Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates
Abstract
1. Introduction
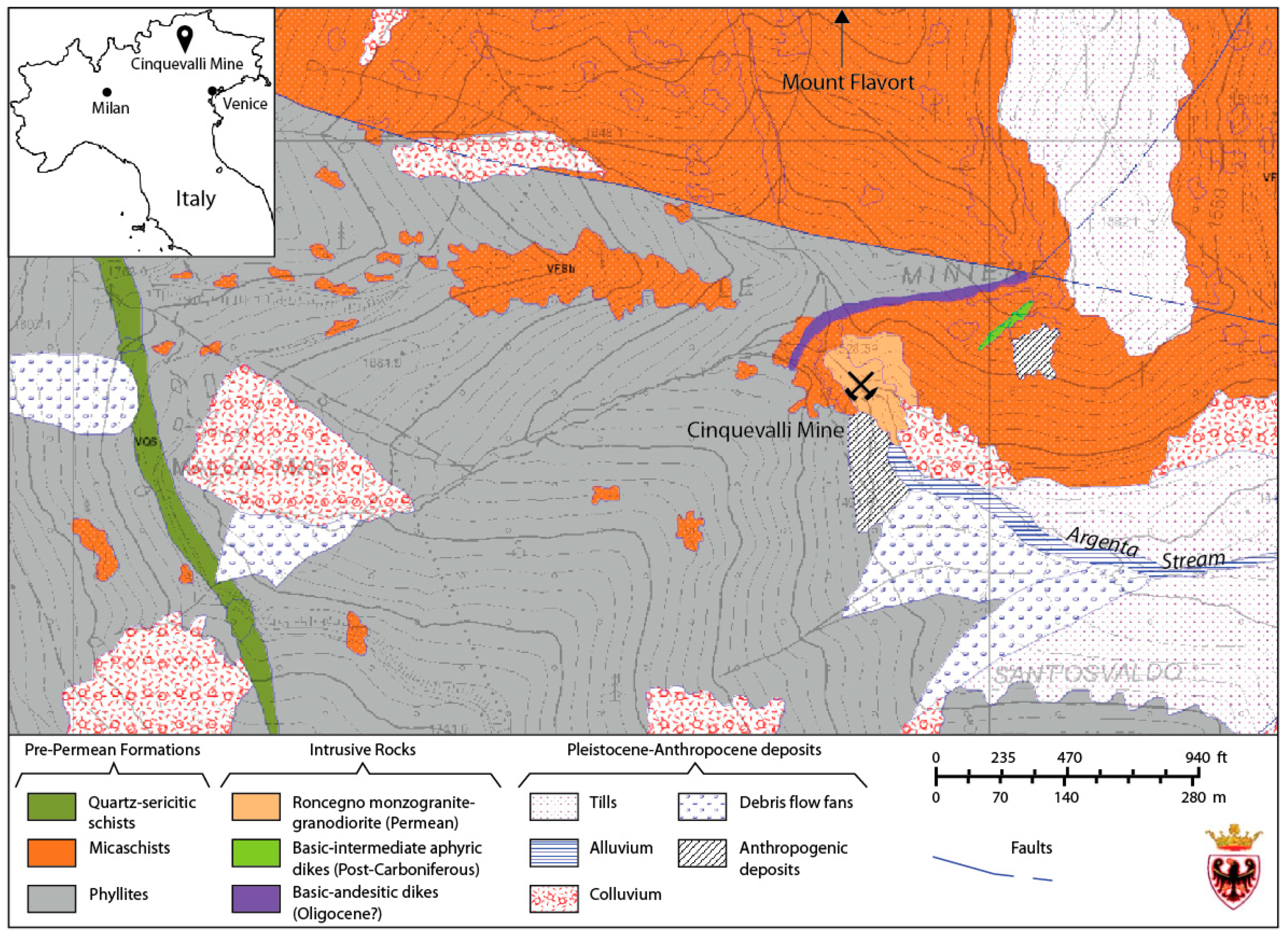
2. Materials and Methods
3. Results
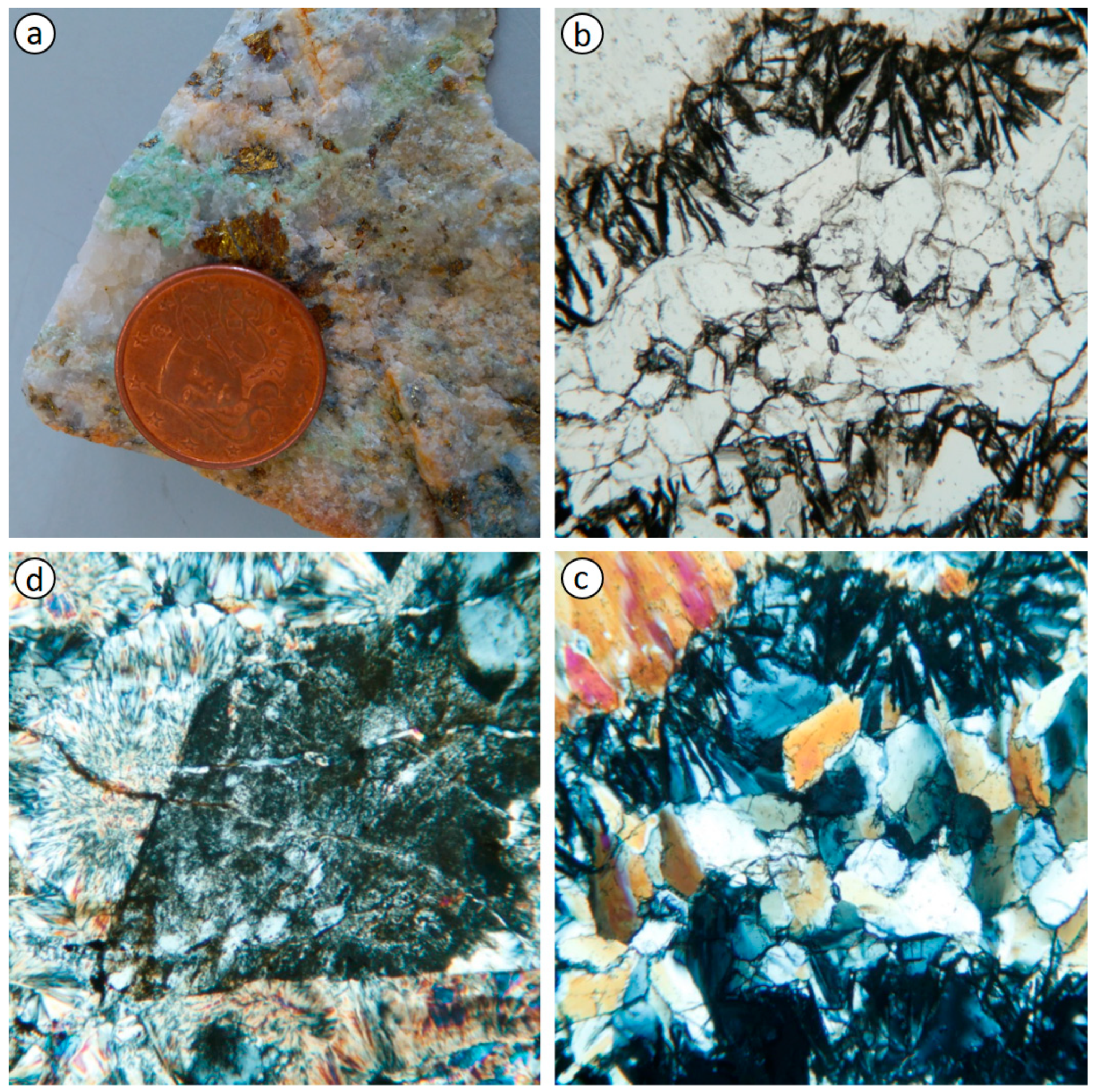
3.1. Rock Mineral Assemblage and Composition
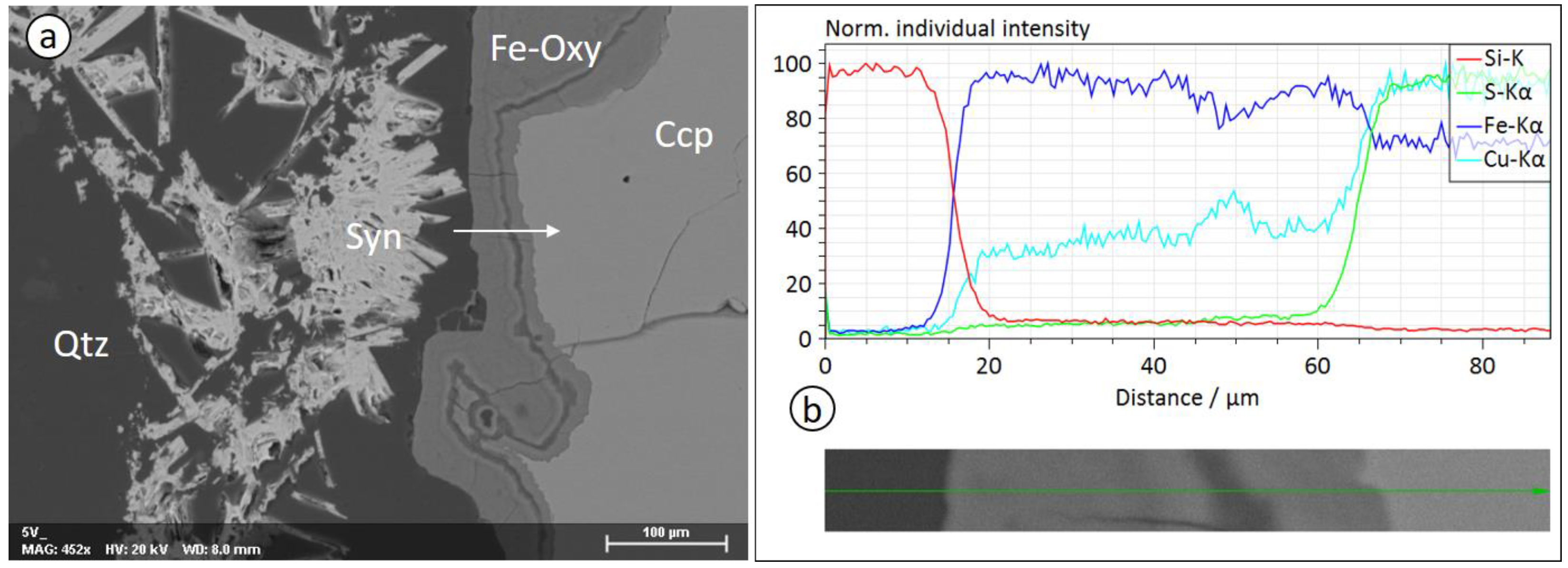
3.2. Microstructure and Mineral Composition
3.3. Observations at the Nanoscale
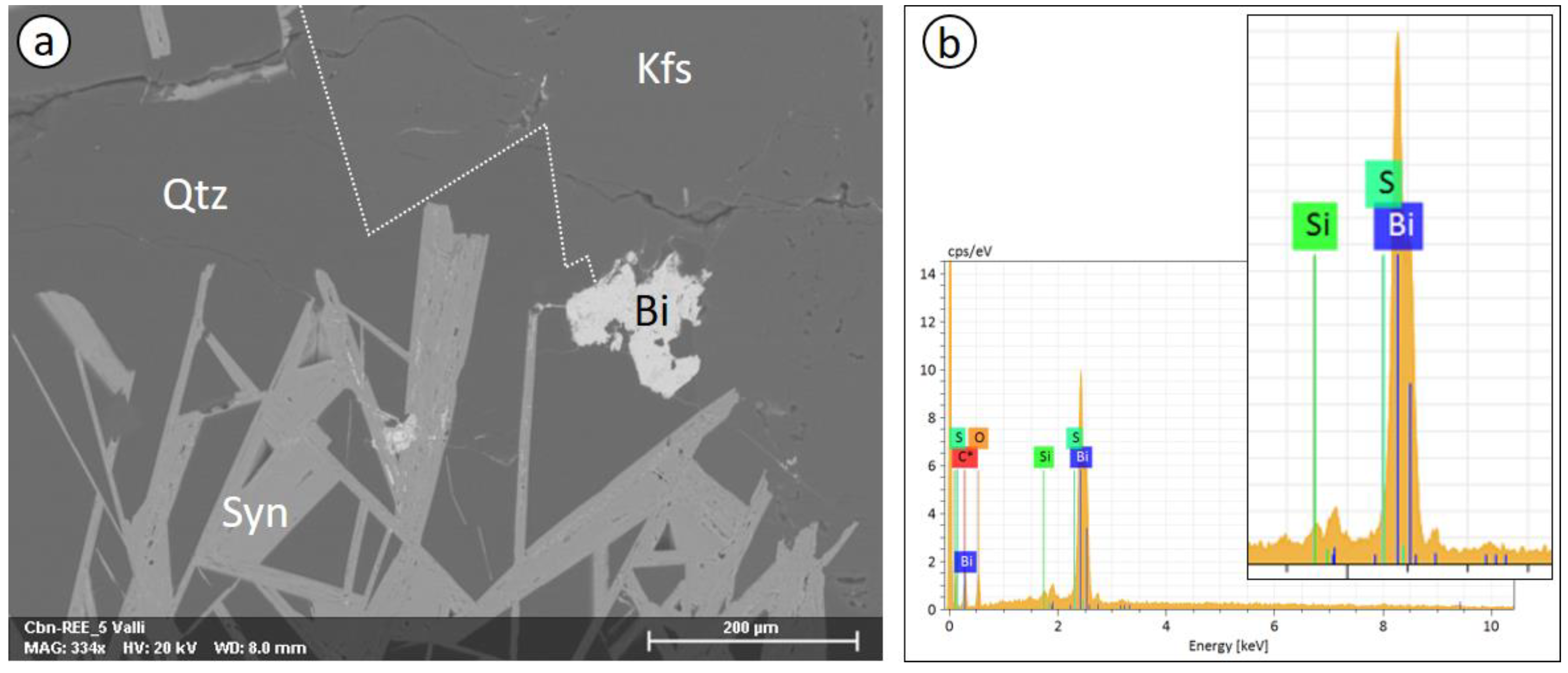
3.3.1. EDS Analysis
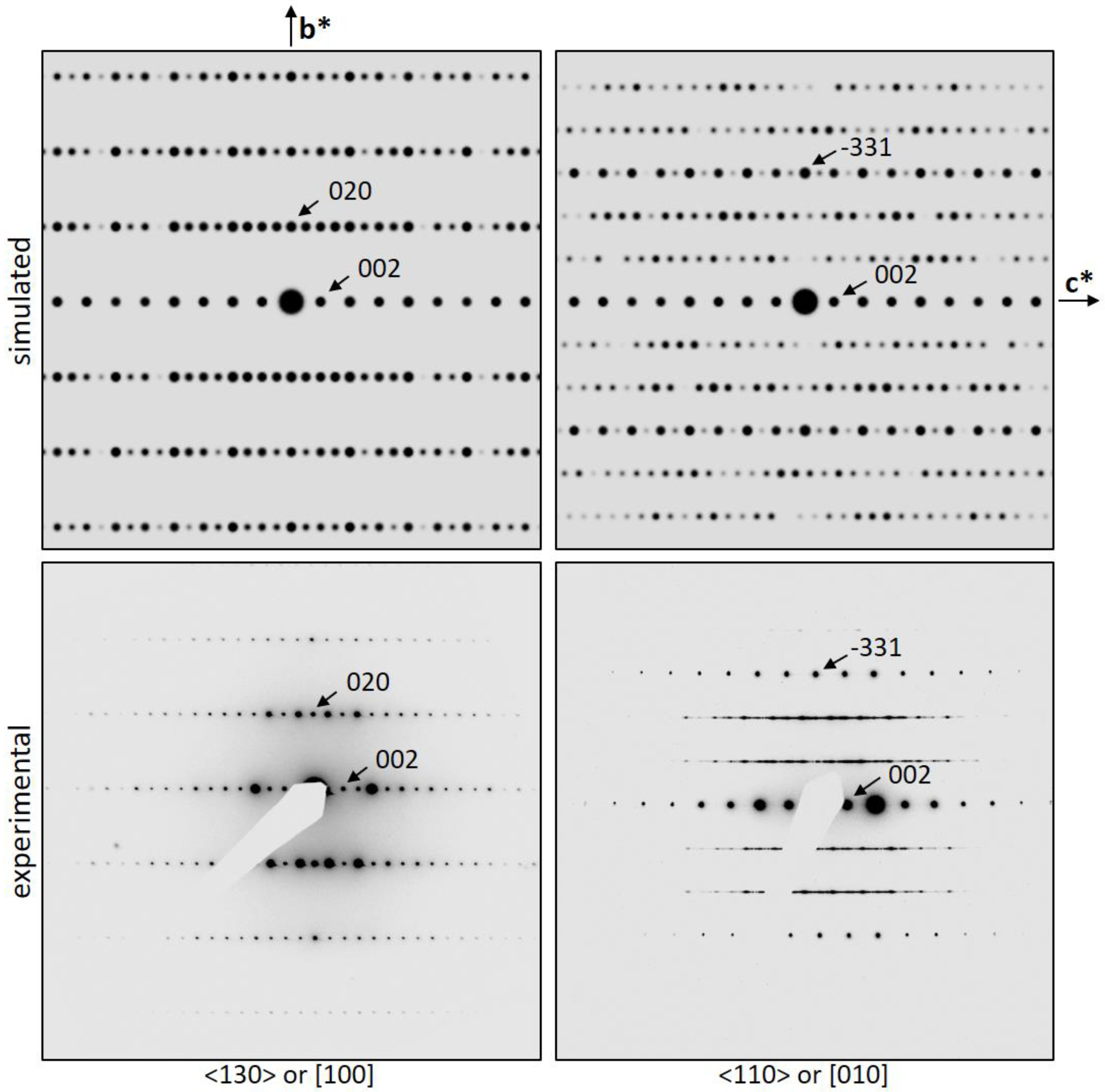
3.3.2. Suitable Crystal Orientation for Order/Disorder Assessment
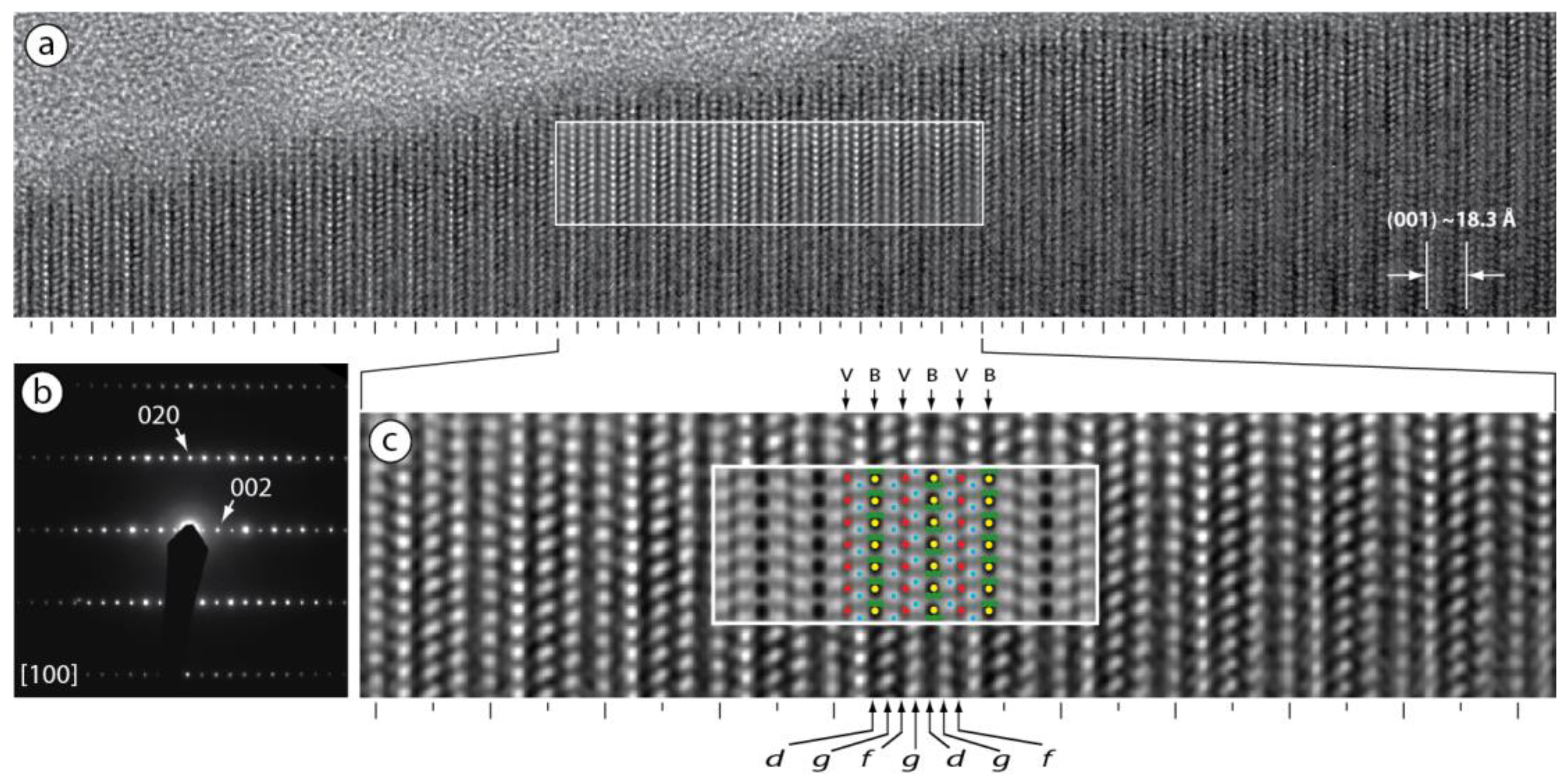
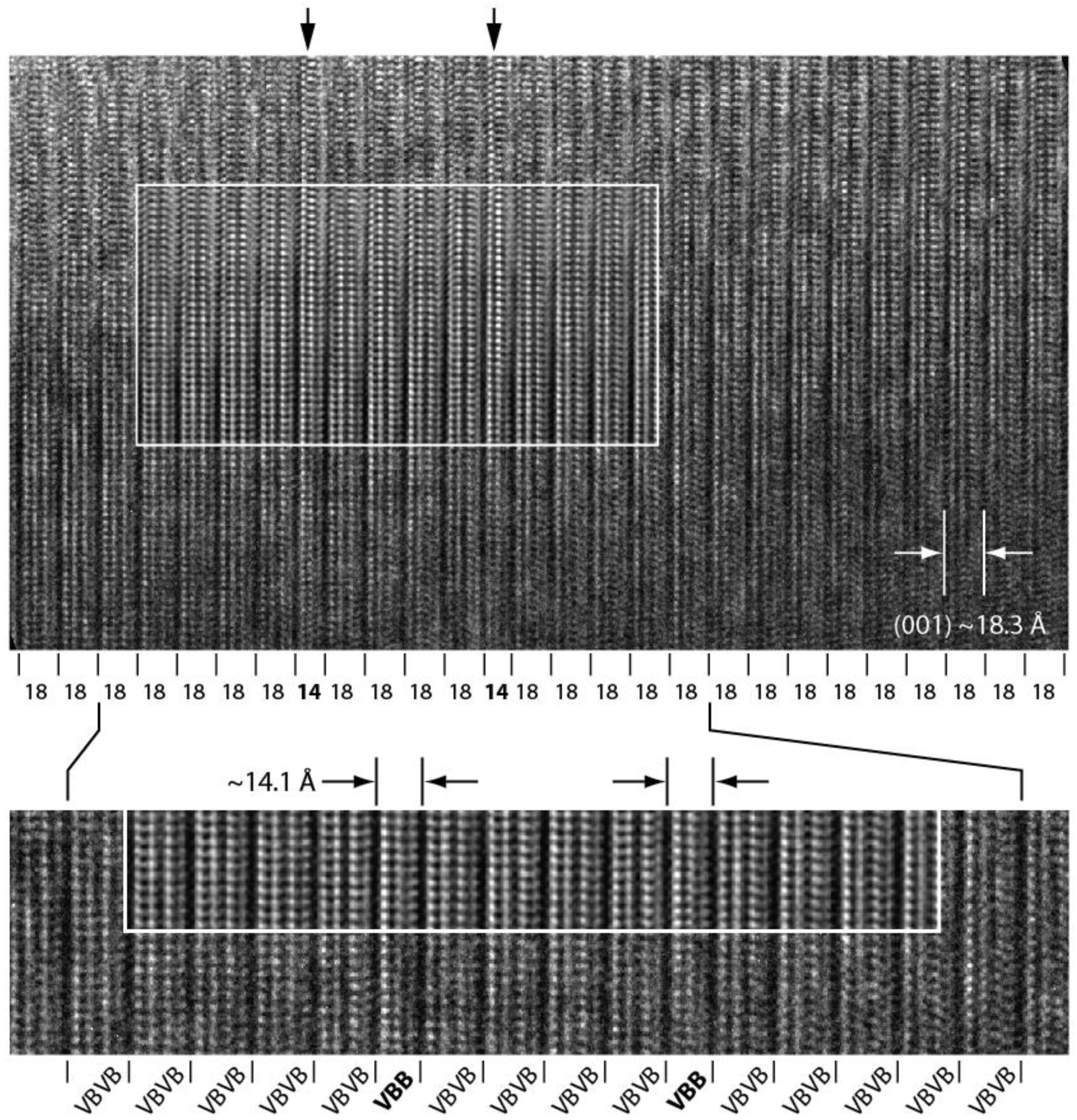
3.3.3. [100] HRTEM Observations
3.3.4. [110] HRTEM Observations
4. Discussion
4.1. Rock Mineral Association
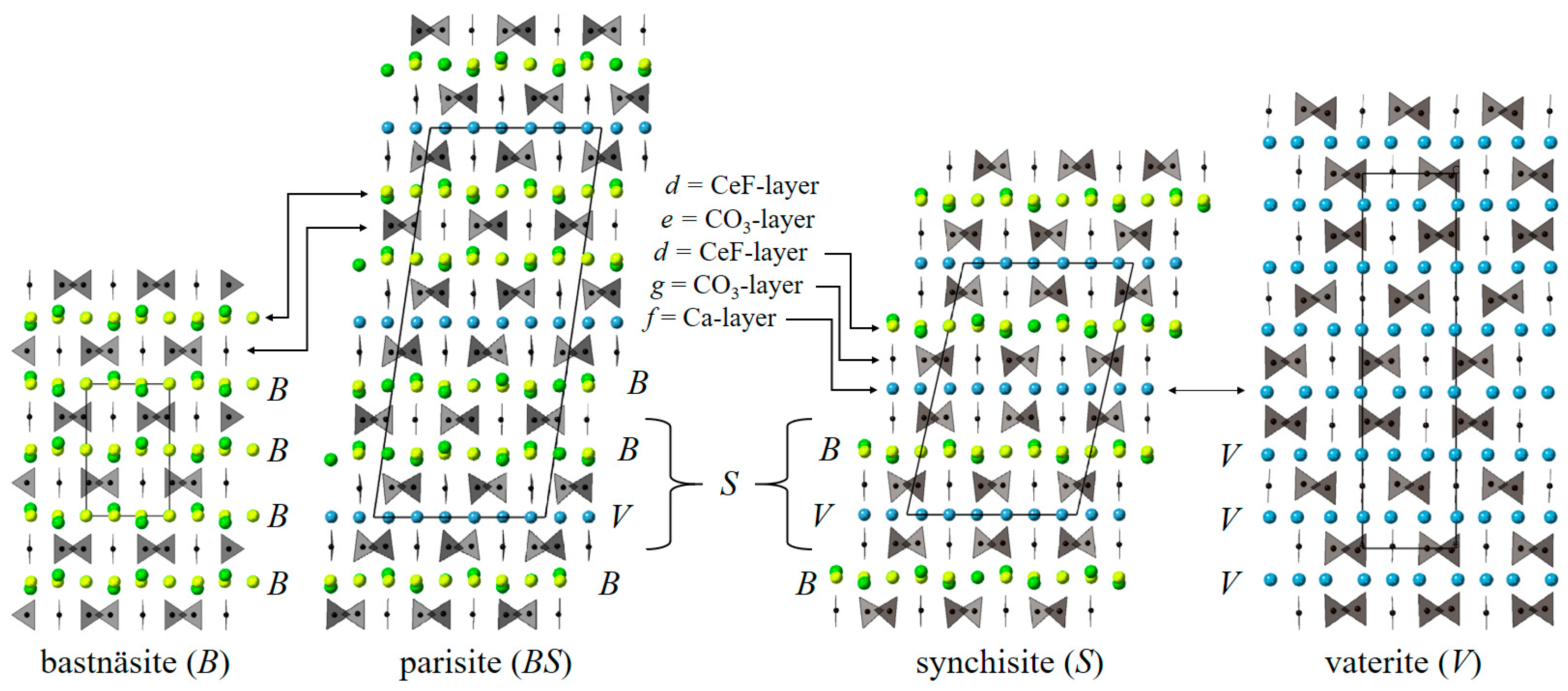
4.2. Polytypism of Synchysite
4.3. A Brief Comparison with the Polytypism of Mica
Funding
Acknowledgments
Conflicts of Interest
References
- Van Landuyt, J.; Amelinckx, S. Multiple beam direct lattice imaging of new mixed-layer compounds of the bastnäsite-synchysite series. Am. Mineral. 1975, 60, 351–358. [Google Scholar]
- Wu, X.; Meng, D.; Pan, Z.; Yang, G. Transmission electron microscope study of new, regular, mixed-layer structures in calcium-rare earth fluorcarbonate minerals. Mineral. Mag. 1998, 62, 55–64. [Google Scholar]
- Meng, D.; Wu, X.; Mou, T.; Li, D. Determination of six new polytypes in parisite-(Ce) by means of high-resolution electron microscopy. Mineral. Mag. 2001, 65, 797–806. [Google Scholar]
- Meng, D.; Wu, X.; Mou, T.; Li, D. Microstructural investigation of new polytypes of parisite-(Ce) by high-resolution transmission electron microscopy. Can. Mineral. 2001, 39, 1713–1724. [Google Scholar] [CrossRef]
- Meng, D.; Wu, X.; Han, Y.; Meng, X. Polytypism and microstructures of the mixed-layer member B2S, CaCe3(CO3)4F3 in the bastnaesite-(Ce)-synchysite-(Ce) series. Earth Planet. Sc. Lett. 2002, 203, 817–828. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in mixed-layer compounds: A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Capitani, G. HRTEM investigation of bastnäsite–parisite intergrowths from Mount Malosa (Malawi): Ordered sequences, polysomatic faults, polytypic disorder, and a new parisite-(Ce) polymorph. Eur. J. Mineral. 2019, 31, 429–442. [Google Scholar] [CrossRef]
- Smith, W.L.; Stone, J.; Ross, D.R.; Levine, H. Doverite, a possible new yttrium fluocarbonate from Dover, Morris County, New Jersey. Am. Mineral. 1960, 45, 92–98. [Google Scholar]
- Scharm, B.; Kühn, P. Synchysite-(Nd), Ca(Nd,Y,Gd,...)[F,(CO3)2], a new mineral. Neues Jahrb. Mineral. 1983, 5, 201–210. [Google Scholar]
- Wall, F.; Le Bas, M.J.; Srivastava, R.K. Calcite and Carbocernaite Exsolution and Cotectic Textures in a Sr,REE-Rich Carbonatite Dyke from Rajasthan, India. Mineral. Mag. 1993, 57, 495–513. [Google Scholar] [CrossRef]
- Forster, H.-J. Synchysite-(Y)-synchysite-(Ce) solid solutions from Markersbach, Erzgebirge, Germany: REE and Th mobility during high-T alteration of highly fractionated aluminous A-type granites. Mineral. Petrol. 2001, 72, 259–280. [Google Scholar] [CrossRef]
- Kalatha, S.; Perraki, M.; Economou-Eliopoulos, M.; Mitsis, I. On the origin of bastnaesite-(La,Nd,Y) in the Nissi (Patitira) bauxite laterite deposit, Lokris, Greece. Minerals 2017, 7, 45. [Google Scholar] [CrossRef]
- Maksimović, Z.; Pantó, G. Hydroxyl-bastnaesite-(Nd), a new mineral from Montenegro, Yugoslavia. Mineral. Mag. 1985, 49, 717–720. [Google Scholar] [CrossRef]
- Michiba, K.; Miyawaki, R.; Minakawa, T.; Terada, Y.; Nakai, I.; Matsubara, S. Crystal structure of hydroxylbastnäsite-(Ce) from Kamihouri, Miyazaki Prefecture, Japan. J. Mineral. Petrol. Sc. 2013, 108, 326–334. [Google Scholar] [CrossRef]
- Grice, J.D.; Maisonneuve, V.; Leblanc, M. Natural and synthetic fluoride carbonates. Chem. Rev. 2007, 107, 114–132. [Google Scholar] [CrossRef]
- Donnay, G.; Donnay, J.D.H. The crystallography of bastnaesite-(Ce), parisite-(Ce), roentgenite-(Ce) and synchisite-(Ce). Am. Mineral. 1953, 38, 932–963. [Google Scholar]
- Wang, L.; Ni, Y.; Hughes, J.M.; Bayliss, P.; Drexler, J.W. The atomic arrangement of synchysite-(Ce), CeCaF(CO3)2. Can. Mineral. 1994, 32, 865–871. [Google Scholar]
- Ni, Y.; Post, J.E.; Hughes, J.M. The crystal structure of parisite-(Ce), Ce2CaF2(CO3). Am. Mineral. 2000, 85, 251–258. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. The atomic arrangement of bastnäsite-(Ce), Ce(CO3)F, and structural elements of synchysite-(Ce), röntgenite-(Ce), and parisite-(Ce). Am. Mineral. 1993, 78, 415–418. [Google Scholar]
- Wang, J.; Becker, U. Structure and carbonate orientation of vaterite (CaCO3). Am. Mineral. 2009, 94, 380–386. [Google Scholar] [CrossRef]
- Maiello, F. Molti, diversi per origine, sorprendenti… I minerali del Trentino. Nat. Alp. 2006, 1, 23–52. (In Italian) [Google Scholar]
- Autonomous Province of Trento, Environment and Territory Information System. Available online: https://patn.maps.arcgis.com/apps/webappviewer/index.html?id=8e6cda8cc23844c9a6d3484f9bbd20f0 (accessed on 3 December 2019).
- Hill, R.J. Expanded use of the Rietveld method in studies of phase abundance in multiphase mixtures. Powder Diffr. 1991, 6, 74–77. [Google Scholar] [CrossRef]
- Bish, D.L.; Post, J.E. Quantitative mineralogical analysis using the Rietveld full-pattern fitting method. Am. Mineral. 1993, 78, 932–940. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004. [Google Scholar]
- Kilaas, R. Optimal and near-optimal filters in high-resolution electron microscopy. J. Microsc. 1998, 190, 45–51. [Google Scholar] [CrossRef]
- Mitchell, D.R. HRTEM Filter. Digital Micrograph Script Database. Available online: https://www.felmi-zfe.at/dm_script/ (accessed on 6 December 2019).
- Stadelmann, P.J. Electron Microscopy Software, Java Version 3.6322U2011; CIME-EPFL: Ecublens, Switzerland, 1999–2011. [Google Scholar]
- Cliff, G.; Lorimer, G.W. The quantitative analysis of thin specimens. J. Microsc. 1975, 103, 203–207. [Google Scholar] [CrossRef]
- Smith, J.V.; Yoder, H.S. Experimental and theoretical studies of the micas polymorphs. Mineral. Mag. 1956, 31, 209–235. [Google Scholar] [CrossRef]
- Bailey, S.W. Crystal chemistry of the true micas. In Micas; Bailey, S.W., Ed.; Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 13, pp. 13–60. [Google Scholar]
- Birle, I.D.; Tettenhorst, R. Refined muscovite structure. Mineral. Mag. 1968, 36, 883–886. [Google Scholar] [CrossRef][Green Version]
- Hazen, R.M.; Burnham, C.W. The crystal structures of one-layer phlogopite and annite. Am. Mineral. 1973, 58, 889–900. [Google Scholar]
- Zhoukhlistov, A.P.; Zvyagin, B.B.; Lazarenko, E.K.; Pavlishin, V.I. Refinement of the crystal structure of ferrous celadonite. Sov. Phys. Crystallogr. 1977, 22, 284–288. [Google Scholar]
- Backhaus, K.O. Structure refinement of a lepidolite-1M. Cryst. Res. Technol. 1983, 18, 1253–1260. [Google Scholar] [CrossRef]
- Joswig, W.; Amthauer, G.; Takeuchi, Y. Neutron diffraction and Mössbauer spectroscopic study of clintonite (xanthophyllite). Am. Mineral. 1986, 71, 1194–1197. [Google Scholar]
- Tyrna, P.L.; Guggenheim, S. The crystal structure of norrishite, KLiMn3+2 Si4O12: An oxygen-rich mica. Am. Mineral. 1991, 76, 266–271. [Google Scholar]
- Oberti, R.; Ungaretti, L.; Tlili, A.; Smith, D.C.; Robert, J. The crystal structure of preiswerkite. Am. Mineral. 1993, 78, 1290–1298. [Google Scholar]
- Evsyunin, V.G.; Kashaev, A.A.; Rastsvetaeva, R.K. Crystal structure of a new representative of Cr micas. Cryst. Rep. 1997, 42, 571–574. [Google Scholar]
- Brigatti, M.F.; Lugli, C.; Poppi, L.; Foord, E.E.; Kile, D.E. Crystal chemical variations in Li- and Fe-rich micas from Pikes Peak Batholith (central Colorado). Am. Mineral. 2000, 85, 1275–1286. [Google Scholar] [CrossRef]
- Guggenheim, S.; Bailey, S.W. Refinement of the margarite structure in subgroup symmetry. Am. Mineral. 1975, 60, 1023–1029. [Google Scholar]
- Sidorenko, O.V.; Zvyagin, B.B.; Soboleva, S.V. Refinement of the crystal structure of 2M1 paragonite by the method of high-voltage electron diffraction. Sov. Phys. Crystallogr. 1977, 22, 554–556. [Google Scholar]
- Swanson, T.H.; Bailey, S.W. Redetermination of the lepidolite-2M1 structure. Clays Clay Miner. 1981, 29, 81–90. [Google Scholar] [CrossRef]
- Lin, J.-C.; Guggenheim, S. The crystal structure of a Li, Be-rich brittle mica: A dioctahedral-trioctahedral intermediate. Am. Mineral. 1983, 68, 130–142. [Google Scholar]
- Gnos, E.; Armbruster, T. Kinoshitalite, Ba(Mg)3(Al2Si2)O10(OH,F)2, a brittle mica from a manganese deposit in Oman: Paragenesis and crystal chemistry. Am. Mineral. 2000, 85, 242–250. [Google Scholar] [CrossRef]
- Sidorenko, O.V.; Zvyagin, B.B.; Soboleva, S.V. Crystal structure of 3T paragonite. Sov. Phys. Crystallogr. 1977, 22, 557–559. [Google Scholar]
- Brown, B.E. The crystal structure of 3T lepidolite. Am. Mineral. 1978, 63, 332–336. [Google Scholar]
- Amisano-Canesi, A.; Chiari, G.; Ferraris, G.; Ivaldi, G.; Soboleva, S.V. Muscovite- and phengite-3T: Crystal strutture and conditions of formation. Eur. J. Mineral. 1994, 6, 489–496. [Google Scholar] [CrossRef]
- Levinson, A.A. Studies in the mica group; relationship between polymorphism and composition in the muscovite-lepidolite series. Am. Mineral. 1953, 38, 88–107. [Google Scholar]
- Shimoda, S. A hydromuscovite from the Shakanai Mine, Akita Prefecture, Japan. Clays Clay Miner. 1970, 18, 269–274. [Google Scholar] [CrossRef]
- Sartori, F. The crystal structure of a 1M lepidolite. Tscher. Miner. Petrog. 1976, 23, 65–75. [Google Scholar] [CrossRef]
- Zhoukhlistov, A.P.; Zvyagin, B.B.; Soboleva, S.V.; Fedotov, A.F. The crystal structure of the dioctahedral mica 2M2 determined by high voltage electron diffraction. Clays Clay Miner. 1973, 21, 465–470. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M. The crystal structure of nanpingite-2M2, the Cs end-member of muscovite. Am. Mineral. 1996, 81, 105–110. [Google Scholar] [CrossRef]
- Mesto, E.; Scordari, F.; Lacalamita, L.; Schingaro, E. Tobelite and NH4-rich muscovite single crystals from Ordovician Armorican sandstones (Brittany, France): Structure and crystal chemistry. Am. Mineral. 2012, 97, 1460–1468. [Google Scholar] [CrossRef]
- Giuseppetti, G.; Tadini, C. The crystal structure of 2O brittle mica: Anandite. Tscher. Miner. Petrog. 1972, 18, 169–184. [Google Scholar] [CrossRef]
- Ferraris, G.; Gula, A.; Ivaldi, G.; Nespolo, M.; Sokolova, E.; Uvarova, Y.; Khomyakov, A.P. First structure determination of an MDO-2O mica polytype associated with a 1M polytype. Eur. J. Mineral. 2001, 13, 1013–1023. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Guggenheim, S. Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. In Micas: Crystal Chemistry and Metamorphic Petrology; Mottana, A., Sassi, F.P., Thompson, J.B., Jr., Guggenheim, S., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 2002; Volume 46, pp. 1–97. [Google Scholar]
- Ferraris, G.; Ivaldi, G. Structural features of micas. In Micas: Crystal Chemistry and Metamorphic Petrology; Mottana, A., Sassi, F.P., Thompson, J.B., Jr., Guggenheim, S., Eds.; Reviews in Mineralogy and Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 2002; Volume 46, pp. 117–153. [Google Scholar]




| MgO | 0.12 | Rb2O | 0.01 |
| Al2O3 | 7.68 | Y2O3 | 0.15 |
| SiO2 | 74.62 | SnO2 | 0.01 |
| P2O5 | 0.04 | La2O3 | 0.76 |
| SO3 | 2.35 | CeO2 | 1.28 |
| K2O | 3.39 | Nd2O3 | 0.65 |
| CaO | 0.70 | PbO | 0.25 |
| TiO2 | 0.08 | Bi2O3 | 0.04 |
| Fe2O3 | 3.57 | LOI | 2.64 |
| CuO | 1.47 | Tot. | 99.8 |
| 1 | 2 | 3 | Mean | 4 | 5 | 6 | 7 | 8 | 9 | Mean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Syn | Syn | Syn | Syn | Mix | Bas | Bas | Bas | Bas | Bas | Bas | ||||||||||||
| F | 0.539 | (17) | 0.656 | (18) | 0.546 | (16) | 0.580 | (17) | 0.642 | (19) | 1.283 | (23) | 1.652 | (25) | 1.648 | (24) | 1.507 | (24) | 1.390 | (23) | 1.496 | (24) |
| Ca | 0.987 | (9) | 0.987 | (9) | 1.012 | (9) | 0.996 | (9) | 0.754 | (9) | 0.168 | (7) | 0.098 | (6) | 0.063 | (6) | 0.144 | (6) | 0.080 | (6) | 0.111 | (6) |
| Y | 0.061 | (5) | 0.082 | (5) | 0.079 | (5) | 0.074 | (5) | 0.094 | (5) | 0.114 | (7) | 0.136 | (7) | 0.109 | (7) | 0.104 | (7) | 0.109 | (7) | 0.114 | (7) |
| La | 0.273 | (6) | 0.263 | (6) | 0.255 | (6) | 0.264 | (6) | 0.322 | (7) | 0.475 | (9) | 0.496 | (9) | 0.520 | (9) | 0.496 | (9) | 0.505 | (9) | 0.498 | (9) |
| Ce | 0.431 | (7) | 0.422 | (7) | 0.421 | (7) | 0.425 | (7) | 0.468 | (8) | 0.704 | (10) | 0.751 | (11) | 0.666 | (10) | 0.659 | (10) | 0.662 | (10) | 0.688 | (10) |
| Pr | 0.036 | (5) | 0.046 | (5) | 0.038 | (4) | 0.040 | (5) | 0.046 | (5) | 0.075 | (7) | 0.063 | (7) | 0.083 | (7) | 0.087 | (6) | 0.075 | (7) | 0.077 | (7) |
| Nd | 0.181 | (6) | 0.163 | (5) | 0.158 | (5) | 0.167 | (5) | 0.237 | (6) | 0.303 | (8) | 0.319 | (8) | 0.348 | (8) | 0.328 | (8) | 0.355 | (8) | 0.331 | (8) |
| Sm | 0.017 | (5) | 0.021 | (5) | 0.020 | (4) | 0.019 | (5) | 0.024 | (5) | 0.033 | (7) | 0.036 | (7) | 0.038 | (7) | 0.037 | (7) | 0.035 | (7) | 0.036 | (7) |
| Gd | 0.009 | (5) | 0.016 | (5) | 0.018 | (5) | 0.014 | (5) | 0.023 | (5) | 0.024 | (7) | 0.006 | (7) | 0.021 | (7) | 0.012 | (7) | 0.006 | (7) | 0.014 | (7) |
| Pb | 0.004 | (3) | - | - | - | 0.033 | (3) | 0.104 | (4) | 0.094 | (4) | 0.152 | (5) | 0.134 | (4) | 0.174 | (5) | 0.132 | (4) | |||
| ΣREE | 1.009 | 1.013 | 0.988 | 1.003 | 1.214 | 1.728 | 1.808 | 1.785 | 1.722 | 1.746 | 1.758 | |||||||||||
| Ca/Ca + REE | 0.49 | 0.49 | 0.51 | 0.50 | 0.38 | 0.09 | 0.05 | 0.03 | 0.08 | 0.04 | 0.06 | |||||||||||
| 1(3) | 2(3) | 3(3) | 4(3) | 5(3) | 6(4) | 7(2) | 8 | 9 | 10 | 11 | 12 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Syn | Syn | Syn | Syn | Syn | Syn | Syn | Syn | Bas | Bas | Bas | Bas | |||||||||||||
| Ca | 1.05 | (4) | 0.98 | (6) | 1.08 | (7) | 0.96 | (3) | 1.10 | (3) | 1.01 | (7) | 1.00 | (5) | 1.04 | (7) | 0.18 | (7) | 0.14 | (4) | 0.19 | (5) | 0.20 | (2) |
| Y | 0.05 | (1) | 0.03 | (1) | 0.06 | (2) | 0.05 | (1) | 0.04 | (1) | 0.04 | (2) | 0.02 | (2) | 0.03 | (2) | 0.13 | (8) | 0.11 | (5) | 0.14 | (5) | 0.13 | (2) |
| La | 0.27 | (2) | 0.27 | (3) | 0.25 | (3) | 0.30 | (2) | 0.26 | (1) | 0.29 | (4) | 0.31 | (3) | 0.28 | (4) | 0.52 | (15) | 0.43 | (9) | 0.48 | (10) | 0.47 | (3) |
| Ce | 0.45 | (2) | 0.47 | (4) | 0.41 | (4) | 0.49 | (2) | 0.42 | (1) | 0.48 | (4) | 0.49 | (3) | 0.48 | (4) | 0.80 | (17) | 0.80 | (10) | 0.94 | (10) | 0.79 | (3) |
| Nd | 0.18 | (2) | 0.24 | (3) | 0.20 | (3) | 0.19 | (1) | 0.19 | (1) | 0.18 | (3) | 0.19 | (3) | 0.17 | (3) | 0.37 | (13) | 0.40 | (8) | 0.24 | (8) | 0.34 | (3) |
| Pb | - | - | - | - | - | - | - | - | - | 0.12 | (3) | - | 0.08 | (1) | ||||||||||
| Ca/Ca + REE | 0.53 | 0.49 | 0.54 | 0.48 | 0.55 | 0.51 | 0.50 | 0.52 | 0.09 | 0.07 | 0.10 | 0.10 | ||||||||||||
| [110] (1) | [110] (2) | [010] (3) | [110] (4) | [110] (5) | [010] (6) | |
|---|---|---|---|---|---|---|
| [110] | –a/6 | 0 | a/6 | a/6 | 0 | –a/6 |
| [100] | –b/6 | b/6 | b/3 | b/6 | –b/6 | –b/3 |
| [110] | 0 | a/6 | a/6 | 0 | –a/6 | –a/6 |
| [130] | –b/6 | –b/3 | –b/6 | b/6 | b/3 | b/6 |
| [010] | –a/6 | –a/6 | 0 | a/6 | a/6 | 0 |
| [130] | –b/3 | –b/6 | b/6 | b/3 | b/6 | –b/6 |
| Polytype Symbol | Vector Rotations | Symbolic Charts | Notable Examples | |
|---|---|---|---|---|
| Micas * | (Ca,REE)-Fluorcarbonates | |||
| 1M | 0° | 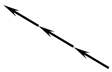 | Muscovite [32]; Phlogopite [33] Annite [33]; Celadonite [34] Polylithionite [35]; Clintonite [36] Norrishite [37]; Preiswerkite [38] Chromophyllite [39]; Siderophyllite [40] | Should not be possible |
| 2M1 | ± 120° | 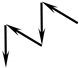 | Margarite [41]; Paragonite [42] Polylithionite [43]; Bityite [44] Kinoshitalite [45] | Should not be possible |
| 3T | + 120° |  | Paragonite [46] Polylithionite [47] Muscovite [48] | Should not be possible |
| 2M2 | ± 60° |  | Lepidolite [49]; Illite [50] Polylithionite [51]; Muscovite [52] Nanpingite [53]; Tobelite [54] | Synchysite-(Ce) [17] Parisite-(Ce) [18] |
| 2O | ± 180° |  | Anandite [55] Phlogopite [56] | Possible, few unit cells observed at the TEM scale (this work, Figure 11). |
| 6H | +60° |  | Possible, but never observed as ordered single crystal. | Possible, should have a “0 + − 0 − + 0” sequence on [110] HR images, not found yet. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitani, G. Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates. Minerals 2020, 10, 77. https://doi.org/10.3390/min10010077
Capitani G. Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates. Minerals. 2020; 10(1):77. https://doi.org/10.3390/min10010077
Chicago/Turabian StyleCapitani, Giancarlo. 2020. "Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates" Minerals 10, no. 1: 77. https://doi.org/10.3390/min10010077
APA StyleCapitani, G. (2020). Synchysite-(Ce) from Cinquevalli (Trento, Italy): Stacking Disorder and the Polytypism of (Ca,REE)-Fluorcarbonates. Minerals, 10(1), 77. https://doi.org/10.3390/min10010077




