Thermal Properties and Burial Alteration of Deep-Sea Sediments: New Indicators of Oxic−Suboxic Diagenesis
Abstract
1. Introduction
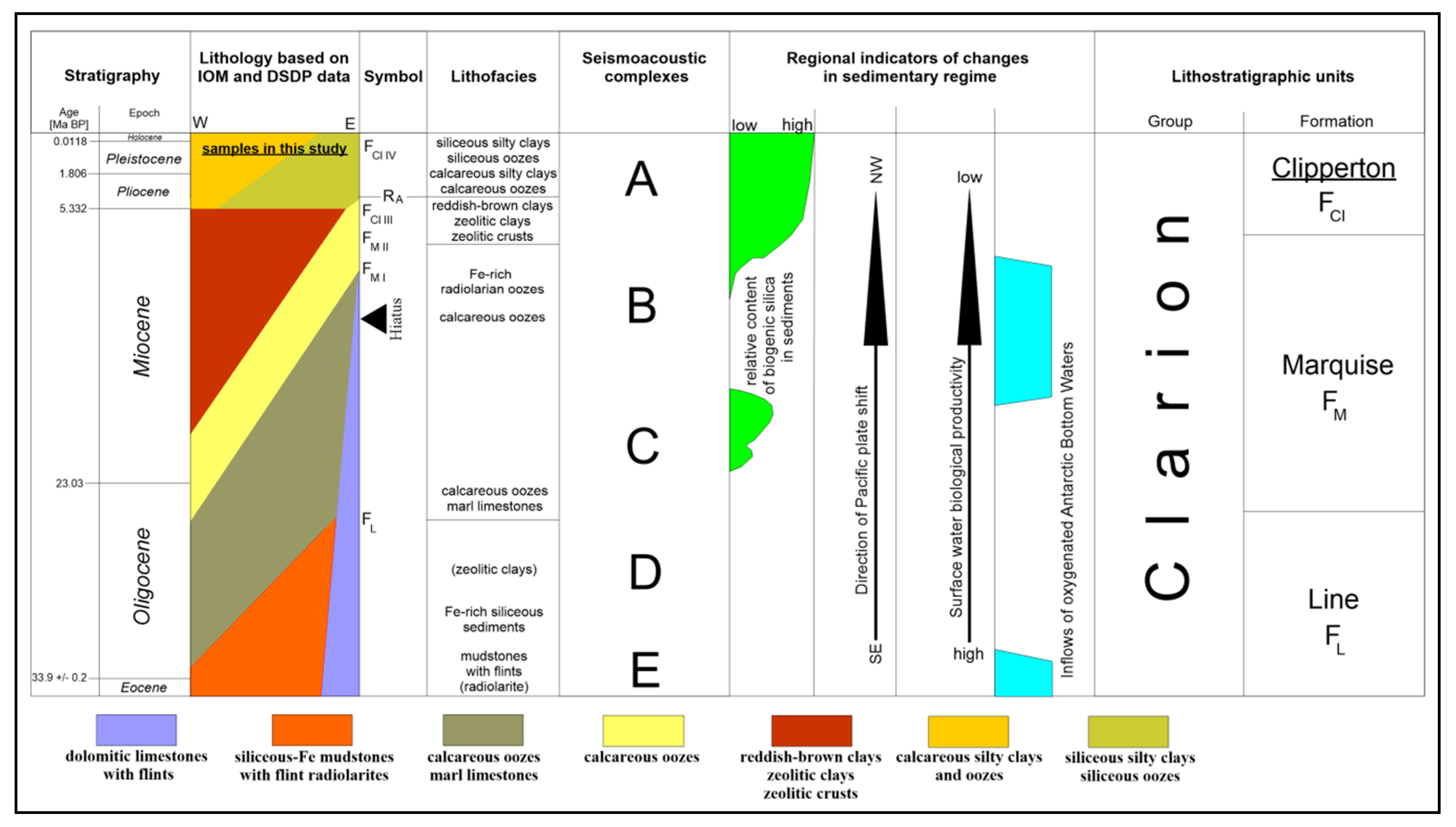
2. Geological Setting
- The low rate of mixed-type (biogenic–clayey) sedimentation.
- Mineralogy and geochemistry of sediments, characterised by the occurrence of amorphous opaline biosilica, clay mineral admixtures and oxygen non-resistible elements, such as Fe, Mn, Ni, Cu, Co, Zn, or rare earth elements (REE).
- Diagenesis occurring under oxic–suboxic conditions, related with a relatively low thickness of sedimentary cover.
- The presence/absence and thickness of the so-called “active layer”, which is the uppermost part of semiliquid sediments, indicating the greatest amount of polymetallic nodules.
- The equatorial tropical climate zonation and high bioproductivity.
- Relatively plain seafloor topography.
- Distinctive changes in physical and chemical parameters of seawater, affecting sediment zonation, including regional and local patterns of palaeocurrents and bottom currents.
- Spatial and temporal variability of geochemical barriers, including oxygen minimum zone and carbonate compensation depth.
- Geochemistry of the underlying sediments and basement rocks.
- The proximity of volcanic activity areas and volcanic and hydrothermal material supply, as well as the lack of distinctive terrestrial input (besides low aeolian influx).
- Relatively low tectonic and seismic activity of the crust.
3. Materials and Methods
3.1. Samples
3.2. Methods
3.2.1. Rock–Eval 6 Pyrolysis (R–E6)
3.2.2. Thermal Analysis (TA)
3.2.3. Nitrogen−Sulphur Combustion Spectrometry (NS)
3.3. Grain Size Analysis
3.4. Geochemistry
3.5. Mineralogy
4. Results
4.1. Thermal Proxies
4.1.1. The R–E6 and Selected Geochemical Signatures of Sedimentary Environment
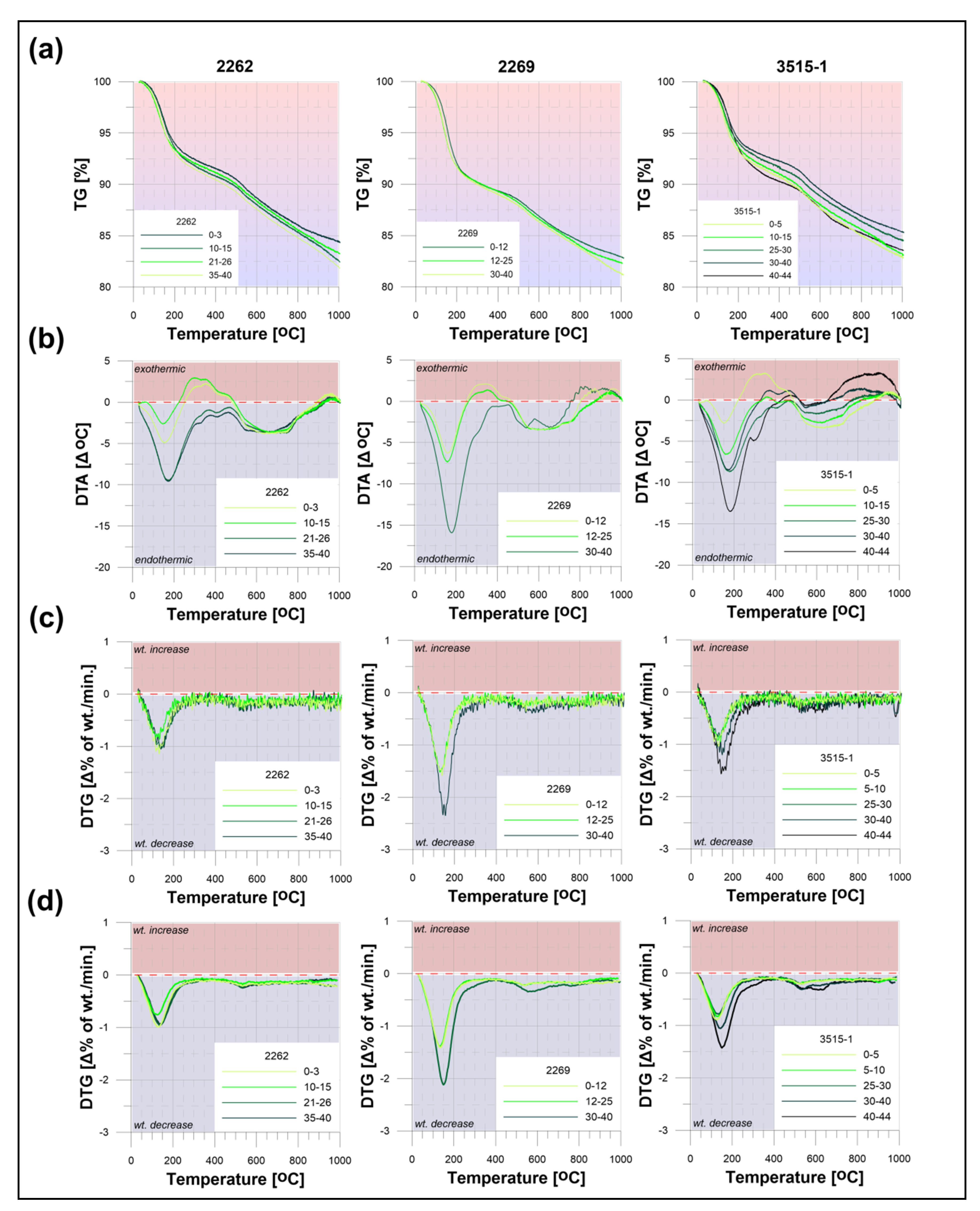
4.1.2. Thermal Analysis (TA)
4.1.3. Nitrogen and Sulphur Combustion Spectrometry (NS)
4.2. Grain Size
4.3. Geochemistry
4.4. Mineralogy
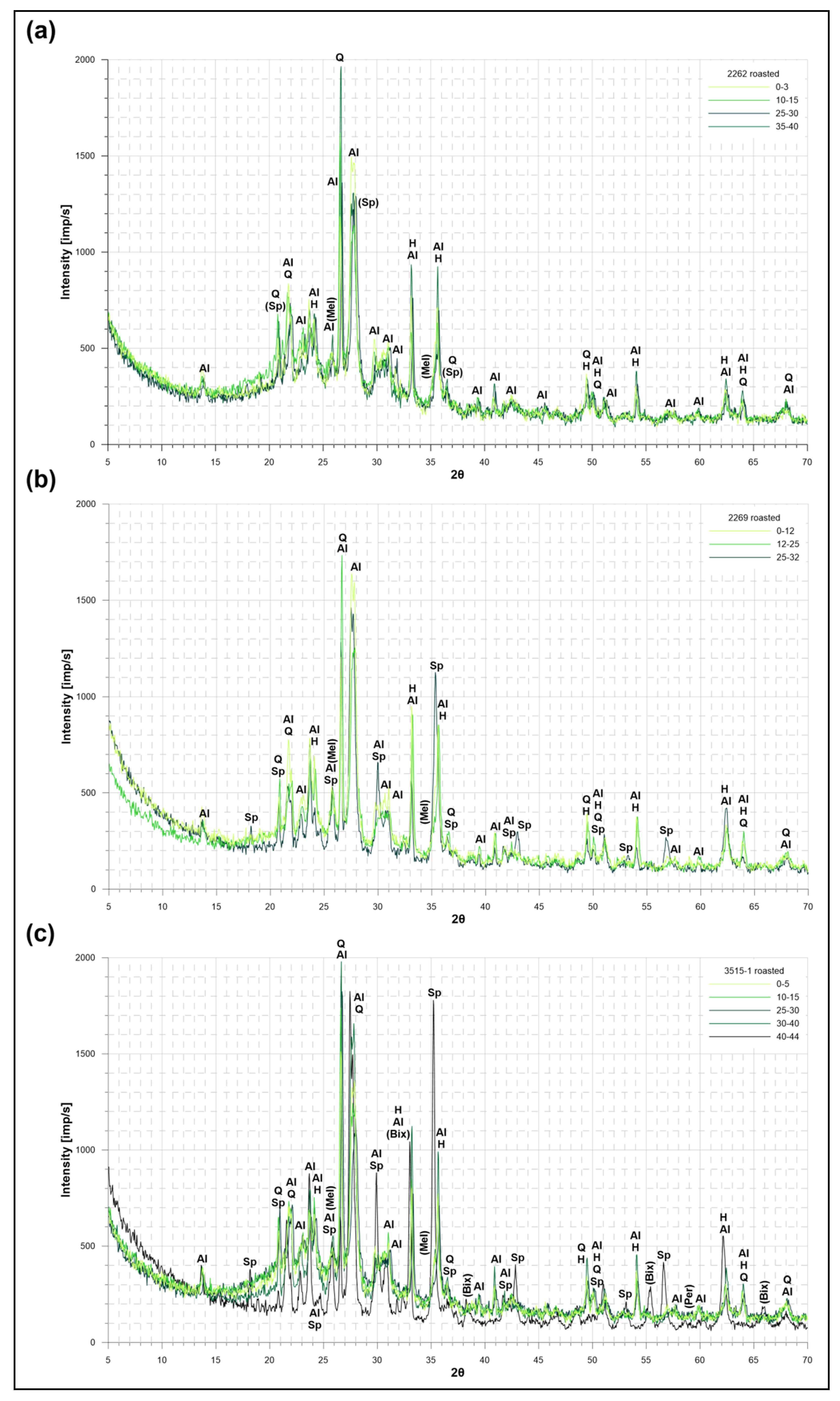
4.4.1. Bulk XRD Analysis of Non-Heated Sediment Samples
4.4.2. Bulk XRD Analysis of Combustion Products
5. Discussion
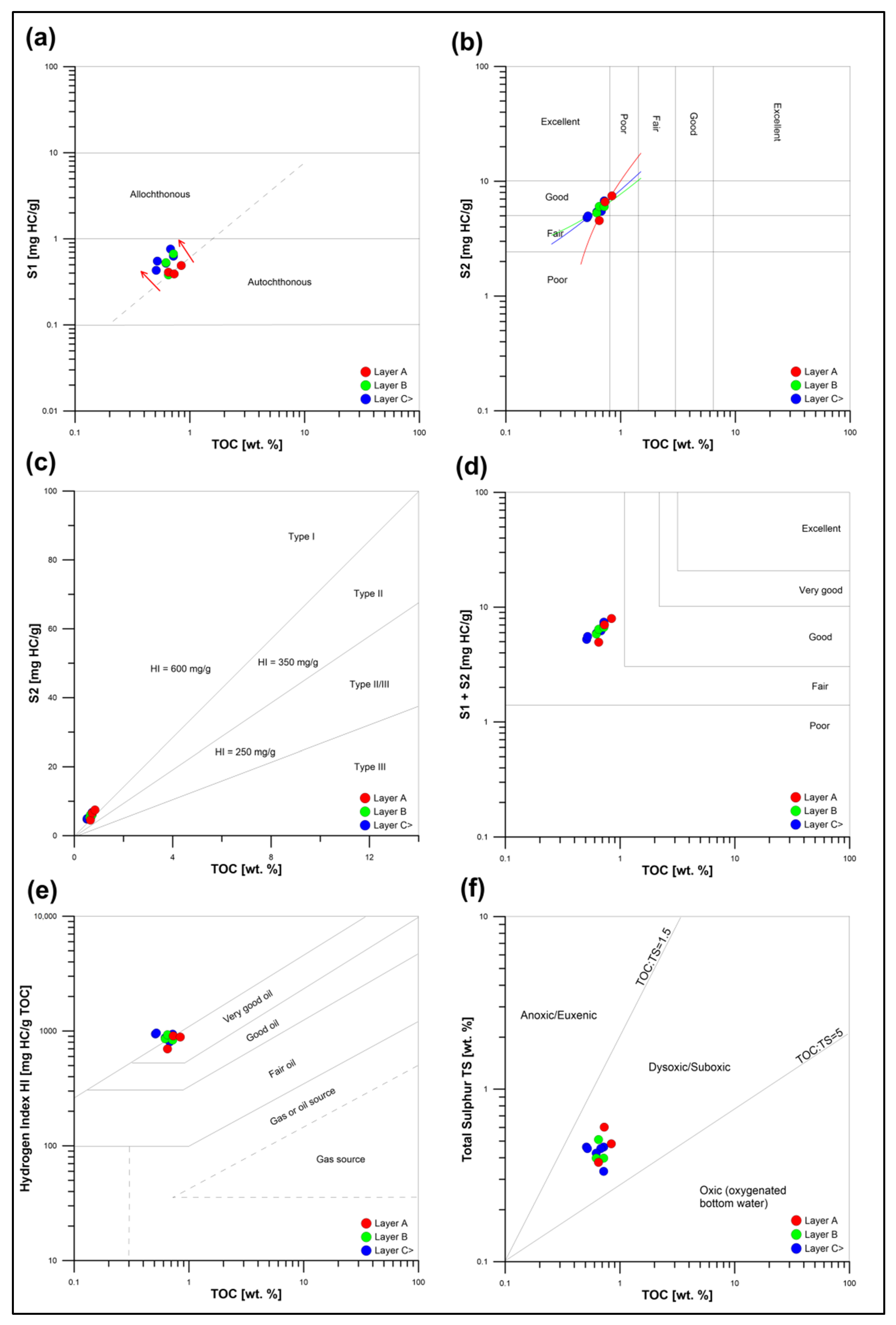
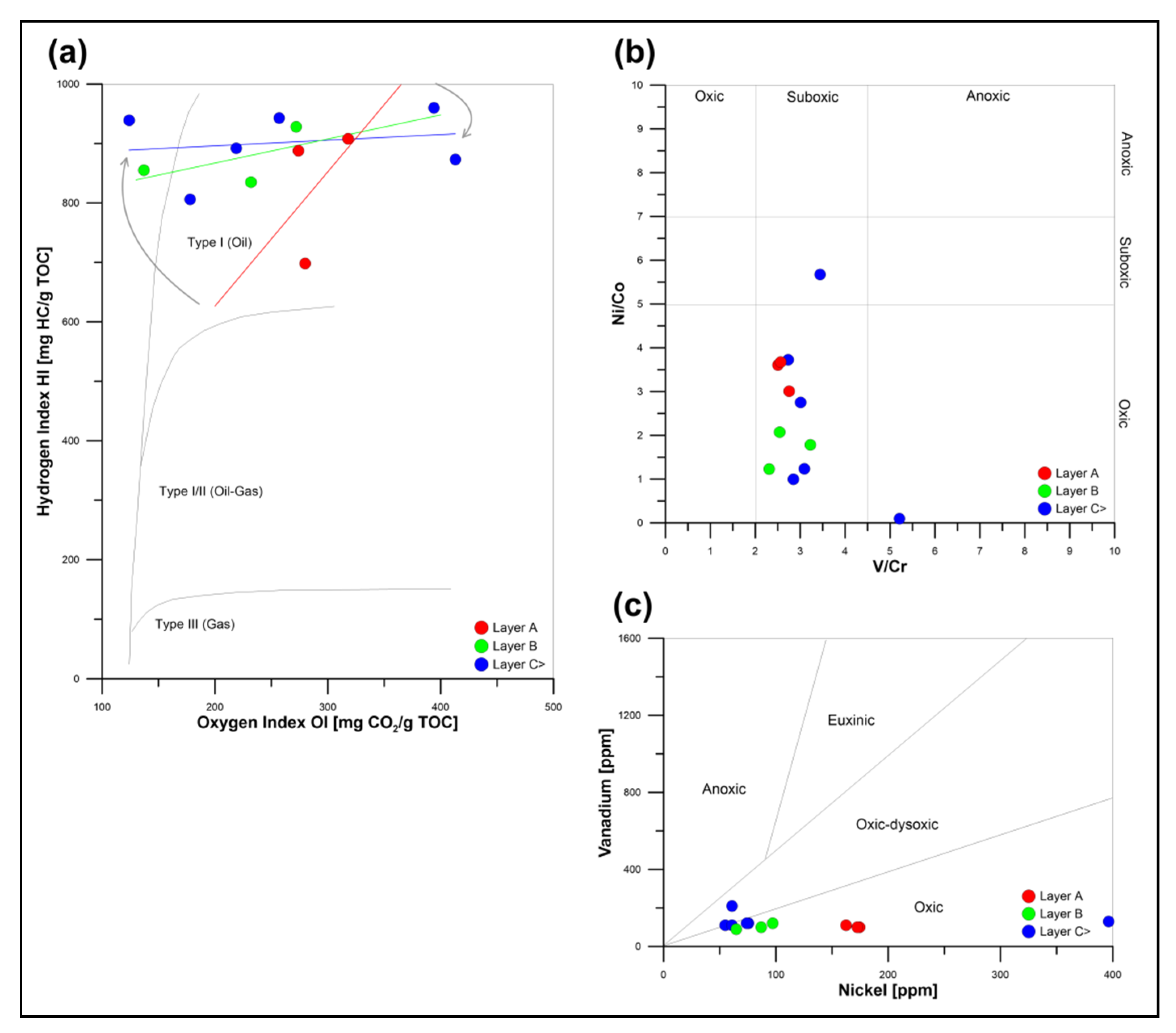
5.1. Hydrocarbon Potential and Maturity of Pelagic Sediments from the CCFZ
5.2. Thermal Behaviour and Combustion Products
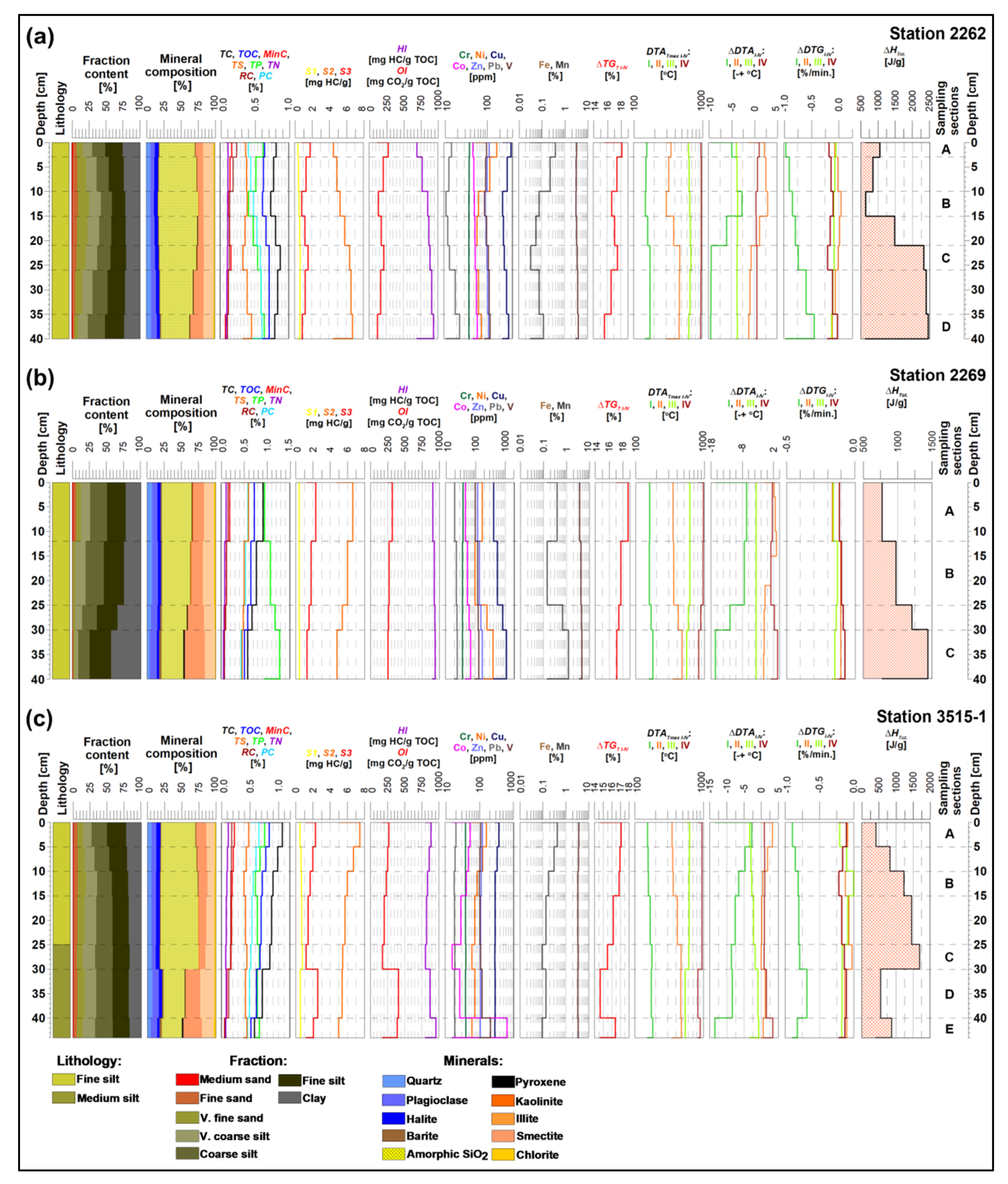
5.3. Comparison of Thermal Proxies of Oxic–Suboxic Burial Diagenesis with Lithological Data
6. Conclusions
- Quaternary pelagic sediments from the Clarion–Clipperton Fracture Zone reveal fair hydrocarbon potential, related to the burial diagenesis of bio-siliceous organic matter, and its further degradation occurring in oxic–suboxic conditions.
- Due to the intensive matrix effects, the occurrence of organic oxygen compounds, and catalytic influences of dispersed metals, the Tmax and Vr0 parameters estimated during Rock–Eval pyrolysis are not applicable for determining the thermal maturity of modern pelagic sediments.
- The total enthalpy ΔHTot. calculated by the use of differential thermal analysis (DTA) is a new indicator of redox potential and alteration processes occurring during the early diagenesis stage. The distinctive increase in negative enthalpy is observed with burial depth, especially below the “active layer” (10 cm and deeper), where sediments show strong depletion of oxygen and intensive phase transformations occur.
- The total activation energy Ea varied from +423 to +2449 J/g (=heat flux), with extremes found below 20 cm, where strong oxygen deficiency occurred, and an increase in clay mineral content was observed. Additionally, the elevated Ea values were related to “active layer A”, mainly due to high oxygenation and increased contents of hydrated biosilica. Increased Ea values were also found in the samples from station 3515-1 (below 30 cm), which showed high contents of clay minerals, finely dispersed metals, and increased R−E6 indices (mainly S2, OI, and HI).
- Mineral products of combustion synthesis are related with the primary components of the CCFZ pelagic sediments and include: albite, Fe–Mn spinels (with potential vacancy of Cu, Ni, Co, Zn, and other metals), protohematite–hematite, and quartz–crystobalite. Traces of bixbyite, melilite, and periclase were also identified. Material enriched with Fe indicated weak paramagnetic properties and an increased degree of crystallinity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Stackelberg, U.; Beiersdorf, H. The formation of manganese nodules between the Clarion and Clipperton Fracture-Zones south east of Hawaii. Mar. Geol. 1991, 98, 411–423. [Google Scholar] [CrossRef]
- Kotliński, R.A. Deep-sea sediments. In Mineral Resources of the Seas and Oceans; Kotliński, R., Szamałek, K., Eds.; Scholar: Warszawa, Poland, 1998; pp. 101–121, (In Polish with English Abstract). [Google Scholar]
- Kotliński, R.A. Relationships between nodule genesis and topography in the eastern area of the Clarion-Clipperton region. In Establishment of a Geological Model of Polymetallic Nodule Deposits in the Clarion-Clipperton Fracture Zone of the Equatorial North Pacific Ocean; ISA: Kingston, Jamaica, 2009; pp. 203–221. [Google Scholar]
- Radziejewska, T. Characteristics of the sub-equatorial north-eastern Pacific Ocean’s abyss, with a particular reference to the Clarion-Clipperton Fracture Zone. In Meiobenthos in the Sub-Equatorial Pacific Abyss; Springer Briefs in Earth System Sciences: Berlin/Heidelberg, Germany, 2014; pp. 13–28. [Google Scholar] [CrossRef]
- Zawadzki, D.; Maciąg, Ł.; Abramowski, T.; McCartney, K. Fractionation Trends and Variability of Rare Earth Elements and Selected Critical Metals in Pelagic Sediment from Abyssal Basin of NE Pacific (Clarion-Clipperton Fracture Zone). Minerals 2020, 10, 320. [Google Scholar] [CrossRef]
- Zawadzki, D.; Kotliński, R.A. Conditions of the occurrence and distribution prospective ferromanganese oxide deposits. Górnictwo i Geoinżynieria 2011, 35, 427–439, (In Polish with English Abstract). [Google Scholar]
- Zonneveld, K.A.F.; Versteegh, G.J.M.; Kasten, S.; Eglinton, T.I.; Emeis, K.-C.; Huguet, C.; Koch, B.P.; de Lange, G.J.; de Leeuw, J.W.; Middelburg, J.J.; et al. Selective preservation of organic matter in marine environments; processes and impact on the sedimentary record. Biogeosciences 2010, 7, 483–511. [Google Scholar] [CrossRef]
- Holtvoeth, J. Terrigenous Organic Matter in Sediments of the Eastern Equatorial Atlantic—Distribution, Reactivity, and Relation to Late Quaternary Climate. Ph.D. Thesis, Faculty of Geosciences, Bremen University, Bremen, Germany, 2004; pp. 1–135. [Google Scholar]
- A Geological Model of Polymetallic Nodule Deposits in the Clarion–Clipperton Fracture Zone; International Seabed Authority Technical Study No. 6; ISA: Kingston, Jamaica, 2010; pp. 1–211. Available online: https://ran-s3.s3.amazonaws.com/isa.org.jm/s3fs-public/files/documents/tstudy6.pdf (accessed on 12 February 2020).
- Hu, W.; Jin, Z.; Yao, S.; Lu, Z.; Chen, Z.; Zhang, L.; Zhang, X.; Zhou, H. Discovery of low-mature hydrocarbon in manganese nodules and ooze from the Central Pacific deep sea floor. Chin. Sci. Bull. 2002, 47, 939–944. [Google Scholar] [CrossRef]
- Volz, J.B.; Mogollón, J.M.; Geibert, W.; Arbizu, P.M.; Koschinsky, A.; Kasten, S. Natural spatial variability of depositional conditions, biogeochemical processes and element fluxes in sediments of the eastern Clarion-Clipperton Zone, Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018, 140, 159–172. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Espitalié, J.; Laporte, J.L.; Madec, M.; Marquis, F.; Leplat, P.J.; Paulet, F.; Boutefeu, A. Rapid Method for Source Rocks Characrerysation and for Determination of Petroleum Potential and Degree of Evolution. Rev. Inst. Fr. Petrol. 1977, 32, 23–42, (In French with English Abstract). [Google Scholar] [CrossRef]
- Espitalié, J.; Deroo, G.; Marquis, F. Rock-Eval pyrolysis and its application. Rev. Inst. Fr. Petrol. 1985, 40, 755–784. [Google Scholar] [CrossRef]
- Baudin, F.; Disnar, J.-R.; Aboussou, A.; Savignac, F. Guidelines for Rock-Eval analysis of recent marine sediments. Org. Geochem. 2015, 86, 71–80. [Google Scholar] [CrossRef]
- Hazra, B.; Wood, D.A.; Mani, D.; Singh, P.K.; Singh, A.K. Evaluation of Shale Source Rocks and Reservoirs; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1–142. [Google Scholar] [CrossRef]
- Shulga, N.A. Characteristics of Alkanes in Ferromanganese Nodules of the Clarion–Clipperton Fracture Zone. Oceanology 2018, 58, 672–678. [Google Scholar] [CrossRef]
- Shulga, N.A. Distribution of n-alkanes in the ferromanganese nodule–sediment–pore water system (Clarion–Clipperton Fracture Zone). Lithol. Miner. Resour. 2017, 52, 435–441. [Google Scholar] [CrossRef]
- Bąk, K. Sedimentological, geochemical and microfaunal responses to environmental changes around the Cenomanian-Turonian boundary in the Outer Carpathian Basin; a record from the Subsilesian Nappe, Poland. Palaeogeogr. Palaeoecol. Palaeoclimatol. 2006, 237, 335–358. [Google Scholar] [CrossRef]
- Littke, R.; Lückge, A.; Welte, D.H. Quantification of organic matter degradation by microbial sulfate reduction for Quaternary sediments from the Northern Arabian Sea. Naturwissenschaften 1997, 84, 312–315. [Google Scholar] [CrossRef]
- Law, A.C. Evaluating source rocks. In Treatise of Petroleum Geology. Handbook of Petroleum Geology: Exploring for Oil and Gas Traps; Beaumont, E.A., Foster, N.H., Eds.; The American Association of Petroleum Geologists: Matarza, OK, USA, 1999; pp. 1–41. [Google Scholar] [CrossRef]
- Johannes, I.; Kruusement, K.; Veski, R.; Bojesen-Koefoed, J.A. Characterisation of pyrolysis kinetics by Rock-Eval basic data. Oil Shale 2006, 23, 249–257. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 2nd ed.; Springer: New York, NY, USA, 1984. [Google Scholar] [CrossRef]
- Labus, M.; Matyasik, I.J. Application of different thermal analysis techniques for the evaluation of petroleum source rocks. J. Therm. Anal. Calorim. 2019, 136, 1185–1194. [Google Scholar] [CrossRef]
- El Nady, M.M.; Hammad, M.M. Organic richness, kerogen types and maturity in the shales of the Dakhla and Duwi formations in Abu Tartur area, Western Desert, Egypt: Implication of Rock–Eval pyrolysis. Egypt. J. Pet. 2015, 24, 423–428. [Google Scholar] [CrossRef]
- Kontakiotis, G.; Karakitsios, V.; Cornée, J.-J.; Moissette, P.; Zarkogiannis, S.D.; Pasadakis, N.; Koskeridou, E.; Manoutsoglou, E.; Drinia, H.; Antonarakou, A. Preliminary results based on geochemical sedimentary constraints on the hydrocarbon potential and depositional environment of a Messinian sub-salt mixed siliciclastic-carbonate succession onshore Crete (Plouti section, eastern Mediterranean). Mediterr. Geosci. Rev. 2020. [Google Scholar] [CrossRef]
- Grohmann, S.; Fietz, S.W.; Littke, R.; Daher, S.B.; Romero-Sarmiento, M.F.; Nader, F.H.; Baudin, F. Source rock characterization of mesozoic to cenozoic organic matter rich marls and shales of the Eratosthenes Seamount, Eastern Mediterranean Sea. Oil Gas Sci. Technol. Rev. d’IFP Energ. Nouv. 2018, 73. [Google Scholar] [CrossRef]
- Kotliński, R.A. Clarion-Clipperton nodule field—A future source of mineral deposits. Górnictwo i Geoinżynieria 2011, 35, 195–214, (In Polish with English Abstract). [Google Scholar]
- Mewes, K.; Mogollón, J.M.; Picard, A.; Rühlemann, C.; Kuhn, T.; Nöthen, K.; Kasten, S. Impact of depositional and biogeochemical processes on small scale variations in nodule abundance in the Clarion-Clipperton Fracture Zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 2014, 91, 125–141. [Google Scholar] [CrossRef]
- Yubko, V.; Kotliński, R. Volcanic, tectonic and sedimentary factors. In Prospectors Guide for the Clarion–Clipperton Zone Polymetallic Nodule Deposits, Development of a Geological Models for the Clarion-Clipperton Zone Polymetallic Nodule Deposit, 1st ed.; Morgan, C., Ed.; ISA: Kingston, Jamaica, 2009; Volume 1, pp. 11–34. [Google Scholar]
- International Seabed Authority. Available online: https://www.isa.org.jm/ (accessed on 10 July 2020).
- Dreiseitl, I. Deep sea exploration for metal reserves—Objectives, methods and look into the future. In Deep Sea Mining Value Chain: Organization, Technology and Development; Abramowski, T., Ed.; IOM: Szczecin, Poland, 2016; pp. 105–118. [Google Scholar]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. In The Treatise on Geochemistry; Scott, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 12, pp. 273–290. [Google Scholar] [CrossRef]
- Zawadzki, D. The state of knowledge and possibilities to extract strategic raw materials from the ocean polymetallic deposits. Przegląd Geol. 2013, 61, 45–53, (In Polish with English Abstract). [Google Scholar]
- Kotliński, R.A.; Maciąg, Ł.; Zawadzki, D. Potential and Recent Problems of the Possible Polymetallic Sources in the Oceanic Deposits. Geol. Miner. Resour. World Ocean 2015, 40, 65–80. [Google Scholar]
- Petersen, S.; Krätschell, A.; Augustin, N.; Jamieson, J.; Hein, J.; Hannington, M.D. News from the seabed—Geological characteristics and resource potential of deepsea mineral resources. Mar. Policy 2016, 70, 175–187. [Google Scholar] [CrossRef]
- Morgan, C. Resource estimates of the clarion-clipperton manganese nodule deposits. In Handbook of Marine Mineral Deposits; Cronan, D., Ed.; CRC Press: London, UK, 2000; pp. 145–170. [Google Scholar]
- Kotliński, R.A.; Stoyanova, V. Control factors of polymetallic nodules distribution within the Eastern area of the Clarion-Clipperton Zone (CCZ). In Proceedings of the 2005 VI ISOPE Ocean Mining Symposium, Changsha, China, 9–13 October 2005; pp. 1–11. [Google Scholar]
- Zawadzki, D.; Maciąg, Ł.; Kotliński, R.A. Eupelagic sediments as a potential resource for rare earth elements. Biul. Państwowego Inst. Geol. 2015, 465, 131–142, (In Polish with English Abstract). [Google Scholar] [CrossRef]
- Maciąg, Ł.; Zawadzki, D. Spatial variability and resources estimation of selected critical metals and rare earth elements in surface sediments from the Clarion-Clipperton Fracture Zone, equatorial Pacific Ocean, Interoceanmetal claim area. In Proceedings of the IAMG 2019—20th Annual Conference of the International Association for Mathematical Geosciences, State College, PA, USA, 10–16 August 2019; pp. 174–178. [Google Scholar]
- Szamałek, K. The state of knowledge concerning on oceanic mineral resources. Przegląd Geol. 2018, 66, 189–194, (In Polish with English Abstract). [Google Scholar]
- Kotliński, R.A.; Piestrzyński, A.; Maciąg, Ł.; Zawadzki, D. Metallogenic potential of the oceans. In Aktualia i Perspektywy Gospodarki Surowcami Mineralnymi; Instytut Gospodarki Surowcami Mineralnymi i Energią PAN: Kraków, Poland, 2019; pp. 117–144, (In Polish with English Abstract). [Google Scholar]
- Kazmin, Y. Geology of the Clarion-Clipperton Zone: Existing geological information in respect of polymetallic nodules. In Estabilishment of a Geological Model of Polymetallic Nodule Deposits in the Clarion-Clipperton Fracture Zone of the Equatorial North Pacific Ocean; ISA: Kingston, Jamaica, 2009; pp. 106–144. [Google Scholar]
- Maciąg, Ł.; Harff, J. Application of multivariate geostatistics for local-scale lithological mapping—Case study of pelagic surface sediments from the Clarion–Clipperton Fracture Zone, north-eastern equatorial Pacific (Interoceanmetal claim area). Comput. Geosci. 2020, 104474. [Google Scholar] [CrossRef]
- ODP—Ocean Drilling Program. Leg 199 Preliminary Report, Shipboard Scientific Party. 2002. Available online: http://www-odp.tamu.edu/publications/prelim/199_prel/199PREL.PDF (accessed on 14 June 2020).
- Maciąg, Ł.; Kotliński, R.A.; Borówka, R.K. Lithological Variability of Siliceous Clayey Silts from IOM Area (Clarion–Clipperton Fracture Zone, East Pacific). Górnictwo i Geoinżynieria 2011, 4, 243–255, (In Polish with English Abstract). [Google Scholar]
- Schultze, D. Differentialthermoanalyse; VEB: Berlin, Germany, 1971; pp. 1–335, (In German with English Abstract). [Google Scholar]
- Thompson, M.; Owen, L.; Wilkinson, K.; Wood, R.; Damant, A. A comparison of the Kjeldahl and Dumas methods for the determination of protein in foods, using data from a proficiency testing scheme. Analyst 2002, 127, 1666–1668. [Google Scholar] [CrossRef]
- Szyduk, A.; Maciąg, Ł.; Skowronek, A.; Mianowicz, K.; Strzelecka, A. Numerical data extraction and evaluation of grain shape factor using laser diffraction method—Case study from Szczecin Lagoon, Northern Poland. In The International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management; SGEM: Albena, Bulgaria, 2019; Volume 19, pp. 385–390. [Google Scholar] [CrossRef]
- Folk, R.L.; Ward, W.C. Brazos River bar: A study in the significance of grain size parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. GRADISTAT: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Donoghue, J.F. Phi scale. In Encyclopedia of Estuaries; Encyclopedia of Earth Sciences Series; Kennish, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Sindern, S. Analysis of Rare Earth Elements in Rock and Mineral Samples by ICP-MS and LA-ICP-MS. Phys. Sci. Rev. 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Eberl, D.D. User’s Guide to Rockjock—A Program For Determining Quantitative Mineralogy From Powder X-ray Diffraction Data; Open-File Report 03-78; U.S. Geological Survey: Boulder, CO, USA, 2003; pp. 1–48. [CrossRef]
- Muszyński, M. Skały krzemionkowe. In Przewodnik do Petrografii; Manecki, A., Muszyński, M., Eds.; Uczelniane Wydawnictwa Naukowo-Dydaktyczne AGH: Kraków, Poland, 2008; pp. 336–348. (In Polish) [Google Scholar]
- Togunwa, O.; Abdullah, W. Geochemical characterization of Neogene sediments from onshore West Baram Delta Province, Sarawak: Paleoenvironment, source input and thermal maturity. Open Geosci. 2017, 9, 302–313. [Google Scholar] [CrossRef]
- Cuadros, J.; Altaner, S. Compositional and structural features of the octahedral sheet in mixed-layer illite/smectite from bentonites. Eur. J. Miner. 1998, 10, 111–124. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Thermal behavior of a bentonite. J. Therm. Anal. Calorim. 2007, 90, 167–172. [Google Scholar] [CrossRef]
- Panna, W.; Wyszomirski, P.; Myszka, R. Characteristics of the clayey-siliceous rock from the Dylągówka–Zapady deposit (polish flysch Carpathians) as a mineral raw material. Gospod. Surowcami Miner. Miner. Resour. Manag. 2014, 30, 85–102. [Google Scholar] [CrossRef]
- Muller, F.; Drits, V.; Plançon, A.; Robert, J.-L. Structural Transformation of 2:1 Dioctahedral Layer Silicates during Dehydroxylation-Rehydroxylation Reactions. Clays Clay Miner. 2000, 48, 572–585. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of thermogravimetric system of minerals and its use in geological practice. Occas. Pap. Geol. Inst. Hung. 2011, 213, 1–180. [Google Scholar]
- Húlan, T.; Trník, A.; Medveď, I. Kinetics of thermal expansion of illite-based ceramics in the dehydroxylation region during heating. J. Therm. Anal. Calorim. 2017, 127, 291–298. [Google Scholar] [CrossRef]
- Wyrwicki, R. Analiza Derywatograficzna Skał Ilastych; Wydawnictwo Uniwersytetu Warszawskiego: Warszawa, Poland, 1988; pp. 1–228. (In Polish) [Google Scholar]
- Earnest, C.M. Thermal analysis of selected illite and smectite clay minerals. Part II. Smectite clay minerals. In Thermal Analysis in the Geosciences; Smykatz-Kloss, W., Warne, S.S.J., Eds.; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 1991; Volume 38, pp. 288–312. [Google Scholar] [CrossRef]
- Mendioroz, S.; Belzunce, M.J.; Pajares, J.A. Thermogravimetric study of diatomites. J. Therm. Anal. 1989, 35, 2097–2104. [Google Scholar] [CrossRef]
- Jones, J.B.; Segnit, E.R. The nature of opal I. nomenclature and constituent phases. J. Geol. Soc. Aust. 1971, 18, 57–68. [Google Scholar] [CrossRef]
- Kasten, S.; Haese, R.R.; Zabel, M.; Ruhlemann, C.; Schulz, H.D. Barium peaks at glacial terminations in sediments of the equatorial Atlantic Ocean—Relicts of deglacial productivity pulses? Chem. Geol. 2001, 175, 635–651. [Google Scholar] [CrossRef]
- Maciąg, Ł.; Zawadzki, D.; Kotliński, R.A.; Borówka, R.K.; Wróbel, R. Authigenic barite crystals from the eupelagic sediments of CCFZ (IOM H22 area, Clarion-Clipperton Fracture Zone, Pacific). In Joint 5th Central-European Mineralogical Conference and 7th Mineral Sciences in the Carpathians Conference. Book of Contributions and Abstracts; Ondrejka, M., Cempírek, J., Bačík, P., Eds.; Comenius University: Bratislava, Slovakia, 2018; pp. 122–124. [Google Scholar]
- Prewitt, C.T.; Sueno, S.; Papike, J.J. The crystal structures of high albite and monalbite at high temperatures. Am. Miner. 1976, 61, 1213–1225. [Google Scholar]
- Gualtieri, A.; Venturelli, P. In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction. Am. Miner. 1999, 84, 895–904. [Google Scholar] [CrossRef]
- Kihara, K. An X-ray study of the temperature dependence of the quartz structure. Eur. J. Miner. 1990, 2, 63–77. [Google Scholar] [CrossRef]
- Dera, P.; Lazarz, J.D.; Prakapenka, V.B.; Barkley, M.; Downs, R.T. New insights into the high-pressure polymorphism of SiO2 cristobalite. Phys. Chem. Miner. 2011, 38. [Google Scholar] [CrossRef]
- Bolewski, A.; Wyderko-Delekta, M. Mineralogia Spieków i Grudek Rudnych; Wydawnictwo AGH: Kraków, Poland, 1995; pp. 1–275. (In Polish) [Google Scholar]
- Hillier, S.; Lumsdon, D. Distinguishing opaline silica from cristobalite in bentonites: A practical procedure and perspective based on NaOH dissolution. Clay Miner. 2008, 43, 477–486. [Google Scholar] [CrossRef]
- Hunt, J.M. Distribution of hydrocarbons in sedimentary rocks. Geochim. Cosmochim. Acta 1961, 22, 37–49. [Google Scholar] [CrossRef]
- Hatcher, P.G. The Organic Geochemistry of Mangrove Lake, Bermuda (NOAA Professional Paper); U.S. Dept. of Commerce, National Oceanic and Atmospheric Administration: Washington, DC, USA, 1978; pp. 1–92.
- Waples, D. Geochemistry in Petroleum Exploration Boston; IHRDC: Boston, MA, USA, 1985; pp. 1–232. [Google Scholar] [CrossRef]
- Haq, B.U.; Hardenbohl, J.; Vail, P.K. Mesozoic and Cenozoic chronostratigraphy and cycles of sea-level change. In Sea-Level Changes: An Integrated Approach; Wilgus, C.K., Hastings, B.S., Posamentier, H., Wagoner, J.V., Ross, C.A., Kendall, C.G.S.C., Eds.; SEPM Special Publication: Tulsa, OK, USA, 1988; Volume 42, pp. 71–108. [Google Scholar] [CrossRef]
- Peters, K.E.; Curry, D.J.; Kacewicz, M. An overview of basin and petroleum system modeling: Definitions and concepts. In Basin Modeling: New Horizons in Research and Applications; Peters, K.E., Curry, D.J., Kacewicz, M., Eds.; AAPG Hedberg Series; No. 4; American Association of Petroleum Geologists: Tulsa, OK, USA, 2012; pp. 1–16. [Google Scholar] [CrossRef]
- Tissot, B.P.; Pelet, R.; Ungerer, P. Thermal history of sedimentary basins, maturation indices, and kinetics of oil and gas generation. AAPG Bull. 1987, 71, 1445–1466. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Claxton, B.L.; Henk, F.; Breyer, J.T. Oil and shale gas from the Barnett Shale, Fort Worth basin, Texas. AAPG National Convention, 3–6 June 2001, Denver, CO. AAPG Bull. 2001, 85 (Suppl. 13), A100. [Google Scholar]
- Dembicki, H. An interlaboratory comparison of source rock data. Geochim. Cosmochim. Acta 1984, 48, 2641–2649. [Google Scholar] [CrossRef]
- Jarvie, D.M. Factors affecting Rock-Eval derived kinetic parameters. Chem. Geol. 1991, 93, 79–99. [Google Scholar] [CrossRef]
- Anderson, O.R. Protozoa, radiolarians. In Encyclopedia of Ocean Sciences, 1st ed.; Steele, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2001; Volume 4, pp. 613–617. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Di, X.; Sigurdur, B.; Fu, W. Exploring Valuable Lipids in Diatoms. Front. Mar. Sci. 2017, 7. [Google Scholar] [CrossRef]
- Peters, K.E.; Walters, C.C.; Moldowan, M. The Biomarker Guide; Biomarkers and Isotopes in the Environment and Human History; Cambridge University Press: Cambridge, UK, 2005; Volume 1–2. [Google Scholar]
- Espitalié, J.; Madec, M.; Tissot, B. Role of mineral matrix in kerogen pyrolysis: Influence on petroleum generation and migration. Am. Assoc. Pet. Geol. Bull. 1980, 64, 59–66. [Google Scholar] [CrossRef]
- Horsfield, B.; Douglas, A.G. The influence of minerals on the pyrolysis of kerogens. Geochim. Cosmochim. Acta 1980, 44, 1110–1131. [Google Scholar] [CrossRef]
- Voorhees, K.J. Analytical Pyrolysis—Techniques and Applications; Butterworth-Heinemann: Southampton, UK, 1984; pp. 1–496. [Google Scholar] [CrossRef]
- Dembicki, H.; Horsfield, B.; Ho, T.Y. Source rock evaluation by pyrolysis-gas chromatography. AAPG Bull. 1983, 67, 1094–1103. [Google Scholar] [CrossRef]
- Dembicki, H. Three common source rock evaluation errors made by geologists during prospect or play appraisals. AAPG Bull. 2009, 93, 341–356. [Google Scholar] [CrossRef]
- Jacobson, S.R. Petroleum source rocks and organic facies. In Source and Migration Processes and Evaluation Techniques; Merill, E.K., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1991; pp. 3–11. [Google Scholar] [CrossRef]
- Arora, A.; Banerjee, S.; Dutta, S. Black shale in late Jurassic Jhuran Formation of Kutch: Possible indicator of oceanic anoxic event? J. Geol. Soc. India 2015, 85, 265–278. [Google Scholar] [CrossRef]

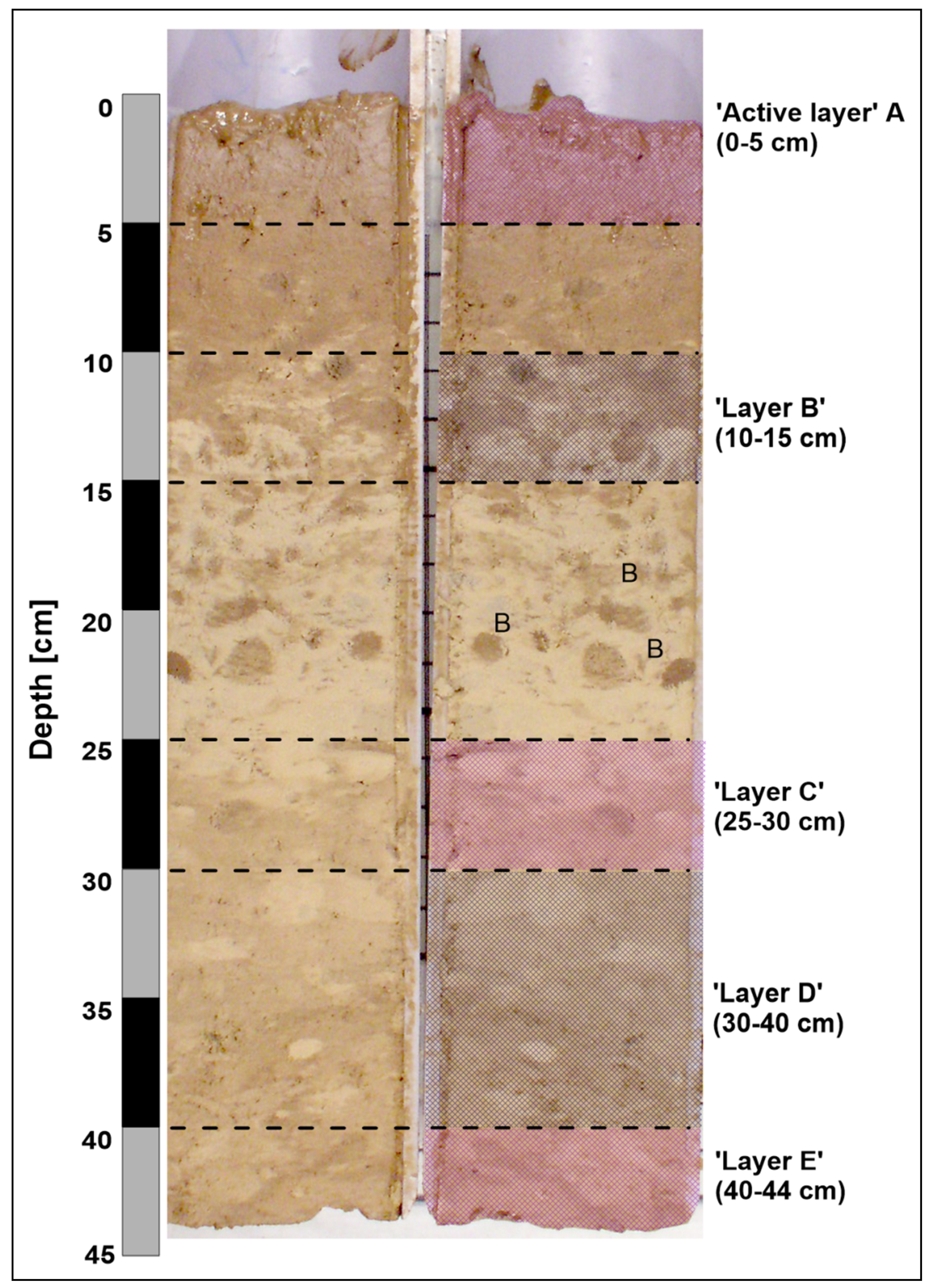
| Station ID (Box Corer Sampling Depth) | Sediment Depth (cm) | Description |
|---|---|---|
| Station 2262 (4529 m bsl) | ||
| 1 | 0–3 | Active “layer A”: highly liquid, brownish, containing small amounts of nodules (3.9 kg/m2), siliceous clayey silt. |
| 2 | 10–15 | Intermediate upper “layer B”: semiliquid, more compacted than “layer A”, light brown-yellowish, siliceous clayey silt with patchy texture, without nodules but with several bioturbations. |
| 3 | 21–26 | Intermediate bottom “layer C”: more compacted, brown-yellowish, siliceous clayey silt, without nodules, less bioturbation. |
| 4 | 35–40 | Bottom “layer D”: more compacted, brown-yellowish, siliceous clayey silt, less bioturbation, without nodules. |
| Station 2269 (4313 m bsl) | ||
| 5 | 0–12 | Active “layer A”: highly liquid, brownish, containing small amounts of nodules (4.0 kg/m2), siliceous clayey silt. |
| 6 | 12–25 | Intermediate “layer B”: semiliquid, more compacted than “layer A”, light brown-yellowish, siliceous clayey silt with patchy texture, without nodules but with several bioturbations. |
| 7 | 30–40 | Bottom “layer D”: more compacted, brown-yellowish, siliceous silty clay without bioturbations, containing several micronodules. |
| Station 3515-1 (4342 m bsl) | ||
| 8 | 0–5 | Active “layer A”: highly liquid, brownish, containing large amounts of nodules (18.3 kg/m2), siliceous clayey silt. |
| 9 | 10–15 | Intermediate upper “layer B”: semiliquid, more compacted than “layer A”, light brown-yellowish, patchy texture, without nodules, several bioturbations. |
| 10 | 25–30 | Intermediate middle “layer C”: more compacted, light brown-yellowish, patchy texture with several bioturbations, without nodules. |
| 11 | 30–40 | Intermediate bottom “layer D”: more compacted, brown-yellowish and patchy, less bioturbation, without nodules. |
| 12 | 40–44 | Bottom “layer E”: highly compacted, brownish, siliceous clayey silt with almost no bioturbation, nodules, or micronodules. |
| Parameters and Indices | Unit | Description | Measured | Calculated |
|---|---|---|---|---|
| TC | wt.% | Total carbon. | v | |
| TOC | wt.% | Total organic carbon. | v | |
| MinC | wt.% | Mineral carbon. | v | |
| Tmax | °C | The maximal temperature of hydrocarbon generation. | v | |
| S1 | mg HC/g | Free hydrocarbons. | v | |
| S2 | mg HC/g | Hydrocarbons generated during pyrolysis. | v | |
| S3 | mg CO2/g | CO2 from organic matter. | v | |
| GP | mg HC/g | Hydrocarbon generation potential. | v | |
| PI | - | Productivity index. | v | |
| HI | mg HC/g TOC | Hydrogen index. | v | |
| OI | mg CO2/g TOC | Oxygen index. | v | |
| RC | wt.% | Total residual carbon. | v | |
| PC | wt.% | Pyrolytic carbon. | v |
| No. | Depth (cm) | TC (wt.%) | TOC (wt.%) | MinC (wt.%) | RC (wt.%) | PC (wt.%) | Tmax (°C) | S1 (mg HC/g) | S2 (mg HC/g) | S3 (mg CO2/g) | S2/S3 (-) | GP (mg HC/g) | PI (-) | HI (mg HC/g TOC) | OI (mg CO2/g TOC) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Station 2262 | |||||||||||||||
| 1 | 0–3 | 0.82 | 0.65 | 0.17 | 0.24 | 0.41 | 604 | 0.41 | 4.54 | 1.82 | 2.49 | 4.95 | 0.08 | 698 | 280 |
| 2 | 10–15 | 0.74 | 0.62 | 0.12 | 0.14 | 0.48 | 603 | 0.53 | 5.30 | 0.85 | 6.24 | 5.83 | 0.09 | 855 | 137 |
| 3 | 21–26 | 0.88 | 0.72 | 0.16 | 0.13 | 0.59 | 603 | 0.65 | 6.42 | 1.58 | 4.06 | 7.07 | 0.09 | 892 | 219 |
| 4 | 35–40 | 0.80 | 0.72 | 0.08 | 0.11 | 0.61 | 605 | 0.63 | 6.76 | 0.89 | 7.60 | 7.39 | 0.08 | 939 | 124 |
| Station 2269 | |||||||||||||||
| 5 | 0–12 | 0.92 | 0.73 | 0.19 | 0.15 | 0.58 | 605 | 0.39 | 6.63 | 2.32 | 2.86 | 7.02 | 0.06 | 908 | 318 |
| 6 | 12–25 | 0.77 | 0.65 | 0.12 | 0.12 | 0.53 | 604 | 0.38 | 6.03 | 1.77 | 3.41 | 6.41 | 0.06 | 928 | 272 |
| 7 | 30–40 | 0.58 | 0.51 | 0.07 | 0.08 | 0.43 | 604 | 0.43 | 4.81 | 1.31 | 3.67 | 5.24 | 0.08 | 943 | 257 |
| Station 3515-1 | |||||||||||||||
| 8 | 0–5 | 1.07 | 0.84 | 0.23 | 0.18 | 0.66 | 604 | 0.49 | 7.46 | 2.30 | 3.24 | 7.95 | 0.06 | 888 | 274 |
| 9 | 10–15 | 0.91 | 0.72 | 0.19 | 0.17 | 0.55 | 604 | 0.67 | 6.01 | 1.67 | 3.60 | 6.68 | 0.10 | 835 | 232 |
| 10 | 25–30 | 0.85 | 0.68 | 0.17 | 0.16 | 0.52 | 603 | 0.76 | 5.48 | 1.21 | 4.53 | 6.24 | 0.12 | 806 | 178 |
| 11 | 30–40 | 0.72 | 0.62 | 0.10 | 0.13 | 0.49 | 602 | 0.52 | 5.41 | 2.56 | 2.11 | 5.93 | 0.09 | 873 | 413 |
| 12 | 40–44 | 0.58 | 0.52 | 0.06 | 0.06 | 0.46 | 601 | 0.55 | 4.99 | 2.05 | 2.43 | 5.54 | 0.10 | 960 | 394 |
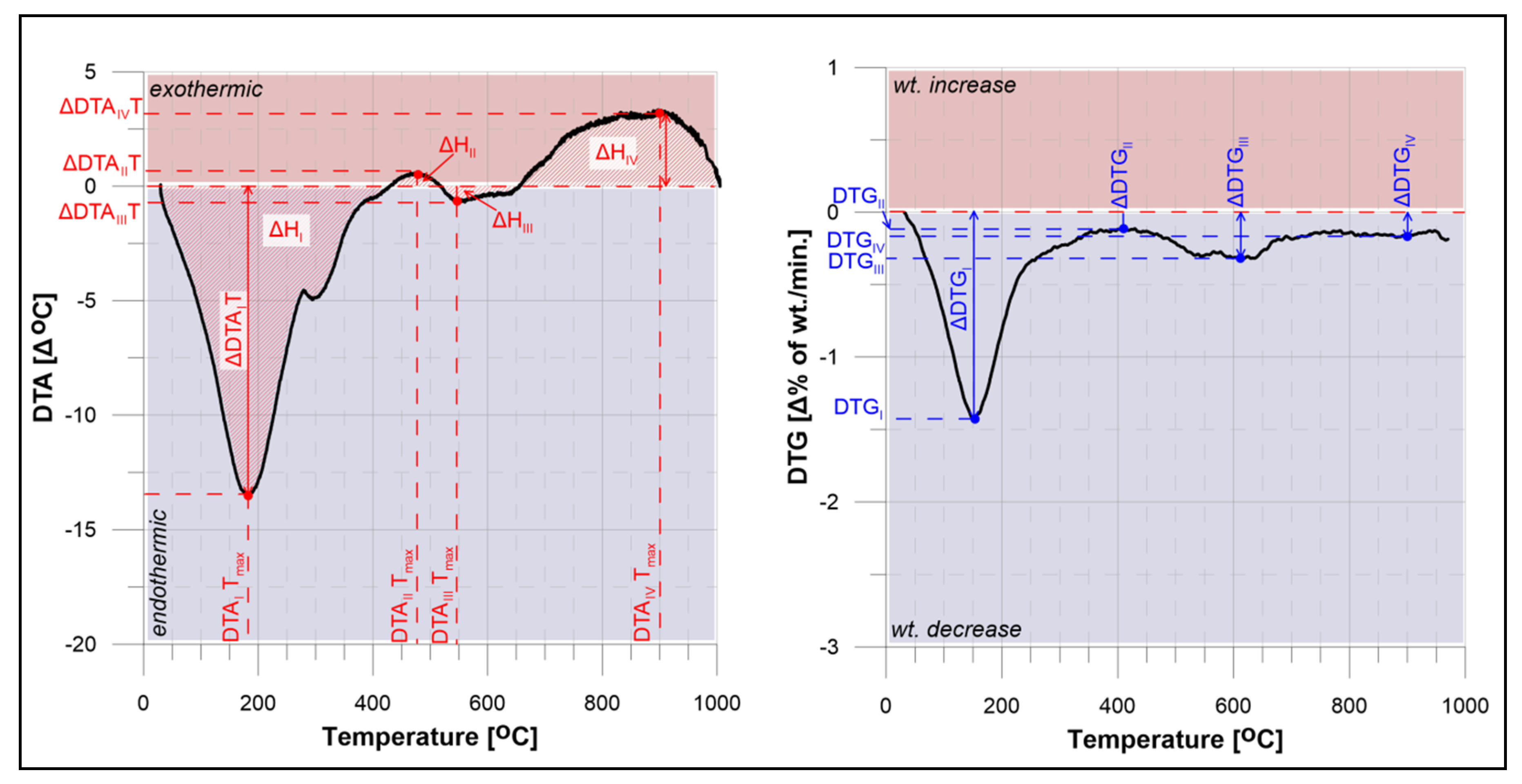
| No. | Depth (cm) | ΔTGT (%) | ΔHTot. (J/g) | DTAI Tmax (°C) | ΔDTAI (°C) | ΔDTGI (%/min.) | ΔHI (J/g) | DTAII Tmax (°C) | ΔDTAII (°C) | ΔDTGII (%/min.) | ΔHII (J/g) | DTAIII Tmax (°C) | ΔDTAIII (°C) | ΔDTGIII (%/min.) | ΔHIII (J/g) | DTAIV Tmax (°C) | ΔDTAIV (°C) | ΔDTGIV (%/min.) | ΔHIV (J/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Station 2262 | |||||||||||||||||||
| 1 | 0–3 | 18.18 | 1050.98 | 156 | −4.86 | −0.96 | 437.77 | 353 | 2.23 | −0.07 | −303.93 | 638 | −3.83 | −0.04 | 958.77 | 960 | 1.09 | −0.17 | −59.66 |
| 2 | 10–15 | 16.70 | 645.58 | 149 | −2.63 | −0.84 | 201.88 | 300 | 2.94 | 0.05 | −582.75 | 661 | −3.62 | −0.09 | 1137.57 | 968 | 0.37 | −0.10 | −18.75 |
| 3 | 21–26 | 17.55 | 2333.13 | 174 | −9.41 | −0.73 | 916.00 | 469 | −0.68 | −0.01 | - | 691 | −3.72 | −0.08 | 840.48 | 968 | 0.56 | −0.20 | −23.64 |
| 4 | 35–40 | 15.62 | 2449.43 | 174 | −9.53 | −0.45 | 877.49 | 461 | −1.20 | −0.11 | - | 642 | −3.68 | −0.13 | 708.38 | 954 | 0.59 | −0.02 | −21.91 |
| Station 2269 | |||||||||||||||||||
| 5 | 0–12 | 18.78 | 772.98 | 159 | −6.59 | −1.06 | 453.37 | 349 | 2.14 | −0.17 | −257.56 | 614 | −3.46 | −0.16 | 667.72 | 960 | 1.59 | −0.11 | −84.16 |
| 6 | 12–25 | 17.69 | 975.01 | 158 | −7.27 | −1.12 | 575.80 | 355 | 1.38 | −0.11 | −121.99 | 612 | −3.45 | −0.13 | 772.57 | 945 | 1.20 | −0.10 | −71.30 |
| 7 | 30–40 | 17.10 | 1440.40 | 177 | −16.50 | −1.14 | 1080.21 | 472 | −0.81 | −0.11 | - | 549 | −3.46 | −0.15 | 374.71 | 818 | 3.32 | −0.07 | −136.52 |
| Station 3515-1 | |||||||||||||||||||
| 8 | 0–5 | 17.10 | 422.53 | 154 | −2.76 | −0.89 | 206.39 | 349 | 3.27 | −0.09 | −560.36 | 633 | −3.31 | −0.20 | 872.48 | 950 | 0.93 | −0.11 | −69.97 |
| 9 | 10–15 | 16.86 | 1242.59 | 158 | −6.56 | −0.81 | 834.53 | 361 | 0.41 | −0.12 | −12.01 | 619 | −2.76 | 0.00 | 594.90 | 946 | 0.97 | −0.22 | −85.98 |
| 10 | 25–30 | 15.50 | 1692.26 | 178 | −8.66 | −0.79 | 1428.15 | 469 | −0.08 | −0.02 | - | 612 | −1.70 | −0.17 | 193.14 | 953 | 0.80 | −0.14 | −33.44 |
| 11 | 30–40 | 14.65 | 560.49 | 174 | −8.44 | −0.68 | 777.05 | 465 | 1.15 | −0.13 | −29.97 | 543 | −0.94 | −0.17 | 58.15 | 832 | 1.46 | −0.10 | −183.65 |
| 12 | 40–44 | 16.42 | 876.95 | 183 | −13.48 | −0.82 | 1318.03 | 475 | 0.59 | −0.10 | −20.78 | 543 | −0.69 | −0.17 | 37.58 | 897 | 3.35 | −0.13 | −467.63 |
| No. | Depth (cm) | Textural Name | Mean Grain Size (φ) | Sorting (φ) | Skewness (-) | Kurtosis (-) | Dist. Shape | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Station 2262 | ||||||||||
| 1 | 0–3 | Fine silt | 6.78 | 1.68 | −0.24 | 0.93 | Mesokurtic | 7.4 | 67.1 | 25.5 |
| 2 | 10–15 | Fine silt | 6.39 | 1.77 | −0.05 | 0.76 | Platykurtic | 9.4 | 68.9 | 21.7 |
| 3 | 21–26 | Fine silt | 6.52 | 1.65 | −0.13 | 0.83 | Platykurtic | 7.2 | 72.3 | 20.4 |
| 4 | 35–40 | Fine silt | 6.97 | 1.41 | −0.15 | 1.04 | Mesokurtic | 4.3 | 72.3 | 23.4 |
| Station 2269 | ||||||||||
| 5 | 0–12 | Fine silt | 6.86 | 1.50 | −0.18 | 1.07 | Mesokurtic | 5.9 | 72.2 | 21.9 |
| 6 | 12–25 | Fine silt | 7.10 | 1.22 | −0.05 | 0.91 | Mesokurtic | 1.0 | 74.5 | 24.5 |
| 7 | 30–40 | Fine silt | 7.73 | 1.21 | −0.14 | 1.11 | Mesokurtic | 1.6 | 55.2 | 43.2 |
| Station 3515-1 | ||||||||||
| 8 | 0–5 | Fine silt | 6.41 | 1.75 | −0.19 | 0.82 | Platykurtic | 6.8 | 71.0 | 22.2 |
| 9 | 10–15 | Fine silt | 5.92 | 1.82 | 0.12 | 0.72 | Platykurtic | 7.3 | 73.4 | 19.2 |
| 10 | 25–30 | Medium silt | 5.76 | 1.77 | 0.22 | 0.79 | Platykurtic | 6.1 | 76.8 | 17.1 |
| 11 | 30–40 | Medium silt | 7.26 | 1.30 | −0.19 | 1.09 | Mesokurtic | 6.7 | 75.1 | 18.2 |
| 12 | 40–44 | Medium silt | 7.99 | 1.35 | −0.01 | 1.23 | Leptokurtic | 6.1 | 76.8 | 17.1 |
| Mean | 6.81 | 1.54 | −0.08 | 0.94 | - | 5.8 | 71.3 | 22.9 | ||
| Std. dev. | 0.64 | 0.22 | 0.13 | 0.16 | - | 2.3 | 5.6 | 6.7 | ||
| No. | Depth (cm) | Fe (%) | Mn (%) | Cu (ppm) | Ni (ppm) | Co (ppm) | Zn (ppm) | Pb (ppm) | V (ppm) | Cr (ppm) | TP (%) | TN (%) | TS (%) | Fe/Mn - | Cu/Zn - | V/Cr - | Ni/Co - | TOC/TS - | TOC/TN - | TOC* - |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | AAS | AAS | AAS | AAS | ICP-MS | AAS | AAS | XRF | AAS | FIA | NS | NS | x | x | x | x | x | x | x | |
| Station 2262 | ||||||||||||||||||||
| 1 | 0–3 | 3.44 | 0.40 | 371.2 | 174.4 | 48.4 | 115.2 | 15.2 | 100 | 39.9 | 0.63 | 0.12 | 0.38 | 8.57 | 3.22 | 2.50 | 3.61 | 1.72 | 5.59 | 0.93 |
| 2 | 10–15 | 3.22 | 0.08 | 238.6 | 64.7 | 52.6 | 93.1 | 11.3 | 90 | 39.0 | 0.42 | 0.11 | 0.40 | 40.34 | 2.56 | 2.31 | 1.23 | 1.56 | 5.40 | 0.92 |
| 3 | 21–26 | 3.65 | 0.03 | 258.9 | 54.8 | 54.9 | 101.6 | 13.6 | 110 | 38.6 | 0.55 | 0.11 | 0.33 | 107.70 | 2.55 | 2.85 | 1.00 | 2.15 | 6.63 | 0.97 |
| 4 | 35–40 | 4.12 | 0.12 | 330.1 | 75.6 | 61.3 | 111.9 | 23.5 | 120 | 38.8 | 0.65 | 0.09 | 0.46 | 33.79 | 2.95 | 3.09 | 1.23 | 1.56 | 8.01 | 1.07 |
| Station 2269 | ||||||||||||||||||||
| 5 | 0–12 | 3.79 | 0.39 | 413.3 | 172.5 | 47.0 | 121.2 | 20.6 | 100 | 39.0 | 0.95 | 0.11 | 0.60 | 9.76 | 3.41 | 2.57 | 3.67 | 1.21 | 6.62 | 1.18 |
| 6 | 12–25 | 4.35 | 0.15 | 551.9 | 97.2 | 54.5 | 136.9 | 23.2 | 120 | 37.2 | 1.07 | 0.08 | 0.51 | 29.95 | 4.03 | 3.23 | 1.78 | 1.28 | 8.39 | 1.03 |
| 7 | 30–40 | 4.70 | 1.22 | 1107.9 | 396.2 | 69.8 | 170.8 | 25.2 | 130 | 37.8 | 1.28 | 0.05 | 0.46 | 3.84 | 6.49 | 3.44 | 5.68 | 1.10 | 9.59 | 0.86 |
| Station 3515–1 | ||||||||||||||||||||
| 8 | 0–5 | 3.78 | 0.43 | 372.9 | 162.5 | 54.0 | 122.1 | 20.8 | 110 | 39.9 | 0.76 | 0.12 | 0.48 | 8.70 | 3.05 | 2.76 | 3.01 | 1.74 | 6.76 | 1.20 |
| 9 | 10–15 | 3.40 | 0.18 | 291.4 | 87.0 | 42.0 | 113.1 | 18.5 | 100 | 39.4 | 0.60 | 0.10 | 0.40 | 18.99 | 2.58 | 2.54 | 2.07 | 1.81 | 7.17 | 1.02 |
| 10 | 25–30 | 3.54 | 0.09 | 285.2 | 61.0 | 16.4 | 107.6 | 19.3 | 110 | 40.3 | 0.67 | 0.08 | 0.45 | 38.28 | 2.65 | 2.73 | 3.73 | 1.50 | 8.31 | 1.02 |
| 11 | 30–40 | 3.47 | 0.14 | 288.3 | 74.0 | 26.9 | 110.3 | 18.9 | 120 | 39.8 | 0.63 | 0.09 | 0.43 | 25.56 | 2.61 | 3.01 | 2.75 | 1.46 | 6.81 | 0.94 |
| 12 | 40–44 | 3.54 | 0.09 | 285.2 | 61.0 | 649.1 | 107.6 | 19.3 | 210 | 40.3 | 0.67 | 0.08 | 0.45 | 38.28 | 2.65 | 5.21 | 0.09 | 1.15 | 6.36 | 0.86 |
| Mean | 3.75 | 0.28 | 399.6 | 123.4 | 98.1 | 117.6 | 19.1 | 118 | 39.2 | 0.74 | 0.10 | 0.45 | 30.31 | 3.23 | 3.02 | 2.49 | 1.52 | 7.14 | 1.00 | |
| Std. dev. | 0.42 | 0.31 | 228.6 | 92.9 | 166.7 | 19.2 | 3.9 | 30 | 0.9 | 0.23 | 0.02 | 0.07 | 26.52 | 1.07 | 0.73 | 1.48 | 0.30 | 1.17 | 0.11 | |
| No. | Depth (cm) | Quartz (%) | Plagioclase (%) | Halite (%) | Barite (%) | Am. SiO2 (%) | Pyroxene (%) | Illite (%) | Chlorite (%) | Smectite (%) | Kaolinite (%) | ∑Clays (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Station 2262 | ||||||||||||
| 1 | 0–3 | 6.6 | 6.0 | 5.6 | 1.5 | 51.4 | + | 14.8 | 2.1 | 10.8 | + | 28.4 |
| 2 | 10–15 | 6.1 | 5.4 | 5.1 | 1.2 | 58.4 | + | 14.7 | 1.6 | 6.6 | + | 23.6 |
| 3 | 21–26 | 6.8 | 6.2 | 5.1 | 1.2 | 54.1 | + | 14.0 | 1.6 | 9.6 | + | 25.8 |
| 4 | 35–40 | 7.3 | 8.3 | 4.3 | 1.8 | 41.6 | + | 14.5 | 2.1 | 19.2 | + | 36.0 |
| Station 2269 | ||||||||||||
| 5 | 0–12 | 7.5 | 7.6 | 4.9 | 1.7 | 43.5 | + | 14.5 | 2.5 | 16.1 | + | 34.0 |
| 6 | 12–25 | 7.9 | 8.2 | 4.3 | 1.7 | 41.1 | + | 16.2 | 2.4 | 17.1 | + | 36.4 |
| 7 | 30–40 | 4.4 | 10.1 | 3.3 | 3.3 | 31.8 | + | 14.6 | 1.0 | 29.4 | - | 44.9 |
| Station 3515–1 | ||||||||||||
| 8 | 0–5 | 6.3 | 6.8 | 5.8 | 1.3 | 50.1 | + | 12.5 | 1.5 | 14.8 | + | 29.3 |
| 9 | 10–15 | 6.1 | 6.8 | 5.5 | 1.3 | 54.2 | + | 10.3 | 2.0 | 13.0 | + | 25.9 |
| 10 | 25–30 | 6.3 | 6.1 | 5.6 | 1.1 | 55.9 | + | 13.1 | 2.2 | 8.8 | + | 24.9 |
| 11 | 30–40 | 7.5 | 9.3 | 4.8 | 2.0 | 31.2 | + | 21.0 | 2.0 | 21.3 | + | 44.3 |
| 12 | 40–44 | 5.3 | 9.0 | 3.4 | 3.3 | 29.6 | + | 17.4 | 3.0 | 26.9 | - | 47.3 |
| Mean | 6.5 | 7.5 | 4.8 | 1.8 | 45.2 | - | 14.8 | 2.0 | 16.1 | 0.5 | 33.4 | |
| Std. dev. | 0.9 | 1.4 | 0.8 | 0.7 | 9.9 | - | 2.5 | 0.5 | 6.8 | 0.3 | 8.1 | |
| No. | Depth (cm) | Feldspar (Albite) | Hematite (Protohematite) | Spinels | Melilite | Quartz (Crystobalite) | Other |
|---|---|---|---|---|---|---|---|
| Station 2262 | |||||||
| 1 | 0–3 | 53.5 | 7.3 | 5.1 | + | 34.0 | - |
| 2 | 10–15 | 26.2 | 48.3 | 5.0 | + | 20.4 | - |
| 3 | 21–26 | 47.2 | 37.0 | + | + | 15.6 | - |
| 4 | 35–40 | 45.0 | 35.4 | + | + | 19.4 | - |
| Station 2269 | |||||||
| 5 | 0–12 | 63.6 | 15.5 | + | + | 15.6 | - |
| 6 | 12–25 | 50.2 | 12.2 | 15.6 | + | 19.9 | - |
| 7 | 30–40 | 44.1 | 10.7 | 16.0 | + | 17.4 | - |
| Station 3515–1 | |||||||
| 8 | 0–5 | 61.5 | 19.5 | + | + | 16.5 | - |
| 9 | 10–15 | 41.2 | 16.9 | 5.3 | 5.1 | 31.5 | - |
| 10 | 25–30 | 36.3 | 15.1 | 14.9 | 5.2 | 38.0 | Periclase |
| 11 | 30–40 | 68.7 | 12.5 | + | + | 14.9 | Bixbyite |
| 12 | 40–44 | 47.1 | 8.6 | 20.3 | + | 20.9 | Bixbyite |
| Mean | 48.7 | 19.9 | 7.3 | + | 22.0 | - | |
| Std. dev. | 11.4 | 12.5 | 7.1 | + | 7.6 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciąg, Ł.; Zawadzki, D.; Kotarba, M.J.; Piestrzyński, A.; Kotliński, R.A.; Wróbel, R.; Zych, H. Thermal Properties and Burial Alteration of Deep-Sea Sediments: New Indicators of Oxic−Suboxic Diagenesis. Minerals 2020, 10, 901. https://doi.org/10.3390/min10100901
Maciąg Ł, Zawadzki D, Kotarba MJ, Piestrzyński A, Kotliński RA, Wróbel R, Zych H. Thermal Properties and Burial Alteration of Deep-Sea Sediments: New Indicators of Oxic−Suboxic Diagenesis. Minerals. 2020; 10(10):901. https://doi.org/10.3390/min10100901
Chicago/Turabian StyleMaciąg, Łukasz, Dominik Zawadzki, Maciej J. Kotarba, Adam Piestrzyński, Ryszard A. Kotliński, Rafał Wróbel, and Hieronim Zych. 2020. "Thermal Properties and Burial Alteration of Deep-Sea Sediments: New Indicators of Oxic−Suboxic Diagenesis" Minerals 10, no. 10: 901. https://doi.org/10.3390/min10100901
APA StyleMaciąg, Ł., Zawadzki, D., Kotarba, M. J., Piestrzyński, A., Kotliński, R. A., Wróbel, R., & Zych, H. (2020). Thermal Properties and Burial Alteration of Deep-Sea Sediments: New Indicators of Oxic−Suboxic Diagenesis. Minerals, 10(10), 901. https://doi.org/10.3390/min10100901







