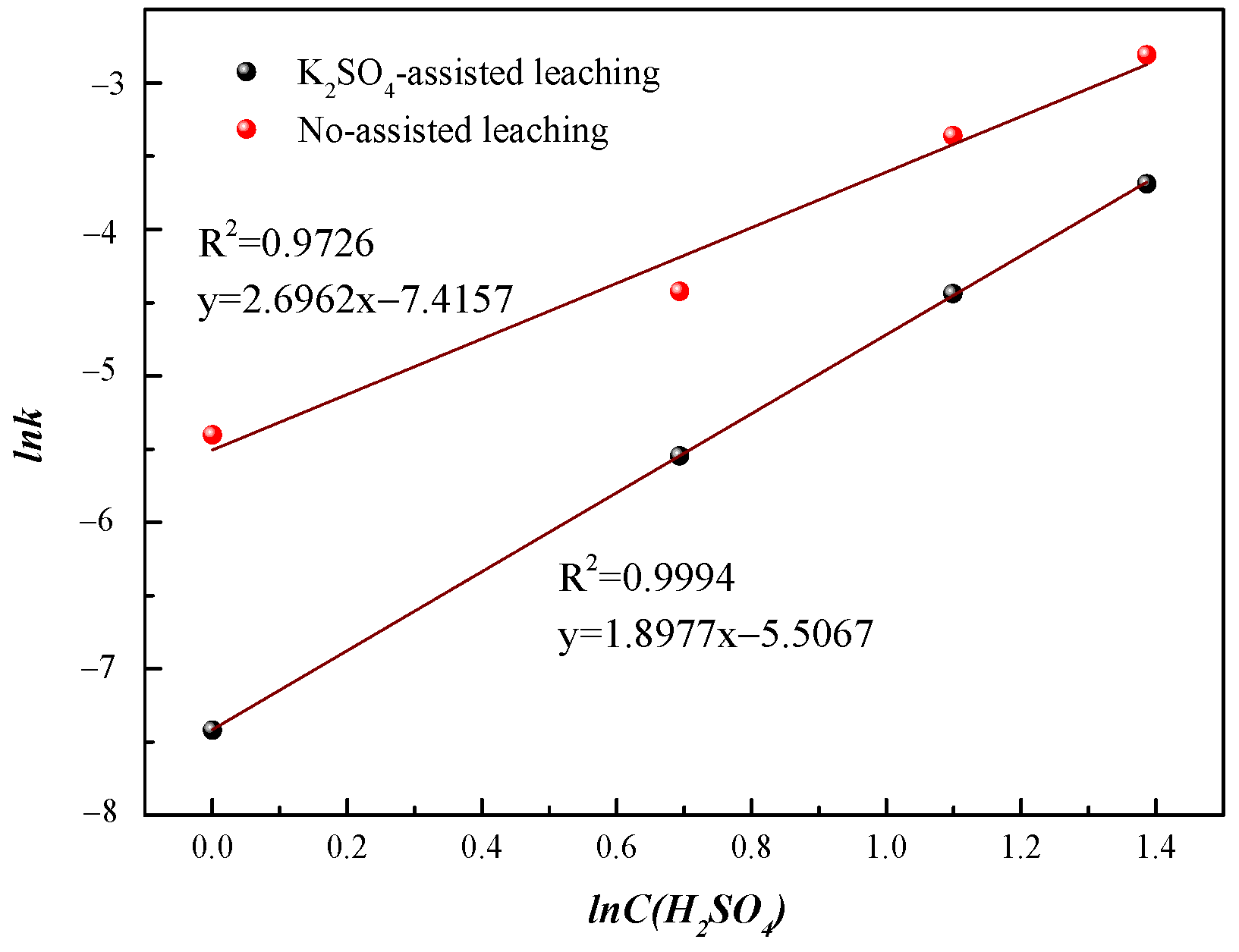
3.2.2. Apparent Reaction Order of Sulfuric Acid
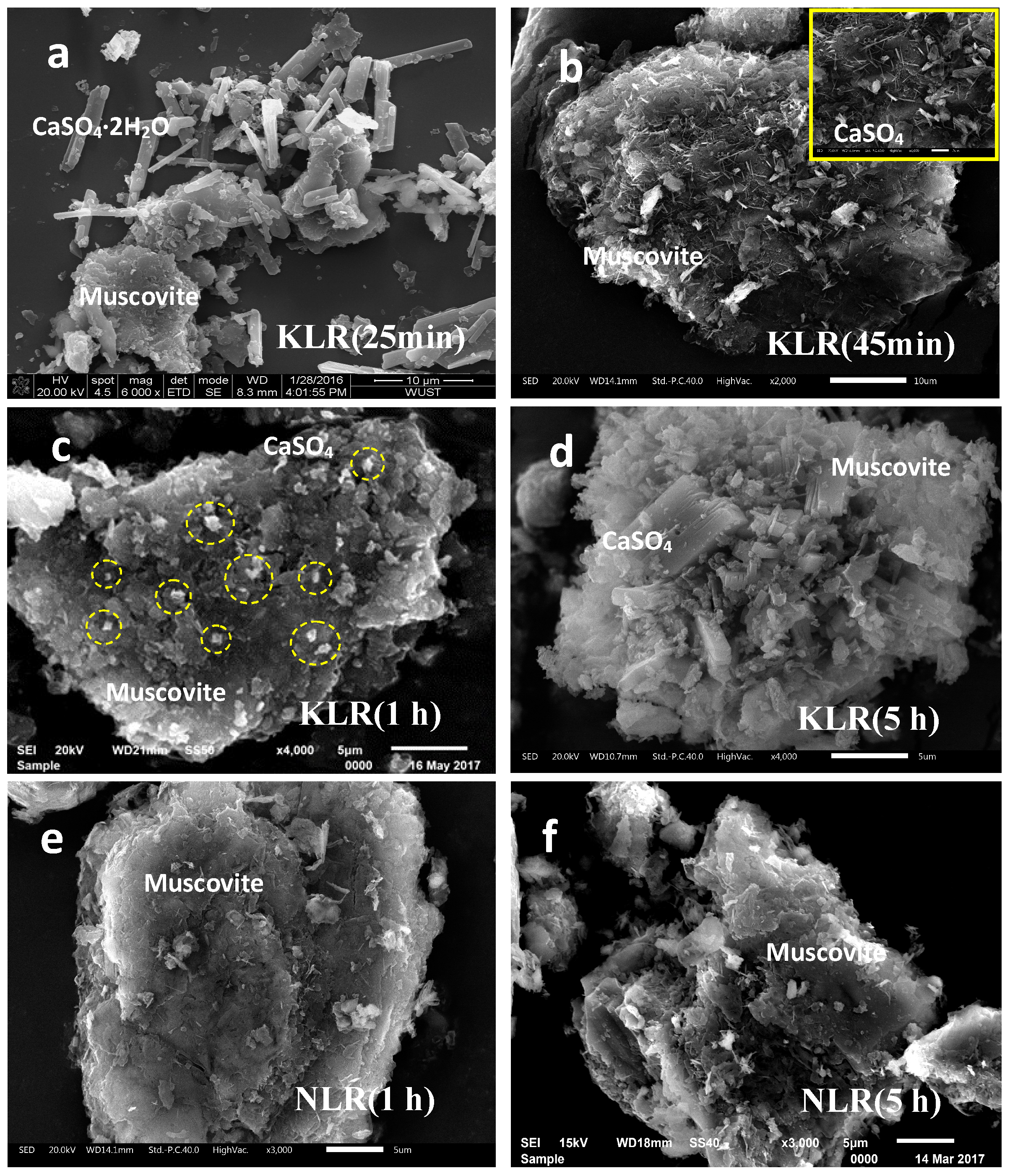
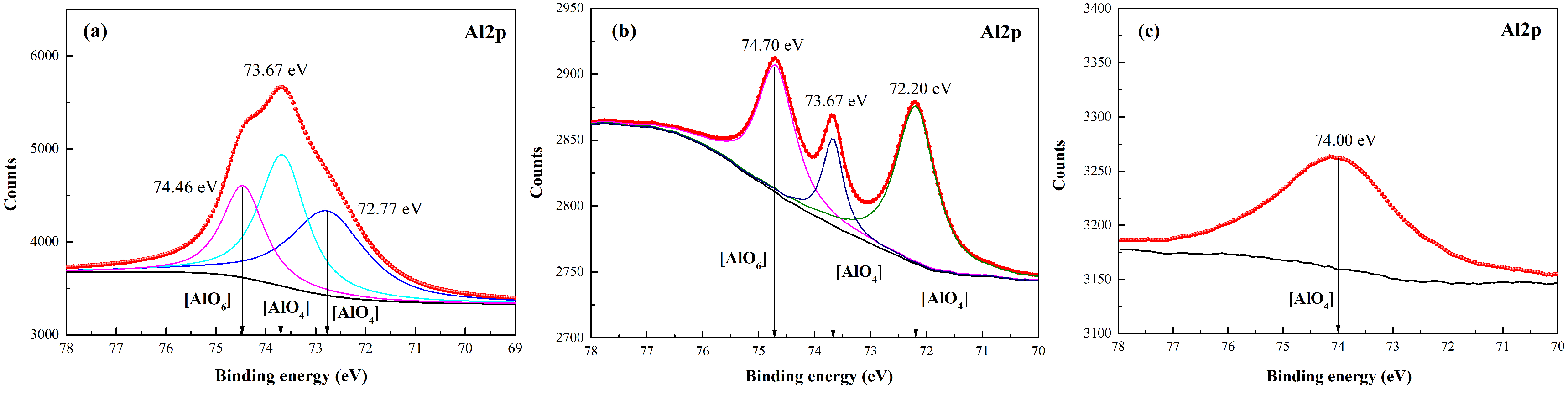
3.3. Surface Condition of Muscovite after K2SO4-Assisted Leaching
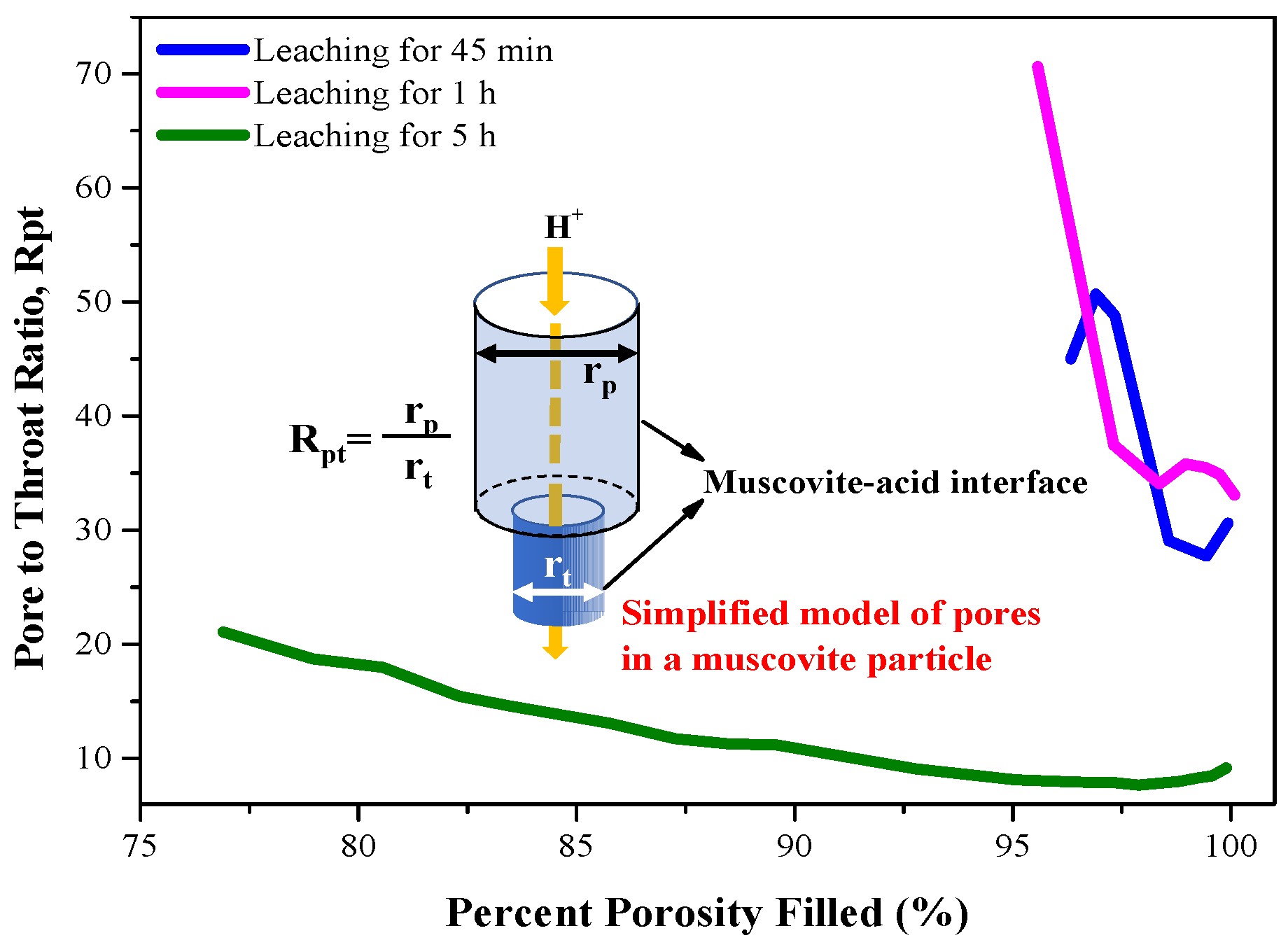
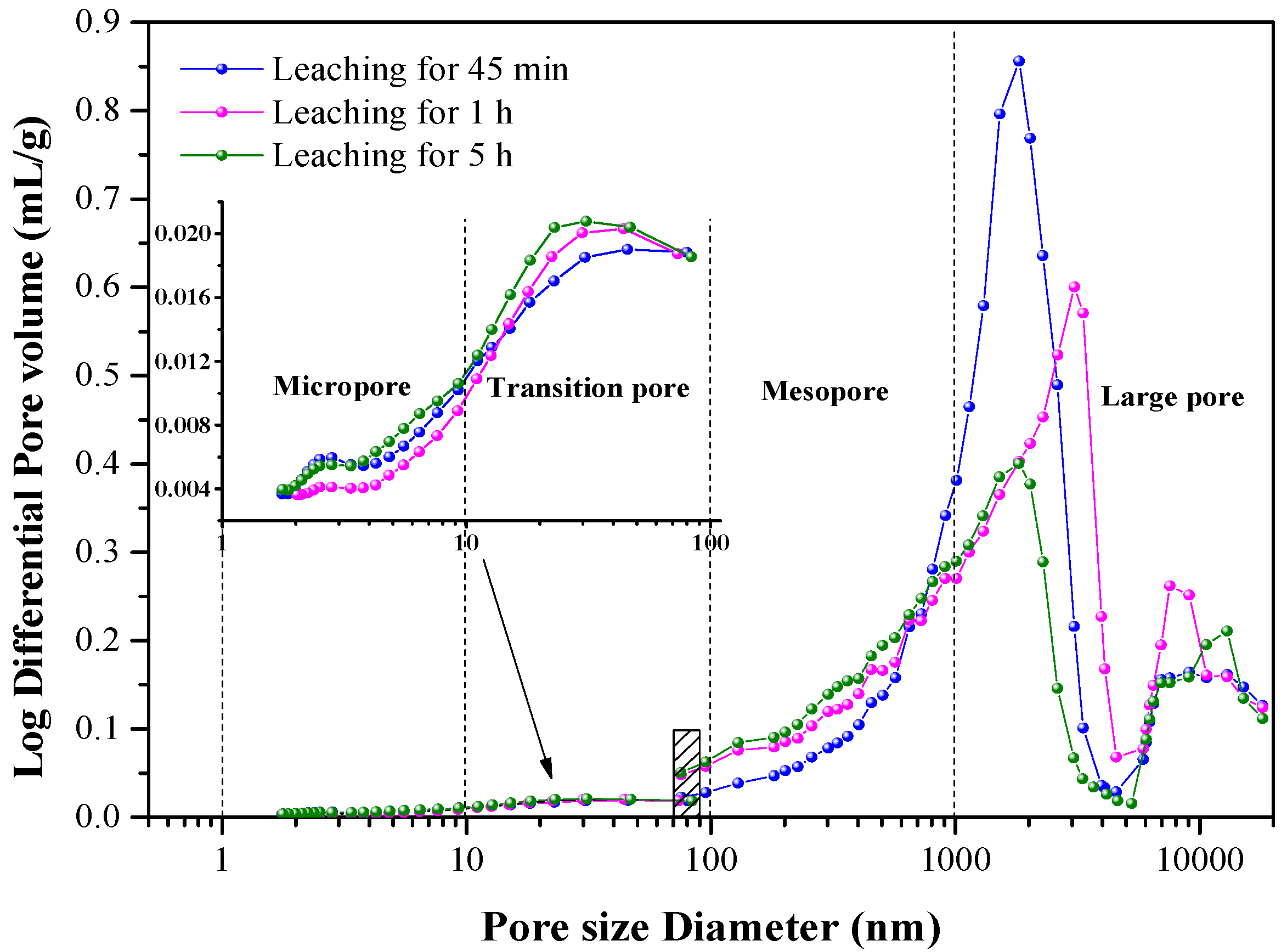
3.4. Pore Distribution in the KLR and the NLR
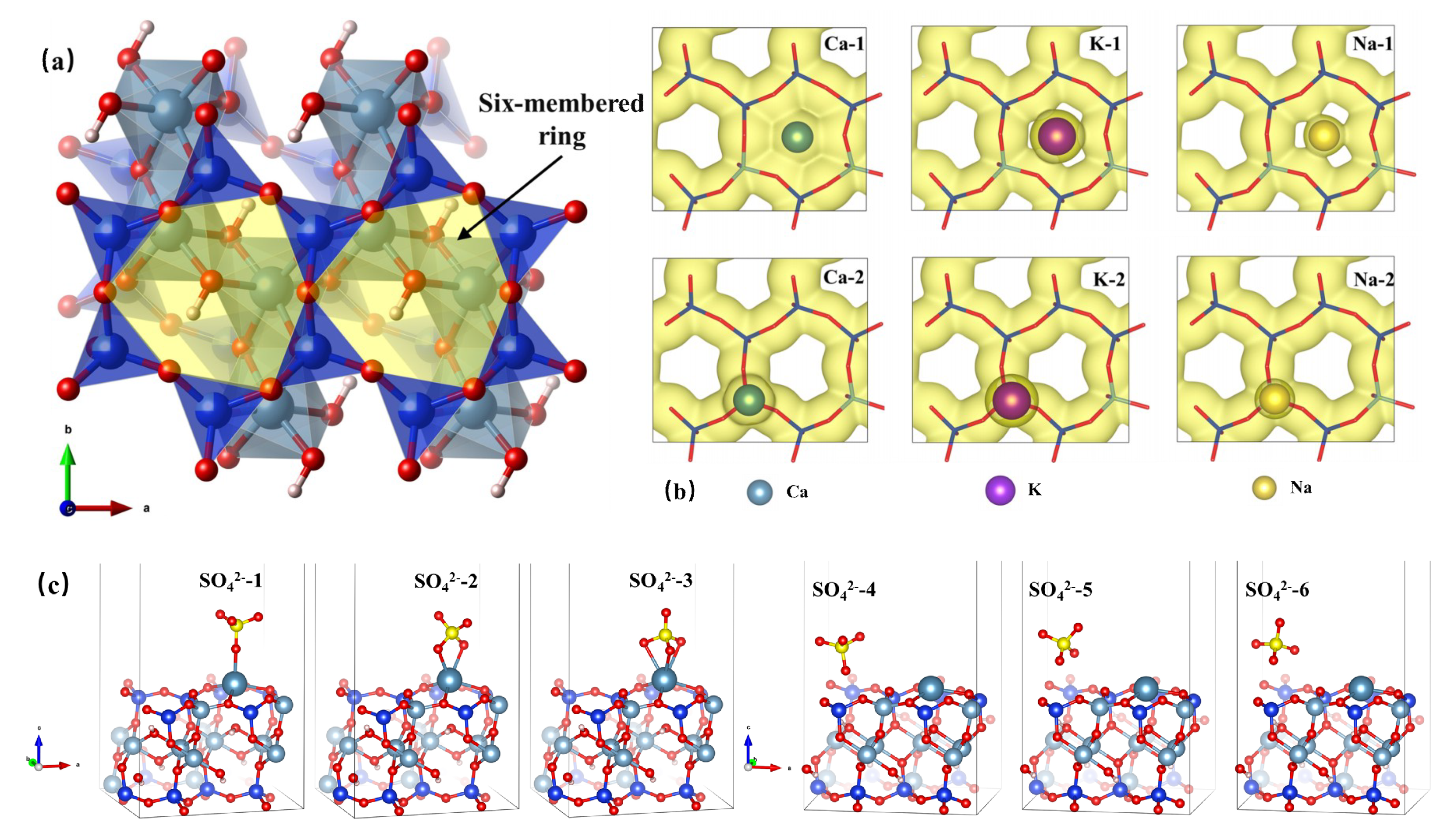
3.5. Mechanism of CaSO4 Deposition on Muscovite and Its Function
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J.Q.; Chen, G.R.; Guo, F.; Xie, J.Q. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NOx with ammonia: Review. Chem. Eng. J. 2018, 353, 507–518. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Gao, Y.C.; Meng, Y.; Chen, X.J.; Liu, J.W.; Xiang, H.L.; Xu, Y.W.; Jiang, J.G. Review of plant-vanadium physiological interactions, bioaccumulation, and bioremediation of vanadium-contaminated sites. Sci. Total Environ. 2020, 712, 135637. [Google Scholar] [CrossRef] [PubMed]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Gummow, B. Vanadium: Environmental pollution and health effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 19–326. [Google Scholar]
- Dai, S.F.; Zheng, X.; Wang, X.B.; Finkelman, R.B.; Jiang, Y.F.; Ren, D.Y.; Yan, X.Y.; Zhou, Y.P. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Fozia, A.; Muhammad, S.; Ata, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117, 1–12. [Google Scholar]
- Kelley, K.D.; Scott, C.T.; Polyak, D.E.; Kimball, B.E.; Schulz, K.J.; DeYoung, J.H., Jr.; Seal, R.R., II; Bradley, D.C. (Eds.) Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply: U.S. Geological Survey Professional Paper 1802; U.S. Geological Survey: Reston, VA, USA, 2017; pp. U1–U36.
- Northrop, H.R.; Goldhaber, M.B.; Landis, G.P.; Unruh, J.W.; Reynolds, R.L.; Campbell, J.A.; Wanty, R.B.; Grauch, R.I.; Whitney, G.; Rye, R.O. Genesis of the tabular-type vanadium-uranium deposits of the Henry basin. Econ. Geol. Utah 1990, 85, 215–269. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Bian, Y.; Bao, S.X. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Process. 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Wang, X.J.; Zhang, Y.M.; Liu, T.; Cai, Z.L. Influence of metal ions on muscovite and calcite flotation: With respect to the pre-treatment of vanadium bearing stone coal. Coll. Surf. A Physicochem. Eng. Asp. 2019, 564, 89–94. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.M.; Wang, H.; Feng, Q.C. Selective adsorption mechanism of salicylic acid on pyrite surfaces and its application in flotation separation of chalcopyrite from pyrite. Sep. Purif. Technol. 2020, 240, 116650. [Google Scholar] [CrossRef]
- Zhao, W.J.; Liu, D.W.; Feng, Q.C. Enhancement of salicylhydroxamic acid adsorption by Pb(II) modified hemimorphite surfaces and its effect on floatability. Miner. Eng. 2020, 152, 106373. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Bosbach, D.; Hochella, M.F., Jr.; Charlet, L.; Rufe, E. In situ atomic force microscopy study of hectorite and nontronite dissolution: Implications for phyllosilicate edge surface structures and dissolution mechanisms. Am. Mineral. 2001, 86, 411–423. [Google Scholar] [CrossRef]
- Rufe, E.; Hochella, M. Quantitative assessment of reactive surface area of phlogopite during acid dissolution. Science 1999, 285, 874–876. [Google Scholar] [CrossRef]
- Turpault, M.P.; Trotignon, L. The dissolution of biotite single crystals in dilute HNO3 at 24 °C: Evidence of an anisotropic corrosion process of micas in acidic solutions. Geochim. Cosmochim. Acta 1994, 58, 2761–2775. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Zhang, Y.M.; Liu, T.; Huang, J.; Xue, N.N. Removal process of structural oxygen from tetrahedrons in muscovite during acid leaching of vanadium-bearing shale. Minerals 2018, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Azimi, G.; Papangelakis, V.G. The solubility of gypsum and anhydrite in simulated laterite pressure acid leach solutions up to 250 °C. Hydrometallurgy 2010, 102, 1–13. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J.; Liu, H.; Chen, F. Mechanism of vanadium extraction from stone coal via hydrating and hardening of anhydrous calcium sulfate. Hydrometallurgy 2016, 166, 48–56. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N.N.; Shi, Q.H. Optimal location of vanadium in muscovite and its geometrical and electronic properties by DFT calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Arabi-Karasgani, M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Ke, Y.; Min, X.B.; Chai, L.Y.; Zhou, B.S.; Xue, K. Sulfidation behavior of Zn and ZnS crystal growth kinetics for Zn(OH)2-S-NaOH hydrothermal system. Hydrometallurgy 2016, 161, 166–173. [Google Scholar] [CrossRef]
- Eric, H.O.; Jacques, S.; Gauthier, M.J.; Teresa, H.R. An experimental study of the dissolution mechanism and rates of muscovite. Geochimica et Cosmochimica Acta 2008, 72, 4948–4961. [Google Scholar]
- Xoaotb, B.B. Coal and Gas Outburst; Song, S.Z.; Wang, Y.A., Translators; Industry Press: Beijing, China, 1966. [Google Scholar]





| V2O5 | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | Na2O | BaO | TiO2 | S | C | Else |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.72 | 53.53 | 9.21 | 6.35 | 5.82 | 2.44 | 2.00 | 1.09 | 0.75 | 0.42 | 3.60 | 10.30 | 3.73 |
| V(III) | V(IV) | V(V) | Total V |
|---|---|---|---|
| 75.4 | 24.6 | 0 | 100 |
| Temperature | Reactions |
|---|---|
| T < 100 °C | CaCO3(s) + H+ = Ca2+ + H2O + CO2 |
| Ca2+ + SO42− + K2SO4 + H2O = K2Ca(SO4)2·H2O | |
| K2Ca(SO4)2·H2O + H2O = CaSO4·2H2O(s) + K2SO4 | |
| T > 100 °C | CaSO4·2H2O(s) = Ca2++SO42− + 2H2O |
| Ca2+ + SO42− = CaSO4(s) |
| Pore | Leaching Time | Proportion/% | Pore Volume/mL·g−1 | Pore Surface Area/m2·g−1 |
|---|---|---|---|---|
| Large pore | 45 min | 75.91 | 0.3807 | 0.3757 |
| 1 h | 70.97 | 0.3462 | 0.2744 | |
| 5 h | 59.45 | 0.2294 | 0.2037 | |
| Mesopore | 45 min | 19.70 | 0.0988 | 0.4788 |
| 1 h | 24.11 | 0.1176 | 0.6532 | |
| 5 h | 34.21 | 0.1320 | 0.7418 | |
| Transition pore | 45 min | 3.41 | 0.0171 | 2.8821 |
| 1 h | 4.18 | 0.0204 | 2.9374 | |
| 5 h | 5.00 | 0.0193 | 3.2427 | |
| Micropore | 45 min | 0.98 | 0.0049 | 5.1398 |
| 1 h | 0.74 | 0.0036 | 3.5296 | |
| 5 h | 1.35 | 0.0052 | 5.3163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, N.; Zheng, Q.; Zhang, Y. Function of Interface Deposition of Calcium Sulfate in Pressure Acid Leaching of Black Shale-Hosted Vanadium. Minerals 2020, 10, 952. https://doi.org/10.3390/min10110952
Xue N, Zheng Q, Zhang Y. Function of Interface Deposition of Calcium Sulfate in Pressure Acid Leaching of Black Shale-Hosted Vanadium. Minerals. 2020; 10(11):952. https://doi.org/10.3390/min10110952
Chicago/Turabian StyleXue, Nannan, Qiushi Zheng, and Yimin Zhang. 2020. "Function of Interface Deposition of Calcium Sulfate in Pressure Acid Leaching of Black Shale-Hosted Vanadium" Minerals 10, no. 11: 952. https://doi.org/10.3390/min10110952
APA StyleXue, N., Zheng, Q., & Zhang, Y. (2020). Function of Interface Deposition of Calcium Sulfate in Pressure Acid Leaching of Black Shale-Hosted Vanadium. Minerals, 10(11), 952. https://doi.org/10.3390/min10110952




