Crystal Chemistry of Carnotite in Abandoned Mine Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electron Microprobe Analysis
2.3. X-ray Diffraction (XRD)
2.4. Focused Ion Beam (FIB) Sample Preparation
2.5. Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM)
2.6. Raman Spectroscopy
3. Results and Discussion
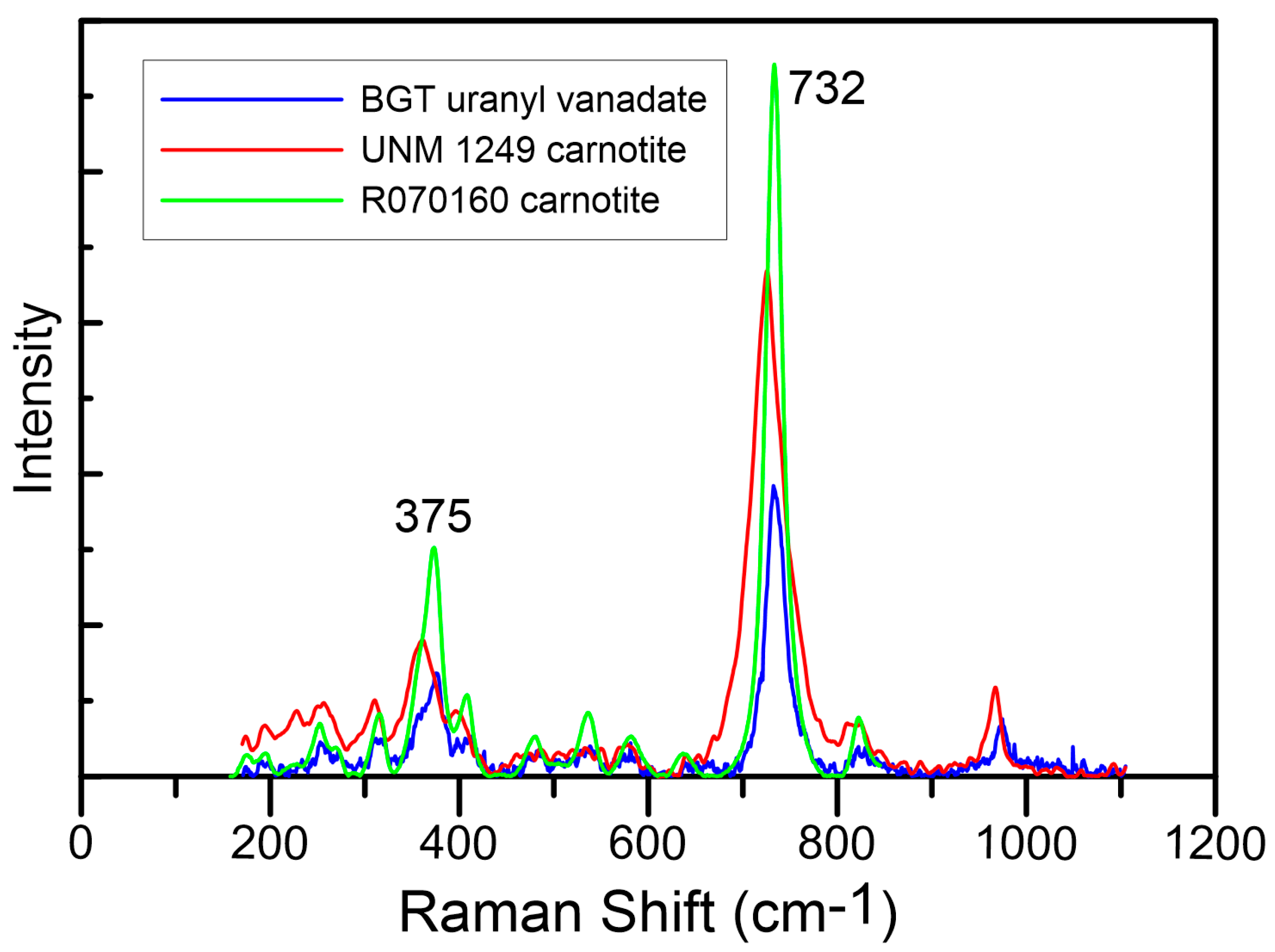
3.1. Raman Spectroscopy
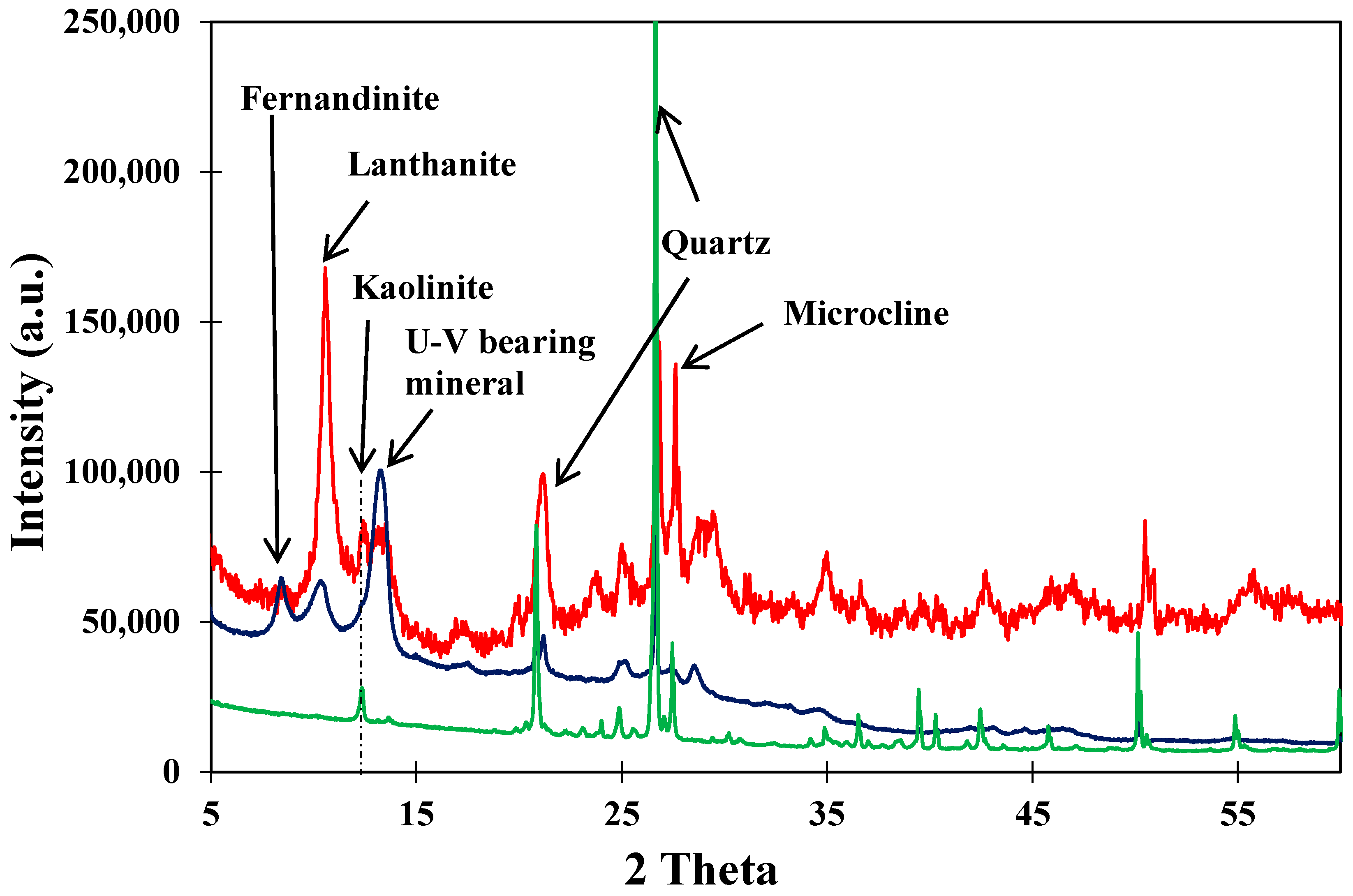
3.2. X-ray Diffraction (XRD)
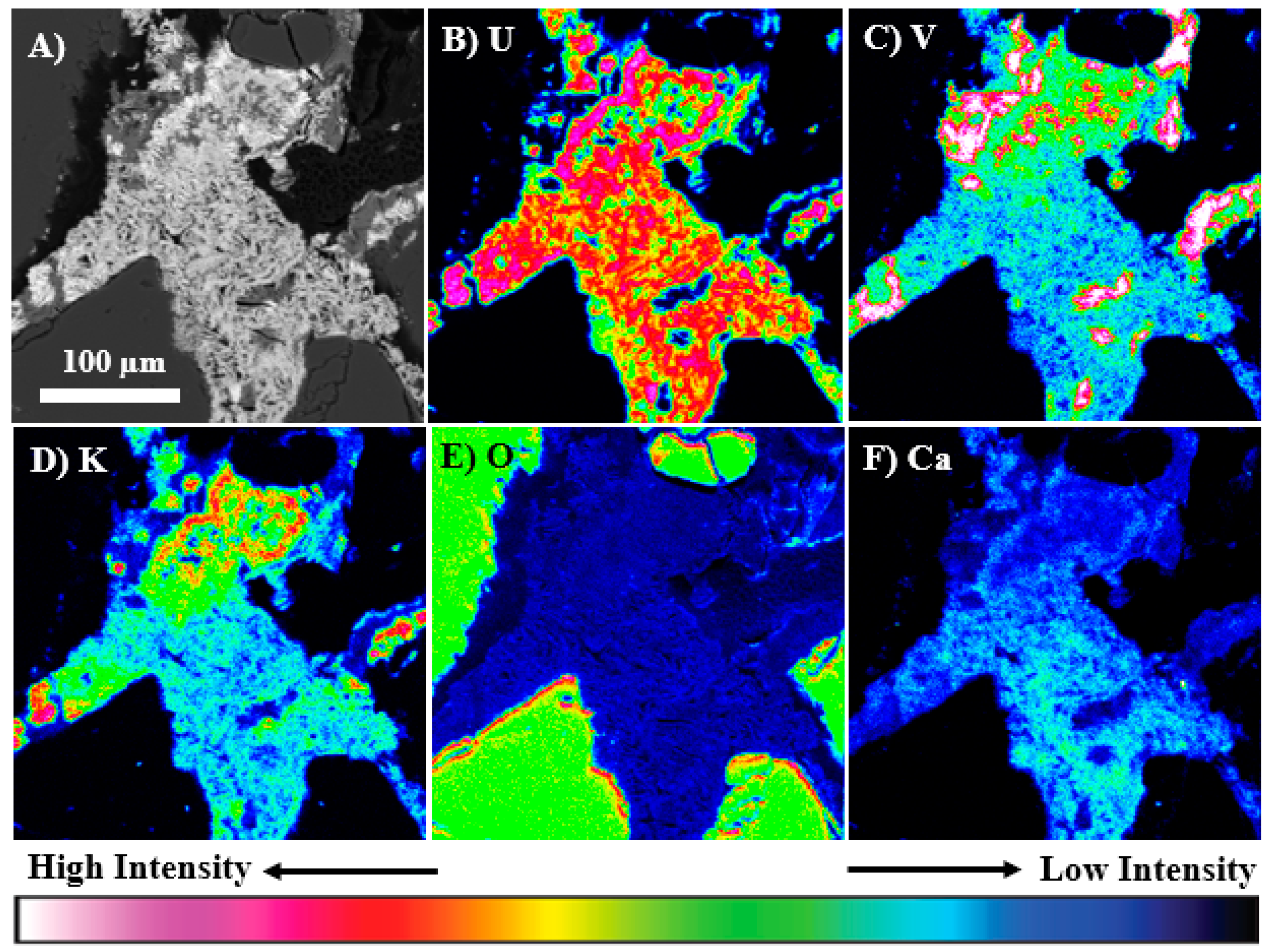
3.3. Electron Microprobe Analysis (Imaging, Qualitative, and Quantitative X-ray Mapping)
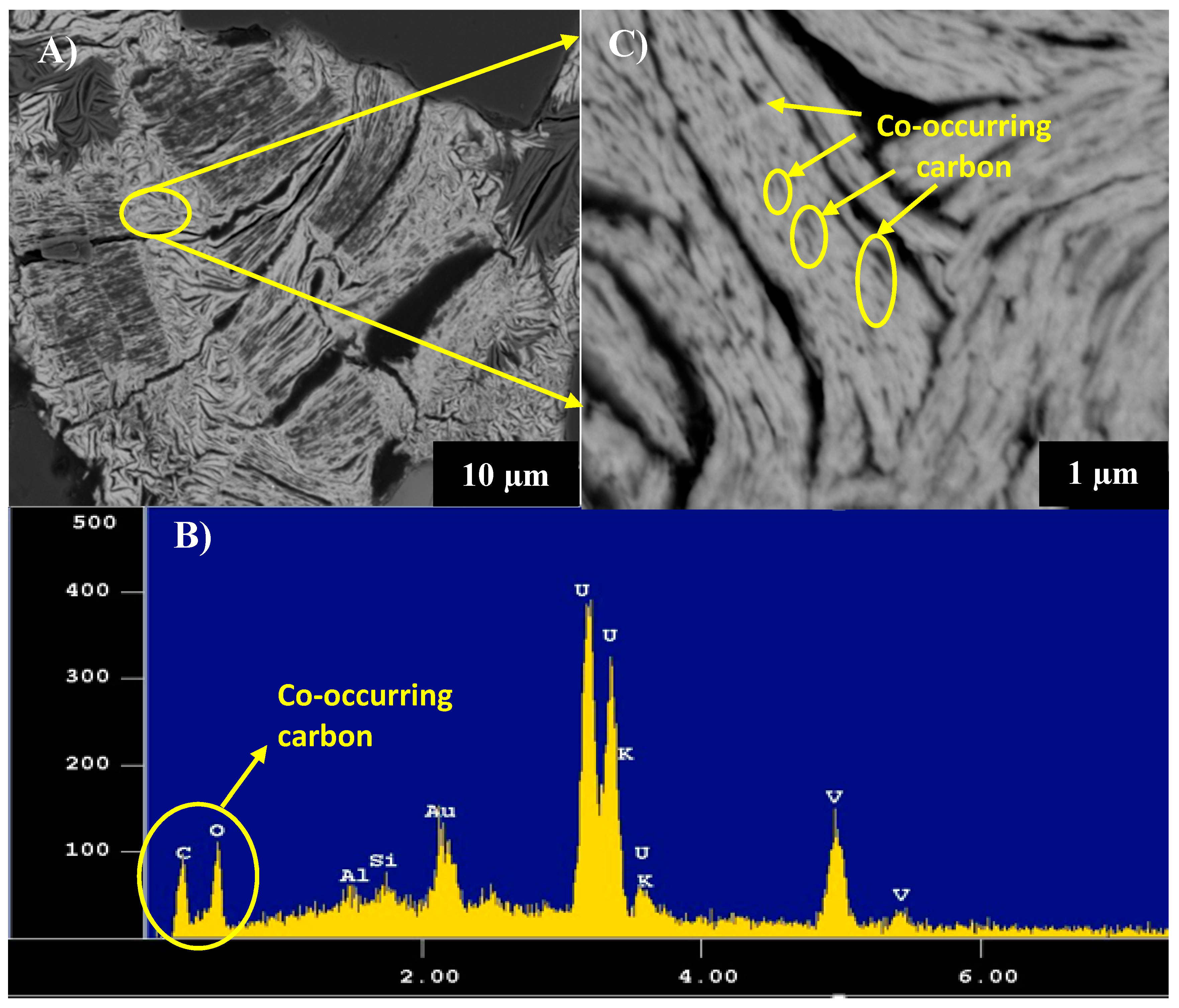
3.4. Scanning Transmission Electron Microscopy (STEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA. Addressing Uranium Contamination on the Navajo Nation. U.S. Environmental Protection Agency, Pacific Southwest, Region 9; USEPA: San Francisco, CA, USA, 2015.
- USEPA. Technical Report on Technologically Enhanced Naturally Occurring Radioactive Materials from Uranium Mining Vol. 1: Mining and Reclamation Background; EPA 402-R-08-005; U.S. Environmental Protection Agency, Office of Radiation and Indoor Air: San Francisco, CA, USA, 2008.
- Blake, J.M.; Avasarala, S.; Artyushkova, K.; Ali, A.-M.S.; Brearley, A.J.; Shuey, C.; Robinson, W.P.; Nez, C.; Bill, S.; Lewis, J. Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a northeastern Arizona Native American community. Environ. Sci. Technol. 2015, 49, 8506–8514. [Google Scholar] [CrossRef]
- Lewis, J.; Hoover, J.; MacKenzie, D. Mining and environmental health disparities in Native American communities. Curr. Environ. Health Rep. 2017, 4, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Avasarala, S.; Lichtner, P.C.; Ali, A.-M.S.; González-Pinzón, R.; Blake, J.M.; Cerrato, J.M. Reactive Transport of U and V from Abandoned Uranium Mine Wastes. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.M.; Avasarala, S.; Ali, A.-M.S.; Spilde, M.; Lezama-Pacheco, J.S.; Latta, D.; Artyushkova, K.; Ilgen, A.G.; Shuey, C.; Nez, C. Reactivity of As and U co-occurring in Mine Wastes in northeastern Arizona. Chem. Geol. 2019, 522, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, P.; Garrels, R. Transportation and precipitation of uranium and vanadium at low temperatures, with special reference to sandstone-type uranium deposits. Econ. Geol. 1962, 57, 137–167. [Google Scholar] [CrossRef]
- Mann, A. Chemical Ore Genesis Models for the Precipitation of Carnotite in Calcrete; Commonwealth Scientific and Industrial Research Organisation (CSIRO): Canberra, Australia, 1974. [Google Scholar]
- Kim, C.; Chi, C.; Miller, S.; Rosales, R.; Sugihara, E.; Akau, J.; Rytuba, J.; Webb, S. (Micro) spectroscopic analyses of particle size dependence on arsenic distribution and speciation in mine wastes. Environ. Sci. Technol. 2013, 47, 8164–8171. [Google Scholar] [CrossRef]
- Hillebrand, W. Carnotite and tyuyamunite and their ores in Colorado and Utah. Am. J. Sci. 1924, 45, 201–216. [Google Scholar] [CrossRef]
- Crook, T.; Blake, G. On carnotite and an associated mineral complex from South Australia. Mineral. Mag. 1910, 15, 271–284. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, S.-Y.; Luo, T.-Y.; Zhou, W.; Li, Y.-L. Uranium mineralization and its radioactive decay-induced carbonization in a black shale-hosted polymetallic sulfide ore layer, southwest China. Econ. Geol. 2015, 110, 1643–1652. [Google Scholar] [CrossRef]
- Abed, A.M.; Saffarini, G.A.; Sadaqah, R.M. Spatial distribution of uranium and vanadium in the upper phosphorite member in Eshidiyya basin, southern Jordan. Arab. J. Geosci. 2014, 7, 253–271. [Google Scholar] [CrossRef]
- Chenoweth, W. Vanadium Mining in the Carrizo Mountains, 1942–1947; Open-File Report 378; New Mexico Bureau of Mines and Mineral Resources: Socorro, NM, USA, 1991; p. 33. [Google Scholar]
- Sharma, R.K.; Putirka, K.D.; Stone, J.J. Stream sediment geochemistry of the upper Cheyenne River watershed within the abandoned uranium mining region of the southern Black Hills, South Dakota, USA. Environ. Earth Sci. 2016, 75, 823. [Google Scholar] [CrossRef]
- Hilpert, L.S. Uranium Resources of Northwestern New Mexico; Series 603; U.S. Geological Survey: Reston, VA, USA, 1969; 166p.
- McLemore, V.T.; Donahue, K.; Krueger, C.B.; Rowe, A.; Ulbricht, L.; Jackson, M.L.; Breese, M.R.; Jones, G.; Wilks, M. Database of the Uranium Mines, Prospects, Occurrences, and Mills in New Mexico; Open File Report 461; New Mexico Bureau of Geology and Mineral Resources: Socorro, NM, USA, 2002. [Google Scholar]
- Caldwell, S. Paragenesis of Uranium Minerals in the Grants Mineral Belt, New Mexico: Applied Geochemistry and the Development of Oxidized Uranium Mineralization; New Mexico Institute of Mining and Technology: Socorro, NM, USA, 2018. [Google Scholar]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 42, 547–569. [Google Scholar] [CrossRef]
- Tokunaga, T.K.; Kim, Y.; Wan, J. Potential remediation approach for uranium-contaminated groundwaters through potassium uranyl vanadate precipitation. Environ. Sci. Technol. 2009, 43, 5467–5471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abraham, F.; Dion, C.; Saadi, M. Carnotite analogues: Synthesis, structure and properties of the Na1–x KxUO2VO4 solid solution (0 ≤ x ≤ 1). J. Mater. Chem. 1993, 3, 459–463. [Google Scholar] [CrossRef]
- Weigel, F.; Hoffmann, G. The phosphates and arsenates of hexavalent actinides. Part, I. Uranium. J. Less Common Met. 1976, 44, 99–123. [Google Scholar] [CrossRef]
- Plášil, J.; Veselovský, F.; Hloušek, J.; Škoda, R.; Novák, M.; Sejkora, J.; Čejka, J.; Škácha, P.; Kasatkin, A.V. Mathesiusite, K5(UO2)4(SO4)4 (VO5)(H2O)4, a new uranyl vanadate-sulfate from Jachymov, Czech Republic. Am. Mineral. 2014, 99, 625–632. [Google Scholar] [CrossRef]
- Karyakin, N.; Chernorukov, N.; Suleimanov, E.; Alimzhanov, M. Chemical thermodynamics of alkaline-earth metal uranovanadates. Radiochemistry 2003, 45, 457–468. [Google Scholar] [CrossRef]
- Burns, P.C. U6+ minerals and inorganic compounds: Insights into an expanded structural hierarchy of crystal structures. Can. Mineral. 2005, 43, 1839–1894. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Plášil, J. Mineralogy and Crystallography of Uranium. In Uranium: From Cradle to Grave; Mineralogical Association of Canada Short Courses 43; Mineralogical Association of Canada: Winnipeg, MB, Canada, 2013; pp. 15–119. [Google Scholar]
- Plasil, J.; Kampf, A.R.; Skoda, R.; Cejka, J. Vandermeerscheite, a new uranyl vanadate related to carnotite, from Eifel, Germany. J. Geosci. 2019, 64, 219–227. [Google Scholar] [CrossRef]
- Senchyk, G.; Wylie, E.; Prizio, S.; Szymanowski, J.; Sigmon, G.; Burns, P. Hybrid uranyl–vanadium nano-wheels. Chem. Commun. 2015, 51, 10134–10137. [Google Scholar] [CrossRef] [PubMed]
- Barton, P.B., Jr. Synthesis and Properties of Carnotite and Its Alkali Analogues. Am. Mineral. 1958, 43, 799. [Google Scholar]
- Abraham, F.; Dion, C.; Tancret, N.; Saadi, M. Ag2(UO2)2V2O8: A new compound with the carnotite structure. Synthesis, structure and properties. In Advanced Materials Research; Trans Tech Publication: Stafa-Zurich, Switzerland, 1994; pp. 511–520. [Google Scholar]
- Dickens, P.G.; Stuttard, G.P.; Ball, R.G.; Powell, A.V.; Hull, S.; Patat, S. Powder neutron diffraction study of the mixed uranium–vanadium oxides Cs2(UO2)2(V2O8) and UVO5. J. Mater. Chem. 1992, 2, 161–166. [Google Scholar] [CrossRef]
- Spano, T.L.; Dzik, E.A.; Sharifironizi, M.; Dustin, M.K.; Turner, M.; Burns, P.C. Thermodynamic investigation of uranyl vanadate minerals: Implications for structural stability. Am. Mineral. 2017, 102, 1149–1153. [Google Scholar] [CrossRef]
- Stubbs, J.E.; Post, J.E.; Elbert, D.C.; Heaney, P.J.; Veblen, D.R. Uranyl phosphate sheet reconstruction during dehydration of metatorbernite [Cu (UO2)2(PO4)2·8H2O]. Am. Mineral. 2010, 95, 1132–1140. [Google Scholar] [CrossRef]
- Graham, J.; Butt, C.; Vigers, R. Sub-surface charging, a source of error in microprobe analysis. X-ray Spectrom. 1984, 13, 126–133. [Google Scholar] [CrossRef]
- Burns, P.C.; Ewing, R.C.; Hawthorne, F.C. The crystal chemistry of hexavalent uranium: Polyhedron geometries, bond-valence parameters, and polymerization of polyhedra. Can. Mineral. 1997, 35, 1551–1570. [Google Scholar]
- Catalano, J.G.; Brown, G.E. Analysis of uranyl-bearing phases by EXAFS spectroscopy: Interferences, multiple scattering, accuracy of structural parameters, and spectral differences. Am. Mineral. 2004, 89, 1004–1021. [Google Scholar] [CrossRef]
- Franczyk, K.J. Stratigraphic Revision and Depositional Environments of the Upper Cretaceous Toreva Formation in the Northern Black Mesa Area, Navajo and Apache Counties, Arizona; U.S. Geological Survey: Reston, VA, USA, 1988.
- Chenoweth, W.L. The Geology and Production History of the Uranium Deposits in the Toreva Formation, Black Mesa, Apaciie County, Arizona; Arizona Geological Survey: Tucson, AZ, USA, 1990. [Google Scholar]
- McGuire, A.V.; Francis, C.A.; Dyar, M.D. Mineral standards for electron microprobe analysis of oxygen. Am. Mineral. 1992, 77, 1087–1091. [Google Scholar]
- Jarosewich, E. Smithsonian microbeam standards. J. Res. Nat. Inst. Stan. 2002, 107, 681. [Google Scholar] [CrossRef]
- Donovan, J.J.; Snyder, D.A.; Rivers, M.L. An improved interference correction for trace element analysis. In Proceedings of the Annual Meeting-Electron Microscopy Society of America; San Francisco Press: San Francisco, CA, USA; p. 1646.
- Donovan, J.J.; Lowers, H.A.; Rusk, B.G. Improved electron probe microanalysis of trace elements in quartz. Am. Mineral. 2011, 96, 274–282. [Google Scholar] [CrossRef]
- Bastin, G.; Heijligers, H. Quantitative electron probe microanalysis of carbon in binary carbides: Principles and procedures. X-ray Spectrom. 1986, 15, 135–141. [Google Scholar] [CrossRef]
- Jones, A.; Palmer, D.; Islam, M.; Mortimer, M. Alkali ion migration in albite and K-feldspar. Phys. Chem. Miner. 2004, 31, 313–320. [Google Scholar] [CrossRef]
- Spray, J.G.; Rae, D.A. Quantitative electron-microprobe analysis of alkali silicate glasses; a review and user guide. Can. Mineral. 1995, 33, 323–332. [Google Scholar]
- Fournelle, J.; Moy, A.; Nachlas, W.; Donovan, J. The EPMA matrix correction: All elements must be present for accuracy: Four examples with B., C., O and F. Microsc. Microanal. 2020. [Google Scholar] [CrossRef]
- Poppe, L.; Paskevich, V.; Hathaway, J.; Blackwood, D. A Laboratory Mmanual for X-ray Powder Diffraction; US Geological Survey Open File Report 01-041; U.S. Geological Survey: Woods Hole, MA, USA, 2001; pp. 1–88.
- Abreu, N.M.; Brearley, A.J. Early solar system processes recorded in the matrices of two highly pristine CR3 carbonaceous chondrites, MET 00426 and QUE 99177. Geochim. Cosmochim. Acta 2010, 74, 1146–1171. [Google Scholar] [CrossRef]
- Frost, R.L.; Čejka, J.; Weier, M.L.; Martens, W.; Henry, D.A. Vibrational spectroscopy of selected natural uranyl vanadates. Vib. Spectrosc. 2005, 39, 131–138. [Google Scholar] [CrossRef][Green Version]
- Anthony, J.W. Handbook of Mineralogy: Arsenates, Phosphates, Vanadates. Arsenates, Phosphates, Vanadates; Mineral Data Publisher: Tuscon, AZ, USA, 2000; Volume 4. [Google Scholar]
- Palache, C.; Berman, H.; Frondel, C. Dana’s System of Mineralogy, 7th ed.; John Wiley: New York, NY, USA, 1957; Volume II, p. 74. [Google Scholar]
- Nasdala, L.; Kronz, A.; Wirth, R.; Váczi, T.; Pérez-Soba, C.; Willner, A.; Kennedy, A.K. The phenomenon of deficient electron microprobe totals in radiation-damaged and altered zircon. Geochim. Cosmochim. Acta 2009, 73, 1637–1650. [Google Scholar] [CrossRef]
- Craw, D. Oxidation and microprobe-induced potassium mobility in iron-bearing phyllosilicates from the Otago schists, New Zealand. Lithos 1981, 14, 49–57. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Ratcliffe, C.I.; Dutrizac, J.E.; Jambor, J.L. Hydronium ion in the alunite-jarosite group. Can. Mineral. 1986, 24, 435–447. [Google Scholar]
- Papike, J.; Burger, P.; Karner, J.; Shearer, C.; Lueth, V. Terrestrial analogs of martian jarosites: Major, minor element systematics and Na-K zoning in selected samples. Am. Mineral. 2007, 92, 444–447. [Google Scholar] [CrossRef]
- Katrinak, K.; Rez, P.; Buseck, P. Structural variations in individual carbonaceous particles from an urban aerosol. Environ. Sci. Technol. 1992, 26, 1967–1976. [Google Scholar] [CrossRef]
- Comelli, G.; Stöhr, J.; Robinson, C.; Jark, W. Structural studies of argon-sputtered amorphous carbon films by means of extended x-ray-absorption fine structure. Phys. Rev. B 1988, 38, 7511. [Google Scholar] [CrossRef]
- Sundberg, I.; Sillén, L. On the crystal structure of K2(UO2)2(VO4)2 (synthetic anhydrous carnotite). Arkiv Kemi 1950, 1, 337. [Google Scholar]
- Stern, T.; Stieff, L.; Girhard, M.; Meyrowitz, R. The occurrence and properties of metatyuyamunite, Ca(UO2)2(VO4)2.3–5H2O. Am. Mineral. 1956, 41, 187–201. [Google Scholar]
- Donnay, G.; Donnay, J.D.H. Contribution to the Crystallography of Uranium Minerals; Survey TEI-507; US Atomic Energy Commission: Oak Ridge, TN, USA, 1955; Volume 507, p. 42.

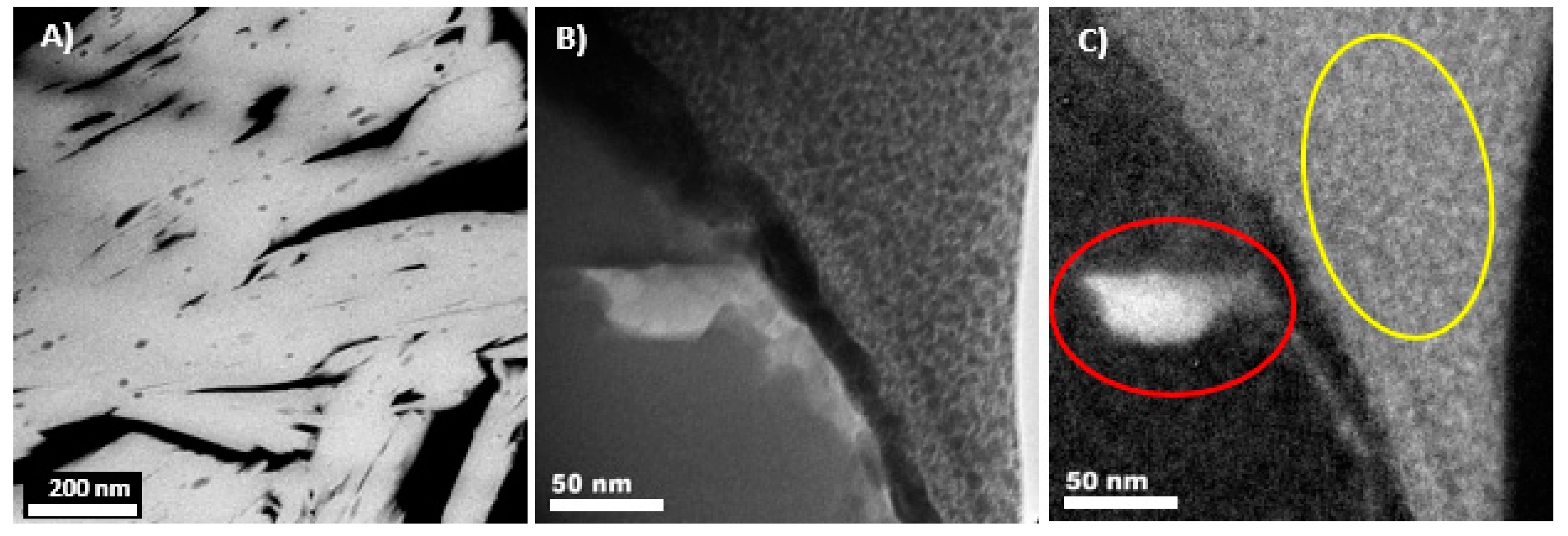
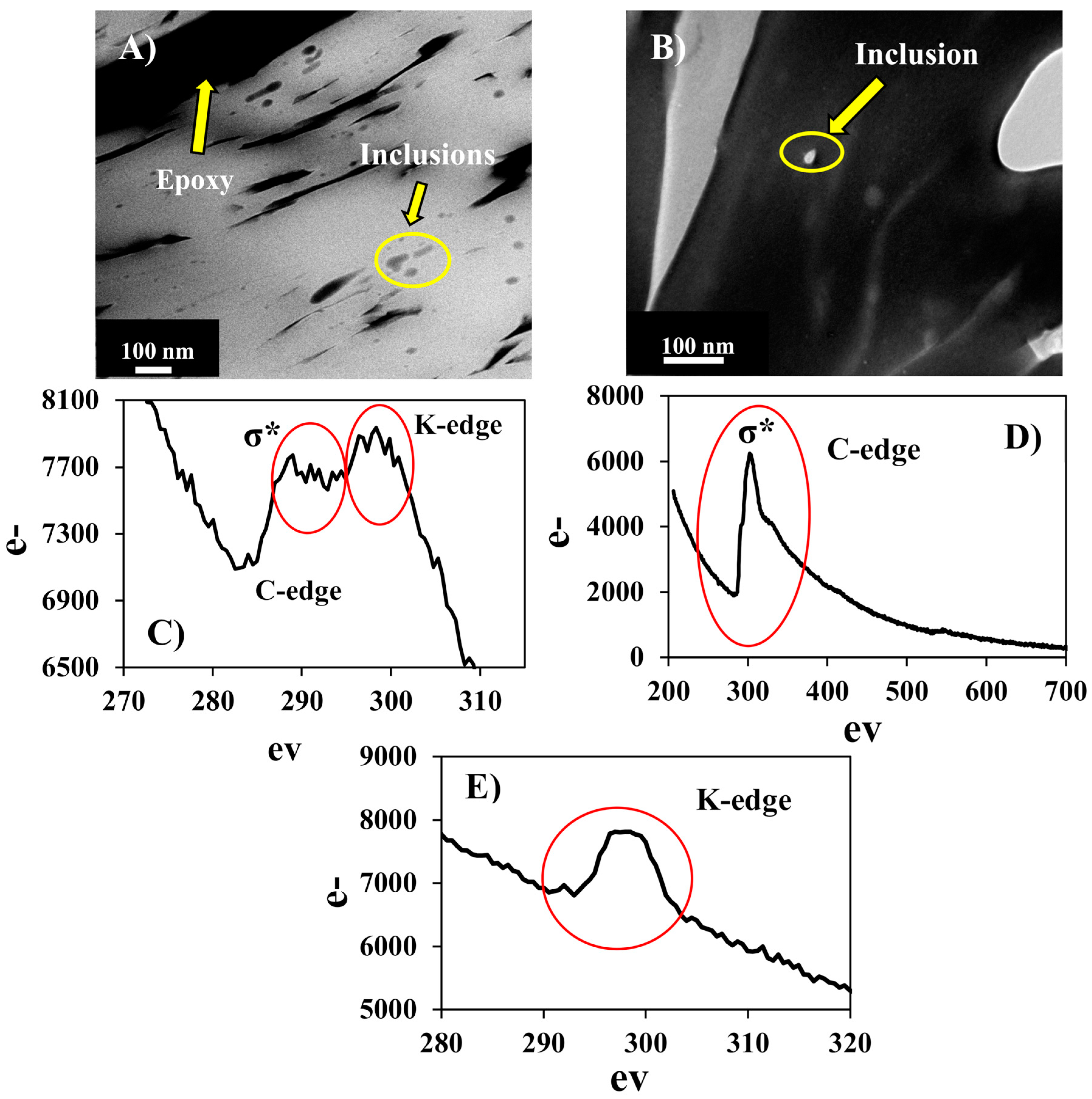
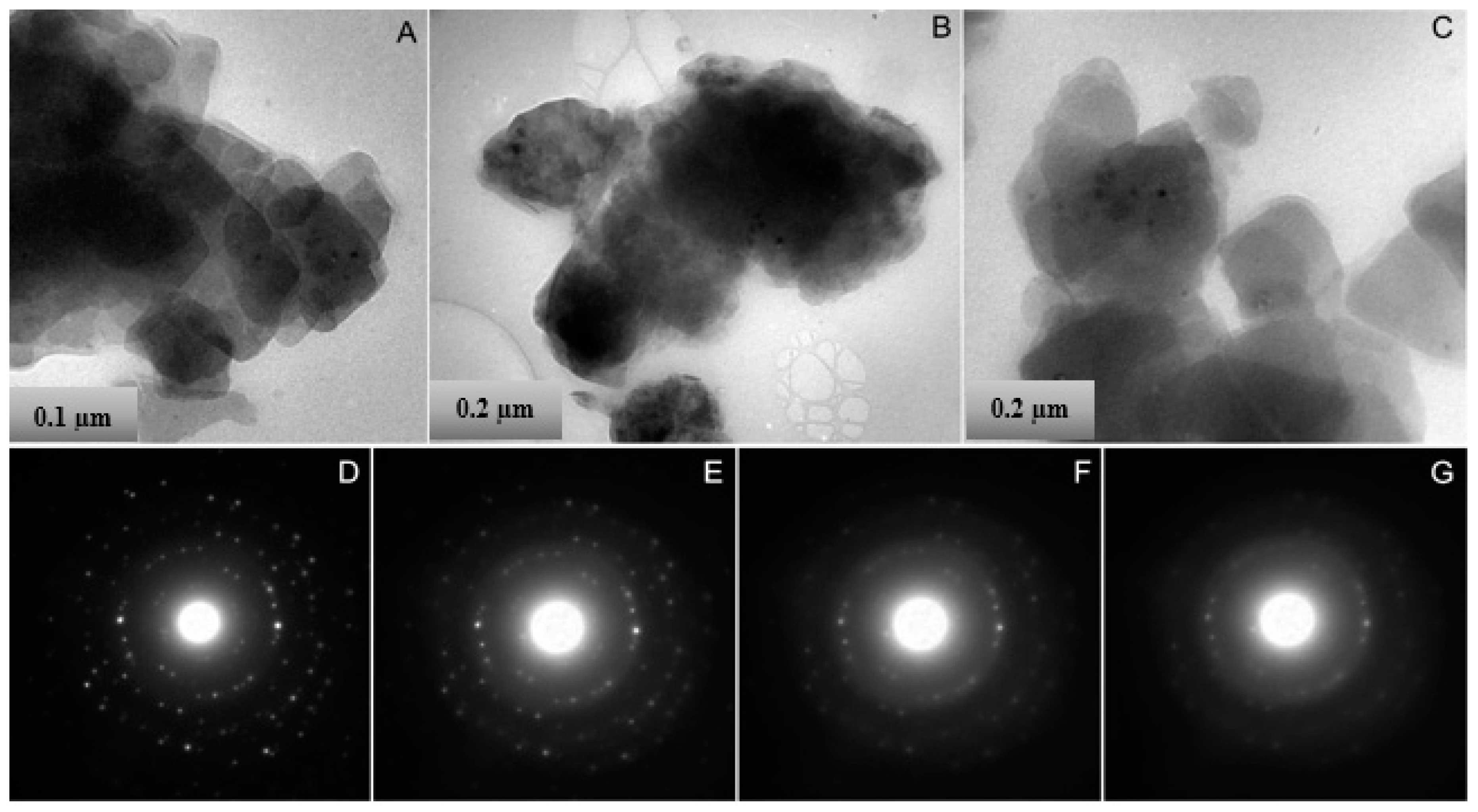
| Element | Element Weight% | Oxide | Oxide Weight% | Element | Formula Based on Stoichiometrically Estimated O (w/o H2O) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (1) | (2) | (3) | (1) | (2) | (3) | |||
| K | 3.54 | 7.17 | 7.95 | K2O | 4.45 | 8.64 | 10.00 | K+ | 0.881 | 1.677 | 1.887 |
| Ca | 0.37 | 0.19 | 0.46 | CaO | 0.53 | 0.26 | 0.66 | Ca2+ | 0.088 | 0.043 | 0.105 |
| Na | 0.03 | 0 | 0.12 | Na2O | 0.041 | 0 | 0.16 | Na+ | 0.012 | - | 0.046 |
| U | 52.20 | 51.12 | 52.58 | Al2O3 | - | 0.81 | - | Al(OH)2+ | - | 0.145 | - |
| V | 11.50 | 11.35 | 11.46 | Fe2O3 | 0.56 | 0.38 | 0.55 | Fe(OH)2+ | 0.022 | 0.044 | 0.02 |
| Sum A-site | 5.581 | 10.09 | 10.82 | Sum A-site | 1.003 | 1.909 | 2.038 | ||||
| Al | - | 0.43 | - | UO3 | 62.72 | 61.43 | 62.26 | U | 2.046 | 1.948 | 1.935 |
| Fe | 0.41 | 0.27 | 0.40 | V2O5 | 20.65 | 20.26 | 20.57 | V | 2.118 | 2.018 | 2.011 |
| H | 0.92 | 0.55 | H2O | 1.7 | 4.08 | 4.90 | H | 0.981 | 1.705 | 2.038 | |
| O | 17.80 | 28.56 | 26.53 | ||||||||
| Total without C | 85.90 | 100.01 | 100.05 | Total | 90.65 | 95.86 | 99.50 | ||||
| C | 4.81 | - | - | 4.81 | - | ||||||
| Total with C | 90.71 | 95.46 | |||||||||
| Calculated | h | k | l | Measured [57] |
|---|---|---|---|---|
| 6.4626 | 0 | 1 | 1 | 6.427 |
| 6.3886 | 1 | 0 | 0 | 6.318 |
| 5.75 | ||||
| 5.0857 | 1 | 1 | 0 | 5.17 |
| 4.9478 | 1 | 1 | 1 | 4.92 |
| 4.68 | ||||
| 4.5438 | 1 | 0 | 2 | 4.58 |
| 4.3320 | 0 | 1 | 2 | |
| 4.2243 | 1 | 1 | 1 | 4.27 |
| 4.2015 | 0 | 2 | 0 | |
| 3.9969 | 1 | 1 | 2 | 3.95 |
| 3.8799 | 0 | 2 | 1 | 3.79 |
| 3.5620 | 1 | 0 | 2 | |
| 3.5104 | 1 | 2 | 0 | |
| 3.4640 | 1 | 2 | 1 | 3.41 |
| 3.2795 | 1 | 1 | 2 | |
| 3.2313 | 0 | 2 | 2 | 3.21 |
| 3.1943 | 2 | 0 | 0 | |
| 3.1858 | 1 | 2 | 1 | 3.13 |
| 3.1282 | 0 | 1 | 3 | |
| 3.1022 | 1 | 1 | 3 | |
| 3.0848 | 1 | 2 | 2 | |
| 3.0607 | 2 | 0 | 2 | 3.05 |
| 3.0606 | 2 | 1 | 1 | 2.99 |
| 2.9859 | 2 | 1 | 0 | |
| 2.8759 | 2 | 1 | 2 | 2.90 |
| 2.7170 | 1 | 2 | 2 | 2.71 |
| 2.7004 | 2 | 1 | 1 | |
| 2.6993 | 0 | 3 | 1 | |
| 2.6290 | 0 | 2 | 3 | |
| 2.6136 | 1 | 2 | 3 | 2.603 |
| 2.5885 | 2 | 2 | 1 | 2.596 |
| 2.5865 | 1 | 1 | 3 | |
| 2.5767 | 1 | 0 | 4 | |
| 2.5653 | 1 | 3 | 0 | |
| 2.5470 | 1 | 3 | 1 | 2.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avasarala, S.; J. Brearley, A.; Spilde, M.; Peterson, E.; Jiang, Y.-B.; Benavidez, A.; Cerrato, J.M. Crystal Chemistry of Carnotite in Abandoned Mine Wastes. Minerals 2020, 10, 883. https://doi.org/10.3390/min10100883
Avasarala S, J. Brearley A, Spilde M, Peterson E, Jiang Y-B, Benavidez A, Cerrato JM. Crystal Chemistry of Carnotite in Abandoned Mine Wastes. Minerals. 2020; 10(10):883. https://doi.org/10.3390/min10100883
Chicago/Turabian StyleAvasarala, Sumant, Adrian J. Brearley, Michael Spilde, Eric Peterson, Ying-Bing Jiang, Angelica Benavidez, and José M. Cerrato. 2020. "Crystal Chemistry of Carnotite in Abandoned Mine Wastes" Minerals 10, no. 10: 883. https://doi.org/10.3390/min10100883
APA StyleAvasarala, S., J. Brearley, A., Spilde, M., Peterson, E., Jiang, Y.-B., Benavidez, A., & Cerrato, J. M. (2020). Crystal Chemistry of Carnotite in Abandoned Mine Wastes. Minerals, 10(10), 883. https://doi.org/10.3390/min10100883






