Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials
Abstract
:1. Introduction
2. Materials and Methods
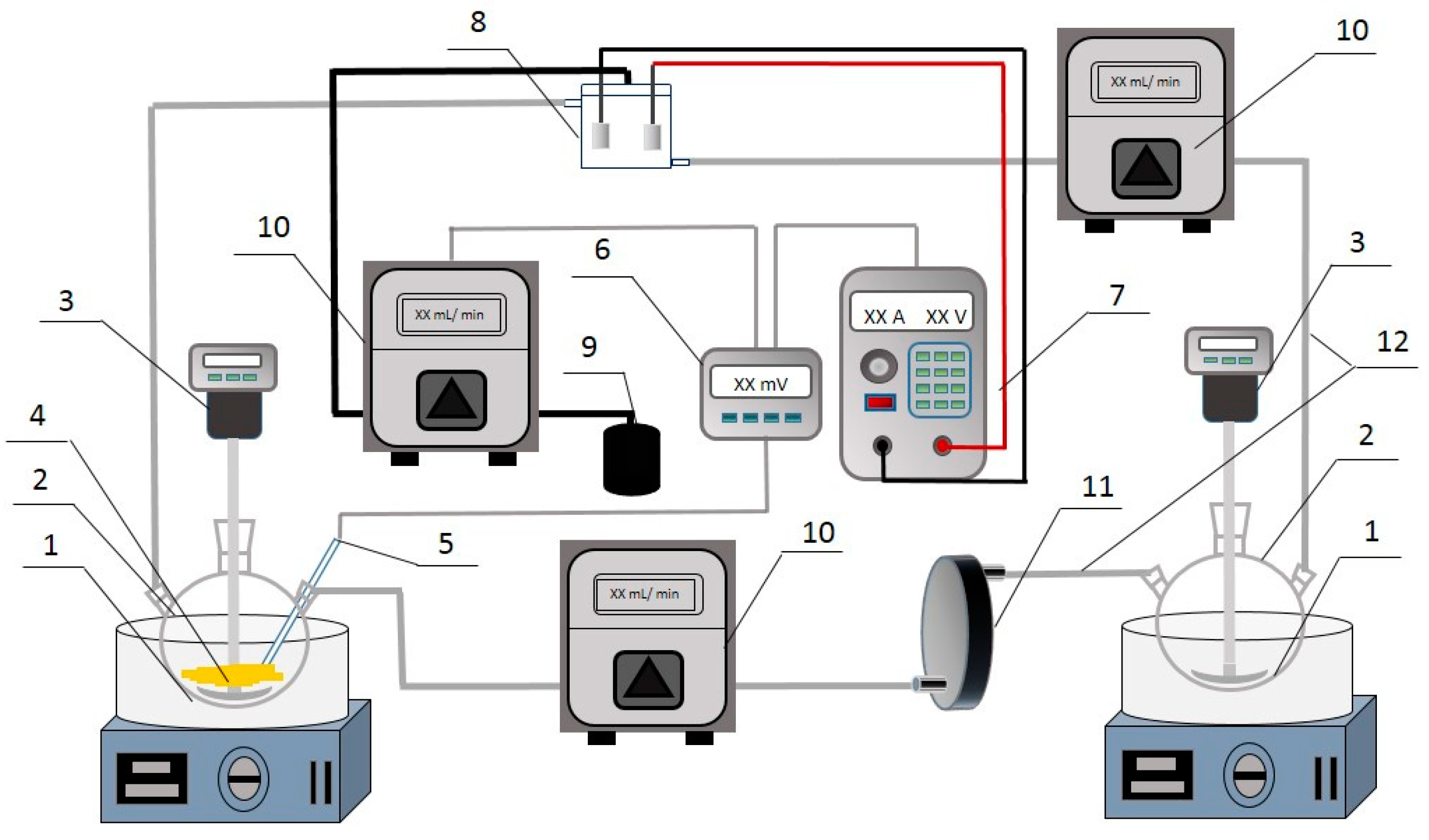
2.1. Apparatus
2.2. Pyrite Samples
2.3. Culture Medium
2.4. Microbial Cultivation and Preparation
2.5. Batch Experiments
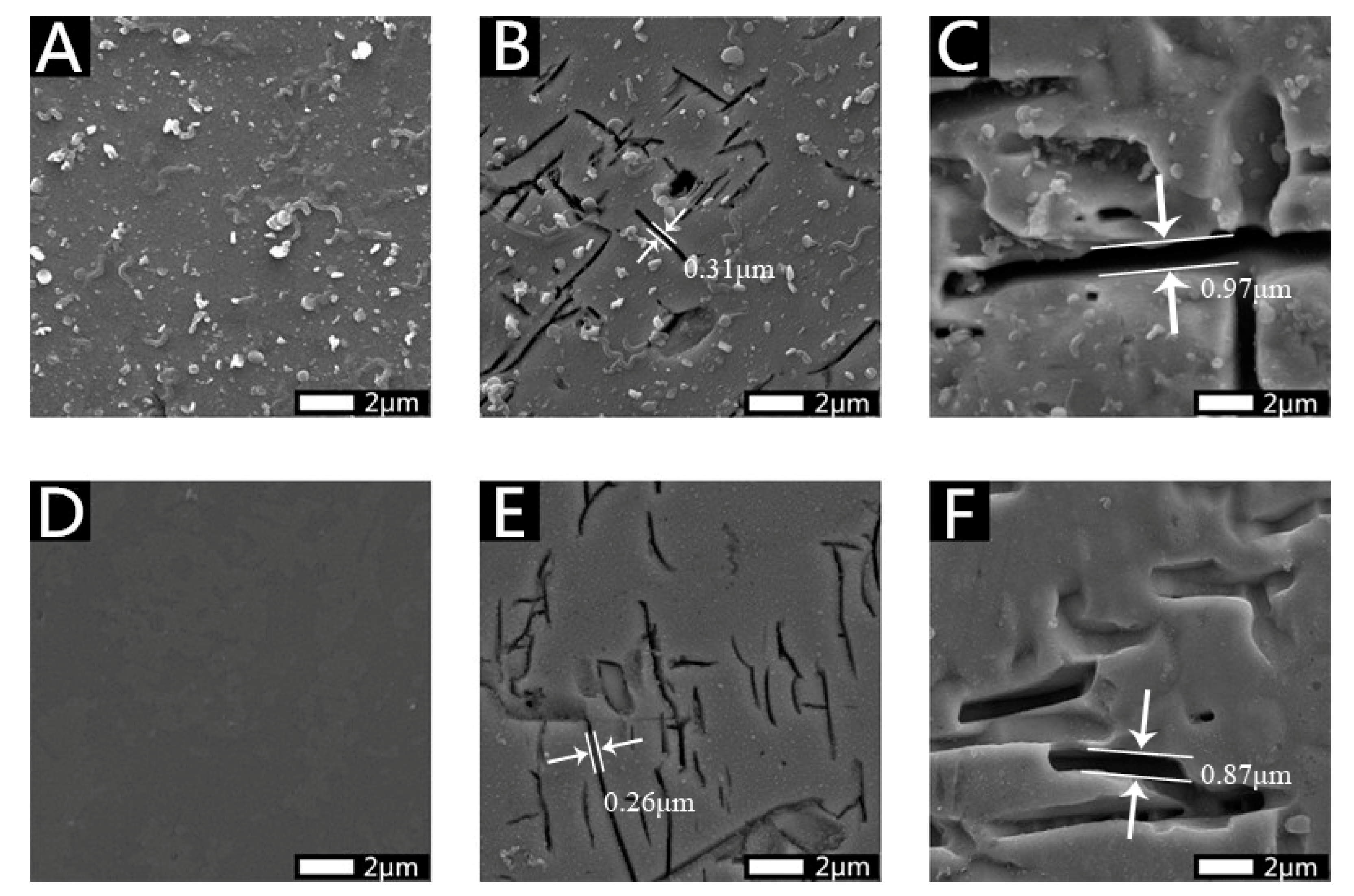
2.6. Pyrite Surface Detection by SEM
2.7. X-Ray Photoelectron Spectroscopy (XPS) Analyses
2.8. Quantification of Elemental Sulfur by HPLC
2.9. DNA Extraction and Microbial Consortium Analysis
3. Results and Discussion
3.1. Morphological Characteristics of Residue Samples
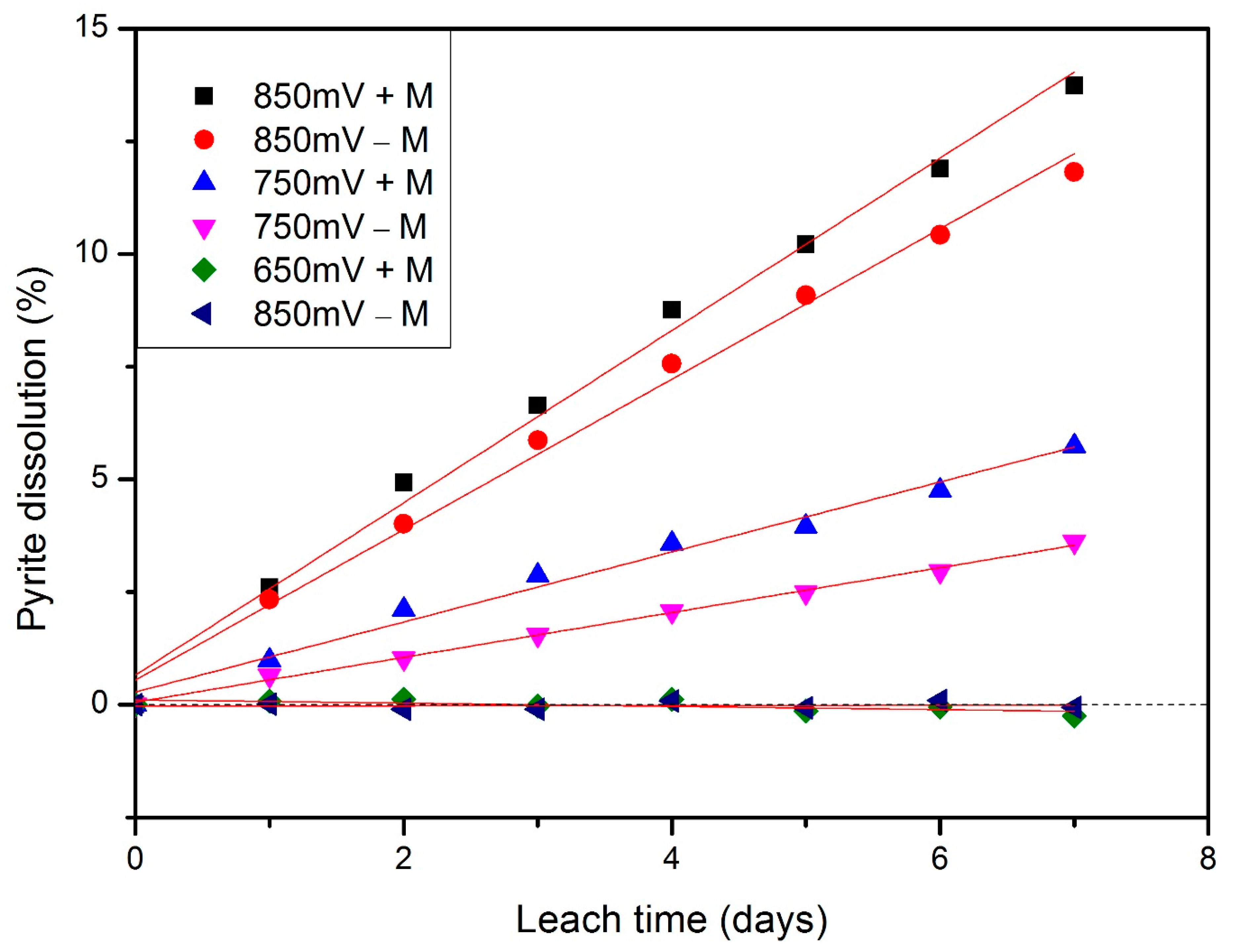
3.2. Kinetics under Different Redox Potentials and Microbial Conditions
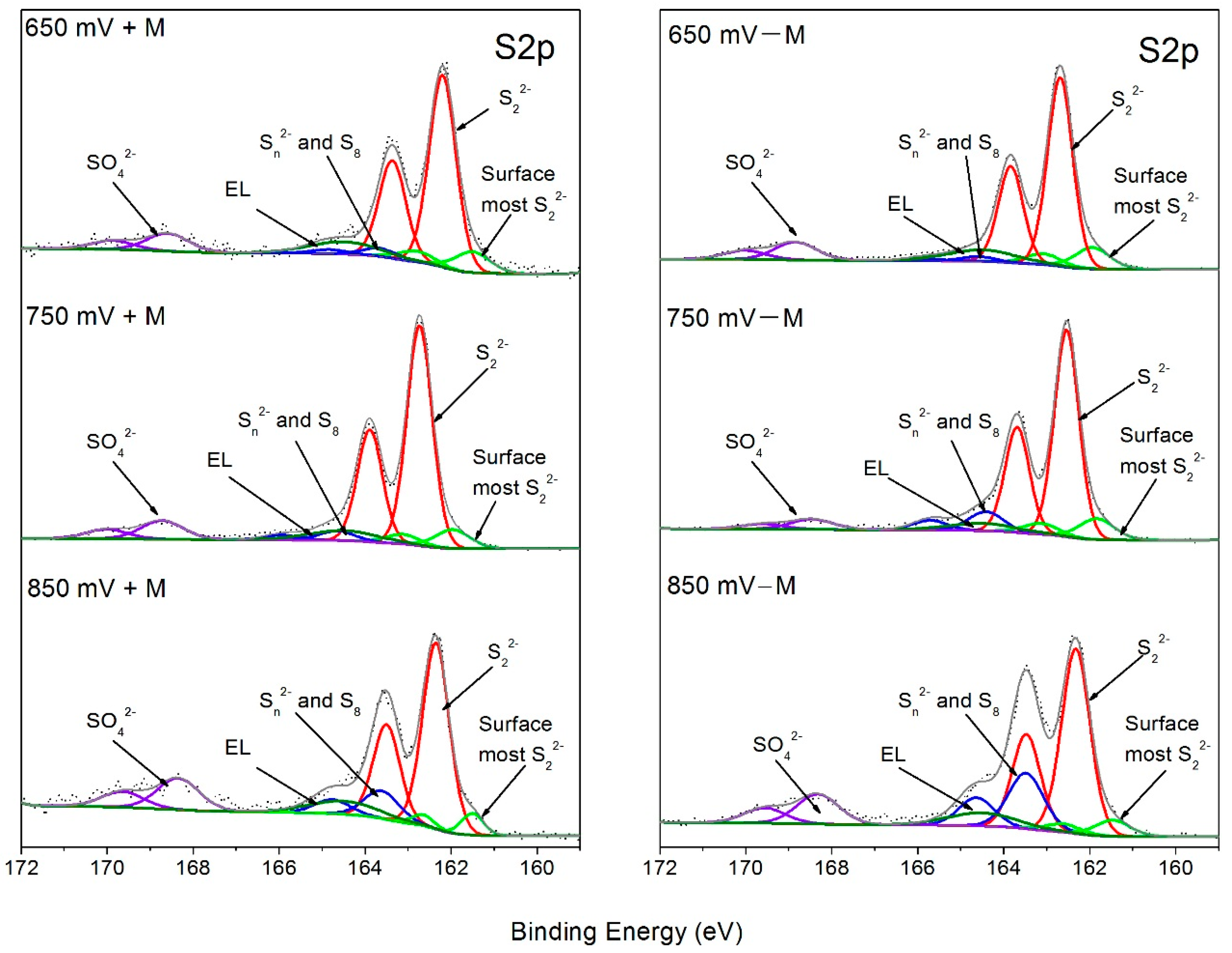
3.3. XPS Characterization
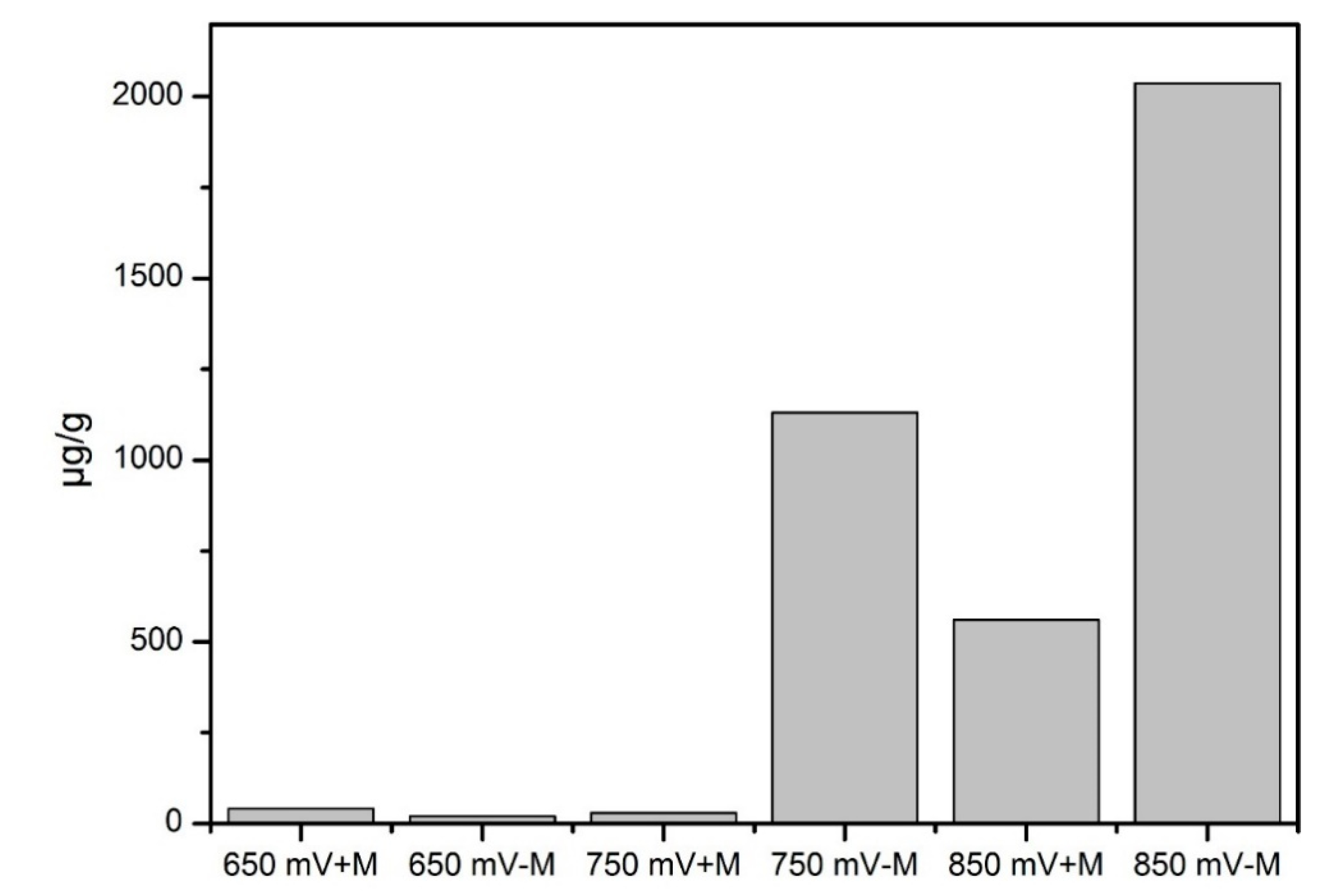
3.4. Elemental Sulfur in Leaching Residue
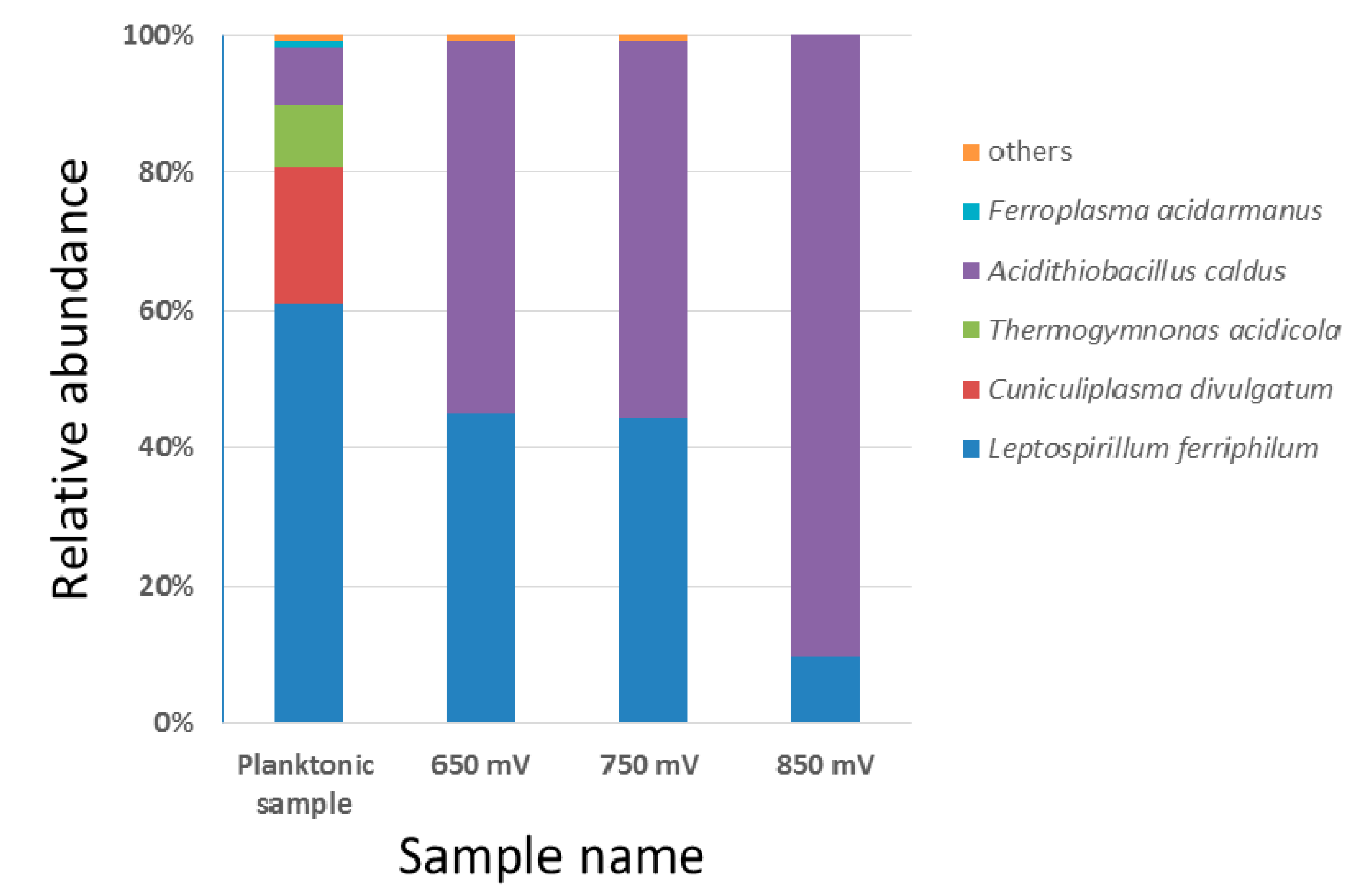
3.5. Attached Microbial Comminity on Pyrite Residual
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundgren, D.; Silver, M. Ore Leaching by Bacteria. Annu. Rev. Microbiol. 1980, 34, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I. Microbial leaching of metals from sulfide minerals. Biotechnol. Adv. 2001, 19, 119–132. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y. Sequential biological process for molybdenum extraction from hydrodesulphurization spent catalyst. Chemosphere 2016, 160, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Ting, Y. Microbial leaching of heavy metals using Escherichia coli and evaluation of bioleaching mechanism. Bioresour. Technol. Rep. 2020, 9, 100368. [Google Scholar] [CrossRef]
- Baker, B.; Banfield, J. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Gerson, A. Redox potential (Eh) and anion effects of pyrite (FeS2) leaching at pH 1. Geochim. Cosmochim. Acta 2011, 75, 6893–6911. [Google Scholar] [CrossRef]
- Sun, H.; Chen, M.; Zou, L.; Shu, R.; Ruan, R. Study of the kinetics of pyrite oxidation under controlled redox potential. Hydrometallurgy 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Ma, J.; Tang, Y.; Yang, D.; Pei, P. Kinetics of advanced oxidative leaching of pyrite in a potassium peroxy-disulphate solution. J. S. Afr. Inst. Min. Metall. 2020, 120, 165–172. [Google Scholar] [CrossRef]
- Bouffard, S.; Riveravasquez, B.; Dixon, D. Leaching kinetics and stoichiometry of pyrite oxidation from a pyrite–marcasite concentrate in acid ferric sulfate media. Hydrometallurgy 2006, 84, 225–238. [Google Scholar] [CrossRef]
- Qian, G.; Fan, R.; Short, M.; Schumann, R.; Li, J.; Smart, R.; Gerson, A. The Effects of Galvanic Interactions with Pyrite on the Generation of Acid and Metalliferous Drainage. Environ. Sci. Technol. 2018, 52, 5349–5357. [Google Scholar] [PubMed]
- Liu, C.; Jia, Y.; Sun, H.; Tan, Q.; Niu, X.; Leng, X.; Ruan, R. Limited role of sessile acidophiles in pyrite oxidation below redox potential of 650 mV. Sci. Rep. 2017, 7, 5032–5040. [Google Scholar] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [PubMed]
- Rodrıguez, Y.; Ballester, A.; Blazquez, M.; González, F.; Munoz, J. New information on the pyrite bioleaching mechanism at low and high temperature. Hydrometallurgy 2003, 71, 37–46. [Google Scholar]
- Sand, W.; Gehrke, T.; Jozsa, P.G.; Schippers, A. (Bio) chemistry of bacterial leaching—Direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Silverman, M.; Ehrlich, H. Microbial formation and degradation of minerals. Adv. Appl. Microbiol. 1964, 6, 153–206. [Google Scholar]
- Tributsch, H. Direct versus indirect bioleaching. Hydrometallurgy 2001, 59, 177–185. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef]
- McKibben, M.; Barnes, H. Oxidation of pyrite in low temperature acidic solutions: Rate laws and surface textures. Geochim. Cosmochim. Acta 1986, 50, 1509–1520. [Google Scholar] [CrossRef]
- Govender, E.; Kotsiopoulos, A.; Bryan, C.; Harrison, S. Modelling microbial transport in simulated low-grade heap bioleaching systems: The hydrodynamic dispersion model. Chem. Eng. Sci. 2017, 172, 545–558. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Sand, W.; Zhang, R. Influence of Sulfobacillus thermosulfidooxidans on initial attachment and pyrite leaching by thermoacidophilic archaeon Acidianus sp. DSM 29099. Minerals 2016, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhu, J.; Li, S.; Zhang, R.; Xiao, T.; Sand, W. Interactions between cells of Sulfobacillus thermosulfidooxidans and Leptospirillum ferriphilum during pyrite bioleaching. Front. Microbiol. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, P.; Smriga, S.; Banfield, J. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 2000, 66, 3842–3849. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hu, M.; Huang, L.; Hua, Z.; Kuang, J.; Li, S.; Shu, W. Comparative metagenomic and metatranscriptomic analyses of microbial communities in acid mine drainage. ISME J. 2015, 9, 1579–1592. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: Significance of microbial interactions. Biotechnol. Bioeng. 2004, 87, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, X.; Ma, L.; Liang, Y.; Niu, J.; Gu, Y.; Zhang, X.; Hao, X.; Dong, W.; She, S. Microbial communities from different subsystems in biological heap leaching system play different roles in iron and sulfur metabolisms. Appl. Microbiol. Biotechnol. 2016, 100, 6871–6880. [Google Scholar] [CrossRef]
- Xian, H.; Zhu, J.; Tan, W.; Tang, H.; Liu, P.; Zhu, R.; Liang, X.; Wei, J.; He, H.; Henry, T. The mechanism of defect induced hydroxylation on pyrite surfaces and implications for hydroxyl radical generation in prebiotic chemistry. Geochim. Cosmochim. Acta 2018, 244, 263–272. [Google Scholar] [CrossRef]
- Caldeira, C.; Ciminelli, V.; Osseoasare, K. The role of carbonate ions in pyrite oxidation in aqueous systems. Geochim. Cosmochim. Acta 2010, 74, 1777–1789. [Google Scholar] [CrossRef]
- Silverman, M. Studies on the chemoautotrophic iron bacterium ferrobacillus ferrooxidans. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Shu, W.; Hallberg, K.; Li, F.; Lan, C.; Zhou, W.; Huang, L. Culturable and molecular phylogenetic diversity of microorganisms in an open-dumped, extremely acidic Pb/Zn mine tailings. Extremophiles 2008, 12, 657. [Google Scholar] [CrossRef]
- Bates, S.; Berglyons, D.; Caporaso, J.; Walters, W.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Xu, Y.; Dong, W.; Liang, Y.; Fan, F.; Zhang, X.; Zhang, X.; Niu, J.; Ma, L.; She, S. The complicated substrates enhance the microbial diversity and zinc leaching efficiency in sphalerite bioleaching system. Appl. Microbiol. Biotechnol. 2015, 99, 10311–10322. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Pena, A.; Goodrich, J.; Gordon, J. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.; Gish, W.; Miller, W.; Myers, E.; Lipman, D. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- McGuire, M.; Edwards, K.; Banfield, J.; Hamers, R. Kinetics, surface chemistry, and structural evolution of microbially mediated sulfide mineral dissolution. Geochim. Cosmochim. Acta 2001, 65, 1243–1258. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, S.; Glenn, A.; Harmer, S.; Bhargava, S.; Chen, M. A direct observation of bacterial coverage and biofilm formation by Acidithiobacillus ferrooxidans on chalcopyrite and pyrite surfaces. Biofouling 2015, 31, 575–586. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 167, 58–65. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.; Gerson, A. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197, 1–32. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [CrossRef] [Green Version]
- Becker, T.; Gorham, N.; Shiers, D.; Watling, H. In situ imaging of Sulfobacillus thermosulfidooxidans on pyrite under conditions of variable pH using tapping mode atomic force microscopy. Process Biochem. 2011, 46, 966–976. [Google Scholar] [CrossRef]
- Bellenberg, S.; Barthen, R.; Boretska, M.; Zhang, R.; Sand, W.; Vera, M. Manipulation of pyrite colonization and leaching by iron-oxidizing Acidithiobacillus species. Process Biochem. 2015, 99, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Fowler, T.; Holmes, P.; Crundwell, F. Mechanism of Pyrite Dissolution in the Presence of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 2987–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michels, M.; Bakker, E.P. Generation of a large, protonophore-sensitive proton motive force and pH difference in the acidophilic bacteria Thermoplasma acidophilum and Bacillus acidocaldarius. J. Bacteriol. 1985, 161, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Peng, Y.; Bradshaw, D. Effect of regrinding conditions on pyrite flotation in the presence of copper ions. Int. J. Miner. Process. 2013, 125, 129–136. [Google Scholar] [CrossRef]
- Nesbitt, H.; Bancroft, G.; Pratt, A.; Scaini, M. Sulfur and iron surface states on fractured pyrite surfaces. Am. Mineral. 1998, 83, 1067–1076. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The surface properties of pyrite coupled with gold in the presence of oxygen. Miner. Eng. 2017, 111, 131–139. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The effect of gold coupling on the surface properties of pyrite in the presence of ferric ions. Appl. Surf. Sci. 2019, 488, 277–283. [Google Scholar] [CrossRef]
- Acres, R.; Harmer, S.; Beattie, D. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite. Int. J. Miner. Process. 2010, 94, 43–51. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y. The effect of regrind mills on the separation of chalcopyrite from pyrite in cleaner flotation. Miner. Eng. 2015, 83, 33–43. [Google Scholar] [CrossRef]
- Schaufuss, A.; Nesbitt, H.; Kartio, I.; Laajalehto, K.; Bancroft, G.; Szargan, R. Reactivity of surface chemical states on fractured pyrite. Surf. Sci. 1998, 411, 321–328. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar]
- Cai, Y.; Pan, Y.; Xue, J.; Sun, Q.; Su, G.; Li, X. Comparative XPS study between experimentally and knaturally weathered pyrites. Appl. Surf. Sci. 2009, 255, 8750–8760. [Google Scholar]
- Niu, X.; Ruan, R.; Tan, Q.; Jia, Y.; Sun, H. Study on the second stage of chalcocite leaching in column with redox potential control and its implications. Hydrometallurgy 2015, 155, 141–152. [Google Scholar]
- Xu, Y.; Schoonen, M.A. The stability of thiosulfate in the presence of pyrite in low-temperature aqueous solutions. Geochim. Cosmochim. Acta 1995, 59, 4605–4622. [Google Scholar]
- Steudel, R.; Holdt, G.; Göbel, T.; Hazeu, W. Chromatographic separation of higher polythionates SnO (n= 3… 22) and their detection in cultures of Thiobacillus ferroxidans; molecular composition of bacterial Sulfur secretions. Angew. Chem. Int. Edit. 1987, 26, 151–153. [Google Scholar]
- De Jong, G.; Hazeu, W.; Bos, P.; Kuenen, J. Polythionate degradation by tetrathionate hydrolase of Thiobacillus ferrooxidans. Microbiology 1997, 143, 499–504. [Google Scholar]
- Mcguire, M.; Jallad, K.; Ben-Amotz, D.; Hamers, R. Chemical mapping of elemental sulfur on pyrite and arsenopyrite surfaces using near-infrared Raman imaging microscopy. Appl. Surf. Sci. 2001, 178, 105–115. [Google Scholar]
- Tu, Z.; Wan, J.; Guo, C.; Fan, C.; Zhang, T.; Lu, G.; Reinfelder, J.; Dang, Z. Electrochemical oxidation of pyrite in pH 2 electrolyte. Electrochim. Acta 2017, 239, 25–35. [Google Scholar]
- Schippers, A.; Rohwerder, T.; Sand, W. Intermediary sulfur compounds in pyrite oxidation: Implications for bioleaching and biodepyritization of coal. Appl. Microbiol. Biotechnol. 1999, 52, 104–110. [Google Scholar]
- Panyushkina, A.; Tsaplina, I.; Kondrat’Eva, T.; Belyi, A.; Bulaev, A. Physiological and Morphological Characteristics of Acidophilic Bacteria Leptospirillum ferriphilum and Acidithiobacillus thiooxidans, Members of a Chemolithotrophic Microbial Consortium. Microbiology 2018, 87, 326–338. [Google Scholar]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.-U.; Golyshin, P.N. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order Thermoplasmatales. Int. J. Syst. Evol. Microbiol. 2016, 66, 332. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.; Lindstrom, E. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology 1994, 140, 3451–3456. [Google Scholar] [PubMed] [Green Version]
- Yelton, A.; Thomas, B.; Simmons, S.; Wilmes, P.; Zemla, A.; Thelen, M.; Justice, N.; Banfield, J. A semi-quantitative, synteny-based method to improve functional predictions for hypothetical and poorly annotated bacterial and archaeal genes. PLoS Comput. Biol. 2011, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.; Pivovarova, T.; Karavaiko, G.; Kondratéva, T.; Moore, E.; Abraham, W.; Lünsdorf, H.; Timmis, K.; Yakimov, M.; Golyshin, P. Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. nov., comprising a distinct lineage of the Archaea. Int. J. Syst. Evol. Microbiol. 2000, 50, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Mutch, L.; Watling, H.; Watkin, E. Microbial population dynamics of inoculated low-grade chalcopyrite bioleaching columns. Hydrometallurgy 2010, 104, 391–398. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Feng, X.; Liang, Y.; Xiao, Y.; Hao, X.; Yin, H.; Liu, H.; Liu, X. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession. Bioresour. Technol. 2017, 223, 121–130. [Google Scholar]
- Dopson, M.; Lindström, E. Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl. Environ. Microbiol. 1999, 65, 36–40. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.; Jia, Y.; Tan, Q.; Sun, H.; Ruan, R. Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials. Minerals 2020, 10, 856. https://doi.org/10.3390/min10100856
Dong B, Jia Y, Tan Q, Sun H, Ruan R. Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials. Minerals. 2020; 10(10):856. https://doi.org/10.3390/min10100856
Chicago/Turabian StyleDong, Bingxu, Yan Jia, Qiaoyi Tan, Heyun Sun, and Renman Ruan. 2020. "Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials" Minerals 10, no. 10: 856. https://doi.org/10.3390/min10100856
APA StyleDong, B., Jia, Y., Tan, Q., Sun, H., & Ruan, R. (2020). Contributions of Microbial “Contact Leaching” to Pyrite Oxidation under Different Controlled Redox Potentials. Minerals, 10(10), 856. https://doi.org/10.3390/min10100856



