Abstract
The structure of hydrous amorphous SiO2 is fundamental in order to investigate the effects of water on the physicochemical properties of oxide glasses and magma. The hydrous SiO2 glass with 13 wt.% D2O was synthesized under high-pressure and high-temperature conditions and its structure was investigated by small angle X-ray scattering, X-ray diffraction, and neutron diffraction experiments at pressures of up to 10 GPa and room temperature. This hydrous glass is separated into two phases: a major phase rich in SiO2 and a minor phase rich in D2O molecules distributed as small domains with dimensions of less than 100 Å. Medium-range order of the hydrous glass shrinks compared to the anhydrous SiO2 glass by disruption of SiO4 linkage due to the formation of Si–OD deuterioxyl, while the response of its structure to pressure is almost the same as that of the anhydrous SiO2 glass. Most of D2O molecules are in the small domains and hardly penetrate into the void space in the ring consisting of SiO4 tetrahedra.
1. Introduction
Silica glass is the most fundamental, fully polymerized network glass whose structure and properties under pressure have long been of interest as an important analog material of silicate magma [1,2,3,4,5,6,7,8,9]. For the structural aspect, SiO2 glass is densified by compression with the change in medium-range order in the pressure region up to about 10 GPa [2,4,8]. Namely, reduction of the Si–O–Si bond angle between SiO4 tetrahedra shrinks the ring and squeezes the interstitial void, leading to permanent densification of SiO2 glass. SiO2 glass becomes fully densified glass by room temperature compression to the pressure of 9 to 13 GPa, of which the density is about 20% higher than ordinary SiO2 glass at ambient conditions [8].
Water in silicate glass has a large effect on the structure as well as on the properties such as viscosity and glass transition temperature [10,11]. Water can react with SiO2 glass to form hydroxyl species (Si–OH), disrupting the linkage of SiO4 tetrahedra. As a result, the medium-range order of the SiO2 glass is also affected by the addition of water in which the size of the ring of the SiO2 glass containing water becomes smaller than that of ordinary SiO2 glass [12]. The molecular water, as well as the hydroxyl, is also present in the silicate glasses. It is known that the molecular water becomes the dominant water species in the silicate glasses with increasing total water content [10,11]. However, it is not yet well understood how the molecular water is incorporated into the silicate glasses.
In this study, the hydrous SiO2 glass containing 13 wt.% of D2O was synthesized under high-pressure and high-temperature conditions and small angle X-ray scattering (SAXS), X-ray diffraction (XRD), and neutron diffraction (ND) have been applied in order to investigate the short-range order, medium-range order, and phase separation of this hydrous glass. In addition to this, in-situ high-pressure XRD and ND measurements of this hydrous glass have been conducted up to about 10 GPa to clarify the pressure response of its structure. Effects of the pressure and the water dissolution on the medium-range order of the SiO2 glass and the state of molecular water in the SiO2 glass are also discussed.
2. Hydrous SiO2 Glass Sample
The glass sample with a composition of SiO2–13wt.% D2O was synthesized using a Kawai-type high-pressure apparatus driven by the 3000 ton press installed at GRC, Ehime University. Powdered quartz enclosed in a Pt capsule together with 13 wt.% D2O was melted at 3 GPa and 1873 K for 30 min, then it was quenched to room temperature by turning off the electric power supply. The quenching rate was approximately 1000 K/s. Subsequently, the applied pressure was released slowly in several hours, and then the sample was recovered in ambient conditions (Figures S1 and S2). Since we have not measured the content of heavy water in SiO2 glass, the 13 wt.% D2O content is a nominal value. The recovered glass is optically clear and homogeneous. Density of this hydrous SiO2 glass was determined to be 2.239 g/cm3 by Archimedes’ method. This value is slightly higher than the density of ordinary SiO2 glass (2.20 g/cm3), although it contains a large amount of heavy water. This means that this high-pressure hydrous SiO2 glass is an intermediately densified glass.
Raman spectroscopy analysis has been applied to clarify the species of water dissolved in the SiO2 glass. Raman spectrum shows that the hydrous SiO2 glass has some additional peaks except those assigned to silica glass (Figure 1). Those are a sharp peak around 960 cm−1 and a broad peak from 2100 to 2750 cm−1. The former is assigned to the Si–OD stretching vibrations and the latter to OD stretching vibrations in D2O molecules and Si–OD groups [13,14]. Heavy water in the hydrous SiO2 glass is thought to take two states, OD deuterioxyl and D2O molecule. In general, the amount of OH hydroxyls dissolved in the silicate glass increases with the total H2O content, but reaches a limit of about 3 wt.% [10,11]. Thus, this hydrous SiO2 glass may contain about 10 wt.% of the heavy water as molecular species.
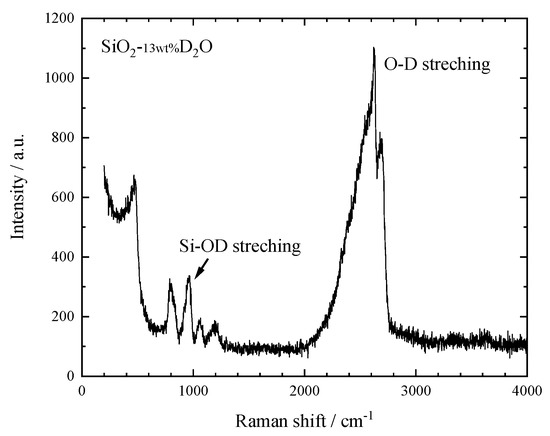
Figure 1.
Raman spectrum of the hydrous SiO2 glass at ambient conditions. Spectrum is unpolarized.
3. Experiments
3.1. Small Angle X-ray Scattering at Ambient Conditions
SAXS experiments were performed at the BL18C of the Photon Factory (PF) of the High Energy Accelerator Research Organization (KEK), Japan. Incident X-rays were monochromatic X-rays of 15.28 keV with a beam diameter of 35 micrometers, and scattered X-rays were detected in Q-range from 0.02 to 4.0 Å−1 with an image plate. The detail of experimental setup is described elsewhere [15].
3.2. Angle-Dispersive X-ray Diffraction at Ambient Conditions
Angle-dispersive XRD experiment at ambient conditions was carried out using two-axis diffractometer at the BL04B2 beamline of SPring-8, Japan. We acquired diffraction data up to Q = 25 Å−1 by angle dispersive method using the monochromatic X-rays of 61.37 keV. The detail of the experimental setup is described elsewhere [16,17].
3.3. High-Pressure X-ray Diffraction
XRD experiments on the hydrous glass during compression were performed using the DIA-type cubic press MAX80 installed at the AR-NE5C beamline of the PF. Tungsten carbide anvils and a boron-epoxy pressure transmitting medium were used. A powdered sample was pressed into a pellet with a diameter of 2 mm and enclosed in a boron nitride (BN) capsule. Sample pressure was determined from the volume of the NaCl pressure marker [18]. XRD profiles were acquired by an energy-dispersive method in a transmitting geometry with an intrinsic Ge detector at room temperature and pressures of up to 9.6 GPa. White X-rays up to 120 keV were used and the data was collected at 11 fixed angles ranging from 3° to 30° to cover a wide Q range. Background intensities were also measured at each angle using an empty cell.
3.4. High-Pressure Neutron Diffraction
ND experiments of the hydrous SiO2 glass under pressure were conducted by the time-of-flight (TOF) method combined with a multi-anvil press ATSUHIME [19] at the PLANET beamline [20] of the spallation neutron source of the Materials and Life Science Experimental Facility (MLF) at the J-PARC, Japan. A multi-anvil 6-6 type high-pressure apparatus was used [21]. The second-stage Ni-bound WC anvils were used. Clumps of SiO2–13wt.%D2O glasses were directly packed in the ZrO2 pressure medium. The size of the hydrous SiO2 glass sample was 4.7 mm in diameter and 6.7 mm in height. Sample pressures were calculated on the basis of the pressure-load calibration curves which were determined in separated runs beforehand. The diffracted neutrons were detected by a pair of 90° detector banks consisting of 3He position sensitive detectors equipped with receiving radial collimators. The data were acquired for 15–33 h at the proton beam power of 300 kW. ND profiles were acquired at room temperature and with the same pressure conditions as those of XRD experiments. Diffraction profiles of a vanadium pellet in a high-pressure cell and an empty cell were also acquired for the correction of scattering intensity.
4. Results and Discussion
4.1. Phase Separation of Hydrous SiO2 Glass
It is known that the silicate glass with high water content undergoes the glass-in-glass phase separation at a low temperature [11]. Due to the limitation of mutual solubility, it separates into a silica-rich and a water-rich phase. We performed the SAXS measurements on several parts of the hydrous SiO2 glass to clarify the possible phase separation. The hydrous SiO2 glass clearly shows significant scattering intensity compared with anhydrous glass (Figure 2). The scattering intensity, however, shows a difference depending on location; some regions have strong scattering intensity, while others only show very weak scattering intensity. This indicates that the distribution of scattering entities in the hydrous glass is heterogeneous in the dimension on the order of from μm to mm. The SAXS patterns of the hydrous glass has a broad peak at Q = 0.05–0.1 Å−1, which indicates the existence of an average distance frequently realized between neighboring scattering entities. The average distance is estimated to be about 100 Å from the length scale (2π/Q) corresponding to the position of the peaks. It is, therefore, considered that the size of the scattering entities is less than 100 Å. The similar SAXS pattern was observed in hydrated Na-silicate glass, and it was interpreted by the glass-in-glass phase separation [11]. In our hydrous SiO2 glass, similar phase separation may occur. When combined with the results of the XRD and ND described below, the most likely candidate for this scattering entities in hydrous SiO2 glass is a phase rich in molecular D2O. Although this hydrous glass is optically homogeneous, it is considered to be the mixture of SiO2-rich glass part and D2O-rich domain with the dimensions of less than 100 Å.
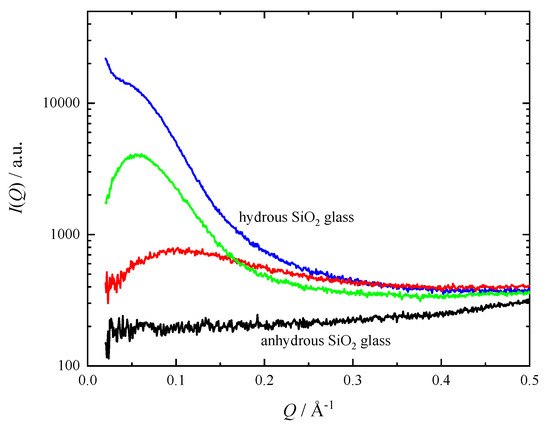
Figure 2.
Small angle X-ray scattering intensity from the hydrous SiO2 glass at ambient conditions. Scattering intensity from anhydrous SiO2 glass (black curve) is also shown for comparison. Three curves were observed at locations from a few hundred micrometers to a millimeter apart.
4.2. Comparison with Dry SiO2 Glass at Ambient Conditions
We compare the structure of the hydrous glass and the anhydrous glass at atmospheric pressure using the results of XRD and ND. The structure factors S(Q) and the total correlation functions T(r) for the hydrous and the anhydrous glass are shown in Figure 3. The S(Q) and T(r) for the anhydrous SiO2 glass were reported by Kohara et al. [22] for X-ray and Hannon [23] for neutron. Here, we consider what the S(Q) and the T(r) of the hydrous SiO2 glass represent. XRD and ND measurements were performed on the bulk hydrous SiO2 glass. As shown in the results of the SAXS measurements, the hydrous glass is separated into two phases. Therefore, the S(Q) and T(r) obtained from XRD and ND are the sum of contributions from the SiO2-rich glass part and the D2O-rich domains. Hereafter, we discuss the structure of the hydrous SiO2 glass based on this view.
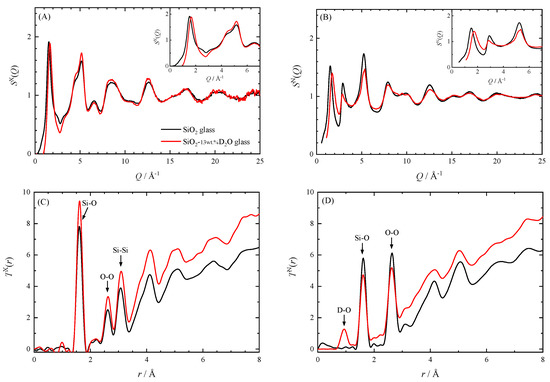
Figure 3.
X-ray and neutron diffraction data of the hydrous SiO2 glass and the anhydrous SiO2 glass at ambient conditions. (A) The X-ray structure factor SX(Q), (B) the neutron structure factor SN(Q), (C) the X-ray total correlation function TX(r), and (D) the neutron total correlation function TN(r).
As discussed below, both S(Q) and T(r) show that the short-range order composed of SiO4 tetrahedra of the hydrous SiO2 glass is almost the same as that of anhydrous glass, while the medium-range order related to the ring of the SiO4 tetrahedra is diminished by addition of water. The first sharp diffraction peak (FSDP) observed at Q ~ 1.5–1.7 Å−1 in both the SX(Q)and SN(Q) of hydrous glass clearly shifts to the higher-Q side than those of anhydrous glass. The second peak is observed at Q = 2.9 Å−1 only in neutron data. In the other peaks, the intensity and the position of SX(Q) are almost the same between the hydrous and the anhydrous glass, whereas the SN(Q) shows a difference. This is due to the very small X-ray atomic scattering factor of D and the relatively large neutron scattering length of D. The similarity of SX(Q) means the Si–O correlation between the hydrous and anhydrous glass is similar. The X-ray total correlation function TX(r) of hydrous glass shows almost the same peaks positions as those of anhydrous glass, but the height of TX(r) of hydrous glass is higher than that of anhydrous glass because of its higher number density. The Si coordination number of the hydrous glass calculated from the area of the first peak at 1.6 Å is about 3.9, which is almost the same as that for the anhydrous glass. On the other hand, the neutron total correlation function TN(r) of hydrous glass has a different shape from that of anhydrous glass because of the presence of peaks due to the OD deuterioxyl and the D2O molecules. The peak at about 0.93 Å for hydrous glass corresponds to the D–O distance. In the TN(r), it is difficult to identify peaks originating from deuterium other than this peak, but the effect of overlapping peaks is recognized. Since the Si-O correlation typically located around 1.6 Å in the TN(r) is superimposed on the intramolecular D–D correlation (1.56 Å) and the intermolecular O–D correlation (1.92 Å) of D2O [24], the coordination number of Si cannot be determined.
FSDP of S(Q) is thought to be related to the formation of medium-range order, although its origin has been still under debate [25,26,27,28,29]. The position of FSDP, Q1, corresponds to the length scale of periodicity in real space l1 (=2π/Q1). Thus, the shift of the FSDP toward high-Q side by dissolution of water means the shrinkage of the medium-range order. This is attributed to the decrease of the size of the SiO4 ring by breaking SiO4 linkages with the OH (OD) group. In ordinary SiO2 glass, the six-membered ring of SiO4 tetrahedra is most frequent followed by the five-membered ring [30], but the population of a smaller ring, such as five- and four-membered rings, may increase by water dissolution. The shift of the FSDP toward high-Q side due to water dissolution has been reported for rhyolitic glasses containing more than 70 wt.% of SiO2 [31]. The displacement of the FSDP of SX(Q) in rhyolitic glass is about 1.5 Å−1 by the 7.5 wt.% dissolution of H2O, which is comparable to that of our hydrous silica glass containing 13 wt.% of D2O.
On the other hand, the FSDP of densified SiO2 glasses is known to be on the high-Q side compared to ordinary SiO2 glass [32]. This is considered to correspond to the shrinkage of the medium-range order, which is related to a reduction of Si–O–Si bond angle associated with the reduction of interstitial voids by compression [4]. Our hydrous SiO2 glass was prepared by quenching from the melt at 3 GPa and is thought to partially retain the structure of intermediately densified glass. Therefore, the position of FSDP in our hydrous glass can be attributed to the effects of both water addition and densification.
As revealed by SAXS, XRD, and ND, the hydrous glass synthesized in this study is a mixture of SiO2-rich glass parts and a D2O-rich domain. By assuming that the structure of each phase is the same as that for pure SiO2 glass and pure liquid D2O, the S(Q) for hydrous glass can be calculated from those for pure phases [22,33,34,35] (the detailed method is described in Appendix B). Comparison of the SX(Q) and the SN(Q) of the hydrous SiO2 with those calculated from the previously reported S(Q) are shown in Figure 4. They showed good agreement except the position of FSDP of the SX(Q) and the SN(Q), and the height of the second peak of the SN(Q). The difference may be attributed to the shrinkage of the medium-range order of the SiO2 glass by dissolution of water.
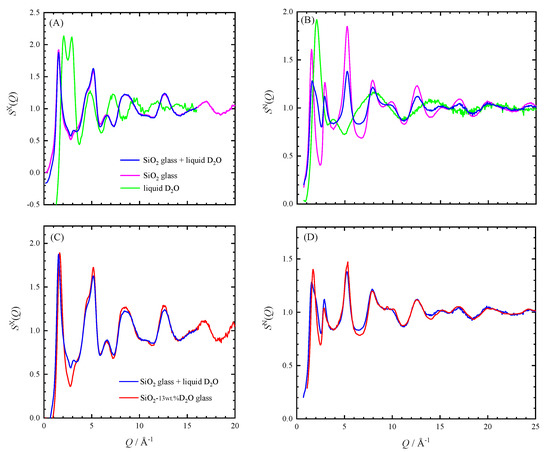
Figure 4.
(A) SX(Q)’s of SiO2 glass and liquid D2O at ambient conditions [22,34], and that simulated for the hydrous glass using them. (B) Corresponding S(Q) for neutron, SN(Q) [33,35]. (C) Comparison of the observed and simulated SX(Q)s for the hydrous SiO2 glass. (D) Comparison of the observed and simulated SN(Q)s.
4.3. Hydrous SiO2 Glass under Pressure
We consider the response of the structure of the hydrous SiO2 glass to pressure. The S(Q) and T(r) for the hydrous glass during compression to about 10 GPa are shown in Figure 5. In XRD, the SX(Q) of hydrous SiO2 glass changes with pressure in the same way as anhydrous SiO2 glass reported by Inamura et al. [4], except for the position of the FSDP. This means that the response to the pressure of the short-range order of the hydrous glass is the same as that of the anhydrous glass. This is consistent with the structure deduced from SAXS showing that the hydrous SiO2 glass is mainly composed of relatively dry parts rich in SiO2.
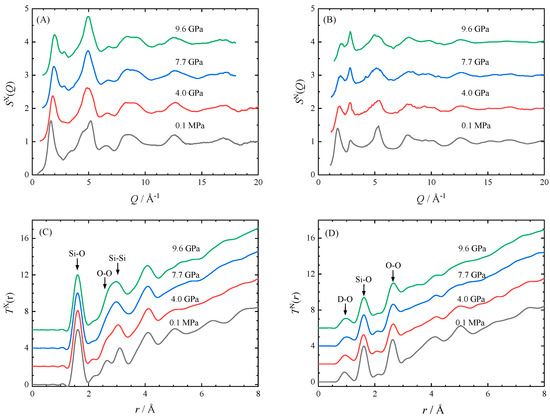
Figure 5.
X-ray and neutron diffraction data of the hydrous SiO2 glass with 13 wt.% D2O during room temperature compression to 10 GPa. (A) The X-ray structure factor SX(Q), (B) the neutron structure factor SN(Q), (C) the X-ray total correlation function TX(r), and (D) the neutron total correlation function TN(r).
Figure 6 compares the pressure dependence of the position of FSDP of hydrous SiO2 glass obtained from both XRD and ND with that of anhydrous glass [4,9]. The FSDP of SX(Q) and SN(Q) shifts in parallel to the high-Q side in proportion to the pressure up to 10 GPa. Those shifts are also parallel to the FSDP of ordinary anhydrous glass. In addition, the intensity of the FSDP of the hydrous glass decreases with pressure in the same way as anhydrous glass for both the SX(Q) and SN(Q) [4,9]. Shrinkage of the medium-range order is also found in T(r). The Si–O distance is almost constant with up to 10 GPa, but the Si–Si distance decreases with increasing pressure (Figure 5). Thus, the Si–O–Si bond angle decreases, and the network linkage of the SiO4 tetrahedra is distorted with increasing pressure. These show that even though the medium-range order of the hydrous SiO2 glass is partly disrupted by OD deuterioxyl, it shrinks with pressure just like the anhydrous glass.
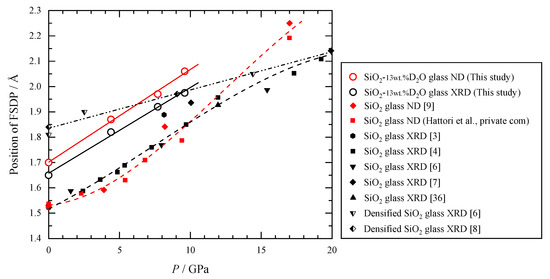
Figure 6.
Comparison of the position of the first sharp diffraction peak (FSDP) of SX(Q) and SN(Q) between the hydrous and anhydrous SiO2 glass [3,4,6,7,8,36].
We estimate the density of hydrous SiO2 glass under pressure in order to derive the total correlation function T(r). Since the FSDP of the hydrous glass changes with pressure at almost the same rate as that of the anhydrous glass, and the shape of the structure factors resemble each other except the FSDP, we assume that the densities of hydrous glass and anhydrous glass would change in the same rate with pressure. We evaluate the T(r) using the densities of hydrous glass at high pressures, which is estimated from the relationships between density and pressure of ordinary SiO2 glass shown in Figure 1 of Wakabayashi et al. [8]. The coordination number of Si calculated from TX(r) is about four up to 10 GPa, which is consistent with that of anhydrous SiO2 glass. This shows that our estimation of high-pressure density of hydrous SiO2 glass is fairly good, because the similarity of the short-range order between hydrous glass and anhydrous glass is expected.
What happens to the D2O-rich phase in the hydrous SiO2 glass during compression? Unfortunately, neutron diffraction data does not give us any information except nearly constant D–O distance in TN(r) up to 10 GPa. When evaluated using the partial molar volume of H2O at 3 GPa [37], at which the hydrous SiO2 glass was synthesized, 10 wt.% D2O is equivalent to about 14% in volume. It is interesting that such an amount of molecular D2O does not greatly affect the compression behavior of SiO2 glass.
4.4. Molecular Water in the Hydrous SiO2 Glass
The SAXS strongly suggests that most of the D2O molecules may form the separate phase in small domains distributed in the hydrous SiO2 glass. Can all D2O molecules in the hydrous glass be incorporated into the small domains? Here, we consider the possibility that D2O molecules are contained in the voids characteristic of the ring structure made by connecting SiO4 tetrahedra.
Sato et al. [38] showed that when He penetrates into SiO2 glass, SiO2 glass becomes rigid. Since the interstitial voids in the SiO2 glass are occupied by He, the voids are not squeezed even when pressure is applied and the bulk modulus of the SiO2 glass with He becomes larger than that of the ordinary SiO2 glass. It is shown that the FSDP position of the SiO2 glass saturated with He does not change up to 10 GPa. If D2O molecules penetrate the interstitial voids formed by the SiO4 linkage, the bulk modulus of the hydrous glass should be larger than that of ordinary glass, as shown in the case of SiO2 glass with He. The position of the FSDP of our hydrous glass, however, changes with pressure in the same way as the anhydrous glass (Figure 6), and its bulk modulus is estimated to be almost the same as that of the anhydrous glass. Thus, it is considered that the interstitial void space in the hydrous SiO2 glass is almost empty, and D2O does not penetrate into the SiO4 network. It is supported from void size: The interstitial void in the SiO4 network of SiO2 glass is too small to accommodate D2O molecule in it. In the size distribution model of interstitial voids in silica glass estimated from the solubility site density of He and Ne dissolved in cristobalite, about 10% of voids can contain He and only 6% of voids can contain Ne [39]. According to Zhang and Xu [40], the molecular diameter of H2O is 2.74 Å (that of D2O is also expected to be about the same), which is larger than He (2.16 Å) and Ne (2.42 Å), so that it seems difficult to incorporate the H2O (or D2O) molecules in the interstitial voids of SiO2 glass. Further, the void size in the hydrous glass is smaller than that of anhydrous glass due to the reduction of size of the SiO4 ring, and it is more difficult for the H2O (or D2O) molecule to be incorporated into the interstitial void.
5. Conclusions
SAXS, XRD and ND show that the SiO2–13wt.% D2O composition glass, which was synthesized by quenching from melt at 3 GPa is separated into two phases: SiO2-rich glass phase and D2O-rich minor phase were distributed as small domains with dimensions of less than 100 Å. Both the SX(Q) and the SN(Q) of the hydrous SiO2 glass can be reproduced from those of the SiO2 glass and liquid D2O. Medium-range order and short-range order derived from XRD and ND mainly reflect the structure of the SiO2-rich glass part. The FSDP of hydrous glass shifts to the higher-Q side than those of anhydrous glass at ambient conditions. This corresponds to the shrinkage of the medium-range order associated with the decrease of the size of SiO4 rings by breaking the SiO4 linkage with the OD group, as well as by distorting the network linkage of SiO4 with a pressure of 3 GPa during glass synthesis. The response of the structure of the SiO2-rich glass part to pressure is almost the same as that of the anhydrous SiO2 glass. The FSDP of the hydrous glass shifts to the high-Q side with increasing pressure in parallel with that of the anhydrous glass. The Si–O–Si bond angle decreases with pressure, although the coordination number of Si is about four at the pressures of from 0.1 MPa to 10 GPa. Thus, the medium-range order shrinks with pressure associated with distortion of network linkage of SiO4 tetrahedra. Most of D2O molecules are in the small domains of the D2O-rich phase and hardly penetrate into the void space in the ring consisting of SiO4 tetrahedra of SiO2-rich glass part.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-163X/10/1/84/s1, Figure S1: Cell assemble for the synthesis of hydrous SiO2 glass at 3 GPa and 1873 K, Figure S2: Recovered Pt capsule and hydrous SiO2 glass quenched from the conditions of 3 GPa and 1873 K.
Author Contributions
Conceptualization, S.U. and T.I.; formal analysis, S.U., T.H., S.K.; investigation, S.U., T.I., T.H., A.S.-F., S.K., D.W., T.S., N.F., K.-i.F.; writing—original draft preparation, S.U.; All author contribute review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part supported by the JSPS KAKENHI Grant (19GS0205, 18K03805), the Earthquake Research Institute Joint Usage/Research Program (2010-G-01, 2011-G-05, 2012-G-02), and the Joint Usage/Research Center PRIUS, Ehime University.
Acknowledgments
We thank Y. Katayama and T. Kimura for help with the ND experiments at J-PARC, and K. Mibe and M. Kanzaki for help with the synthesis of hydrous SiO2 glass. We are grateful to A. Zeidler and P. S. Salmon for providing us with high-pressure neutron diffraction data for SiO2 glass. We are also grateful to two anonymous reviewers for their thoughtful formal reviews. The XRD experiments were conducted under approval of PF Proposal Advisory Committee (2011G652, 2017G135) and the JASRI (2014B1480), and the ND experiments were performed with the approval of the Neutron Science Proposal Review Committee of J-PARC, MLF (2013I0011).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Data Reduction of X-ray and Neutron Diffraction
Appendix A.1.1. Energy Dispersive X-ray Diffraction at High Pressures
We used a Faber-Ziman definition for X-ray structure factor SX(Q) which is defined as follows.
where icohX(Q) is a coherent scattering intensity per atom, ,, ci and fi(Q) are an atomic fraction and an atomic scattering factor of the ith atom, respectively.
The SX(Q) was evaluated by the procedure developed by Funakoshi [41]. Energy profiles of XRD pattern obtained at various Bragg angles can be expressed by the following equation.
where N is the number of atom, A(E,θ) is the absorption factor, P(E,θ) is the polarization factor, I0X(E) is the intensity of incident X-rays. iinc(E,θ)X and im(E,θ)X are incoherent and multiple scattering intensity per atom, respectively. IBGX(E,θ) is background intensity. At first, IBGX(E,θ)’s were subtracted from IobX(E,θ)’s. As the energy spectrum of incident X-rays from the synchrotron source I0X(E) is unknown, we evaluated simultaneously both I0X(E) and icohX(E,θ)’s from IobX(E,θ)’s by simulation using the Monte Carlo method [41]. Here, we ignored the differences of absorption and polarization effects and multiple scattering terms among data taken with different Bragg angles. The incoherent scattering term was corrected using the formulation of Hajdu [42] and Pálinkás [43]. Then coherent scattering intensities at each Bragg angle were unified to obtain IcohX(Q). The Faber–Ziman total structure factor SX(Q) was evaluated from Equation (A1) in which the atomic scattering factor was calculated using the approximation formula given by Doyle and Turner [44].
Appendix A.1.2. Time-of-Flight Neutron Diffraction at High Pressures
Structure factor for neutron SN(Q) was evaluated from a ND profile obtained by the TOF method combined with a multi-anvil press. Observed ND profile is described as follows.
where N is the number of atoms, A(λ,θ) is the absorption factor, I0N(λ) is the intensity of incident neutron, icohN(λ,θ), iincN(λ,θ), and imN(λ,θ) are coherent, inelastic, incoherent, and multiple scattering cross section per one atom, respectively. IBGN(λ,θ) is background intensity. Observed scattering intensities IobN(λ,θ) are normalized by I0N(λ) obtained from the scattering intensity of a vanadium pellet after background subtraction and absorption correction. Absorption correction was performed according to the method of Paalman and Pings [45]. Then, incoherent scattering and multiple scattering terms were corrected. The incoherent term was evaluated by a composition average of incoherent scattering length binc of the elements, which are listed in the table of Sears [46]. The incoherent scattering for D atom was significantly deviated from the value listed in the table due to the inelastic effect. Therefore, we corrected it by empirical method employed by Kameda et al. [47] (as described below). The multiple scattering term was corrected by the method of Blech and Averbach [48]. Thus, we calculated the structure factor SN(Q) from the corrected intensity IcorN(λ,θ), which is the sum of icohN(λ,θ) and iineN(λ,θ).
Inelastic effect for incoherent scattering of deuterium has a small but non-negligible effect on the total scattering intensity of the hydrous SiO2 glass. The effect was corrected based on the empirical method by Kameda et al. [48] in which incoherent scattering was estimated from the self term of the liquid null-H2O using a known O–O correlation. The applicability of this method at PLANET has been confirmed from the good coincidence of the S(Q) for D2O water with that reported so far in the literature (Hattori et al., private com).
Definition of Faber–Ziman total structure factor for the SN(Q) is as follows.
where , , ci, and bi are an atomic fraction and a coherent scattering length of the ith atom [46], respectively.
Appendix A.1.3. Fourier Analysis
The S(Q) data in reciprocal space can be transformed into total correlation function T(r) in real space by means of a Fourier transform.
where ρ0 is a number density of atoms and M(Q) is a modification function. We used a Lorch function as M(Q) [49].
Appendix B
Faber–Ziman Structure Factor of a Two-Phase Mixture can be Written as Follows for X-ray
References
- Bridgman, P.W. Effects of very high pressures on glass. J. Appl. Phys. 1953, 24, 405–413. [Google Scholar] [CrossRef]
- Hemley, R.J.; Mao, H.K.; Bell, P.M.; Mysen, B.O. Raman spectroscopy of SiO2 glass at high pressure. Phys. Rev. Lett. 1986, 57, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.; Hemley, R.J.; Mao, H.K. High-pressure X-ray diffraction of SiO2 glass. Pys. Rev. Lett. 1992, 69, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Inamura, Y.; Katayama, Y.; Ustumi, W.; Funakoshi, K. Transformations in the intermediate-range structure of SiO2 glass under high pressure and temperature. Phys. Rev. Lett. 2004, 93, 015501. [Google Scholar] [CrossRef]
- Sato, T.; Funamori, N. Sixfold-coordinated amorphous polymorph of SiO2 under high pressure. Phy. Rev. Lett. 2008, 101, 255502. [Google Scholar] [CrossRef]
- Benmore, C.J.; Soignard, E.; Amin, S.A.; Guthrie, M.; Shastri, S.D.; Lee, P.L.; Yarger, J.L. Structural and topological changes in silica glass at pressure. Phys. Rev. B 2010, 81, 054105. [Google Scholar] [CrossRef]
- Sato, T.; Funamori, N. High-pressure structural transformation of SiO2 glass up to 100 GPa. Phys. Rev. B 2010, 82, 184102. [Google Scholar] [CrossRef]
- Wakabayashi, D.; Funamori, N.; Sato, T.; Taniguchi, T. Compression behavior of densified SiO2 glass. Phys. Rev. B 2011, 84, 144103. [Google Scholar] [CrossRef]
- Zeidler, A.; Wezka, K.; Rowlands, R.F.; Whittaker, D.A.J.; Salmon, P.S.; Polidori, A.; Drewitt, J.W.E.; Klotz, S.; Fischer, H.E.; Wilding, M.C.; et al. High-pressure transformation of SiO2 glass from a tetrahedral to an octahedral network: A joint approach using neutron diffraction and molecular dynamics. Phys. Rev. Lett. 2014, 113, 13501. [Google Scholar] [CrossRef]
- Stolper, E. Water in silicate glasses: An infrared spectroscopic study. Contrib. Mineral. Petrol. 1982, 81, 1–17. [Google Scholar] [CrossRef]
- Tomozawa, M. Water in glass. J. Non-Cryst. Solids 1985, 73, 197–204. [Google Scholar] [CrossRef]
- Zotov, N.; Keppler, H.; Hannon, A.C.; Soper, A.K. The effect of water on the structure of silicate glasses—A neutron diffraction study. J. Non-Cryst. Solids 1996, 202, 153–163. [Google Scholar] [CrossRef]
- Van der Steen, G.H.A.M.; van den Boom, H. Raman spectroscopic study of hydrogen-containing vitreous silica. J. Non-Cryst. Solids 1977, 23, 279–286. [Google Scholar] [CrossRef]
- McMillan, P.F.; Remmele, R.L. Hydroxyl sites in SiO2 glass: A note on infrared and Raman spectra. Am. Mineral. 1986, 71, 772–778. [Google Scholar]
- Sato, T.; Funamori, N.; Wakabayashi, D.; Nishida, K.; Kikegawa, T. Coexistence of two states in optically homogeneous silica glass during the transformation in short-range order. Phys. Rev. B 2018, 98, 144111. [Google Scholar] [CrossRef]
- Kohara, S.; Suzuya, K.; Kashihara, K.; Matsumoto, N.; Umesaki, N.; Sakai, I. A horizontal two-axis diffractometer for high-energy X-ray diffraction using synchrotron radiation on bending magnet beamline BL04B2 at SPring-8. Nucl. Instrum. Methods. Phys. Res. 2001, A467–A468, 1030–1033. [Google Scholar] [CrossRef]
- Kohara, S.; Suzuya, K. High-energy X-ray diffraction studies of disordered materials. Nucl. Instrum. Methods. Phys. Res. 2003, B199, 23–28. [Google Scholar] [CrossRef]
- Decker, D.L. High-pressure equation of state for NaCl, KCl, and CsCl. J. Appl. Phys. 1971, 42, 3239–3244. [Google Scholar] [CrossRef]
- Sano-Furukawa, A.; Hattori, T.; Arima, H.; Yamada, A.; Tabata, S.; Kondo, M.; Nakamura, A.; Kagi, H.; Yagi, T. Six-axis multi-anvil press for high-pressure, high-temperature neutron diffraction experiments. Rev. Sci. Instrum. 2014, 85, 113905. [Google Scholar] [CrossRef]
- Hattori, T.; Sano-Furukawa, A.; Arima, H.; Komatsu, K.; Yamada, A.; Inamura, Y.; Nakatani, T.; Seto, Y.; Nagai, T.; Utsumi, W.; et al. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. Nucl. Instrum. Methods Phys. Res. 2015, A780, 55–67. [Google Scholar] [CrossRef]
- Nishiyama, N.; Wang, Y.; Sanehira, T.; Irifune, T.; Rivers, M.L. Development of the multi-anvil assembly 6-6 for DIA and D-DIA type high-pressure apparatuses. High Press. Res. 2008, 28, 307–314. [Google Scholar] [CrossRef]
- Kohara, S.; Ito, M.; Suzuya, K.; Inamura, Y.; Sakurai, Y.; Ohishi, Y.; Takata, M. Structural studies of disordered materials using high-energy X-ray diffraction from ambient to extreme conditions. J. Phys. Condens. Matter 2007, 19, 506101. [Google Scholar] [CrossRef]
- Hannon, A.C. Unpublished GEM Datra Examples: Silica Glass, Diffraction Data for Vitreous SiO2, Oxide Glass Data. 1990. Available online: https://www.isis.stfc.ac.uk/Pages/Oxide-Glass-Data.aspx (accessed on 3 December 2019).
- Kameda, Y.; Amo, Y.; Usui, T.; Umebayashi, Y.; Ikeda, K.; Otomo, T. Neutron diffraction study on partial pair correlation functions of water at ambient temperature. Bull. Chem. Soc. Jpn. 2018, 91, 1586–1595. [Google Scholar] [CrossRef]
- Elliot, S.R. Medium-range structural order in covalent amorphous solids. Nature 1991, 354, 445–452. [Google Scholar] [CrossRef]
- Gaskell, P.H. Medium-range structure in glasses and low-Q structure in neutron and X-ray scattering data. J. Non-Cryst. Solids 2005, 351, 1003–1013. [Google Scholar] [CrossRef]
- Crupi, C.; Carini, G.; González, M.; D’Angelo, G. Origin of the first sharp diffraction peak in glasses. Phys. Rev. B 2015, 92, 134206. [Google Scholar] [CrossRef]
- Zeidler, A.; Salmon, P.S. Pressure-driven transformation of the ordering in amorphous network-forming materials. Phys. Rev. B 2016, 93, 214204. [Google Scholar] [CrossRef]
- Onodera, Y.; Kohara, S.; Tahara, S.; Masuno, A.; Inoue, H.; Shiga, M.; Hirata, A.; Tsuchiya, K.; Hiraoka, Y.; Obayashi, I.; et al. Understanding diffraction patterns of glassy, liquid and amorphous materials via persistent homology analyses. J. Ceram. Soc. Jpn. 2019, 127, 853–863. [Google Scholar] [CrossRef]
- Pasquarello, A.; Car, R. Identification of Raman defect lines as signatures of ring structures in vitreous silica. Phys. Rev. Lett. 1998, 80, 5145–5147. [Google Scholar] [CrossRef]
- Zotov, N.; Yanev, Y.; Epelbaum, M.; Konstantinov, L. Effect of water on the structure of rhyolite glasses—X-ray diffraction and Raman spectroscopy studies. J. Non-Cryst. Solids 1992, 142, 234–246. [Google Scholar] [CrossRef]
- Susman, S.; Volin, K.J.; Price, D.L.; Grimsditch, M.; Rino, J.P.; Kalia, R.K.; Vashishta, P.; Gwanmessia, G.; Wang, Y.; Libermann, R.C. Intermediate-range order in permanently densified vitreous SiO2: A neutron-diffraction and molecular-dynamics study. Phys. Rev. B 1991, 43, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Takimoto, Y.; Hijiya, H.; Taniguchi, T.; Urata, S.; Inaba, S.; Fujita, S.; Obayashi, I.; Hiraoka, Y.; Kohara, S. Origin of the mixed alkali effect in silicate glass. NPG Asia Mater. 2019, 11, 75. [Google Scholar] [CrossRef]
- Hart, R.T.; Benmore, C.J.; Neuefeind, J.; Kohara, S.; Tomberli, B.; Egelstaff, P.A. Temperature dependence of isotropic quantum effects in water. Phys. Rev. Lett. 2005, 94, 047801. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Uemura, O. The intramolecular structure of oxonium ion in concentrated aqueous deuterochloric acid solutions. Bull. Chem. Soc. Jpn. 1992, 65, 2021–2028. [Google Scholar] [CrossRef]
- Funamori, N.; Sato, T. A cubic boron nitride gasket for diamond-anvil experiments. Rev. Sci. Instrum. 2008, 79, 053903. [Google Scholar] [CrossRef]
- Sakamaki, T. Density of hydrous magma. Chem. Geol. 2017, 475, 135–139. [Google Scholar] [CrossRef]
- Sato, T.; Funamori, N.; Yagi, T. Helium penetrates into silica glass and reduces its compressibility. Nat. Commun. 2011, 2, 345. [Google Scholar] [CrossRef]
- Shacckelford, J.F.; Masaryk, J.S. The interstitial structure of vitreous silica. J. Non-Cryst. Solids 1978, 30, 127–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z. Atomic radii of noble gas elements in condensed phases. Am. Mineral. 1995, 80, 670–675. [Google Scholar] [CrossRef]
- Funakoshi, K. Energy-dispersive X-ray Diffraction Study for Alkali Silicate Melts Using Synchrotron Radiation under High Pressure and Temperature. Ph.D. Thesis, Tokyo Institute of Technology, Tokyo, Japan, March 1997. [Google Scholar]
- Hajdu, F. Analytic approximation for incoherent scattering X-ray intensities. Acta Crystallogr. 1971, A27, 73–74. [Google Scholar] [CrossRef]
- Pálinkás, G. Analytic approximation for the incoherent X-ray intensities of the atoms from Ca to Am. Acta Crystallogr. 1973, A29, 10–12. [Google Scholar] [CrossRef]
- Doyle, P.A.; Turner, P.S. Relativistic Hartree-Fock X-ray and electron scattering factors. Acta Crystallogr. 1968, A24, 390–397. [Google Scholar] [CrossRef]
- Paalman, H.H.; Pings, C.J. Numerical evaluation of X-ray absorption Factors for cylindrical samples and annular sample cells. J. App. Phys. 1962, 33, 2635–2639. [Google Scholar] [CrossRef]
- Sears, V.F. Neutron scattering lengths and cross sections. Neutron News 1992, 3, 26–37. [Google Scholar] [CrossRef]
- Kameda, Y.; Sasaki, M.; Usuki, T.; Otomo, T.; Itoh, K.; Suzuya, K.; Fukunaga, T. Inelasticity effect on neutron scattering intensities of the null-H2O. J. Neutron Res. 2003, 11, 153–163. [Google Scholar] [CrossRef]
- Blech, I.A.; Averbach, B.L. Multiple scattering of neutrons in vanadium and copper. Phys. Rev. 1965, 137, A113–A116. [Google Scholar] [CrossRef]
- Lorch, E. Neutron diffraction by germania, silica and radiation-damaged silica glasses. J. Phys. C Solid State Phys. 1969, 2, 229–237. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).