Mineral Inventory of the Algares 30-Level Adit, Aljustrel Mine, Iberian Pyrite Belt, Portugal
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Mineral Inventory of the Algares Adit
4.1.1. Alunite/Jarosite
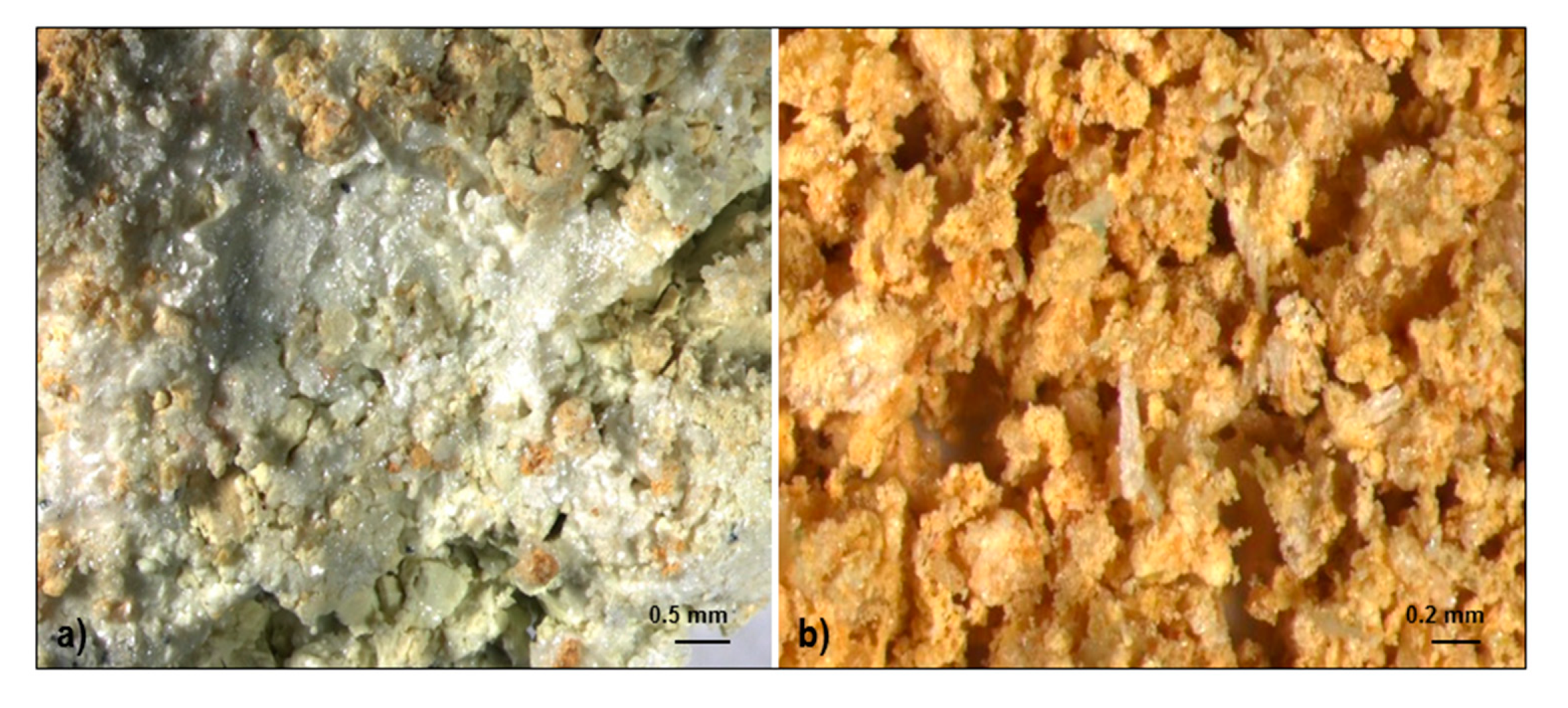
4.1.2. Alunogen
4.1.3. Antlerite
4.1.4. Atacamite
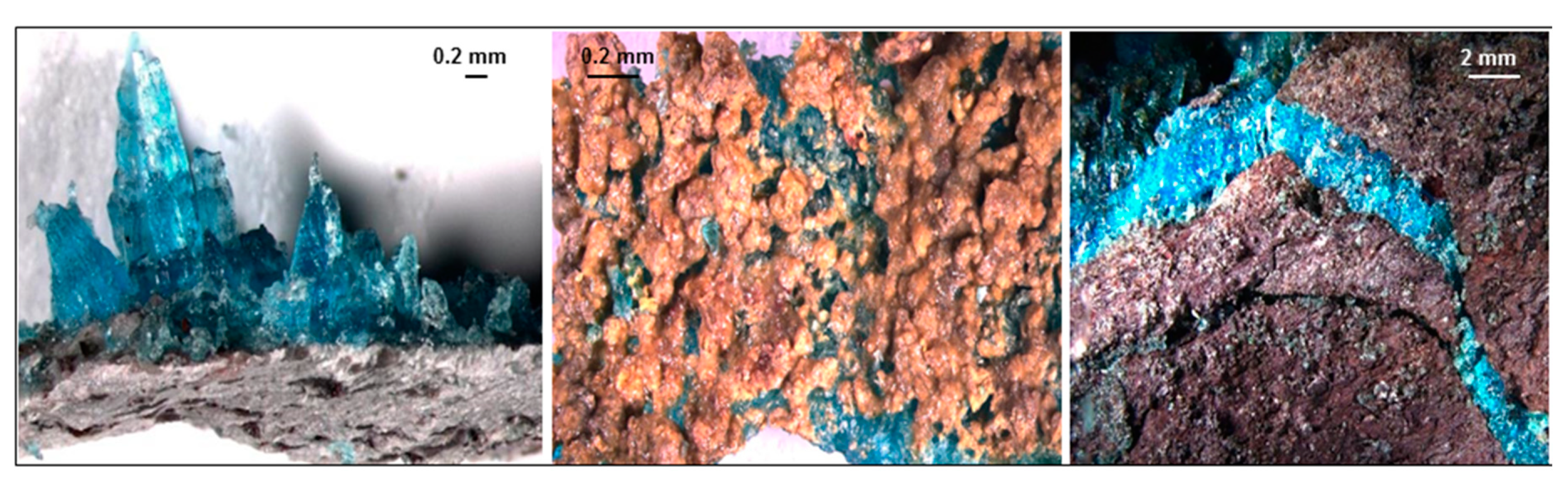
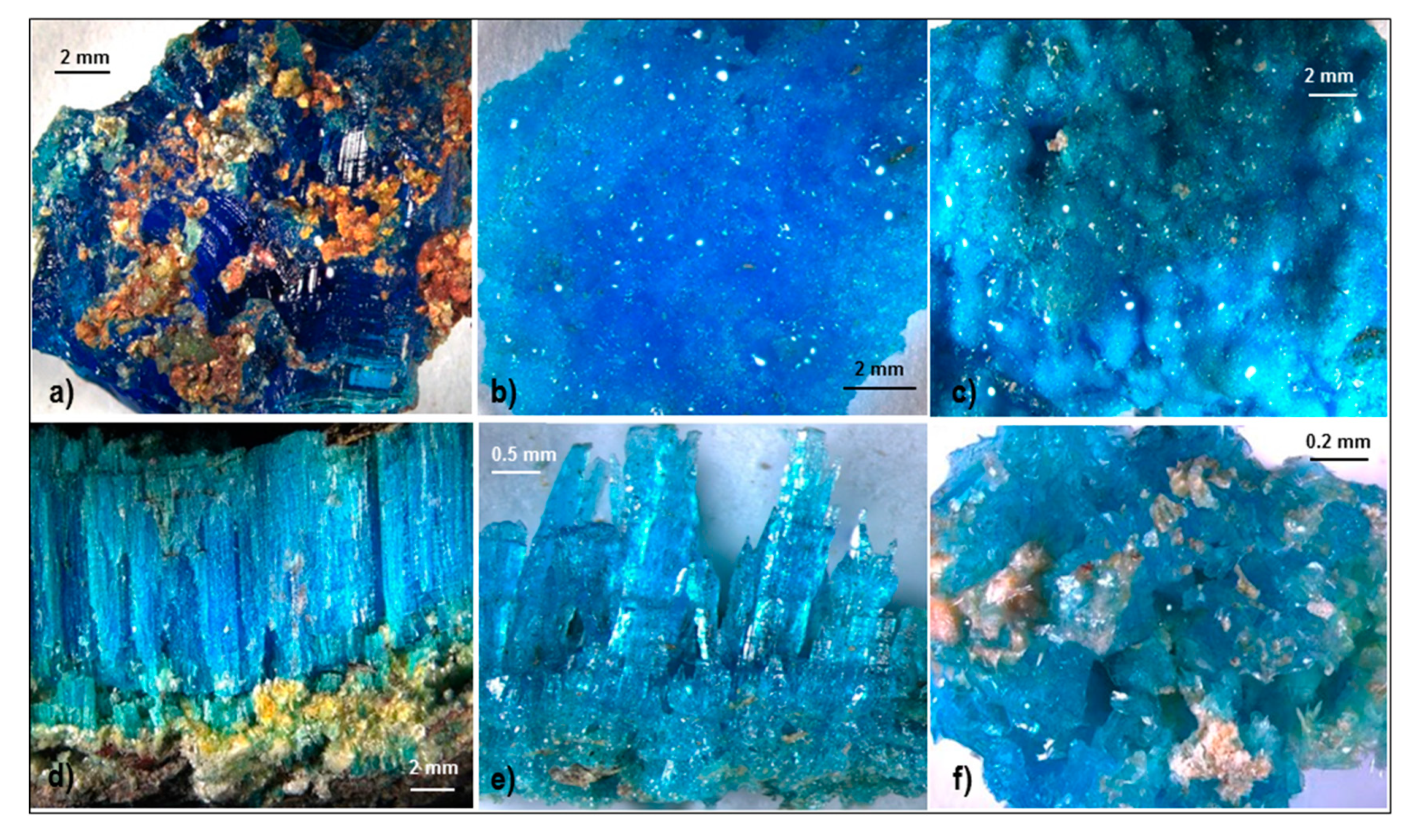
4.1.5. Chalcanthite
4.1.6. Copiapite
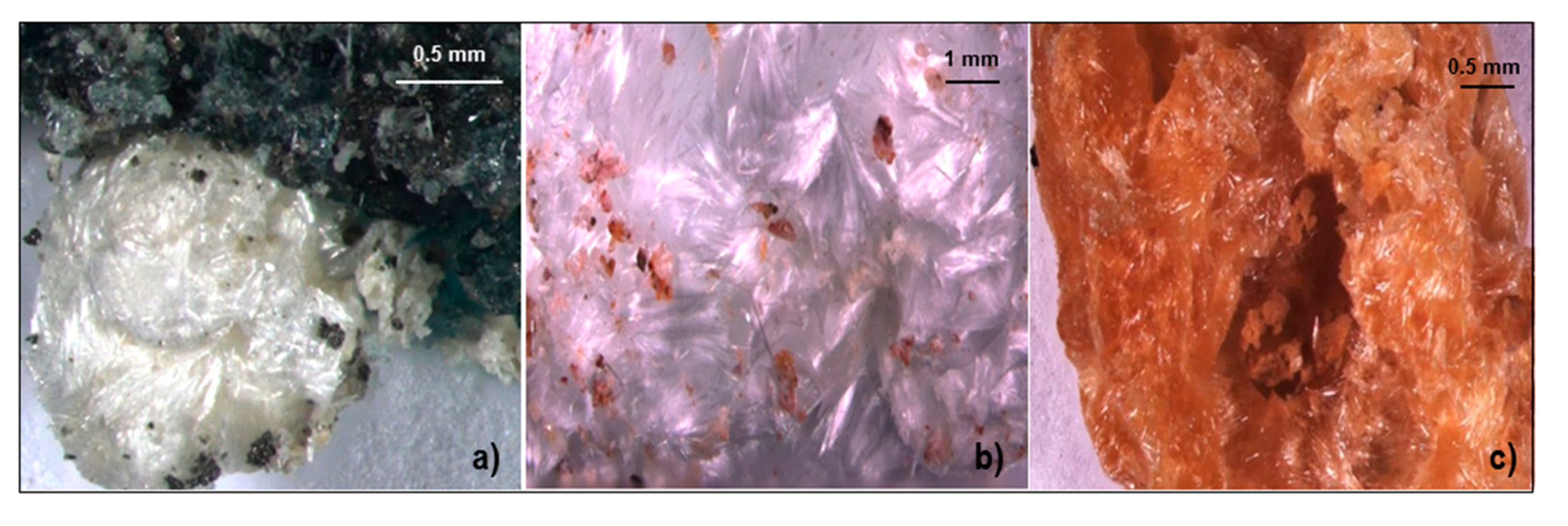
4.1.7. Fibroferrite
4.1.8. Gypsum
4.1.9. Halotrichite/Pickeringite
4.1.10. Melanterite
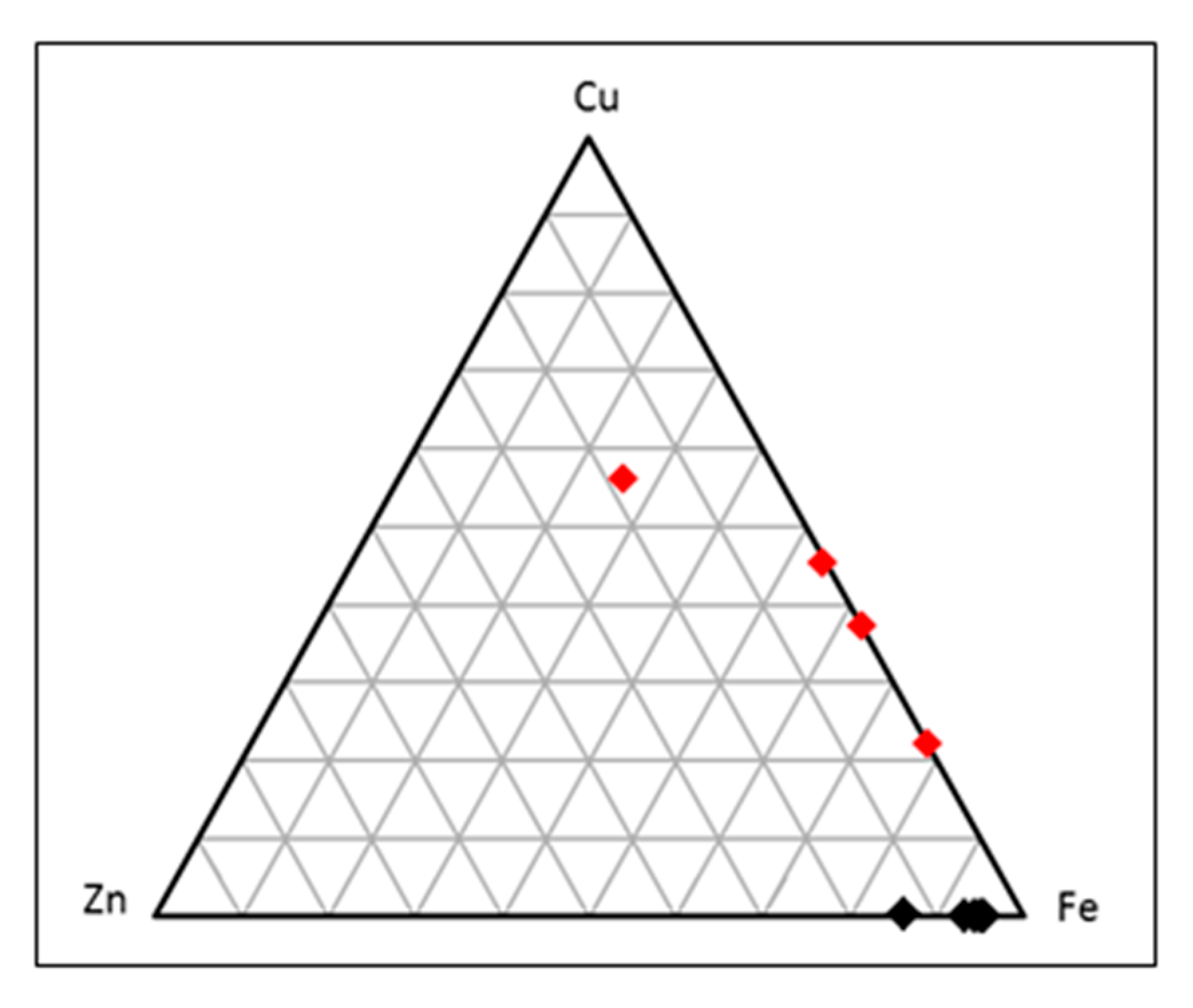
5. Discussion
Chemistry of Sulphate Minerals
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barriga, F.J.A.S.; Carvalho, D.; Ribeiro, A. Introduction to the Iberian Pyrite Belt. In Geology and VMS Deposits of the Iberian Pyrite Belt; Society of Economic Geologists: Littleton, CO, USA, 1997; Volume 27, pp. 1–20. [Google Scholar]
- Almodóvar, G.R.; Yesares, L.; Sáez, R.; Toscano, M.; González, F.; Pons, J.M. Massive sulfide ores in the Iberian Pyrite Belt: Mineralogical and textural evolution. Minerals 2019, 9, 653. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.X.; Martins, A.; Rego, M.; Mateus, A.; Pinto, A.; Figueiras, J.; Silva, E. Roman Slag Distribution in the Portuguese Sector of the Iberia Pyrite Belt. In Actas of the “V Congreso Internacional sobre Minería y Metalurgia Históricas en el Suroeste Europeo (León 2008)”; Mata-Perelló, J.M., Iabat, L.T., Prieto, M.N.F., Eds.; SEDPGYM: Madrid, Spain, 2011; pp. 567–576. [Google Scholar]
- Matos, J.X.; Martins, L.P.; Oliveira, J.T.; Pereira, Z.; Batista, M.J.; Quental, L. Pyrite Route in the Portuguese Sector of the Iberian Pyrite Belt, Challenges for a Sustained Development of Geological Tourism and Mining. In Rutas Minerales en Iberoamérica; Carrion, P., Ed.; Escuela Superior Politécnica del Litoral: Guayaquil, Equador, 2008; pp. 136–155. (In Portuguese) [Google Scholar]
- Matos, J.X. Expansion and Development of the Pyrite Route through the inclusion of the Geological Gardens of Algares and Lousal, Iberian Pyirite Belt, Portugal. In Rutas Minerales en Iberoamérica; Carrion, P., Ed.; Escuela Superior Politécnica del Litoral: Guayaquil, Equador, 2009; pp. 113–121. (In Portuguese) [Google Scholar]
- Andrade, R.F. The mine of Aljustrel. Bol. Minas 1967, 4, 73–90. (In Portuguese) [Google Scholar]
- Andrade, R.; Schermerhorn, L. Aljustrel and Gavião. In Livro Guia da Excursão 4. I Congresso Hipano-Luso-Americano de Geologia Económica; Carvalho, D., Goinhas, J., Schermerhorn, L.J.S., Eds.; Direcção-Geral de Minas e Serviços Geológicos: Lisbon, Portugal, 1971; pp. 32–59. [Google Scholar]
- Matos, J.X.; Martins, L.P. Environmental rehabilitation of mining areas in the Portuguese sector of the Iberian Pyrite Belt: State of the art and future perspectives. Boletín Geológico Min. 2006, 117, 289–304. (In Portuguese) [Google Scholar]
- Matos, J.X.; Barriga, F.; Oliveira, V. Alunite veins versus supergene kaolinite-halloysite alteration in the Lagoa Salgada, Algares and S. João, Aljustrel, and S. Domingos massive sulphide deposits, Iberian Pyrite Belt, Portugal. In Proceedings of the VI Congresso Nacional de Geologia, Caparica, Portugal, 4–6 June 2003; pp. B56–B59. [Google Scholar]
- Matos, J.X.; Barriga, F.J.A.S.; Relvas, J.M.R.S. Acid Sulphate Alteration in the Iberian Pyrite Belt. In Proceedings of the 14th SGA Biennial Meeting, Mineral Resources to Discover, Quebec City, QC, Canada, 20–23 August 2017; Volume II, pp. 625–628. [Google Scholar]
- Matos, J.X.; Barriga, F.J.A.S.; Relvas, J.M.R.S. Acid Sulphate Alteration at the Lagoa Salgada, Aljustrel and São Domingos VMS Deposits, Iberian Pyrite Belt, Portugal: Mineral Exploration Guidelines. Geophys. Res. Abstr. 2019, 21, EGU2019-9641-1. [Google Scholar]
- Brilha, J.; Andrade, C.; Azerêdo, A.; Barriga, F.J.A.S.; Cachão, M.; Couto, H.; Cunha, P.P.; Crispim, J.A.; Dantas, P.; Duarte, L.V.; et al. Definition of the Portuguese frameworks with international relevance as an input for the European geological heritage characterization. Episodes 2005, 28, 177–186. [Google Scholar] [CrossRef]
- Matos, J.X.; Pereira, Z. The LNEG ATLANTERRA South Portuguese Zone Geosite characterization Program. In Proceedings of the 11th European Geoparks Conference, Arouca, Portugal, 19–21 September 2012; Sá, A., Rocha, D., Paz, A., Correia, V., Eds.; AGA—Associação Geoparque Arouca: Arouca, Portugal, 2012; pp. 189–190. [Google Scholar]
- Silva, T.P.; Matos, J.X.; De Oliveira, D.; Morais, I.; Gonçalves, P.; Albardeiro, L.; Veiga, J.P. Mineralogical Characterization of the Algares 30-level Adit for Cultural Tourism, Aljustrel Mine, Iberian Pyrite Belt. In Book of Abstracts of “Materiais 2019”; Rectorate of the NOVA University of Lisbon: Lisbon, Portugal, 2019; p. 353. [Google Scholar]
- Leitão, J. Geology of the Aljustrel Massive Sulfides Deposits. Seg Guideb. Ser. V 1997, 27, 82–97. [Google Scholar]
- Leitão, J. Geology of the Massive Sulphide Deposits of Aljustrel. In Livro Guia das Excursões do V Congresso Nacional de Geologia; IGM-Instituto Geológico e Mineiro: Lisbon, Portugal, 1998; pp. 91–100. (In Portuguese) [Google Scholar]
- Luís, A.T.; Durães, N.; Silva, E.F.; Ribeiro, S.; Silva, A.J.F.; Patinha, C.; Almeida, S.F.P.; Azevedo, M.R. Tracking multiple Sr sources through variations in 87Sr/86Sr ratios of surface waters from the Aljustrel massive sulphide mining area: Geological versus anthropogenic inputs. Appl. Geochem. 2019, 102, 108–120. [Google Scholar] [CrossRef]
- Gaspar, O.C. Microscopy and petrology of ores applied to the genesis, exploration and mineralogy of the massive sulphides of the Aljustrel and Neves-Corvo deposits. Estud. Notas E Trab. Inst. Geológico E Min. 1996, 38, 3–195. (In Portuguese) [Google Scholar]
- Schermerhorn, L.; Zbyzewski, G.; Ferreira, V. Notícia Explicativa da Carta Geológica 42D Aljustrel; SGP: Lisbon, Portugal, 1987; p. 55. [Google Scholar]
- Rosa, D.; Finch, A.; Andersen, T.; Inverno, C. U–Pb geochronology and Hf isotope ratios of magmatic zircons from the Iberian Pyrite Belt. Min. Pet. 2009, 95, 47–69. [Google Scholar] [CrossRef]
- Matos, J.X.; Pereira, Z.; Fernandes, P.; Rosa, D.; Oliveira, J.T. Contribution to the understanding of the structure of Aljustrel mine Iberian Pyrite Belt, based in new palynostratigraphical data obtained in the Volcano-Sedimentary Complex and Mértola Formation. VIII Congr. Nac. De Geol. E Terra 2010, 21, 1–4. (In Portuguese) [Google Scholar]
- Hammarstrom, J.M.; Seal, R.R., II; Meier, A.L.; Kornfeld, J.M. Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef] [Green Version]
- Stoffregen, R.E.; Alpers, C.N.; Jambor, J.L. Alunite-Jarosite Crystallography, Thermodynamics, and Geochronology. In Reviews in Mineralogy and Geochemistry, Sulfate Minerals-Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2000; Volume 40, pp. 453–479. [Google Scholar]
- Fang, J.H.; Robinson, P.D. Alunogen, Al2(H2O)12(SO4)3.5H2O; its atomic arrangement and water content. Am. Min. 1976, 61, 311–317. [Google Scholar]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Kiadó, F., Ed.; Geological Institute of Hungary: Budapest, Hungary, 2011. [Google Scholar]
- Palache, C.; Berman, H.; Frondel, C. Dana’s System of Mineralogy, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1951; Volume II. [Google Scholar]
- Lima-de-Faria, J. Structural Mineralogy—An Introduction; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; p. 317. [Google Scholar]
- Scordari, F. Fibroferrite: A mineral with a {Fe(OH)(H2O)2SO4} spiral chain and its relationship to Fe(OH)SO4, butlerite and parabutlerite. TMPM Tschermaks Petr. Mitt. 1981, 28, 17–29. [Google Scholar] [CrossRef]
- Menchetti, S.; Sabelli, C. The halotrichite group: The crystal structure of apjohnite. Min. Mag. 1976, 40, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Parafiniuk, J.; Siuda, R.; Borkowski, A. Sulphate and arsenate minerals as environmental indicators in the weathering zones of selected ore deposits, Western Sudetes, Poland. Acta Geol. Pol. 2016, 66, 493–508. [Google Scholar] [CrossRef]
- Peterson, R.C. The relationship between Cu content and distortion in the atomic structure of melanterite from the Richmond mine, Iron Mountain, California. Can. Mineral. 2003, 41, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Mauro, D.; Biagioni, C.; Pasero, M. Crystal-chemistry of sulfates from Apuan Alps (Tuscany, Italy). I. Crystal structure and hydrogen bond system of melanterite, Fe(H2O)6(SO4)·H2O. Periodico Mineralogia 2018, 87, 89–96. [Google Scholar] [CrossRef]
- Baur, W.H. On the crystal chemistry of salt hydrates. III. The determination of the crystal structure of FeSO4·7H2O (melanterite). Acta Crystallogr. 1964, 17, 1167–1174. [Google Scholar] [CrossRef]
- Município de Aljustrel. History of Mining, Câmara Municipal de Aljustrel. Available online: http://www.mun-aljustrel.pt/menu/114/historiada-mineracao.aspx (accessed on 23 April 2020). (In Portuguese).
- Cruz, A.J. The Provenance of the Pigments used in Painting in Portugal Before the Invention of Paint Tubes: Problems and Perspectives. In As Preparações na Pintura Portuguesa. Séculos XV e XVI; Serrão, V., Antunes, V., Seruya, A.I., Eds.; Faculty of Letters/Lisbon University: Lisbon, Portugal, 2013; (In Portuguese). [Google Scholar] [CrossRef]
- Gil, M.; Seruya, A.I.; Aguiar, J.; Candeias, A.; Frade, J.C.; Valadas, S.; Alves, P.; Ribeiro, I. Colored limewash paintings in Alentejo (Part 1): Pigments’ identification and stratigraphic analysis (in 2004–2006). Conservar Património 2009, 10, 19–38. (In Portuguese) [Google Scholar] [CrossRef]
- Rebollar, M.C.; Díaz, F.G.; Oliá, J.V. Mineralogy of the Iberian Pyrite Belt. Bocamina 1999, 4, 50–85. (In Spanish) [Google Scholar]
- Buckby, T.; Black, S.; Coleman, M.L.; Hodson, M.E. Fe-sulphate-rich evaporative mineral precipitates from the Rio Tinto, southwest Spain. Min. Mag. 2003, 67, 263–278. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Intern. 2007, 33, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-España, J. Acid mine drainage in the Iberian Pyrite Belt: An overview with special emphasis on generation mechanisms, aqueous composition and associated mineral phases. Macla 2008, 10, 34–43. [Google Scholar]
- Abreu, M.M.; Batista, M.J.; Magalhães, M.C.F.; Matos, J.X. Acid Mine Drainage in the Portuguese Iberian Pyrite Belt. In Mine Drainage and Related Problems; Robinson, B.C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 71–118. [Google Scholar]
- Gallo, A.A. Mineralogía y Geoquímica de Sulfatos Secundarios en Ambientes de Drenaje Ácido de Mina. Área Minera del Yacimiento de San Miguel (Faja Pirítica Ibérica). Ph.D. Thesis, Universidad del País Vasco, Leioa, Spain, October 2010; p. 273. [Google Scholar]
- Bobos, I.; Durães, N.; Noronha, F. Mineralogy and geochemistry of mill tailings impoundments from Algares (Aljustrel), Portugal: Implications for acid sulfate mine waters formation. J. Geochem. Explor. 2006, 88, 1–5. [Google Scholar] [CrossRef]
- Alpers, C.N.; Nordstrom, D.K.; Thompson, J.M. Seasonal Variations of Zn/Cu Ratios in Acid Mine Water from Iron Mountain, California. In Environmental Geochemistry of Sulfide Oxidation; ACS Symposium Series 550; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 324–344. [Google Scholar]
- De Waele, J.; Forti, P. Mineralogy of Mine Caves in Sardinia (Italy). In Proceedings of the 14th International Congress of Speleology, Kalamos, Greece, 21–28 August 2005; pp. 306–311. [Google Scholar]
- Verhaert, M.; Bernard, A.; Saddiqi, O.; Dekoninck, A.; Essalhi, M.; Yans, J. Mineralogy and genesis of the polymetallic and polyphased low grade Fe-Mn-Cu ore of Jbel Rhals deposit (Eastern High Atlas, Morocco). Minerals 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Parafiniuk, J. Sulfate minerals and their origin in the weathering zone of the pyrite-bearing schists at Wiesciszowice (Rudawy Janowickie Mts, Western Sudetes). Acta Geol. Pol. 1996, 46, 353–414. [Google Scholar]
- Bigham, J.M.; Nordstrom, D.K. Iron and Aluminium Hydroxysulfates from Acid Sulfate Waters. In Reviews in Mineralogy and Geochemistry, Sulfate Minerals-Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2000; Volume 40, pp. 351–403. [Google Scholar]
- Borek, S.L. Effect of Humidity on Pyrite Oxidation. In Environmental Geochemistry of Sulfide Oxidation; ACS Symposium Series 550; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society: Washington, DC, USA, 1993; pp. 31–44. [Google Scholar]
- Frau, F. The formation-dissolution-precipitation cycle of melanterite at the abandoned pyrite mine of Genna Luas in Sardinia, Italy: Environmental implications. Min. Mag. 2000, 64, 995–1006. [Google Scholar] [CrossRef]
- Fanfani, L.; Nunzi, A.; Zanazzi, P.F.; Zanzari, A.R. The copiapite problem: The crystal structure of a ferrian copiapite. Am. Min. 1973, 58, 314–322. [Google Scholar]
- Parafiniuk, J. Fibroferrite, slavikite and pickeringite from the oxidation zone of pyrite-bearing schists in Wiesciszowice (Lower Silesia). Min. Pol. 1991, 22, 3–15. [Google Scholar]
- Valente, T.; Grande, J.A.; Torre, M.L. Magnesium and Aluminium Sulfates in Salt Efflorescences from Acid Mine Drainage in the Iberian Pyrite Belt (SW Spain). In Proceedings of the IMWA 2016, Leipzig, Germany, 11–15 July 2016; Drebenstedt, C., Paul, M., Eds.; Technische Universität Bergakademie Freiberg: Freiberg, Germany, 2016; pp. 445–450. [Google Scholar]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A. The stabilities of antlerite and Cu3SO4(OH)4·2H2O: Their formation and relationships to other copper(II) sulfate minerals. Min. Mag. 1992, 56, 359–365. [Google Scholar] [CrossRef]
- Fleet, M.E. The crystal structure of paratacamite, Cu2(OH)3Cl. Acta Cryst. 1975, B31, 183–187. [Google Scholar] [CrossRef]
- Cameron, E.M.; Leybourne, M.I.; Palacios, C. Atacamite in the oxide zone of copper deposits in northern Chile: Involvement of deep formation waters? Min. Depos. 2007, 42, 205–218. [Google Scholar] [CrossRef]
- Reich, M.; Palacios, C.; Parada, M.A.; Fehn, U.; Cameron, E.M.; Leybourne, M.I.; Zúñiga, A. Atacamite formation by deep saline waters in copper deposits from the Atacama Desert, Chile: Evidence from fluid inclusions, groundwater geochemistry, TEM, and 36Cl data. Min. Depos. 2008, 43, 663–675. [Google Scholar] [CrossRef]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate Salts from Sulfide Mineral Oxidation. In Reviews in Mineralogy and Geochemistry, Sulfate Minerals-Crystallography, Geochemistry, and Environmental Significance; Alpers, C.N., Jambor, J.L., Nordstrom, D.K., Eds.; Mineralogical Society of America: Chantilly, VA, USA; Volume 40, pp. 303–350.









| Name | Chemical Formula | Habit | Colour | JCPDF Card Number |
|---|---|---|---|---|
| Alunite | (K,Na)Al3(SO4)2(OH)6 | massive cryptocrystalline aggregates | yellowish | 14–136 |
| Alunogen (s) | Al2(SO4)3·17H2O | massive macrocrystalline aggregates | white | 26–1010 |
| Antlerite (i) | Cu3(SO4)(OH)4 | massive aggregates | pale green | 7–407 |
| Atacamite | Cu2Cl(OH)3 | massive | dark green | 25–269 |
| Chalcanthite (s) | CuSO4·5H2O | massive, crystalline, fibrous columnar, crusts | deep or light blue | 11–646 |
| Chalcopyrite | CuFeS2 | - | - | 37–471 |
| Chlorite/Clinochlore | (Mg,Fe)5Al2Si3O10(OH)8 | massive | grey | 24–506 |
| Copiapite (s) | Fe2+Fe3+4(SO4)6(OH)2·20H2O | macro crystalline aggregates | deep yellow | 35–583 |
| Fibroferrite | Fe3+(OH)SO4·5H2O | acicular crystals | whitish | 83–1803 |
| Gypsum (i) | CaSO4·2H2O | radial aggregates of crystals | white, greyish | 33–311 |
| Halotrichite (s) | FeAl2(SO4)4·22H2O | aggregates of acicular crystals | white | 39–1387 |
| Hematite | α-Fe2O3 | massive | reddish | 33–664 |
| Jarosite (i) | KFe3(SO4)2(OH)6 | massive aggregates | brownish | 22–827 |
| Kaolinite | Al2Si2O5(OH)4 | massive | yellowish | 75–938 |
| Melanterite (s) | FeSO4·7H2O | massive, crystalline, fibrous columnar, crusts | greenish, yellow, bluish | 22–633 |
| Mica group minerals | (K,H3O)Al2Si3AlO10(OH)2 | - | - | 26–911 |
| Pyrite | FeS2 | - | - | 42–1340 |
| Quartz | α-SiO2 | crystalline | white | 46–1045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.P.; Matos, J.X.; De Oliveira, D.; Veiga, J.P.; Morais, I.; Gonçalves, P.; Albardeiro, L. Mineral Inventory of the Algares 30-Level Adit, Aljustrel Mine, Iberian Pyrite Belt, Portugal. Minerals 2020, 10, 853. https://doi.org/10.3390/min10100853
Silva TP, Matos JX, De Oliveira D, Veiga JP, Morais I, Gonçalves P, Albardeiro L. Mineral Inventory of the Algares 30-Level Adit, Aljustrel Mine, Iberian Pyrite Belt, Portugal. Minerals. 2020; 10(10):853. https://doi.org/10.3390/min10100853
Chicago/Turabian StyleSilva, Teresa P., João X. Matos, Daniel De Oliveira, João P. Veiga, Igor Morais, Pedro Gonçalves, and Luís Albardeiro. 2020. "Mineral Inventory of the Algares 30-Level Adit, Aljustrel Mine, Iberian Pyrite Belt, Portugal" Minerals 10, no. 10: 853. https://doi.org/10.3390/min10100853
APA StyleSilva, T. P., Matos, J. X., De Oliveira, D., Veiga, J. P., Morais, I., Gonçalves, P., & Albardeiro, L. (2020). Mineral Inventory of the Algares 30-Level Adit, Aljustrel Mine, Iberian Pyrite Belt, Portugal. Minerals, 10(10), 853. https://doi.org/10.3390/min10100853









